Abstract
In the late 1980s, the finding that the dentate gyrus contains more granule cells in the male than in the female of certain mouse strains provided the first indication that the dentate gyrus is a significant target for the effects of sex steroids during development. Gonadal hormones also play a crucial role in shaping the function and morphology of the adult brain. Besides reproduction-related processes, sex steroids participate in higher brain operations such as cognition and mood, in which the hippocampus is a critical mediator. Being part of the hippocampal formation, the dentate gyrus is naturally involved in these mechanisms and as such, this structure is also a critical target for the activational effects of sex steroids. These activational effects are the results of three major types of steroid-mediated actions. Sex steroids modulate the function of dentate neurons under normal conditions. In addition, recent research suggests that hormone-induced cellular plasticity may play a larger role than previously thought, particularly in the dentate gyrus. Specifically, the regulation of dentate gyrus neurogenesis and synaptic remodeling by sex steroids received increasing attention lately. Finally, the dentate gyrus is influenced by gonadal hormones in the context of cellular injury, and the work in this area demonstrates that gonadal hormones have neuroprotective potential. The expression of estrogen, progestin and androgen receptors in the dentate gyrus suggests that sex steroids, which could be of gonadal origin and/or synthesized locally in the dentate gyrus, may act directly on dentate cells. In addition, gonadal hormones could also influence the dentate gyrus indirectly, by subcortical hormone-sensitive structures such as the cholinergic septohippocampal system. Importantly, these three sex steroid-related themes, functional effects in the normal dentate gyrus, mechanisms involving neurogenesis and synaptic remodeling, as well as neuroprotection, have substantial implications for understanding normal cognitive function, with clinical importance for epilepsy, Alzheimer's disease and mental disorders.
Keywords: androgen, estrogen, progesterone, sex difference, electrophysiology, neurogenesis, synaptic remodeling, neuroprotection
Introduction
In 1989, Wimer and Wimer (1989) reported that in certain mouse strains, the dentate gyrus contains more granule cells in the male than in the female. A few years later, the dentate granule cell layer was shown to be larger in males relative to females, both in adult and prepubescent rats, which is well correlated with performance in spatial memory tasks (Roof and Havens, 1992; Roof, 1993). Later, in the hilus of the dentate gyrus, the number of synapses formed by mossy fibers, the axons of dentate granule cells, was demonstrated to be higher in male rats than in females, consistent with the idea that more granule cells in males provide a more robust input to CA3 pyramidal neurons (Parducz and Garcia-Segura, 1993). Using rigorous stereology techniques, however, two studies performed later have found no sexual dimorphism in the volume of the dentate granule cell layer in Sprague-Dawley (Isgor and Sengelaub, 1998) and Long-Evans rats (Jones and Watson, 2005). Paying particular attention to rodent strains in this respect is emphasized by another mouse study, demonstrating that the overall volume of the dentate granule cell layer in A/J mice is larger in males than in females, while there is no such sexual dimorphism in C57Bl/6J mice (Tabibnia et al., 1999). These key developments in our understanding of sex differences in the dentate gyrus were followed by other examples of sexual dimorphism in certain aspects of dentate gyrus function and morphology. For example, female rats produce more newly-born cells than males in the dentate gyrus, but not in the subventricular zone (Tanapat et al., 1999).
Expression of proteins regulated by estrogen, such as brain-derived neurotrophic factor (BDNF) (Sohrabji et al., 1995), also differs in males vs. females. Using immunocytochemistry, it appears that the mossy fibers contain the vast majority of BDNF protein (Conner et al., 1997). When a proestrous or estrous female rat with high estrogen levels was compared to a metestrous female, ovariectomized female, or male, which have low estrogen blood concentrations, BDNF protein expression in the mossy fibers was relatively low (Scharfman et al., 2003). These studies are consistent with BDNF expression in mossy fiber-containing micropunches of CA3 assayed by ELISA, demonstrating a higher level of BDNF in the female relative to the male rat (Franklin and Perrot-Sinal, 2006).
The finding that prepubescent female rats injected neonatally with testosterone develop a male-like dentate gyrus and perform better in the Morris water maze (Roof, 1993) suggests that the “gender” of the dentate gyrus and many other sexually dimorphic brain functions and structures could be readily manipulated via the hormonal milieu. The window during which these hormonal manipulations can affect dentate gyrus morphology seems to be restricted to the first few postnatal days (Roof, 1993), because no morphological responses to sex steroid treatment in the rat dentate gyrus were found before or after this period (Isgor and Sengelaub, 1998). The findings of Roof (1993) support the so-called “aromatization hypothesis”, i.e., the brain is masculinized by estrogen that is produced by aromatase from testosterone, which is readily available in the developing male but not in the female (Naftolin et al., 1975). Also consistent with this hypothesis, estrogen receptor (ER) mRNA levels in the dentate gyrus increase significantly between birth and postnatal day 4, and then decline by postnatal day 10, while adult male rat ER mRNA levels are similar to those found in newborn and postnatal day 10 animals (O'Keefe et al., 1995). On the other hand, it has been reported that the dentate granule cell layer is significantly larger in testicular feminization mutant (Tfm) male rats than in wild-type females (Jones and Watson, 2005). Because Tfm rats express a dysfunctional androgen receptor (AR), this finding implicates the AR in the development of the dentate gyrus, which contradicts the aromatization hypothesis. It should be noted, however, that Tfm rats retain a considerable portion of AR activity, whereas the dentate granule cell layer is not sexually dimorphic in Tfm mice with complete deletion of AR function (Tabibnia et al., 1999).
Sex Steroids and Dentate Physiology
The findings of sexual dimorphism strongly suggest that sex steroids, and their receptors, are important regulators of dentate gyrus organization, and emphasize their important role in development. The rest of this review, however, will focus on more recent and exciting research on the activational effects of gonadal hormones in the adult brain. In adulthood, sex steroids participate not only in reproduction-related central actions, but also in higher brain functions such as cognition and mood regulation (Korol and Kolo, 2002; Seidman, 2003; Steiner et al., 2003; MacLusky et al., 2006). Because the hippocampal formation plays an essential role in declarative, spatial, and contextual memory, as well as in the regulation of mood and the hypothalamic-pituitary-adrenal axis, this limbic structure is a critical target of hormone action (McEwen and Alves, 1999; McEwen, 2003). As a part of the hippocampal formation, it is highly likely that the dentate gyrus also plays an important role in cognitive function and mood regulation. Although research has made progress in associating hippocampal subregions with specific functional roles in complex topics such as cognition and mood, proving that any specific aspect of these behaviors is dependent on the dentate gyrus has been more difficult, probably because complex hippocampal operations require uncompromised signal flow throughout the entire hippocampal formation, rather than only in select areas.
