Abstract
Our goal was to examine the relationship between early life trauma and the development of visceral hypersensitivity in later life in irritable bowel syndrome (IBS). Rat pups underwent neonatal conditioning: (i) paired odour-shock, where odour is a predictable shock signal, (ii) unpaired odour-shock, where odour is an unpredictable shock signal or (iii) control odour-only with odour presentations and handling without shock. At maturity, colorectal sensitivity was measured as a visceromotor behavioural response. In adulthood, colorectal distension (CRD) induced a pressure-dependent increase in the number of abdominal muscle contractions all three experimental groups. However, compared to animals that had received control odour-only presentations in infancy, there was an attenuated response to CRD in animals previously exposed to neonatal predictable shock pups and an exaggerated response in the animals previously exposed to neonatal unpredictable shock. Adult responses to CRD were altered by infant experience with shock trauma. However, depending on the context of that early life trauma, there are major differences between the long-term effects of that early life trauma on colonic sensitivity compared to controls. These results strengthen the link between early life trauma and adult IBS, and suggest that unpredictable trauma is a critical factor for later life disorders.
Keywords: amygdala, colon, fear conditioning, hypersensitivity, infant trauma, irritable bowel syndrome, predictable, unpredictable
INTRODUCTION
Symptoms of irritable bowel syndrome (IBS) related to gastrointestinal dysfunction include abdominal cramping with pain, and concurrent abnormal bowel habits such as episodes of diarrhoea, constipation or both. One important observation is that the symptoms of IBS are due in part to alterations in visceral perception, which are characterized by heightened awareness of visceral stimuli.1-4 Furthermore, there is sufficient evidence that suggests an important link between the development of heightened visceral perception and psychosocial factors. Thus, IBS is most likely a multi-factorial biopsychosocial disorder in which physiological, psychological, behavioural and environmental factors all contribute to the clinical expression of the disorder.5-9
While many factors contribute to the pathophysiology of IBS and other gut disorders, emotional stress, physical stress, anxiety and more importantly unpredictable stress have been shown to increase the risk for developing IBS.10-16 These stress/anxiety-induced effects are associated with colonic hypersensitivity and abnormal bowel motility in rodents17,18 as well as hyperresponsiveness of the hypothalamic-pituitary-adrenal axis (HPA-stress axis) as demonstrated by corticotropin releasing factor (CRF) hypersecretion and higher release of corticosterone (CORT).19,20 Within the central nervous system (CNS), many structures are involved in stress, anxiety and HPA regulation including the hypothalamus, hippocampus and limbic regions and may play a role in visceral hypersensitivity in rodents.21-23 Thus, while IBS has historically been associated with psychosomatic factors, more recent research has also clearly documented abnormalities in neuroendocrine systems and the brain.24-26
Clinically, IBS is associated with early life trauma as adults who have experienced child abuse exhibit significantly higher rates of depression and IBS.16,17,20,27,28 Early life adverse experience, has been modelled by early life stressors, such as prolonged maternal separation or electric shock, which leads to heightened adult emotionality and anxiety.29-34 Recent work using the maternal separation model has shown that early life manipulation alters adult gut function and worsens IBS symptoms as measured by visceral hypersensitivity.35-41 However, the impact of infant trauma using shock, which enables the effects of early life trauma to be placed in the context of predictable vs unpredictable infant pain, has not been assessed. It is recognized that shock alters later emotionality; with unpredictable shock producing significant enhancement in adult emotionality compared to predictable infant shock, which is consistent with the adult literature on trauma predictability.42,43 In the current study, we assessed the potential effects of predictable (paired odour-shock) and unpredictable infant trauma (unpaired odour-shock) using a Pavlovian olfactory fear-conditioning paradigm44-46 on colonic hypersensitivity in adulthood.
MATERIALS AND METHODS
Experimental animals
Long–Evans hooded male and female rats received infant conditioning (n = 52) and later tested in a two-odour choice test (post-natal (PN)13; n = 18) or in an adult anxiety test (5–6 months: n = 18) at the University of Oklahoma, Norman campus. At 3–4 months, the remaining rats (n = 16) were transferred to the Veteran's Administration (VA) Medical Center animal facility located at the University of Oklahoma Health Science Center for colorectal sensitivity experiments. Animals were handled for 2 weeks before distension experiments. At both facilities, rats were group-housed with ad libitum food and water, in standard rodent cages at 23 °C in a light-dark controlled room. Both animal facilities are AAALAC accredited and experiments were approved by the respective institutional Animal Care and Use Committee. No animals were eliminated from any test and animals only participated in one test.
