Abstract
INTRODUCTION
Insulinoma is a rare, but curable, endocrine tumour. The ability to localise the tumour accurately before or during surgery is an important factor in the management of these elusive lesions, which has been extensively debated. We have reviewed our experience of these lesions to establish the role of localisation tests.
PATIENTS AND METHODS
The medical records of 20 consecutive patients who had surgery for sporadic insulinomas since 1985 at this institution were retrospectively reviewed. All the patients had a definite biochemical diagnosis of endogenous hyperinsulinism. Results of pre-operative and intra-operative localisation tests were compared with the final outcome.
RESULTS
Of the 20 patients with sporadic insulinomas reviewed, 17 patients (85%) had multiple pre-operative localising investigations. Overall accuracy of pre-operative localisation tests was 33%. Non-invasive pre-operative localisation tests (ultrasonography, CT, MRI) had a combined localisation rate of 25% with MRI having the highest sensitivity of 71%. Invasive tests (angiography, transhepatic portal venous sampling [THPVS], endoscopic ultrasound) detected 48% of lesions with THPVS being most sensitive (67%). THPVS was particularly helpful in localising lesions before re-operation. Intra-operative inspection and palpation localised the lesions correctly in 91% and intra-operative ultrasound in 93% of cases. All 5 occult tumours (indeterminate anatomical site before operation) were palpable at surgery and four of these were also correctly identified by intra-operative ultrasound. Site and size of tumour correlated poorly with pre-operative localisation. Operative procedure did not influence outcome with three patients needing re-operation. One patient died (5% mortality) and 9 patients (45%) had complications. Normoglycaemia has been obtained in all but one patient.
CONCLUSIONS
Insulinomas can be readily localised by systematic operative exploration. Non-invasive pre-operative investigations (ultrasonography/MRI) may help identify the location of tumour to determine the appropriate surgical procedure. Invasive pre-operative localisation tests like angiography and THPVS may be a valuable adjunct for re-operations. This also helps reduce the costs.
Keywords: Insulinoma, Whipple's triad, Localisation, Surgical exploration
Insulinoma is a rare and elusive, but the most common, curable endocrine tumour of the pancreas. The incidence is estimated at 4 cases per million-person years.1 The ability to localise the tumour accurately before or during surgery is an important factor in the management of these lesions. In the event of failed localisation, a blind pancreatic resection is no longer recommended. The development of sensitive radioimmunoassays to detect endogenous hyperinsulinaemia resulted in extensive investigations to localise the tumour preoperatively, in the belief that this would reduce morbidity from pancreatic exploration. These pre-operative tests included ultrasound scans (USS), computerised tomography (CT), magnetic resonance imaging (MRI), selective angiography, transhepatic portal venous sampling (THPVS), endoscopic ultrasound (EUS), octreotide scans and calcium stimulation arteriography. There is a wide variation in the sensitivity of these tests and there is no clear consensus in the choice of these tests even amongst enthusiasts.2,3 Some believe that meticulous intra-operative exploration combined with intra-operative ultrasound (IOUS) has a higher sensitivity than pre-operative localisation tests.4,5 We have reviewed our experience of these lesions to assess if pre-operative localisation tests are necessary and to formulate a rational management strategy for these lesions.
Patients and Methods
The medical records of 20 consecutive patients who had surgery for sporadic insulinomas, since 1985 at this institution, by a single surgeon, were retrospectively reviewed. Hypoglycaemia was either spontaneous or confirmed during a supervised fasting test. All patients met the criteria for Whipple's triad (symptoms and signs of hypoglycaemia during a fast, blood glucose of less than 2.5 mmol/l during this episode and relief of symptoms by ingestion of glucose). Definite biochemical diagnosis of endogenous hyperinsulinism (raised serum insulin and C-peptide levels) was obtained in all patients. Results of pre-operative and intra-operative localisation tests were compared with the final outcome (lesions correctly identified and resected, postoperative morbidity and reversal to normoglycaemia).
Pre-operative localisation tests: non-invasive tests
USS
This was performed using a 3.5 MHz sector probe (Acuson XP10, Mountainview, USA).
CT SCAN
An initial unenhanced scan using 10-mm sections followed by dynamic 5-mm contiguous sections after intravenous contrast injection was used. A spiral CT scanner has been used since 1999 (Sytec3000, International General Electric Medical Systems, Slough, UK).
MRI
Images were obtained by specifically tailored sequences with dynamic gadolinium enhancement and fat suppression technique on a high-field machine operating at 1.5 T (Sigma advantage, International General Electric Medical systems).