Electrophysiological studies may provide the most specific insights into the ways sex steroids influence the adult dentate gyrus. Working with rat hippocampal slices in the presence or absence of 17β-estradiol, Kim and colleagues (2006) have reported that 17β-estradiol significantly potentiates the amplitude and slope of field excitatory postsynaptic potentials in dentate gyrus directly, as well as in CA3 following mossy fiber stimulation. Repetitive hilar stimuli frequently evoke multiple population spikes in CA3 at proestrus and estrus, but only rarely at other cycle stages, and never in slices of ovariectomized rats (Scharfman et al., 2003). This hyperexcitability in CA3 at proestrus was blocked by exposure to the high-affinity neurotrophin receptor antagonist K252a, or by an antagonist of the α7 nicotinic cholinergic receptor, whereas it was induced at metestrus by the addition of BDNF to hippocampal slices (Scharfman et al., 2003). These findings indicate that an estrogen-induced interaction of BDNF and α7 nicotinic receptors is important for estrous cycle-related changes in CA3 and dentate gyrus (Scharfman et al., 2003).
Considering androgen, intrahippocampal microinjection of neither dehydroepiandrosterone-sulfate (DHEAS) nor trilostane, an inhibitor of the enzyme that metabolizes DHEAS, alters dentate field excitatory postsynaptic potential slopes or population spike amplitudes, but increases the amplitude of a late component of the postsynaptic potential. Both DHEAS and trilostane abolishes GABA-mediated paired-pulse inhibition. In addition, both DHEAS and trilostane markedly increases the spontaneous firing rate of dentate hilar interneurons and synchronizes their firing during hippocampal theta rhythm induced by tail-pinch (Steffensen, 1995).
Many studies indicate that sex steroid-induced electrophysiological changes may be due to modulation of dentate glutamate and GABA receptors. Indeed, ovariectomy in rats decreases [3H] glutamate binding to N-methyl-D-aspartate (NMDA) receptors in the dentate gyrus, while hormone replacement with estradiol, tamoxifen, or raloxifene prevents this decrease (Cyr et al., 2000; Cyr et al., 2001). On the other hand, [3H] MK-801 binding shows that the density of noncompetitive NMDA antagonist sites is significantly increased in the dentate gyrus of ovariectomized compared to sham-operated rats (El-Bakri et al., 2004). 17β-estradiol returns [3H] MK-801 binding to the normal levels, while progesterone has no effect (Weiland, 1992; El-Bakri et al., 2004). In addition, estradiol treatment of ovariectomized rats significantly increases NMDA R1 subunit protein levels in granule cell somata, in comparison with non-treated animals, without concomitant changes in the corresponding mRNA hybridization signal (Gazzaley et al., 1996). Regarding other receptor types, ovarian steroids have no effect on the density of kainate or α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (Weiland, 1992; Cyr et al., 2000), while estradiol-benzoate increases [3H] muscimol binding in the dentate gyrus (Schumacher et al., 1989). In situ hybridization showed that progesterone suppresses mRNA levels of the α1 GABAA receptor subunit in the dentate gyrus of animals that were pretreated with estradiol (Weiland and Orchinik, 1995). Finally, there is a significant negative correlation between testosterone levels and the mRNA level for the α1 GABAA receptor subunit (Orchinik et al., 1995).
Sex Steroids and Dentate Plasticity
Although numerous findings support the view that molecular mechanisms, including receptor changes, play a critical role in alterations of neuronal activity and functional plasticity (see above), recent evidence suggests that sex steroids may also influence long-term potentiation (Lynch, 2004) and learning/memory via mediating aspects of structural plasticity, such as neurogenesis and synaptic remodeling (Kandel, 2001; Kasai et al., 2003).
Sex steroids and neurogenesis
Due to the recent confirmation that neurogenesis continues throughout the lifespan in the adult dentate gyrus (Altman and Das, 1965), the subgranular zone, where progenitors are primarily generated, has drawn considerable attention. Translational implications are one reason: dentate neurogenesis may be a critical factor in the pathophysiology of depression and in the mechanism of antidepressant action (Santarelli et al., 2003). The first indication that sex steroids may influence adult neurogenesis came from Tanapat and colleagues (1999), who demonstrated that female rats produce more newly-born cells than males in the dentate gyrus (but not in the subventricular zone). They have also reported a fluctuation in cell proliferation during the estrous cycle: females produce more newly-born cells during proestrus (when estrogen levels are highest) compared with estrus and diestrus. Ovariectomy diminishes, while acute treatment with estrogen rapidly increases, cell proliferation in ovariectomized rats, an effect that is reversed by the administration of progesterone (Tanapat et al., 1999; Falconer and Galea, 2003; Tanapat et al., 2005). Both the prolonged absence of ovarian hormones and chronic treatment decreases the potential of estrogen to stimulate cell proliferation (Tanapat et al., 2005), suggesting that both dose-response and temporal characteristics of estrogen treatment may critically influence its neurogenic efficacy. Indeed, Ormerod and colleagues (2003) have reported that relative to vehicle-treated rats, the number of new cells increases following a 4-hr exposure but decreases following a 48-hr exposure to estrogen in ovariectomized animals. This decrease at 48 hr is abolished by adrenalectomy, suggesting a role of adrenal activity (Ormerod et al., 2003).
In addition to being effective under normal conditions, estrogen is capable of influencing neurogenic potential when neurogenesis is examined in other contexts. For example, a strong reduction in cell proliferation occurs in the dentate gyrus and subventricular zone of mice sacrificed 20 days after streptozotocin administration, which induces a diabetic state. This reduction is completely relieved by 10 days of estradiol pellet implantation, which increases the circulating estrogen levels 30-fold (Saravia et al., 2006). In addition, there is a striking effect of aging on cell proliferation that appears to be influenced by estradiol. Perez-Martin and colleagues (2005) have reported that treatment of 22-month-old ovariectomized animals for 10 weeks with a weekly subcutaneous injection of estradiolvalerianate, or with soy extract added to the drinking water, reverses the age-associated decline in dentate granule cell production (Perez-Martin et al., 2005). Similarly, estrogen also normalizes the deficient granule cell proliferation in the dentate gyrus of aging mice (De Nicola et al., 2006).
Several studies have provided insight into the mechanisms underlying the influence of estrogen on neurogenesis. Mazzucco and colleagues (2006) have shown that both diarylpropionitrile, an ERβ agonist, and propyl-pyrazole triol, an ERα agonist, significantly enhances cell proliferation in the dentate gyrus of female rats. Other findings suggest that ERs are involved in the induction of adult neurogenesis by an interaction with insulin-like growth factor-1 (IGF-1), as estradiol and IGF-1 have a cooperative effect to promote neurogenesis (Perez-Martin et al., 2003). Administration of IGF-1 significantly increases dentate neurogenesis compared to rats treated with vehicle; and rats treated with both IGF-1 and estradiol show a higher level of cell proliferation than rats treated with IGF-1 or estradiol alone (Perez-Martin et al., 2003).
Serotonin has also been linked to the effect of estradiol on dentate neurogenesis. Administration of 5-hydroxytryptophan, a precursor to serotonin, restores cell proliferation that was decreased by ovariectomy, whereas estradiol is unable to reverse this change in ovariectomized rats treated with p-chlorophenylalanine, an inhibitor of serotonin synthesis (Banasr et al., 2001). These data implicate the central serotonergic system in the mediation of estrogen effects on dentate neurogenesis. Indeed, several studies indicate that estrogen influences the dentate serotonergic system (Bowman et al., 2002). In case of 5-HT1A receptors, estradiol treatment reduces 5-HT1A gene expression in the dentate gyrus (Birzniece et al., 2001), while ovariectomy increases 5-HT1A receptor stimulation, which is reversed by estradiol (Le Saux and Di Paolo, 2005).