Postnatal conditioning and behavioural testing
Rat pups were trained from PN days 8 through 12 and assigned to one of three training 1-h training sessions: (i) paired odour-shock (predictable shock); (ii) unpaired odour-shock (unpredictable shock); and (iii) odour-only (control). Pups received 11, 30 s odour presentations of a 30 s peppermint odour (CS) with an inter-trial interval of 4 min. Paired odour-shock subjects received the 0.5 mA shock during the last second of the odour presentation, while unpaired odour-shock subjects received a 1-s shock 2 min after an odour presentation. Peppermint odour was presented with a flow-dilution olfactometer at 2 L min−1 at a concentration of 1 : 10 peppermint vapour. Acquisition curves were recorded during conditioning to verify learning by recording behavioural activation.44-46
To verify odour preference learning, some pups were given five trials in a Y-maze test consisting of a habituation chamber (8.5 cm width, 10 cm length, 8 cm height) and two alleys (8.5 × 24 × 8 cm) separated via two doors (familiar pine wood shavings vs 25 mL peppermint). Each pup was placed in the starting chamber and given 5 s for habituation before the doors to the alleys were removed. Testers were unaware of pups training conditions.44-46
Anxiety was assessed in adulthood using a light-dark emergence test, which utilizes rats avoidance of bright areas compared to darker areas and assumes the latency to enter and total time spent in the light box predicts the level of anxiety. The plastic apparatus (53 cm long × 12 cm wide × 18 cm height) was divided into two compartments by a sliding door: one compartment was painted black and shut-off from all light while the light, larger compartment was painted white and brightly lit. At the beginning of the test, the rat was placed in the dark box and given 1 min to habituate before the door separating the dark and light boxes was opened. Each animal was given 10 min to explore. The latency to enter the light box and the total time spent in the light compartment were recorded. Rats were considered to have entered the light compartment when the rat's entire body had passed the separation between the two compartments. The floor was cleaned between each test. During the testing, observers were completely blinded to the training condition.
Animal preparation and instrumentation for colonic sensitivity assessment
After an overnight fast, adult rats were anesthetized by isoflurane inhalation (1.5–3.0% isoflurane) via a small facemask. A visceromotor behavioural response (VMR) was measured as a model for quantifying the level of visceral pain in rats.47 Attachment of a strain gauge force transducer to the abdominal oblique muscle allows direct monitoring of muscle contractile activity and has been used in many related studies.48-51 A colorectal balloon was inserted via the anal canal (11.5 cm) and secured by tape to the base of tail. The rat was then allowed to recover for 30–45 min.
Colorectal distension
After the rat regained consciousness, 30-min recovery periods were allowed before colorectal distensions (CRD) began. A control period with the balloon inserted but not distended was allowed for 10 min. Following the control period, three distensions were performed at 20, 40 and 60 mmHg of balloon pressure. Distension durations were 10 min each followed by a 10-min resting period between distensions. Abdominal muscle contractions were recorded during a basal period of 10-min and continued for all 10-min distension periods. During the testing, observers were completely blinded to the training condition. Immediately following the last CRD, rats were anesthetized using isoflurane inhalation (5%) and then euthanized.
Data analysis
Following odour-shock conditioning, behavioural testing was conducted at PN13 using a Y-maze (conditioned vs the familiar odour). Five consecutive choices were determined for each animal. At adulthood, rats were given either a dark-light emergence testing in which the latency to enter and total time spent in the light compartment were measured or transferred to the VA animal facility to undergo colorectal sensitivity assessment. Data are reported as the mean ± standard error (SEM) for each treatment group and comparisons between the three treatment groups were made using anova F-statistical analysis. Differences between the groups were considered significant at P ≤ 0.05. As adults no animal was used in more than one test.
The VMR to CRD was measured as the number of abdominal muscle contractions recorded during each 10-min distension period. Data are reported as the mean ± standard error (SEM) for each treatment group and comparisons between the three treatment groups were made using anova testing followed by Tukey–Kramer post-test for comparisons between controls, random and paired groups. Significant difference was inferred when the Tukey–Kramer test resulted in a P-value of ≤0.05.
RESULTS
Our goal was to examine the relationship between early life trauma and colonic hypersensitivity in later life using a rodent model.
Behavioural testing
Following shock-odour conditioning treatments and as outlined in the material and methods section, behavioural testing was conducted on PN13 using a Y-maze. As illustrated in Fig. 1, during testing only pups in the paired odour-shock group learned an odour preference [F (2,15) = 12.727, P < 0.001], replicating previous results.44-46 After reaching adulthood, rats were subjected to dark-light emergence testing in which the latency to enter and total time spent in the light compartment were measured (n = 6/group). As illustrated in Fig. 2, adult rats that had received unpaired odour-shock treatment as neonates took significantly longer to emerge from the dark compartment into the light compartment (A) and spent significantly less time in the light (B) than both the paired group and the odour-only control groups [latency F (2,15) = 3.757, P < 0.05; total time F (2,15) = 3.769, P < 0.05].