Pre-operative localisation tests: invasive tests
SELECTIVE ANGIOGRAPHY
Transfemoral selective coeliac axis and superior mesenteric angiography was performed and images obtained using either digital subtraction or conventional angiography (Phillips, Eindhoven, The Netherlands).
THPVS
Following transhepatic catheterisation of portal, superior mesenteric and splenic veins, blood was sampled for insulin levels and the location of each specimen marked. Localisation was made on the basis of the area of highest hormone gradient to one of the three regions of the pancreas (tail, body/neck and head/uncinate process).
EUS, OCTREOTIDE SCAN, CALCIUM STIMULATION TESTS
Only one patient underwent each of these three tests in our study.
Intra-operative localisation tests
A single surgeon performed all operations. Mobilisation and careful bidigital palpation of the entire gland was the mainstay in all explorations. IOUS with a 7 MHz finger probe was used regularly since its introduction into our practice. A clearly demarcated hypo-echoic nodule with a more echogenic surrounding normal pancreas was considered positive for an insulinoma. Frozen section biopsies were routinely employed to confirm the diagnosis.
Results
Twenty patients with twenty-one sporadic insulinomas operated by a single surgeon during the study period were reviewed. There were 12 female and 8 male patients and the mean age was 49 years (range, 28–85 years). The mean duration of neuroglycopenic symptoms was 19 months (range, 0–84 months). All patients satisfied the criteria for Whipple's triad. The mean fasting blood glucose was 1.69 mmol/l (range, 1.0–2.1 mmol/l). The mean insulin levels were 43.2 μU/ml (normal levels, 2–14 μU/ml) and the mean C-peptide levels were 2.2 nmol/l (normal levels, 0.18–0.63 nmol/l).
Of the patients, 85% had multiple pre-operative investigations with an average of three investigations per patient (range, 1–5). Overall accuracy of pre-operative localisation tests was poor (Tables 1–3). Non-invasive tests had a combined localisation rate of 25% only (PPV, 67%). MRI had the highest accuracy whilst USS and CT scan were poor at accurate localisation. Invasive tests on the other hand had an accuracy rate of 48% (PPV, 73%). TVPHS had the most sensitive pre-operative localisation rate at 67%. It was also particularly helpful in localising the lesions before re-exploration in two patients.
Table 1.
Pre-operative non-invasive localisation tests
| Modality | Sensitivity (%) | Accuracy (%) | PPV (%) |
|---|---|---|---|
| USS | 9 | 15 | 50 |
| CT | 12 | 17 | 67 |
| MRI | 71 | 56 | 71 |
| Overall | 24 | 25 | 67 |
| Raw data (n) | ||||
|---|---|---|---|---|
| USS | CT | MRI | Overall | |
| Total | 13 | 18 | 9 | 40 |
| True positive | 1 | 2 | 5 | 8 |
| False positive | 1 | 1 | 2 | 4 |
| True negative | 1 | 1 | 0 | 2 |
| False negative | 10 | 14 | 2 | 26 |
PPV = Positive predictive value
Table 3.
Overall pre-operative localisation rates
| Modality | Sensitivity (%) | Accuracy (%) | PPV (%) |
|---|---|---|---|
| All tests | 36 | 33 | 70 |
| Raw data (n) | |
|---|---|
| Overall | |
| Total | 63 |
| True positive | 19 |
| False positive | 8 |
| True negative | 2 |
| False negative | 34 |
PPV = Positive predictive value
Table 2.
Pre-operative invasive localisation tests
| Modality | Sensitivity (%) | Accuracy (%) | PPV (%) |
|---|---|---|---|
| Angiography | 50 | 44 | 78 |
| THPVS | 67 | 40 | 50 |
| Overall | 65 | 48 | 73 |
| Raw data (n) | ||||
|---|---|---|---|---|
| Angiography | THPVS | Others | Overall | |
| Total | 16 | 5 | 2 | 23 |
| True positive | 7 | 2 | 2 | 11 |
| False positive | 2 | 2 | 0 | 4 |
| True negative | 0 | 0 | 0 | 0 |
| False negative | 7 | 1 | 0 | 8 |
PPV = Positive predictive value
Intra-operative localisation tests were 92% accurate with a positive predictive value of 94% (Table 4). Amongst the intra-operative localisation tests, intra-operative inspection and palpation had an accuracy comparable to IOUS (74% of lesions could be identified on inspection alone). While inspection and palpation had a sensitivity of 95%, IOUS had a sensitivity of 100%.