Besides the plethora of data with respect of estrogen and neurogenesis, there are almost no published work that address the neurogenic effect of androgen except an initial study in songbird suggesting that testosterone promotes neurogenesis (Louissaint et al., 2002). Another study has demonstrated that in a neuronal stem cell culture stimulated with epidermal growth factor, nandrolone, a synthetic androgen reduces cell proliferation (Brannvall et al., 2005). The decrease is abolished by flutamide, an AR antagonist. Nandrolone also reduces new cell production in the dentate gyrus, an effect observed in both female and male rats (Brannvall et al., 2005). For more details on gonadal hormone modulation of hippocampal neurogenesis in the adult, some excellent reviews are available (Gould et al., 2000; Galea et al., 2006). For more details on adult neurogenesis and its role in mood regulation, we refer the reader to other chapters within this volume.
Sex steroids and synaptic remodeling
In 1992, the discovery that estradiol mediates fluctuation in hippocampal CA1 spine synapse density during the estrous cycle in the adult rat marked the beginning of a new era in the research of sex steroids (Woolley and McEwen, 1992). Subsequent extensive work has shown that sex steroids hold an unparalleled synaptogenic power in the adult hippocampus. For example, hormone replacement induces changes on the order of 50-100% in the number of CA1 spine synapses of rats (MacLusky et al., 2006; Parducz et al., 2006). This synaptogenic efficacy seems to be rivaled only by antidepressant drugs (Hajszan et al., 2005). Moreover, estrogen-induced remodeling of hippocampal spine synapses is remarkably rapid, similar to the time course required for long-term potentiation induction (MacLusky et al., 2005). The temporal analogy suggests that formation of spine synapses may be involved in sex steroid-modulated cognitive functions. Indeed, a great deal of evidence has accumulated which suggests that rapid remodeling and stabilization of small spines (and their associated synaptic contacts) may represent a mechanism of memory formation and storage (Sorra and Harris, 2000; Kandel, 2001; Kasai et al., 2003).
Unfortunately, several studies support the view that the dentate gyrus may miss this “synaptogenic party”. Woolley and colleagues (1990) have reported no significant changes in dendritic spine density across the estrous cycle in CA3 pyramidal cells or dentate granule cells of the rat. Using a similar Golgi-impregnation technique, Gould and colleagues (1990) have shown that ovariectomy or gonadal steroid replacement do not affect spine density of CA3 pyramidal cells or granule cells of the dentate gyrus. In a later study, Miranda and colleagues (1999) have demonstrated that there may be effects in the dentate gyrus, but they are likely to be dependent on age and the temporal pattern of estradiol replacement. In addition, Szymczak and colleagues (2006) suggest that ERβ expression is negatively correlated with synapse formation.
Another approach to the topic of synaptic remodeling is to address the expression of proteins that are associated with the pre- or postsynaptic apparatus. This approach has also failed to lead to a compelling body of evidence that hormonal fluctuations modulate dentate synaptic remodeling. For example, immunoreactivity for spinophilin, a marker of dendritic spines, is increased in the hilar region of the dentate gyrus, as well as in CA3, of ovariectomized rats treated with estrogen for 2 days (Brake et al., 2001). However, levels of syntaxin and synaptophysin (presynaptic proteins associated with the transmitter release machinery), as well as spinophilin, are unaltered by hormone treatment in the dentate gyrus of rhesus monkeys (Choi et al., 2003). Although young female rhesus monkeys show a trend toward an estrogen-induced increase in immunoreactive spines in the dentate gyrus outer molecular layer, this effect appears to be statistically insignificant (Hao et al., 2003).
Glia have also been associated with synaptic remodeling, as expansion of the dendritic tree and spine growth may occur at the expense of shrinking glial, primarily astroglial volume. As a result, presumably, abundance of astroglial processes and markers is negatively correlated with dendritic spine density. However, the ways sex steroids alter dentate gyrus glia do not appear to be consistent. Luquin and colleagues (1993) have shown that the surface density of astroglial cells is positively influenced by estrogen and progesterone. The surface density of astroglial cells was significantly increased over control values by 5 hr after the injection of estrogen to ovariectomized rats, and as early as 1 hr after the administration of progesterone; it reached maximal values by 24 hr and returned to control levels by 48 hr (Luquin et al., 1993). In contrast, levels of glial fibrillary acidic protein (GFAP) intron 1, a molecular marker of adult astrocytes, shows that GFAP transcription and mRNA are both decreased in the outer molecular layer of the dentate gyrus on the afternoon of proestrus, when plasma estradiol levels are highest (Stone et al., 1998b). In vitro, astrocytes show interesting bidirectional responses, such that estrogen treatment increases GFAP transcription in monotypic astrocytic cultures but decreases GFAP transcription in astrocytes cocultured with neurons (Stone et al., 1998b). In mice, Lei and colleagues (2003) have reported similar findings, i.e., long-term 17β-estradiol treatment in aged female mice significantly lowered the number of astrocytes in the dentate gyrus and CA1 compared with placebo.
What maybe in the cause of such variable findings? Besides confounding variables such as the age of animals, strain, dose and temporal characteristics of hormone treatment, and/or the dentate area examined, the chosen methodological approach alone may be critical. What measures most reliably the remodeling of synaptic connections, i.e., synaptogenesis or loss of synapses, is debatable. Above, we list several light microscopic approaches, such as Golgi-based estimation of dendritic spine density and histochemical detection of pre- and/or postsynaptic marker molecules, which are widely applied, mainly due to their relative methodological simplicity. However, it is impossible to decide at the light microscopic level what proportion of the measured molecular synaptic markers is actually associated with synapses, and what proportion represents extrasynaptic molecules that are processed and/or stored in different cellular compartments. Therefore, levels of synaptic marker molecules may and do change without alterations in the number of synapses (Li et al., 2004). Although Golgi-based estimation of dendritic spine density is a less controversial approach, it also has several limitations. The most important is that spines may or may not form synapses, and the Golgi method is incapable of differentiating between spines that have synapses and spines that do not. Thus, similar to synaptic marker molecules, measures of dendritic spines do not necessarily reflect the true number of spine synapses.
The most important point in this debate is that the number of actual spine synapses is more relevant to the functional status of neurons than the number of dendritic spines or the levels of any molecular markers. Thus, when the true number of synapses is questioned, one should count synapses themselves using electron microscopic stereological techniques, because the above-discussed light microscopic markers are not reliable. Treating ovariectomized rats with 10 μg/day subcutaneous estradiol-bezoate for 2 days, a similar schedule that has been used in previous studies (Gould et al., 1990), estrogen increased the number of spine synapses in the CA1 stratum radiatum by 71.6% over oil-treated control values, by 50.1% in the CA3 stratum radiatum, and by 99.1% in the molecular layer of the dentate gyrus (Figure 1). It is noteworthy that the number of granule cell spine synapses in the dentate gyrus doubles after estrogen administration.
