Abstract
INTRODUCTION
There is no general consensus amongst orthopaedic surgeons on how best to manage the urinary tract and its complications after lower limb arthroplasty. This prospective audit investigates whether postoperative urinary retention can be predicted pre-operatively using the validated International Prostate Symptom Severity score (IPSS).
PATIENTS AND METHODS
A total of 182 patients undergoing lower limb arthroplasty under spinal anaesthetic were given the IPSS questionnaire to complete pre-operatively and an audit into numbers catheterised postoperatively was performed.
RESULTS
Overall, 69% of males and 39% of females required catheterisation. Following logistic regression analysis there was 0.85 predicted probability that males over 70 years would require catheterisation. The IPSS score was not useful in predicting retention in either sex at any age.
CONCLUSIONS
We propose that all males over 70 years undergoing this type of surgery should be catheterised pre-operatively and all other patients should be catheterised postoperatively with close monitoring of bladder volumes to prevent established urinary retention.
Keywords: Arthroplasty, Urinary retention, Spinal anaesthesia, Audit
The peri-operative management of the urinary tract and its complications following lower limb total joint arthroplasty is still a controversial topic. Orthopaedic surgeons are very aware of the potential for postoperative urinary problems including retention and urinary tract infection (UTI) to affect outcome of total joint replacement adversely but there is no general consensus about how best to manage or prevent them.1
It is well established that spinal anaesthesia is thought to predispose to urinary retention,2 although even this notion has been challenged.3 Previous studies2–7 have failed to demonstrate convincingly a method for predicting preoperatively which patients are likely to develop urinary retention postoperatively. In this study, we have scrutinised a cohort of patients undergoing primary lower limb arthroplasty under spinal anaesthesia, to establish whether postoperative urinary retention could have been predicted using a validated urinary tract symptom score – the International Prostate Symptom Severity Score (IPSS). This scoring system has been developed and validated by the American Urological Association (AUA) in the quantification of lower urinary tract symptoms in men8–10 and has been shown to correlate with obstructive symptoms in women.11
Patients and Methods
The study was carried out between March and October 2003 at the Department of Orthopaedics and Trauma, Derbyshire Royal Infirmary, UK. A total of 182 patients undergoing primary total hip or knee arthroplasty under spinal anaesthesia (either alone or in combination with general anaesthesia) were included in the study. Those patients having revision surgery, hip resurfacing, patients who required a catheter pre-operatively for whatever reason and any patient not having a spinal anaesthetic were excluded.
On admission, the patients were given the IPSS score to complete. This assessed lower urinary tract symptoms based on seven criteria (incomplete emptying, frequency, intermittency, urgency, weak stream, straining and nocturia). A score ranging from 0–5 was assigned to each symptom by the patient to assess increasing degree of severity. An overall total score ranging from 0–35 was thus produced. A subjective quality of life (QoL) score with respect to uri-nary symptoms (range, 0–6; delighted to terrible) was also obtained from each patient pre-operatively. Patients were also asked whether they had been catheterised on any previous occasion or had had any genito-urinary intervention. The diagnosis, operation, method of analgesia and need for catheterisation were recorded postoperatively for all patients who met the inclusion criteria. Catheterisation requirement was assessed by suitably trained ward nursing staff using a Bard™ bladder scanner within 2 h of arrival on the receiving ward from the recovery unit. This was repeated at convenient intervals until spontaneous voiding occurred or greater than 500 ml of residual urine in the bladder was detected. At this point, an indwelling Foley catheter was inserted transurethrally on the ward, using standard aseptic technique. Prophylactic gentamicin cover was used for every catheterisation episode. Catheter insertion was either performed by a member of the medical team or an appropriately trained member of the nursing staff.
Statistical analysis
To evaluate the effects of the symptom score on catheterisation, all variables were entered into a logistic regression with catheterisation (yes/no) as dependent variable. All first-order interactions were fitted along with main effects. The least statistically significant variables were removed in a hierarchical backwards procedure, and a nominal cut off of P = 0.1 was used to retain terms in the model.