Table 4.
Intra-operative localisation tests
| Modality | Sensitivity (%) | Accuracy (%) | PPV (%) |
|---|---|---|---|
| Inspection & palpation | 95 | 91 | 95 |
| IOUS | 100 | 93 | 92 |
| Overall | 97 | 92 | 94 |
| Raw data (n) | |||
|---|---|---|---|
| Inspection & palpation | IOUS | Overall | |
| Total | 22 | 14 | 36 |
| True positive | 19 | 12 | 31 |
| False positive | 1 | 1 | 2 |
| True negative | 1 | 1 | 2 |
| False negative | 1 | 0 | 1 |
PPV = Positive predictive value
All 5 occult tumours (indeterminate anatomical site before operation) were palpable at operation and four of them had IOUS, which correctly localised these lesions. Four patients with a positive pre-operative localisation needed a prolonged search at operation and IOUS was particularly helpful in localising these lesions in three patients (Table 5).
Table 5.
Occult tumours in five patients
| Patient no. | Inspection | Palpation | IOUS | Size (cm) | Site |
|---|---|---|---|---|---|
| 5 | + | + | + | 1.5 | Body |
| 6 | − | + | + | 1.0 | Tail |
| 9 | + | + | + | 1.5 | Body |
| 17 | + | + | NP | 1.3 | Tail |
| 20 | − | + | + | 1.0 | Body |
NP, not performed.
The operative procedure employed was dependent on the location of the tumour: 24% of the lesions were in the head and neck, uncinate process or liver (one patient with metastatic malignant insulinoma); while 76% were in the body or tail of pancreas. A total of 17 enucleations (including enucleation of two liver metastases), 4 distal pancreatectomies and two explorations of pancreas were performed. Three patients required repeat operations for persistent hypoglycaemia. Two were successfully treated. The first patient had a distal pancreatectomy in another hospital and was referred to our institution for persistent hypoglycaemia. The lesion was successfully localised pre-operatively (TVPHS) and the lesion enucleated from the head of the pancreas with complete resolution of symptoms. The second patient also had an enucleation. This patient was, however, persistently hypoglycaemic; MRI and TVPHS showed a further lesion in the tail of pancreas which was successfully removed with a distal pancreatectomy. This patient had microscopic multifocal lesions on histology and remains well 10 years following the resection.
The third patient had a positive pre-operative localisation but the lesion could not be detected by intra-operative localisation tests. On the second exploration, one lesion, which felt like an insulinoma on intra-operative localisation tests with good correlation to pre-operative localisation, was removed. This, however, proved to be normal pancreatic tissue and this is the one false positive that we have had in this study. This patient has recently undergone a third exploration at a different hospital. The lesion was found in the head of the pancreas and was removed by pancreaticoduodenectomy.
The lesions ranged from 0.5–2.2 cm in size; 90% of the lesions removed were less than 2 cm and 52% were less than 1 cm. All the five occult tumours were greater than 1 cm. Size correlated poorly with the pre-operative localisation. CT and MRI were more sensitive (60%) for lesions greater than 1 cm.
The majority of the lesions removed were adenomata. There was one islet cell hyperplasia and one patient had a malignant insulinoma with metastases.
The overall mortality was 5% and the morbidity 45% (9 patients). The only patient who died in this study was 80 years old and had a postoperative myocardial infarction. Five of the nine patients who had a major complication had a positive pre-operative localisation. The types of operations performed on these patients and other complications are outlined in Table 6. Three patients needed re-exploration for complications. Normoglycaemia was obtained in all but one patient.
Table 6.
Major complications in 9 patients
| Complications | Pre-operative localisation of insulinoma | Operative procedure | Re-intervention |
|---|---|---|---|
| Pancreatic fistula | CT | Enucleation | No |
| Pancreatic fistula | CT/MRI/angiography | Exploration of pancreas | No |
| Pancreatic fistula | CT/MRI/angiography | Enucleation | No |
| Haemorrhage | CT/MRI/angiography | Enucleation | No |
| Haemorrhage | Nil | Distal pancreatectomy | Yes |
| Subphrenic abscess | CT/MRI/angiography | Enucleation | No |
| Peritonitis | CT | Enucleation | Yes |
| Obstruction | CT | Enucleation | Yes |
| Venous thrombosis | Angiography | Enucleation | No |
Discussion
Insulinomas are predominantly solitary (90%), small (65% are smaller than 1.5 cm), benign (90%) and intrapancreatic (> 99%) in location.1,6,7 Multiple or diffuse islet cell hyperplasia and malignant tumours are responsible for the remaining 10% of patients with endogenous hyperinsulinism.1,2 Surgical excision is the treatment of choice1 and rates of surgical cure for benign insulinomas vary between 77% and 98%.2 At our institution, 95% of the patients were successfully treated by surgery.