Figure 1.
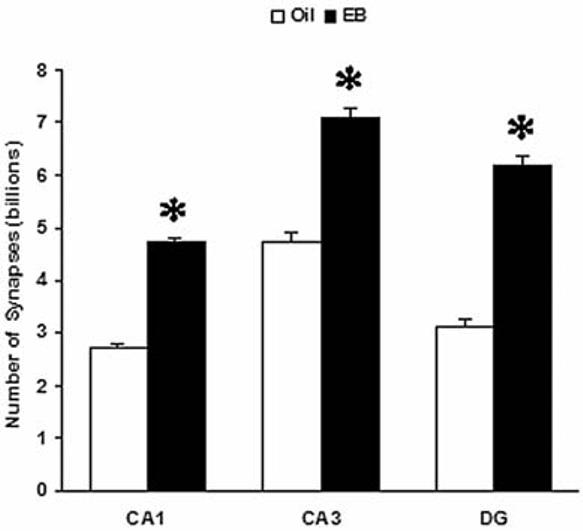
Effects of ovariectomy and estrogen replacement on the number of hippocampal spine synapses. Young adult female Sprague-Dawley rats (250 g) were ovariectomized and one week later, they received either 10 μg/rat/day estradiol-benzoate (EB, solid columns) or 200 μl/rat/day sesame oil vehicle (Oil, open columns) subcutaneously for two days. Two days after the last injection, the animals were sacrificed by transcardial perfusion of fixative, and their brains were processed for electron microscopic stereological analysis. Spine synapses were counted in the CA1 and CA3 strata radiata, and in the molecular layer of the dentate gyrus (DG). *Significantly different from the corresponding Oil group (t-test, p<0.001 in CA1 and DG, p<0.01 in CA3).
Neuroprotective Effects
Due to the vulnerability of some types of neurons in the dentate gyrus to insults, this area is a common subject of neurodegenerative/neuroprotective experiments. The work summarized below is focused around two topics for which neuroprotection is particularly germane: epilepsy and Alzheimer's disease.
Effects of estrogen on seizures vary, depending on the experimental approach, and many other factors. Estrogen may increase neuronal excitability and thus mediate proconvulsant effects that have been reported in the past, but reviews of the clinical and animal data show that estrogen may also have no effect or even anticonvulsant effects (Scharfman et al., 2003; Hajszan and MacLusky, 2006; Veliskova, 2006). The protective role of estrogen in seizure-induced damage is more straightforward. Severe seizures have been shown to trigger excitotoxic cell death in the hilus of the dentate gyrus, and patients with temporal lobe epilepsy often exhibit neuronal loss in the hilus also (Margerison and Corsellis, 1966). Kainic acid is commonly used as a convulsant to elicit severe seizures (status epilepticus) and excitotoxic damage in the dentate gyrus of rats (Ben-Ari and Cossart, 2000). Estrogen administration is capable of preventing kainic acid-induced degeneration (Azcoitia et al., 1998; Veliskova et al., 2000). Estrogen may also mediate the protective actions of other steroids. For example, the neurosteroids pregnenolone and DHEA showed a dose-dependent protective effect of hilar neurons against kainic acid. The administration of the aromatase inhibitor fadrozole, that blocks the conversion of these steroids into estrogen, prevented this effect (Veiga et al., 2003). Interestingly, 2-methoxyestradiol, an estradiol metabolite, induced significant neuronal loss in the hilus, detected 96 hr after the treatment with this steroid. This finding suggests that endogenous metabolism of 17β-estradiol to 2-methoxyestradiol may counterbalance the neuroprotective effects of estrogen (Picazo et al., 2003).
Regarding mechanisms for the neuroprotective effects of estrogen in studies of seizure-induced neuronal damage, Veliskova and colleagues (2000) as well as Haynes and colleagues (2003) suggest that intracellular ERs mediate the neuroprotective effect of estrogen, because tamoxifen pretreatment effectively abolished estrogen-induced neuroprotection. An interaction of ER and IGF-1 receptor signaling may also be important (Azcoitia et al., 1999a). Furthermore, GABAB receptors are likely to play a role, because there was a loss of GABAB receptor-mediated inhibition after kainic acid-induced status epilepticus in the rat dentate gyrus, and pretreatment with estrogen could prevent it (Velisek and Veliskova, 2002).
Other steroids besides estrogen are also likely to reduce seizure-induced damage, and the progesterone metabolite 3α],5α-tetrahyroprogesterone (allopregnanolone) has been shown to be one example. Blocking progesterone's metabolism to 3α,5α-tetrahyroprogesterone reduced progesterone's protective effects in the dentate gyrus (Rhodes et al., 2004). In the kainate model, 3α,5α-tetrahyroprogesterone was able to protect the hilus from kainic acid (Ciriza et al., 2004). Another metabolite was also effective: 5α-hydroxyprogesterone (Ciriza et al., 2004).
Other models of injury, which use adrenalectomy to examine neuronal loss in the dentate gyrus, focus on the granule cells, because adrenalectomy selectively kills granule cells (see chapter by M. Joels in this volume). In this model, estradiol treatment reduced pyknotic cell number compared to vehicle administration (Frye, 2001). Interestingly, a synthetic glucocorticoid, dexamethasone can also induce apoptosis in the dentate gyrus, and pretreatment with estrogen substantially attenuated the dexamethasone-induced neuronal damage (Haynes et al., 2003). Colchicine, a microtubule polymerization inhibitor, also selectively kills granule cells, an effect that is increased by ovariectomy and ameliorated by 17β-estradiol (Liu et al., 2001).
Regarding other sex steroids, treatment of female or male rats with progesterone or its metabolites, 5α-dihydroprogesterone and 3α,5α-tetrahyroprogesterone similarly reduced the total number of adrenalectomy-induced pyknotic cells in the dentate gyrus compared with vehicle administration. In case of androgen, testosterone and its metabolites, 5α-dihydrotestosterone and 5α-androstane-3α,17β-diol significantly reduced the number of pyknotic cells in the dentate gyrus compared to vehicle-administered, adrenalectomized female rats (Frye and McCormick, 2000a, b).
Estrogen also has been found to play a critical neuroprotective role in Azlheimer's disease. Unilateral entorhinal cortex lesion (ECX) is frequently used as a model of Alzheimer's disease-like deafferentation in the dentate gyrus. ECX elicits sprouting in the molecular layer, which is affected by gonadectomy and hormone replacement, but only in female rats: ovariectomy reduces fiber outgrowth and estrogen restores it (Stone et al., 2000). However, testosterone replacement had no effect on sprouting in castrated ECX males (Morse et al., 1986). Sprouting in hippocampal cultures of C57Bl/6J mice was increased by 75% after treatment with 17β-estradiol, which was blocked by an antagonist of nuclear receptors, tamoxifen (Teter et al., 1999). In intact female mice in vivo, lesions of the lateral part of the entorhinal cortex increased axonal sprouting in the outer one-third of the molecular layer of the dentate gyrus. Ovariectomized mice receiving high and moderate estrogen supplementation displayed the same sprouting response. In ovariectomized non-treated mice, however, the sprouting response was significantly reduced (to nearly nothing) (Kadish and van Groen, 2002). Finally, Stone and colleagues (1998a) have shown that in wild-type ECX mice, ovariectomy decreases commissural/associational sprouting to the inner molecular layer of the dentate gyrus, which is reversed by estradiol replacement. In ECX apolipoprotein E-knockout mice, however, estradiol did not enhance sprouting, suggesting that sprouting may be stimulated by estrogen through its up-regulation of apolipoprotein E expression, leading to increased recycling of membrane lipids for use by sprouting neurons (Stone et al., 1998a). Estrogen and apolipoprotein E may therefore interact in their modulation of both Alzheimer's disease risk and recovery from neuronal injury.