Results
The characteristics of patients are shown in Table 1. Catheterisation was more common in men (69% of men were catheterised compared to 39% women), in knee operations (55% catheterised compared to 48% in hip operations), for patients who had had a previous catheter or genito-urinary intervention (64% compared to 48% of patients who had not), and for patients who had an epidural (52% catheterised compared to 51% of patients who had patient-controlled analgesia).
Table 1.
Characteristics of patients by catheterisation
| Characteristic | Catheter (n = 94) | No catheter (n = 88) |
|---|---|---|
| Mean age (SD), years | 69 (9.0) | 68 (11.1) |
| Sex, n (%) | ||
| Female | 42 (45%) | 65 (74%) |
| Male | 52 (55%) | 23 (26%) |
| Operation, n (%) | ||
| Hips | 43 (46%) | 46 (52%) |
| Knees | 51 (54%) | 41 (47%) |
| Previous catheter/genito-urinary intervention, n (%) | 29 (31%) | 16 (18%) |
| Analgesia | ||
| Epidural | 22 (24%) | 20 (23%) |
| Patient-controlled analgesia | 71 (76%) | 68 (77%) |
IPSS scores were slightly higher in patients who had been catheterised, and the distributions of scores were skewed in both groups with most patients scoring below 10. The median IPSS score for patients who were catheterised was 7 (range, 0–24), and for patients who were not catheterised the mean score was 5.5 (range, 0–23). QoL scores were also very similar in both groups, but their distribution was more symmetric. The median QoL score for patients who were catheterised was 1.9 (range, 0–5), and for patients who were not catheterised the median score was 1.8 (range, 0–4). Box-plots of IPSS scores and QoL scores showing medians and interquartile ranges are given in Figure 1.
Figure 1.
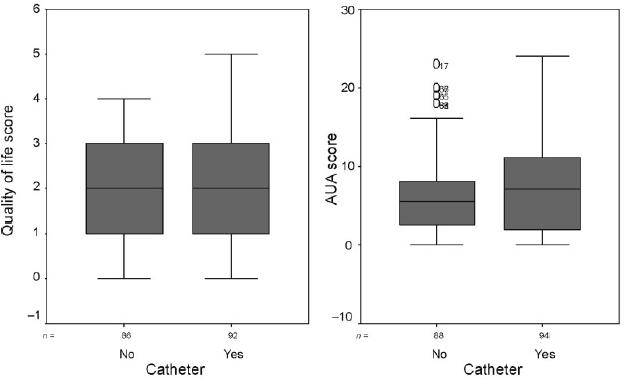
Medians and interquartile ranges for QoL score and IPSS score by catheterisation
The results of the logistic regression are shown in Table 2.
Table 2.
Estimated odds ratios from logistic regression
| Term in model | Estimated odds ratio | Standard error | P-value | 95% confidence interval | |
|---|---|---|---|---|---|
| Constant | 3.51 | ||||
| Sex | Female | 0.34 | 1.02 | 0.29 | (0.05, 2.49) |
| Male | |||||
| Age | ≤ 69 years | 0.12 | 1.01 | 0.03 | (0.02, 0.85) |
| > 69 years | |||||
| Sex* age | |||||
| Female* ≤ 69 years | 5.66 | 0.73 | 0.02 | (1.34, 23.87) | |
The patient factors that have a statistically significant effect on the odds of being catheterised are sex and age. Age has been dichotomised at the median value of 69 years in order to facilitate interpretation of the interaction between age and sex. The predicted probabilities of catheterisation given by the model are shown in Table 3.
Table 3.
Predicted probabilities of catheterisation from logistic regression model
| Age | Sex | Predicted probability of catheterisation |
|---|---|---|
| ≤ 69 years | Female | 0.44 |
| Male | 0.60 | |
| ≥ 70 years | Female | 0.35 |
| Male | 0.85 |
Discussion
An overall 51.6% of patients (men, n = 52 [69%]; women n = 42 [39%]) developed postoperative urinary retention requiring treatment, i.e. an indwelling catheter. It is also clear that the only statistically significant factors predictive of the development of urinary retention after lower limb arthroplasty in patients under spinal anaesthesia in this study are male gender and age. These findings are consistent with previous studies.3 Although IPSS scores do show a tendency to be higher in patients requiring catheterisation postoperatively, this correlation is not statistically significant. We, therefore, suggest that the American Urological Association IPSS score is not useful in the accurate prediction of those patients who are likely to develop postoperative retention and, as a result, require catheterisation.