Before the advent of insulin assays, failed surgical treatment was attributed to inaccurate diagnosis.2 There has been considerable debate regarding the need and type of localisation tests for these lesions pre-operatively and their intra-operative management.2,3,4,5,7,12,14
The results of non-invasive imaging techniques have generally been discouraging. Sensitivities ranging from 9–63% for USS7 and an accuracy of 11–50% for CT scans have been reported.2 In our series, USS had an accuracy of 15% and CT scan 17%; MRI, however, was 56% accurate. This was also reported in a previous study from this institution.10 The overall accuracy of non-invasive tests in our study was only 25% (PPV, 67%) and this compares with other studies.9
The accuracy of selective angiography was 44% and THPVS was 40%. This was similar to the University of Michigan series.2 THPVS has had successful localisation rates ranging between 25–100%.5,8,12 Some authors suggest performing this study selectively prior to surgery.2,12 Vinik et al.12 reported 100% success rate with this technique, but also point out that 81% of tumours would have been correctly identified by intra-operative localisation methods. Others have suggested that it should be performed only prior to re-explorations for failed initial intra-operative localisation.5,14 In this study, it was very helpful in localising the tumour in two patients who had recurrent hyperglycaemia following initial successful enucleation. Sensitivity rates of 57–80% have been reported for endoscopic ultrasound, although in a study by Schumacher et al.11 only 37% of lesions in the tail of the gland could be localised compared to 83% of lesions in the head. Calcium arteriography has been shown to have localisation rates of 67–100%. The greater consistency of pancreatic arterial supply compared to venous drainage gives it a greater predictability for a positive result.7,13 Our experience with endoscopic ultrasound and calcium arteriography was very limited and, as most of the lesions in our study were in the distal half of pancreas, endoscopic ultrasound would have had only a modest sensitivity. It would have been helpful in the one patient in whom the tumour was not found, when subsequently the lesion was discovered in the head of the gland adjacent to the duodenum. Certainly, the ultrasonographic appearances of the endocrine tumours of the pancreas are very specific.
A successful exploration can be achieved by complete mobilisation of the gland, coupled with systematic bidigital palpation and the use of intra-operative ultrasound. These techniques had a high sensitivity and accuracy in this study. It was noteworthy that two-thirds of these lesions were identified on inspection alone. Sensitivity was 100% when inspection and palpation was combined with IOUS.
Our overall complication rate of 45% is higher than that of 32.5% in a multicentre review by Rothmund et al.8 but patients developing pancreatic fistulae (15%) and haemorrhage (10%) were the same as other series.2,4,6,7 The reintervention rates and mortality was, however, similar to other reported series.2,4,6 Correct pre-operative localisation did not reduce the complications as shown in our study and other reports.7
In a world-wide survey of 22 centres, surgical exploration alone was able to detect the tumour in 95% of 375 patients at first operation. Following re-operation, a mere 0.5% of insulinomas remained undetected.8 Van Heerden et al.4 reported 65 patients with insulinomas from the Mayo Clinic, 20 of whom had a negative USS and CT scan. These 20 patients had 38 negative pre-operative imaging tests at a cost of $24,000 and yet all the tumours were resected at laparotomy.4 Our study confirms that systematic inspection and palpation and intra-operative ultrasound helps identify these lesions readily. Intra-operative ultrasound is particularly helpful in delineating the spatial relationship of the tumour with the pancreatic duct and major vessels and helps the surgeon to choose between resection and enucleation. Pre-operative, non-invasive localisation tests may help decide the type of surgical procedure that will be required and thus help in obtaining appropriate consent. Angiography and THPVS would be useful perhaps only prior to re-explorations for missed lesions.