Mediation of Sex Steroid Effects
Effects of sex steroids in the dentate gyrus depend on the location of their action, and the sites of steroid synthesis. Below we discuss the distribution of receptors, which is summarized in Figure 2. Sex steroids are capable of acting directly on granule cells, as well as indirectly via non-granule cells within the dentate gyrus (interneurons, mossy cells). The influence of sex steroids could also be mediated by subcortical, hormone-sensitive structures, such as the septohippocampal cholinergic neurons. Sites of sex steroid synthesis are usually thought to be peripheral, but local synthesis is also possible, and is discussed further below.
Figure 2.
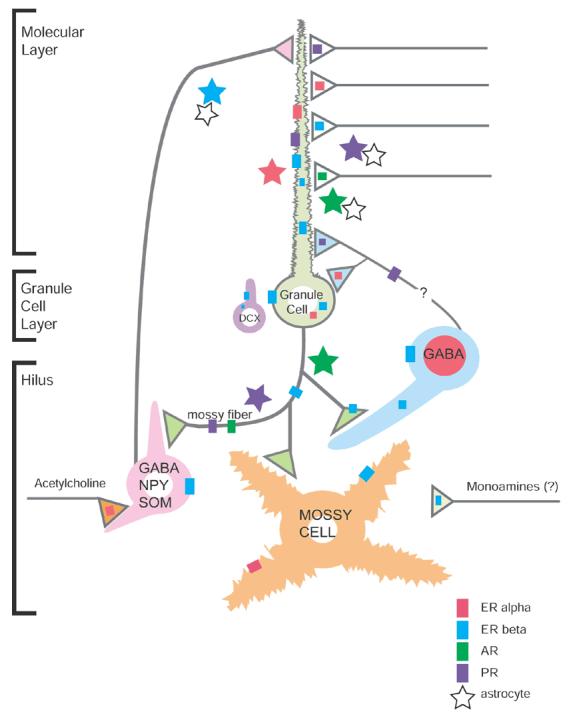
Subcellular localization of estrogen (ER), androgen (AR) and progestin (PR) receptors in the dentate gyrus. A subset of GABAergic interneurons contains nuclear ERα (dark pink). Granule cells, newly born cells (identified by DCX) and some GABAergic interneurons contain cytosolic and plasma membrane-associated ERβ (blue). Dendritic spines, many originating from granule cells contain ERα, ERβ, AR (dark green) and PR (purple). A few dendritic spines in the hilus, likely originating from mossy cells, contain ERα and ERβ. ERα, ERβ, AR and PR are found in axons and axon terminals. Some ERα-containing terminals are cholinergic (acetylcholine, orange); some ERβ-containing terminals resemble monoaminergic boutons. Lot of astrocytes (stars), mostly in the molecular layer, also contain ERα, ERβ, AR and PR.
Distribution of ERα in the dentate gyrus
Estrogen binding, as well as mRNA and immunoreactivity for ERα, have been detected in nuclei of scattered GABAergic interneurons, located predominantly in the subgranular region of the dentate gyrus (Loy et al., 1988; Shughrue et al., 1997; Weiland et al., 1997; Perlman et al., 2005). Nuclear ERα-immunoreactive interneurons co-express neuropeptide Y, calbindin-D28k and calretinin, but not cholecystokinin or parvalbumin (Nakamura and McEwen, 2005). In addition to nuclear receptors, ultrastructural studies have revealed ERα at several extranuclear sites in the dentate gyrus (Milner et al., 2001). Specifically, ERα-immunoreactivity is affiliated with the cytoplasmic plasmalemma of select hilar interneurons and with endosomes of a few granule cell perikarya. Moreover, ERα-labeled profiles are dispersed throughout the dentate gyrus. Approximately half of these labeled profiles are unmyelinated axons and axon terminals that contain numerous small, synaptic vesicles. ERα-labeled terminals form both symmetric and asymmetric synapses on dendritic shafts and spines, suggesting that ERα-positive axons arise from sources in addition to inhibitory interneurons. Dual labeling revealed that ERα-immunoreactivity is contained in axons and terminals labeled with vesicular acetylcholine transporter (Towart et al., 2003), suggesting that estrogen could rapidly and directly affect the local release and/or uptake of acetylcholine. About one-quarter of the ERα-immunoreactive profiles are dendritic spines, many originating from granule cells. In dendritic spines, ERα-immunoreactivity is often associated with the spine apparatus, suggesting that estrogen might act locally through ERα to influence protein synthesis during synaptic remodeling. The remaining one-quarter of ERα-labeled profiles are from glial origin that resemble astrocytes and are often located near the spines of granule cells. Vesicular acetylcholine transporter-containing terminals often abut ERα-positive presynaptic and glial profiles and unlabeled terminals that contact ERα-immunoreactive spines (Towart et al., 2003), suggesting that acetylcholine release might play a critical role in estrogen-modulated structural plasticity. Collectively, these results imply that ERα may serve as both a genomic and non-genomic transducer of estrogen action in the dentate gyrus.