Catheterisation rates in this study are higher than some others in the literature.19,21 Although there is no definite explanation for this, it is possible that the strict criteria used for catheterisation in this study, i.e. 500 ml urine in the bladder detected by regular scanning, produced higher rates than if the patients had been left longer to see if they could pass urine normally. This strategy would, however, have exposed patients to the possible risks associated with uri-nary retention (see later). The figure of 500 ml was chosen as the generally accepted volume of a full, but not over-distended, bladder.22
There is a substantial body of evidence to suggest that urinary tract sepsis can lead to bacteraemia and haematogenous seeding of infection to a prosthesis with the subsequent disastrous result of deep periprosthetic infection.12–15 Michelson et al.16 suggested that use of an in-dwelling Foley catheter during the first 24 h after surgery does not increase the incidence of UTIs, although it is generally accepted that the more prolonged presence of an in-dwelling catheter predisposes to the development of urinary tract infections.12,15,17,18 Urinary retention leads to bladder over-distension, the subsequent urine stasis predisposing to urinary tract infection.5 The increased pressures generated can also lead to the development of acute renal failure if left unchecked. Furthermore, there are significant effects on bladder dynamics in the short- and long-term, potentially culminating in bladder atonia5 and its sequelae.
In patients requiring catheterisation, optimal management would, therefore, be transurethral catheterisation in a sterile environment before the development of retention and before insertion of the prosthesis, i.e. pre-operatively in theatre/anaesthetic room.4,6,12,13,16,17 Some centres advocate this strategy in all patients, but this subjects a significant number of patients to the risks of catheterisation, all be them small, unnecessarily. It would, therefore, be ideal if we could predict pre-operatively those who were going to develop retention postoperatively and catheterise them in a planned manner pre-operatively. This has been attempted before with little success. Various pre-operative tests have been proposed (e.g. Waterhouse et al.19 proposed the ‘bottle test’ and Redfern et al.6 suggested flowmetry as being predictive); we suggest that neither are particularly practical in a busy pre-operative, pre-assessment clinic. Others have questioned their validity.2,7 The only consistent positive predictors to date, including this study's findings, are age and male gender.3
It can be seen from this study that men aged 70 years and over have a 0.85 probability of being catheterised postoperatively and that this is statistically significant. We, therefore, propose that males aged 70 years or over undergoing lower limb arthroplasty under spinal anaesthesia should be catheterised pre-operatively as they have a significant risk of requiring a catheter postoperatively. Although this appears a very narrow group to be concerned with, this does represent a significant number of patients in total every year. The remainder of patients (i.e. men under 70 years and all females) should be catheterised as required, having been monitored closely for the development of postoperative urinary retention.
Conclusions
Although numbers are relatively small, this study confirms with statistical significance that males aged 70 years or over undergoing spinal anaesthesia for lower limb arthroplasty are likely to require postoperative catheterisation and that the IPSS score is not a useful adjunct when trying to predict pre-operatively the need for postoperative catheterisation in these patients.
Acknowledgments
The authors would like to thank Ms Vicky Owen for her assistance with the statistical analysis of the data in this study.