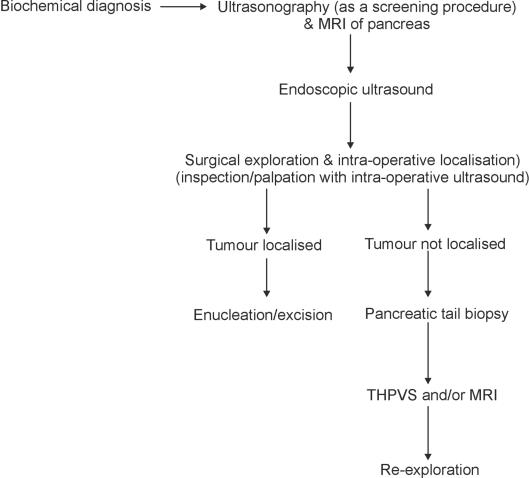
This experience along with support from literature allows us to recommend a simplified localising approach (Fig. 1). In the authors' view, there is no role for blind pancreatic resection. In the rare failures of localisation, a small pancreatic tail resection can help diagnose islet cell hyperplasia without precluding further segmental resection.14
Figure 1.
Management scheme for insulinomas.
References
- 1.Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma: incidence, recurrence and long-term survival of patients: a 60 year study. Mayo Clin Proc. 1991;66:711–9. doi: 10.1016/s0025-6196(12)62083-7. [DOI] [PubMed] [Google Scholar]
- 2.Pasieka JL, McLeod MK, Thompson NW, Burney RE. Surgical approach to insulinomas: assessing the need for preoperative localization. Arch Surg. 1992;12:442–7. doi: 10.1001/archsurg.1992.01420040088015. [DOI] [PubMed] [Google Scholar]
- 3.Bottger TC, Junginger MD. Is preoperative radiographic localization of islet cell tumours in patients with insulinoma necessary? World J Surg. 1993;17:427–32. doi: 10.1007/BF01655099. [DOI] [PubMed] [Google Scholar]
- 4.Van Heerden JA, Grant CS, Czako PF, Service FJ, Charboneau JW. Occult functioning insulinomas: which localizing studies are indicated? Surgery. 1992;112:1010–4. [PubMed] [Google Scholar]
- 5.Daggett PR, Goodburn EA, Kurtz AB, Le Quense LP, Morris DV, Nabarro JDN. Is preoperative localization of insulinomas necessary? Lancet. 1981;1:483–6. doi: 10.1016/s0140-6736(81)91859-6. [DOI] [PubMed] [Google Scholar]
- 6.Stefanini P, Carboni M, Patrassi N, Basoli A. Beta islet cell tumours of the pancreas: results of a study of 1067 cases. Surgery. 1974;75:597–609. [PubMed] [Google Scholar]
- 7.Lo C-Y, Lam K-Y, Kung AWC, Lam KSL, Tung PHM, Fan S-T. Pancreatic insulinomas: a 15 year experience. Arch Surg. 1997;132:926–30. doi: 10.1001/archsurg.1997.01430320128023. [DOI] [PubMed] [Google Scholar]
- 8.Rothmund M, Angelini L, Brunt LM, Farndon JR, Geelhoed G, Grama D, et al. Surgery for benign insulinoma: an international review. World J Surg. 1990;14:393–9. doi: 10.1007/BF01658536. [DOI] [PubMed] [Google Scholar]
- 9.Angeli E, Vanzulli A, Castrucci M, Venturini M, Sironi S, Zerbi A, et al. Value of abdominal sonography and MR imaging at 0.5 T in the preoperative detection of pancreatic insulinoma: a comparison with dynamic CT and angiography. Abdom Imaging. 1997;22:295–303. doi: 10.1007/s002619900193. [DOI] [PubMed] [Google Scholar]
- 10.Moore NR, Rogers CE, Britton BJ. Magnetic resonance imaging of endocrine tumours of pancreas. Br J Radiol. 1995;68:341–7. doi: 10.1259/0007-1285-68-808-341. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher B, Lubke HJ, Frieling T, Strohmeyer G, Starke AAR. Prospective study on the detection of insulinomas by endoscopic ultrasonography. Endoscopy. 1996;28:273–6. doi: 10.1055/s-2007-1005452. [DOI] [PubMed] [Google Scholar]
- 12.Vinik AI, Delbridge L, Moattari R, Cho K, Thomson N. Transhepatic portal vein catheterisation for localization of insulinomas: a ten year experience. Surgery. 1991;109:1–11. [PubMed] [Google Scholar]
- 13.Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991;178:237–41. doi: 10.1148/radiology.178.1.1984311. [DOI] [PubMed] [Google Scholar]
- 14.Pedrazzoli S, Pasquali C, Alfano D'Andrea A. Surgical treatment of insulinoma. Br J Surg. 1994;81:672–6. doi: 10.1002/bjs.1800810513. [DOI] [PubMed] [Google Scholar]