Distribution of ER β in the dentate gyrus
The cellular and subcellular locations of ERβ-immunoreactivity in the dentate gyrus are similar yet distinct from ERα. In monkey, dense ERβ hybridization signal has been seen in the dentate gyrus, CA1, CA2, CA3, CA4, and the prosubiculum/subiculum areas of the hippocampus (Gundlah et al., 2000). In rodents, cells in or near the dentate granule cell layer transiently express high levels of estrogen binding and ERα protein in the nucleus during the first two postnatal weeks (O'Keefe et al., 1995; Solum and Handa, 2001). In adult rats and mice, ERβ mRNA and protein has been found in the perikarya of granule cells as well as cells in the dentate subgranular layer (Li et al., 1997; Shughrue et al., 1997; Mitra et al., 2003; Milner et al., 2005). Szymczak and colleagues (2006) have found that ERβ mRNA and protein are displayed in high levels in the estrus and in low levels in the proestrus phase. Recently, robust mRNA expression for both the α and β subtypes of ERs has been found in proliferating and differentiating cells of neuronal phenotype in the subgranular zone of the dentate gyrus (Isgor and Watson, 2005). Furthermore, ERβ-immunoreactive glia has been observed in the hilus of the dentate gyrus of male and female rats. ERβ-immunoreactivity has been localized in glial processes and perikarya and, in some cases, in glial cell nuclei. Double immunocytochemical labeling of ERβ and the specific astroglial marker, GFAP revealed that the ERβ-immunoreactive glial cells are astrocytes (Azcoitia et al., 1999b). Ultrastructural analysis showed ERβ-immunoreactivity at several extranuclear sites in the dentate gyrus (Milner et al., 2005). ERβ-immunoreactivity is affiliated with cytoplasmic organelles, especially endomembranes and mitochondria, and with the membranes primarily of granule cell perikarya and proximal dendrites. Recent studies revealed that neuronal perikarya and dendrites labeled with doublecortin, a marker of newly-generated cells, also contain extranuclear ERβ-immunoreactivity in both the adult and neonatal dentate gyrus (Herrick et al., 2006). ERβ-labeled dendritic shafts and spines have mostly been found in the molecular layer. In dendritic processes, ERβ-immunoreactivity is near the perisynaptic zone adjacent to synapses formed by unlabeled terminals. The ERβ protein can also be found in preterminal axons and axon terminals, associated with clusters of small, synaptic vesicles. ERβ-labeled axons are particularly dense in the hilus and outer molecular layer, forming both asymmetric and symmetric synapses with dendrites. Finally, ERβ-immunoreactivity has been detected in glial profiles throughout the dentate gyrus, some of which appose doublecortin-labeled perikarya and dendrites (Herrick et al., 2006). These results suggest that ERβ may serve primarily as a non-genomic transducer of estrogen actions in the dentate gyrus.
Distribution of progestin receptor (PR) in the dentate gyrus
Cells containing PR mRNA have been detected in the dentate subgranular zone (Hagihara et al., 1992). By light microscopy, nuclear PR-immunoreactivity is undetectable in the dentate gyrus; however, ultrastructural analysis revealed that the PR protein is found at several extranuclear sites (Waters et al., 2005). In the molecular layer and hilus, PR-immunoreactivity is present in dendritic spines, closely associated with the postsynaptic density. The PR protein is expressed in axons and axon terminals that contain small synaptic vesicles. PR-positive terminals and en passant axonal boutons form synapses with dendritic spines. PR-immunoreactivity has also been found in glia, many resembling astrocytes and some forming presumed gap junctions with other astrocytic profiles. The considerable lack of nuclear PR labeling may indicate that progesterone uses non-genomic signaling mechanism in the dentate gyrus to directly affect dendritic spine morphology and synaptic plasticity.
Distribution of AR in the dentate gyrus
Previous light microscopic studies have shown that AR mRNA, immunoreactivity and binding are present in pyramidal cell nuclei but not granule cells (Commins and Yahr, 1985; Sar et al., 1990; Simerly et al., 1990; Kerr et al., 1995). However, AR-immunoreactivity is present in disperse, punctuate processes that are most dense in the pyramidal cell layer and diffusely distributed in the mossy fiber pathway (Tabori et al., 2005). Electron microscopic analysis revealed AR-immunoreactivity at several extranuclear sites in the dentate gyrus. AR labeling has been found in dendritic spines, many arising from granule cell dendrites. AR is affiliated with clusters of small, synaptic vesicles within preterminal axons and axon terminals, the majority of these being in the central hilus. AR-immunoreactive preterminal axons are most prominent in the CA3 stratum lucidum. AR-labeled terminals exclusively form asymmetric synapses. Throughout the dentate gyrus, AR-immunoreactivity has also been detected in astrocytic profiles; many of them apposing terminals that synapse on unlabeled dendritic spines or forming gap junctions with other AR-positive or unlabeled astrocytes (Tabori et al., 2005). Together, these results suggest that ARs may serve as both a genomic and non-genomic transducer of androgen action in the dentate gyrus.
Role of local steroid synthesis
Recent studies (Rune et al., 2006) suggest that locally synthesized steroids may contribute to hippocampal activational effects. Using slice cultures, Rune and colleagues have reported that the number of dentate proliferative cells decreases, whereas the number of apoptotic cells increases dose-dependently, in response to reduced estradiol release into the medium after treatment with letrozole, an aromatase enzyme inhibitor (Fester et al., 2006). This also holds true for cell cultures transfected with siRNA against steroidogenic acute regulatory protein (StAR). StAR transports cholesterol to the inner mitochondrial membrane, where it is converted by the cytochrome P-450 enzyme complex, and as such, it is the first step in the cascade of estrogen synthesis. Application of estradiol to the medium had no effect on proliferation and apoptosis, whereas the anti-proliferative and pro-apoptotic effects of StAR knockdown and letrozole administration were restored by treatment of the cultures with estradiol (Fester et al., 2006). The data of Rune and colleagues is also supported by other studies. In situ hybridization revealed that StAR and aromatase are highly expressed in neuronal cells of the dentate gyrus. In addition, StAR- and aromatase-positive cells are strictly correlated with steroidogenic factor-1, a regulator of steroid biosynthesis, as shown by computer-assisted confocal microscopy in double labeling experiments (Wehrenberg et al., 2001). Similarly, Hojo and colleagues (2004) have reported in a rather comprehensive study that in the granule cells of the adult male rat dentate gyrus, significant localization is seen for both cytochromes P45017α (dehydroepiandrosterone synthase) and P450 aromatase by means of immunohistochemical staining of slices. Only a weak immunoreaction of these P450s has been observed in astrocytes and oligodendrocytes (Hojo et al., 2004). More importantly, stimulation of hippocampal neurons with NMDA induced a significant net production of estradiol. The analysis of radioactive metabolites demonstrated the conversion from [3H] pregnenolone to [3H] estradiol through dehydroepiandrosterone and testosterone. This activity was abolished by the application of specific inhibitors of cytochrome P450s (Hojo et al., 2004). In summary, although these findings seem compelling, considering the fact that the majority of the above-mentioned data have been obtained from cultures, which may be quite different from conditions in vivo, one should exercise caution in interpreting such results.
Subcortical mediation of sex steroid effects
In addition to direct effects of sex steroids on dentate cells, indirect actions may influence the dentate gyrus. We already mentioned above that the raphe serotonergic system mediates the dentate neurogenic effect of estrogen (Banasr et al., 2001). Consistent with this line of argument, raphe serotonergic neurons express ERα (Leranth et al., 1999). Another structure that appears to be critical is the septohippocampal cholinergic system. Septal cholinergic neurons express nuclear ERs (Shughrue et al., 2000) and hippocampal cholinergic axons and terminals contain extranuclear ERα (Towart et al., 2003). Moreover, estrogen affects septohippocampal cholinergic neurons both genomically and nongenomically (Gibbs and Aggarwal, 1998; Rudick et al., 2003). Androgen also can influence the expression of cholinergic markers in the dentate gyrus. Specifically, gonadectomy reduced the density of choline acetyltransferase immunoreactive fibers in the dentate gyrus, which was reversed by the addition of testosterone propionate (Nakamura et al., 2002). Although there is no direct data from the dentate gyrus, elsewhere in the hippocampus estrogen modulates the inhibition by specific GABAergic interneurons, which is partially dependent on input from basal forebrain cholinergic neurons (Rudick et al., 2003). More evidence is available for the cholinergic role in dentate neurogenesis. Selective neurotoxic lesion of the forebrain cholinergic input with 192 IgG-saporin reduced dentate cell proliferation (Mohapel et al., 2005). Conversely, systemic administration of the cholinergic agonist physostigmine increased dentate neurogenesis. The neurogenic effect of acetylcholine appears to involve nicotinic receptors containing the β2 subunit (Harrist et al., 2004), as well as m2, m3 and m4 muscarinic receptors (Ma et al., 2000; Mohapel et al., 2005). Consistent with these findings, ovariectomy upregulated m4 receptors in the dentate gyrus, whereas estrogen treatment restored m4 binding to the level of the sham group (El-Bakri et al., 2002). However, other results suggest no septohippocampal involvement in the synaptogenic action of estrogen, because rats that received estrogen implants into the medial septum did not exhibit changes in astroglial process density in the dentate gyrus (Lam and Leranth, 2003).