Figure 1.
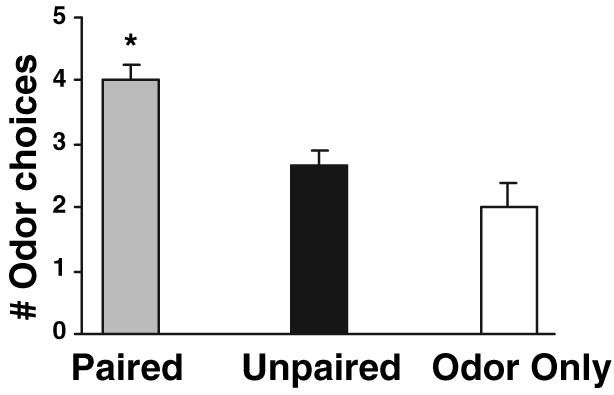
Neonatal experience with paired odour-shock resulted in an odour preference. In neonatal pups, 5 days of pairing odour and aversive shock (0.5 mA) resulted in a relative odour preference during infancy. Unpaired odour-shock and odour-only learning controls did not exhibit learning in the 2-odour choice Y-maze test (*P < 0.01 from both unpaired and odour-only treatment groups).
Figure 2.
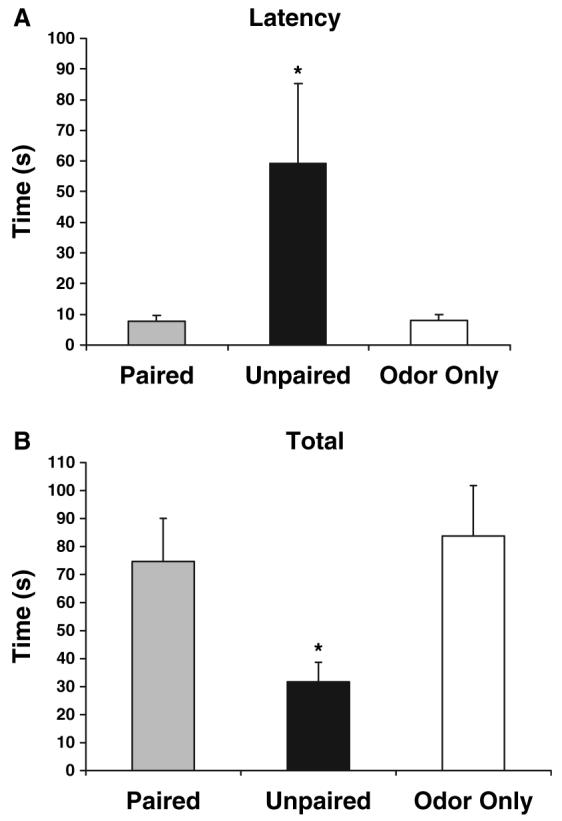
Neonatal experience with unpaired odour-shock enhances anxiety in adult rats. Adult rats previously exposed to neonatal unpredictable neonatal pain showed a significantly higher latency to enter (A) and total time spent (B) in a light box. Adult rats that received paired odour-shock or the odour-only treatments as neonates did not display the same delay to enter and remain in a light box (*P < 0.05 from both paired and odour-only treatment groups).
Colonic sensitivity assessment
In adult male and female rats (age = 6–7 months, weight = 489 ± 28 g), the level of colonic sensitivity was determined in response to mechanical distension of the colon. As illustrated in Fig. 3, in mature rats that received odour-only control treatment as neonates (n = 4), distension of the colon induced a pressure-dependent increase in the VMR quantified as an increase in the number of abdominal muscle contractions in response to CRD. In mature rats that received the unpaired (unpredictable) shock treatment as neonates (n = 6), there was an exaggerated response to CRD that was statistically different at distension pressures of 40 and 60 mmHg from the odour-only control animals. In contrast, in mature rats that received odour-shock treatments as neonates, which were paired (n = 6), there was a trend toward a reduction in the VMR to CRD compared to the odour-only controls, which was statistically significant at the lowest distension pressure of 20 mmHg. In these experiments, there was no difference in the VMR to CRD between males (n = 9) and females (n = 7) [odour only: 20 mmHg male = 8.3 ± 1.2 vs female = 9, 40 mmHg male = 14.7 ± 0.7 vs female = 15, 60 mmHg male = 24.3 ± 0.9 vs female = 22 abdominal contractions/10 min); odour-shock unpaired: 20 mmHg male = 10.5 ± 1.8 vs female = 11.5 ± 1.5, 40 mmHg male = 22.8 ± 2.9 vs female = 19.5 ± 0.5, 60 mmHg male = 32.5 ± 3.7 vs female = 30.0 ± 3.0 abdominal contractions/10 min); odour-shock paired: 20 mmHg male = 5.5 ± 1.8 vs female = 5.3 ± 1.3, 40 mmHg male = 10.5 ± 1.0 vs female = 12.3 ± 1.1, 60 mmHg male = 18.5 ± 3.0 vs female = 21.8 ± 1.2 abdominal contractions/10 min].
