Abstract
INTRODUCTION
Nosocomial infection occurs in 2–9% of patients undergoing vascular surgery and can lead to death, amputation or require complex revision surgery. Neck ties, pagers, stethoscopes and Doppler probes have been shown to carry pathogens. We measured bacterial colonisation of Doppler probes on a vascular unit and audited the effect of staff education at reducing this contamination.
MATERIALS AND METHODS
Bacteriological culture swabs were taken from hand-held Doppler probes on the vascular surgical ward and clinic. There was no protocol for cleaning the Doppler probes, so manufacturers were contacted for their recommendations. The results of cultures were presented to nursing and medical staff, who were then asked to clean the probes with alcohol wipes after each use. After an interval of 1 week, bacteriological cultures from the same Doppler probes was repeated.
RESULTS
Fifty bacteriological cultures were performed from 10 Doppler probes over a 4-week period. Thirteen (26%) cultures were positive for diphtheroids, coliforms, coagulase-negative staphylococci and skin flora. After staff education, 42 further swabs were taken from the same probes; two positive cultures were obtained with scanty growth of skin flora (χ2 P < 0.05).
CONCLUSIONS
Staff education and simple cleaning significantly reduces the contamination of hand-held Doppler probes and may help prevent nosocomial infection.
Keywords: Cross infection, Equipment contamination, Doppler disinfection, Education, Vascular surgery
Nosocomial infections occur at a rate of 5–10 per 100 hospital admissions and cost the NHS £1 billion each year.1 Medical equipment and staff have been implicated as vectors for the transmission of pathogenic organisms. Previous studies have shown contamination of hospital pagers,2 stethoscopes,3 otoscopes,4 bow ties5 and Doppler probes.6
Hand-held Doppler probes are used by doctors and nurses to assess arterial and venous disease. Infections in vascular surgery are potentially serious complications and range from superficial wound infection to deep infections of conventional or endovascular grafts.7,8 Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a major problem in vascular practice9,10 and is likely to become more prevalent. A report of 574 vascular surgical patients in 2001 showed a 9% rate of clinical MRSA infection which resulted in a major increase in mortality and morbidity.11 Nosocomial infections are most commonly caused by methicillin-susceptible S. aureus.12 Other organisms such as Escherichia coli, Enterococcus spp., Staphylococcus epidermidis, Streptococcus spp., Pseudomonas spp. and Candida spp. are also common in surgical patients.13
The aim of this study was to assess contamination of Doppler probes used in the surgical out-patients department and vascular surgical ward of a busy hospital. Using the results of this initial study, we educated staff regarding the need to decontaminate Doppler probes as well as the cleaning methods of Doppler probes recommended by the manufacturers. Following this staff education, we re-audited contamination rates.
Materials and Method
Cultures from the probes of 10 Doppler machines were taken by a single investigator (EJW). Wound swabs were moistened with a small amount of sterile saline and wiped over the area of the Doppler probe that would be expected to come into contact with the patient's skin. Swabs were sent immediately to the microbiology laboratory where direct cultures were performed using routine microbiological media; sheep blood agar incubated aerobically and anaerobically, and CLED agar. This process was repeated at 5 random intervals over a 4-week period on all 10 Doppler probes used by vascular surgical teams. In total, 50 swabs were taken for culture.
The probes were used in the out-patients department to investigate arterial insufficiency and superficial venous incompetence. The same Doppler probes were used to aid assessment of vascular in-patients and in-patient referrals from non-vascular teams. The probes were always used with conducting gel.
Manufacturers (SciMed Ltd, Bristol, UK and Huntleigh Healthcare Ltd, Luton, Bedfordshire, UK) were contacted for advice on disinfection methods. Nursing and medical staff were educated regarding bacterial contamination of the probes and staff were asked to clean the Doppler probes according to the manufacturers' advice, with alcohol impregnated wipes after use. A supply of wipes (‘Tuffie’ hard surface disinfectant wipes; Vernacare, Bolton, Lancashire UK) was provided on the ward and in the out-patients department.
One week later, 42 further cultures were taken from the same Doppler probes in the same locations. The results of cultures before and after staff education were compared.
Results
Fifty direct cultures were obtained from 10 different Doppler probes over a 4-week period. There were 13 positive results. The organisms identified included coliforms, diphtheroids and coagulase negative staphylococci (Table 1).