Concluding Remarks
The studies discussed in this review clearly demonstrate that both the organization and functioning of the dentate gyrus is a significant target of sex steroids. The gonadal hormone modulation of physiological activity, neurogenesis, synaptic remodeling, and neurodegeneration/neuroprotection mechanisms are likely to be relevant to higher brain functions such as cognition and mood, in addition to their clinical implications in epilepsy, Alzheimer's disease and mental disorders. Relative to hippocampal subfield CA1, however, the role of gonadal hormones in the dentate gyrus has not received substantial attention. In particular, our understanding of androgen effects on the dentate gyrus is extremely limited relative to estrogen. The available data suggest potent, complex, and potentially important effects, however, and therefore merit more attention in the future.
Acknowledgements
This work was supported by NIH grants MH074021 (T.H.); DA08259, NS07080 and HL18974 (T.A.M.); MH060858 and NS042644 (C.L.); as well as by a Hungarian National Office for Research and Technology grant RET-08/04.
Abbreviations
- AR
androgen receptor
- BDNF
brain-derived neurotrophic factor
- DHEAS
dehydroepiandrosterone-sulfate
- ECX
entorhinal cortex lesion
- ER
estrogen receptor
- GFAP
glial fibrillary acidic protein
- IGF-1
insulin-like growth factor-1
- NMDA
N-methyl-D-aspartate
- PR
progestin receptor
- StAR
steroidogenic acute regulatory protein
- Tfm
testicular feminization mutant
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. Neuroreport. 1998;9(13):3075–3079. doi: 10.1097/00001756-199809140-00029. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J. Neurosci. Res. 1999a;58(6):815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999b;26(3):260–267. [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur. J. Neurosci. 2001;14(9):1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23(11):580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Johansson IM, Wang MD, Seckl JR, Backstrom T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology. 2001;74(2):135–142. doi: 10.1159/000054679. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113(2):401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142(3):1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Brannvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur. J. Neurosci. 2005;21(4):871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144(11):4734–4738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J. Neuroendocrinol. 2004;16(1):58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J. Comp. Neurol. 1985;231(4):473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 1997;17(7):2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J. Neuroendocrinol. 2000;12(5):445–452. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology. 2001;25(2):242–257. doi: 10.1016/S0893-133X(01)00233-0. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Saravia FE, Beauquis J, Pietranera L, Ferrini MG. Estrogens and neuroendocrine hypothalamic-pituitary-adrenal axis function. Front. Horm. Res. 2006;35:157–168. doi: 10.1159/000094324. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J. Cell. Mol. Med. 2004;8(4):537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bakri NK, Adem A, Suliman IA, Mulugeta E, Karlsson E, Lindgren JU, Winblad B, Islam A. Estrogen and progesterone treatment: effects on muscarinic M(4) receptor subtype in the rat brain. Brain Res. 2002;948(12):131–137. doi: 10.1016/s0006-8993(02)02962-1. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975(12):22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- Fester L, Ribeiro-Gouveia V, Prange-Kiel J, von Schassen C, Bottner M, Jarry H, Rune GM. Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. J. Neurochem. 2006;97(4):1136–1144. doi: 10.1111/j.1471-4159.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31(1):38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estradiol tends to improve inhibitory avoidance performance in adrenalectomized male rats and reduces pyknotic cells in the dentate gyrus of adrenalectomized male and female rats. Brain Res. 2001;889(12):358–363. doi: 10.1016/s0006-8993(00)03236-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM. Androgens are neuroprotective in the dentate gyrus of adrenalectomized female rats. Stress. 2000a;3(3):185–194. doi: 10.3109/10253890009001122. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM. The neurosteroid, 3alpha-androstanediol, prevents inhibitory avoidance deficits and pyknotic cells in the granule layer of the dentate gyrus induced by adrenalectomy in rats. Brain Res. 2000b;855(1):166–170. doi: 10.1016/s0006-8993(99)02208-8. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16(3):225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16(21):6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer's disease-related cognitive decline. Horm. Behav. 1998;34(2):98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry. 2000;48(8):715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res. Mol. Brain Res. 2000;76(2):191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res. Mol. Brain Res. 1992;14(3):239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ. Neurologic links between epilepsy and depression in women: is hippocampal neuroplasticity the key? Neurology. 2006;66(6 Suppl 3):S13–22. doi: 10.1212/wnl.66.66_suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Maclusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur. J. Neurosci. 2005;21(5):1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol. 2003;465(4):540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, Caldarone BJ, Eisch A, Zoli M, Picciotto MR. Alteration of hippocampal cell proliferation in mice lacking the beta 2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse. 2004;54(4):200–206. doi: 10.1002/syn.20081. [DOI] [PubMed] [Google Scholar]
- Haynes LE, Lendon CL, Barber DJ, Mitchell IJ. 17 Beta-oestradiol attenuates dexamethasone-induced lethal and sublethal neuronal damage in the striatum and hippocampus. Neuroscience. 2003;120(3):799–806. doi: 10.1016/s0306-4522(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006 doi: 10.1016/j.brainres.2006.08.084. In press. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A. 2004;101(3):865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm. Behav. 1998;34(2):183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Isgor C, Watson SJ. Estrogen receptor alpha and beta mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134(3):847–856. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol. Behav. 2005;85(2):135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kadish I, van Groen T. Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. J. Neurosci. 2002;22(10):4095–4102. doi: 10.1523/JNEUROSCI.22-10-04095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136(8):3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, Brinton RD. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141(1):391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav. Neurosci. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Gonadal hormones act extrinsic to the hippocampus to influence the density of hippocampal astroglial processes. Neuroscience. 2003;116(2):491–498. doi: 10.1016/s0306-4522(02)00730-3. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment: comparison with tamoxifen and raloxifene. J. Psychiatry Neurosci. 2005;30(2):110–117. [PMC free article] [PubMed] [Google Scholar]
- Lei DL, Long JM, Hengemihle J, O'Neill J, Manaye KF, Ingram DK, Mouton PR. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003;121(3):659–666. doi: 10.1016/s0306-4522(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Estrogen receptor-alpha in the raphe serotonergic and supramammillary area calretinin-containing neurons of the female rat. Exp. Brain Res. 1999;128(3):417–420. doi: 10.1007/s002210050863. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor-beta-like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66(2):63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gastard M, Verina T, Bora S, Mouton PR, Koliatsos VE. Estrogens modulate experimentally induced apoptosis of granule cells in the adult hippocampus. J. Comp. Neurol. 2001;441(1):1–8. doi: 10.1002/cne.1393. [DOI] [PubMed] [Google Scholar]