References
- 1.Goldie A, Campbell A, Muirhead A. The urinary tract and joint replacement surgery: current opinion. J R Coll Surg Edinb. 1995;40:203–5. [PubMed] [Google Scholar]
- 2.Laws G, Ludowski P. Urinary catheterisation after primary total hip replacement. J Bone Joint Surg Br. 1989;71:721. [Google Scholar]
- 3.O'Riordan J, Hopkins P, Ravenscroft A, Stevens J. Patient-controlled analgesia and urinary retention following lower limb joint replacement. Eur J Anaesth. 2000;17:431–5. doi: 10.1046/j.1365-2346.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 4.Ritter M, Faris P, Keating E. Urinary tract catheterisation protocols following total joint arthroplasty. Orthopaedics. 1989;12:1085–7. doi: 10.3928/0147-7447-19890801-08. [DOI] [PubMed] [Google Scholar]
- 5.Oishi C, Williams V, Hanson P, Schneider J, Colwell C, Walker R. Perioperative bladder management after primary total hip arthroplasty. J Arthroplasty. 1995;10:732–6. doi: 10.1016/s0883-5403(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 6.Redfern T, Machin D, Parsons K, Owen R. Urinary retention in men after total hip arthroplasty. J Bone Joint Surg Am. 1986;68:1435–8. [PubMed] [Google Scholar]
- 7.Petersen M, Collins D, Selakovich W, Finkbeiner A. Postoperative urinary retention associated with total hip and total knee arthroplasties. Clin Orthop. 1991;269:102–8. [PubMed] [Google Scholar]
- 8.Barry M, Fowler F, Jr, O'Leary M, Bruskewitz R, Holtgrewe H, Mebust W, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 9.Barry M, Fowler F, Jr, O'Leary M, Bruskewitz R, Holtgrewe H, Mebust W. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol. 1992;148:1558–63. doi: 10.1016/s0022-5347(17)36967-7. [DOI] [PubMed] [Google Scholar]
- 10.Blaivas J. Urinary symptoms and symptom scores. J Urol. 1993;150:1714. doi: 10.1016/s0022-5347(17)35875-5. [DOI] [PubMed] [Google Scholar]
- 11.Groutz A, Blavias J, Fait G, Sassone A, Chaikin D, Gordon D. The significance of the American Urological Association symptom index score in the evaluation of women with bladder outlet obstruction. J Urol. 2000;163:207–11. [PubMed] [Google Scholar]
- 12.Martinez OV, Civetta JM, Anderson K, Roger S, Murtha M, Malinin TI. Bacteriuria in the catheterised surgical intensive care patient. Crit Care Med. 1986;14:188–91. doi: 10.1097/00003246-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Knight R, Pellegrini V. Bladder management after total joint arthroplasty. J Arthroplasty. 1996;11:882–8. doi: 10.1016/s0883-5403(96)80127-6. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M, Kelley K, Thornhill T. Infection as a complication of total knee replacement arthroplasty. J Bone Joint Surg Am. 1990;72:878–83. [PubMed] [Google Scholar]
- 15.Wroblewski B, Del Sel H. Urethral instrumentation and deep sepsis in total hip replacement. Clin Orthop. 1980;146:209–12. [PubMed] [Google Scholar]
- 16.Michelson J, Lotke P, Steinberg E. Urinary-bladder management after total joint replacement surgery. N Engl J Med. 1988;319:321–6. doi: 10.1056/NEJM198808113190601. [DOI] [PubMed] [Google Scholar]
- 17.Carpiniello V, Cendron M, Altman H, Malloy T, Booth R. Treatment of urinary complications in total joint replacement in elderly females. Urology. 1998;32:186–9. doi: 10.1016/0090-4295(88)90381-0. [DOI] [PubMed] [Google Scholar]
- 18.Donovan TL, Gordon RO, Nagel DA. Urinary infections in total hip arthroplasty. J Bone Joint Surg Am. 1976;58:1134–7. [PubMed] [Google Scholar]
- 19.Waterhouse N, Beaumont A, Murray K, Staniforth P, Stone M. Urinary retention after total hip replacement. J Bone Joint Surg Br. 1987;69:64–6. doi: 10.1302/0301-620X.69B1.2434510. [DOI] [PubMed] [Google Scholar]
- 20.Hozack W, Carpiniello V, Booth R. The effect of early bladder catheterisation on incidence of urinary complications after total joint replacement. Clin Orthop. 1988;231:79–82. [PubMed] [Google Scholar]
- 21.MacDowell A, Robinson A, Hill D, Villar R. Is epidural anaesthesia acceptable at total hip arthroplasty? A study of the rates of urinary catheterisation. J Bone Joint Surg Br. 2004;86:1115–7. doi: 10.1302/0301-620x.86b8.14240. [DOI] [PubMed] [Google Scholar]
- 22.Wein AJ, Stephenson TP, Mundy AR. Urodynamics: Principles, Practice and Application. 2nd edn. Edinburgh: Churchill-Livingston; 1994. p. 114. [Google Scholar]