Table 1.
Positive bacteriological cultures from Doppler probes before and after staff education
| Culture | Before staff education (n = 50) | After staff education (n = 42) | χ2P-value |
|---|---|---|---|
| Skin flora only | 5 | 2 | 0.44 |
| Skin flora and coliforms | 1 | 0 | 1 |
| Diphtheroids and coagulase-negative staphylococci | 1 | 0 | 1 |
| Coagulase-negative staphylococci only | 6 | 0 | 0.03 |
| Total | 13 | 2 | 0.009 |
Following staff education about contamination and decontamination of Doppler probes, repeat direct cultures were obtained and only two showed a scanty growth of skin flora (χ2 P < 0.05; Fig. 1). The only significant difference was in reduction of coagulase-negative staphylococci (χ2 P < 0.05).
Figure 1.
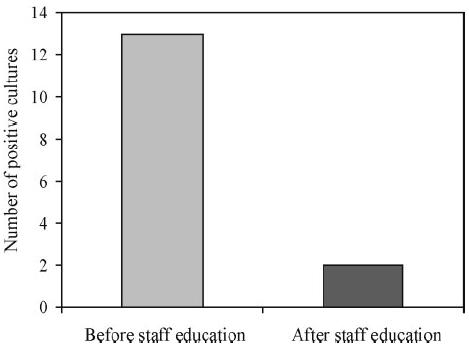
Number of positive bacteriological culture before and after staff education.
Discussion
Direct culture of swabs taken from the Doppler probes used on the vascular unit revealed bacterial contamination in 26% of cases. Although bacteria of differing pathogenicity were isolated, with coliforms being the most and diphtheroids the least pathogenic, all organisms were potentially dangerous. After staff education about contamination of Doppler probes and recommended cleaning methods, the level of contamination fell to less than 5%, which was highly significant (P < 0.05). Coagulase-negative staphylococcus was the most common bacterial pathogen cultured from the swabs, and showed the greatest reduction after staff education (P < 0.05).
The limitations of this study include the method of collecting bacteriological swabs and the timing of the re-audit. Swabs were taken from a selection of different probes in two locations over a period of 2 months. To improve the scientific validity, swabs should have been taken from matched samples, in batches, before and after staff education. The samples should have been taken at the same time on the same days with matched time intervals between obtaining the swabs. Further swabs taken some months subsequent to staff education would have helped confirm whether the impact of staff education was long-lasting. This study did not set out to analyse the overall surgical infection rate on the unit nor did it investigate whether decontamination of Doppler probes affects this rate.
Doppler probes were used with the same conducting gel throughout this study. The gel may have influenced bacterial culture, but the current study was not designed to investigate this. Disposable rubber sheaths have been suggested as a method to reduce contamination but were not available in our hospital and arguably reduce the accuracy of Doppler assessment.
The only previous report that involved bacteriological examination of Doppler probes6 did not document how many different probes were tested or over what time period bacteriological samples were taken. A total of 21 culture results were included of which 38% were positive for bacterial growth. The same study observed that 95% of doctors failed to clean the Doppler probe but did not go on to audit the impact of staff education on the rates of contamination.
Infections that complicate vascular surgery include surgical wound infections, prosthetic graft infections and infected vascular ulcers. The rate and the type of infection varies between different units. Published rates of prosthetic graft infection range from 0.5% for abdominal aortic grafts to 6% for infra-inguinal grafts involving groin dissection.14 Surgical wound infection rates range from 1.8% to 5.1%15,16 but some groups report rates between 6–19%.17 It is recognised that reported rates of infection are dependent on who is reporting the infections and how hard infections are looked for. All infective complications are potentially serious and notoriously difficult or impossible to eradicate in vascular surgical patients.
Conclusions
It is important to identify and implement measures that reduce the risk of nosocomial infection. We have shown that simple cleaning of hand-held Doppler probes with alcohol wipes significantly reduces bacterial contamination of these probes and, therefore, may reduce the rate of infection on vascular surgical units.