- Louissaint AJ, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Loy R, Gerlach JL, McEwen BS. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Brain Res. 1988;467(2):245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Luquin S, Naftolin F, Garcia-Segura LM. Natural fluctuation and gonadal hormone regulation of astrocyte immunoreactivity in dentate gyrus. J. Neurobiol. 1993;24(7):913–924. doi: 10.1002/neu.480240705. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol. Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma W, Maric D, Li BS, Hu Q, Andreadis JD, Grant GM, Liu QY, Shaffer KM, Chang YH, Zhang L, Pancrazio JJ, Pant HC, Stenger DA, Barker JL. Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur. J. Neurosci. 2000;12(4):1227–1240. doi: 10.1046/j.1460-9568.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146(1):287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138(3):957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89(3):499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.05.032. In Press. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429(3):355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J. Neurosci. 1999;19(9):3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging. 2005;26(6):939–946. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Morse JK, Scheff SW, DeKosky ST. Gonadal steroids influence axon sprouting in the hippocampal dentate gyrus: a sexually dimorphic response. Exp. Neurol. 1986;94(3):649–658. doi: 10.1016/0014-4886(86)90244-x. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog. Horm. Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Fujita H, Kawata M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience. 2002;109(3):473–485. doi: 10.1016/s0306-4522(01)00513-9. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: a potential role for neuropeptide Y. Neuroscience. 2005;136(1):357–369. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- O'Keefe JA, Li Y, Burgess LH, Handa RJ. Estrogen receptor mRNA alterations in the developing rat hippocampus. Brain Res. Mol. Brain Res. 1995;30(1):115–124. doi: 10.1016/0169-328x(94)00284-l. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, McEwen BS. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Brain Res. Mol. Brain Res. 1995;34(1):29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J. Neurobiol. 2003;55(2):247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Parducz A, Garcia-Segura LM. Sexual differences in the synaptic connectivity in the rat dentate gyrus. Neurosci. Lett. 1993;161(1):53–56. doi: 10.1016/0304-3940(93)90138-b. [DOI] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138(3):977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur. J. Neurosci. 2003;18(4):923–930. doi: 10.1046/j.1460-9568.2003.02830.x. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Salazar V, Castillo C, Ariznavarreta C, Azcoitia I, Garcia-Segura LM, Tresguerres JA. Estradiol and soy extract increase the production of new cells in the dentate gyrus of old rats. Exp. Gerontol. 2005;40(5):450–453. doi: 10.1016/j.exger.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol. Psychiatry. 2005;58(10):812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Picazo O, Azcoitia I, Garcia-Segura LM. Neuroprotective and neurotoxic effects of estrogens. Brain Res. 2003;990(12):20–27. doi: 10.1016/s0006-8993(03)03380-8. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3alpha,5alpha-THP mediates progestins' effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacol. Biochem. Behav. 2004;78(3):505–512. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Roof RL. The dentate gyrus is sexually dimorphic in prepubescent rats: testosterone plays a significant role. Brain Res. 1993;610(1):148–151. doi: 10.1016/0006-8993(93)91228-k. [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572(12):310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J. Neurosci. 2003;23(11):4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rune GM, Lohse C, Prange-Kiel J, Fester L, Frotscher M. Synaptic plasticity in the hippocampus: effects of estrogen from the gonads or hippocampus? Neurochem. Res. 2006;31(2):145–155. doi: 10.1007/s11064-005-9004-8. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127(6):3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Saravia FE, Beauquis J, Revsin Y, Homo-Delarche F, de Kloet ER, De Nicola AF. Hippocampal neuropathology of diabetes mellitus is relieved by estrogen treatment. Cell. Mol. Neurobiol. 2006 doi: 10.1007/s10571-006-9096-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J. Neurosci. 2003;23(37):11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, McEwen BS. Regulation of high-affinity GABAA receptors in the dorsal hippocampus by estradiol and progesterone. Brain Res. 1989;487(1):178–183. doi: 10.1016/0006-8993(89)90955-4. [DOI] [PubMed] [Google Scholar]
- Seidman SN. The aging male: androgens, erectile dysfunction, and depression. J. Clin. Psychiatry. 2003;64(Suppl 10):31–37. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96(1):41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U. S. A. 1995;92(24):11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res. Dev. Brain Res. 2001;128(2):165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10(5):501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Steffensen SC. Dehydroepiandrosterone sulfate suppresses hippocampal recurrent inhibition and synchronizes neuronal activity to theta rhythm. Hippocampus. 1995;5(4):320–328. doi: 10.1002/hipo.450050405. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J. Affect. Disord. 2003;74(1):67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer's disease. J. Neurosci. 1998a;18(9):3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I. Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology. 1998b;139(7):3202–3209. doi: 10.1210/endo.139.7.6084. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Lopez LM, Shick J, Finch CE. Effects of age on gene expression during estrogen-induced synaptic sprouting in the female rat. Exp. Neurol. 2000;165(1):46–57. doi: 10.1006/exnr.2000.7455. [DOI] [PubMed] [Google Scholar]
- Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, Merchenthaler I, Kaczmarek L. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16(5):453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Cooke BM, Breedlove SM. Sex difference and laterality in the volume of mouse dentate gyrus granule cell layer. Brain Res. 1999;827(12):41–45. doi: 10.1016/s0006-8993(99)01262-7. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130(1):151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 2005;481(3):252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter B, Harris-White ME, Frautschy SA, Cole GM. Role of apolipoprotein E and estrogen in mossy fiber sprouting in hippocampal slice cultures. Neuroscience. 1999;91(3):1009–1016. doi: 10.1016/s0306-4522(98)00630-7. [DOI] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J. Comp. Neurol. 2003;463(4):390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J. Neurobiol. 2003;56(4):398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- Velisek L, Veliskova J. Estrogen treatment protects GABA(B) inhibition in the dentate gyrus of female rats after kainic acid-induced status epilepticus. Epilepsia. 2002;43(Suppl 5):146–151. doi: 10.1046/j.1528-1157.43.s.5.3.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J. The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience. 2006;138(3):837–844. doi: 10.1016/j.neuroscience.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41(Suppl 6):S30–35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Waters EM, Herrick SP, McEwen BS, Milner TA. Subcellular localization of progestin receptors in the rat hippocampus. Society for Neuroscience Abstracts. 2005:403–412. [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J. Neurochem. 2001;76(6):1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-Daspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131(2):662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res. Mol. Brain Res. 1995;32(2):271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J. Comp. Neurol. 1997;388(4):603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wimer CC, Wimer RE. On the sources of strain and sex differences in granule cell number in the dentate area of house mice. Brain Res. Dev. Brain Res. 1989;48(2):167–176. doi: 10.1016/0165-3806(89)90073-4. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10(12):4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