References
- 1.Centers for Disease Control and Prevention, Hospital Infections Program. National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986–April 1996; a report from the NNIS system. Am J Infect Control. 1996;24:380–8. [PubMed] [Google Scholar]
- 2.Singh D, Kaur H, Gardner WG, Treen LB. Bacterial contamination of hospital pagers. Infect Control Hosp Epidemiol. 2002;23:274–6. doi: 10.1086/502048. [DOI] [PubMed] [Google Scholar]
- 3.Bernard L, Kereveur A, Durand D, Gonot J, Goldstein F, Mainardi JL, et al. Bacterial contamination of hospital physicians' stethoscopes. Infect Control Hosp Epidemiol. 1999;20:626–8. doi: 10.1086/501686. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HA, Amir J, Matalon A, Mayan R, Beni S, Barzilai A. Stethoscopes and otoscopes – a potential vector of infection? Fam Pract. 1997;14:446–9. doi: 10.1093/fampra/14.6.446. [DOI] [PubMed] [Google Scholar]
- 5.Biljan MM, Hart CA, Sunderland D, Manasse PR, Kingsland CR. Multicentre randomised double blind crossover trial on contamination of conventional ties and bow ties in routine obstetric and gynaecological practice. BMJ. 1993;307:1582–4. doi: 10.1136/bmj.307.6919.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibria SM, Kerr KG, Dave J, Gough MJ, Homer-Vanniasinkam S, Mavor AI. Bacterial colonization of Doppler probes on vascular surgical wards. Eur J Vasc Endovasc Surg. 2002;23:241–3. doi: 10.1053/ejvs.2001.1552. [DOI] [PubMed] [Google Scholar]
- 7.Ryan SV, Calligaro KD, Scharff J, Dougherty MJ. Management of infected prosthetic dialysis arteriovenous grafts. J Vasc Surg. 2004;39:73–8. doi: 10.1016/j.jvs.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Fiorani P, Speziale F, Calisti A, Misuraca M, Zaccagnini D, Rizzo L, et al. Endovascular graft infection: preliminary results of an international enquiry. J Endovasc Ther. 2003;10:919–27. doi: 10.1177/152660280301000512. [DOI] [PubMed] [Google Scholar]
- 9.Naylor AR, Hayes PD, Darke S. A prospective audit of complex wound and graft infections in Great Britain and Ireland: the emergence of MRSA. Eur J Vasc Endovasc Surg. 2001;21:289–94. doi: 10.1053/ejvs.2001.1311. [DOI] [PubMed] [Google Scholar]
- 10.Earnshaw JJ. Methicillin-resistant Staphylococcus aureus: vascular surgeons should fight back. Eur J Vasc Endovasc Surg. 2002;24:283–6. doi: 10.1053/ejvs.2002.1705. [DOI] [PubMed] [Google Scholar]
- 11.Murphy GJ, Pararajasingam R, Nasim A, Dennis MJ, Sayers RD. Methicillin-resistant Staphylococcus aureus infection in vascular surgical patients. Ann R Coll Surg Engl. 2001;83:158–63. [PMC free article] [PubMed] [Google Scholar]
- 12.Marroni M, Fiorio M, Cao P, Parlani G, Morosi S, Stagni G. Nosocomial infections in vascular surgery: 1-year surveillance. Recenti Prog Med. 2003;94:430–3. [PubMed] [Google Scholar]
- 13.Valero LF, Saenz MC. The etiology of nosocomial infection in surgery: comparison of 2 years (1988 and 1996) Enferm Infecc Microbiol Clin. 1998;16:79–82. [PubMed] [Google Scholar]
- 14.Chiesa R, Astore D, Frigerio S, Garriboli L, Piccolo G, Castellano R, et al. Vascular prosthetic graft infection: epidemiology, bacteriology, pathogenesis and treatment. Acta Chir Belg. 2002;102:238–47. doi: 10.1080/00015458.2002.11679305. [DOI] [PubMed] [Google Scholar]
- 15.Murphy PG, Tadros E, Cross S, Hehir D, Burke PE, Kent P, et al. Skin closure and the incidence of groin wound infection: a prospective study. Ann Vasc Surg. 1995;9:480–2. doi: 10.1007/BF02143863. [DOI] [PubMed] [Google Scholar]
- 16.Cruse PGE, Foord R. A 10 year prospective study of 62,939 surgical wounds. Surg Clin North Am. 1980;69:27–40. doi: 10.1016/s0039-6109(16)42031-1. [DOI] [PubMed] [Google Scholar]
- 17.Bunt TJ. Synthetic vascular graft infections. Surgery. 1983;93:733–46. [PubMed] [Google Scholar]