Figure 3.
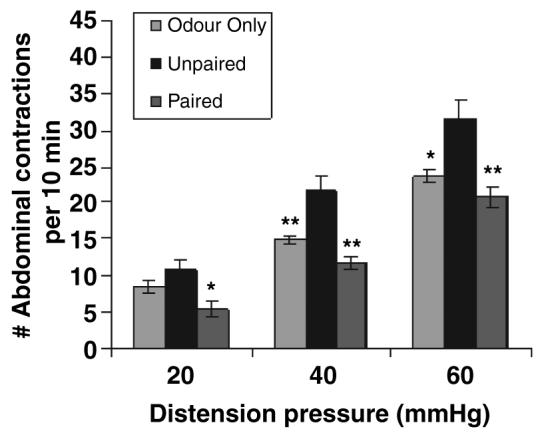
Neonatal experience with unpaired odour-shock enhances colorectal sensitivity in adult rats. In adult rats previously exposed to neonatal unpredictable pain, the level of colorectal sensitivity to luminal distension was assessed. Colorectal distension at pressures of 20, 40 and 60 mmHg for 10 min in the unpaired odour-shock group exhibited a greater visceromotor behavioural response (measured as the number of abdominal contractions per 10 min distension period), when compared to the odour-only and paired odour-shock treatment groups (*P < 0.05; **P < 0.01 from unpaired treatment group).
DISCUSSION
Abdominal pain is the major symptom in IBS and is due in part to a heightened sensitivity to luminal distension.1-3. There is heightened visceral sensory perception (hyperalgesia) in IBS with gastrointestinal distension evoking pain at lower distension pressures compared to asymptomatic control subjects. Visceral hyperalgesia in IBS patients has been postulated to develop as a result of an earlier sensitizing event (i.e. stress/anxiety or peripheral inflammation). As a result, a manifestation of these sensitizing events is that responses to CRD are accentuated in animals and IBS patients with visceral hypersensitivity.4,12
While the causes of IBS remain elusive, an association exists with psychosocial factors such as childhood abuse.6,16,17,27,28,52 In the current study, the IBS-infant trauma relationship, within the context of predictable vs unpredictable trauma, was assessed using a model of odour conditioning. We confirmed that during the testing only pups in the paired odour-shock group learned the odour preferences. This odour conditioning appears to mimic learning the maternal odour, which controls pups orientation to the mother.53 Interestingly, attachment under adverse experiences has also been demonstrated in a wide range of species including chick, dogs, as well as non-human and human primates.54-56 Learning limitations have also been documented in infant dogs, which continue to approach a human attendant who has shocked and mishandles the puppies.57 Similar rough handling by the caregiver in non-human primates produces approach responses in the dependent infant.54,58,59 Finally, similar to our present results, young rat pups show attenuated inhibitory conditioning and passive avoidance learning.60,61
In the current study, colonic sensitivity was measured in the postnatal-treated rats when they reached adulthood. The results show a correlation between early unpredictable trauma and adult heightened sensitivity to luminal distension, characteristic of IBS. Specifically, we demonstrate that there is a significant difference between the long-term effects of predictable vs unpredictable stress as a neonate. The potential mechanisms responsible for the increase in visceral sensitivity in our unpredictable group and the decrease in visceral sensitivity in the predictable shock group require further investigation. However, when our data are integrated into the existing literature, two potential mechanisms emerge to explain our findings. First, differences in emotion/anxiety mediated through the corticotropin (CORT) and CRF-mediated mechanisms could account, at least in part, for group differences. It is likely that effects related to fear-conditioning and pain responses are due to alterations in the brain structures such as the hippocampus, cortex, periaqueductal grey, paraventricular nucleus (PVN) of the hypothalamus, amygdala and brain stem, many of which are altered by pain experienced in early development.8,62,63 In support of a possible involvement of the amygdala, we have shown that direct CORT implantation onto the central amygdaloid nucleus (CeA), which communicates directly with the PVN and other PVN-connected structures, produces colonic hypersensitivity in rodents.24-26
The effects of early life stress and trauma produces profound and enduring effects on both brain and behaviour. These effects have been documented using paradigms such as maternal deprivation and isolation paradigms, where pups are separated from the removed from the nest and mother for prolonged periods of time. These manipulations modify emotional expression, including modifications of fear, learning, CORT/CRF expression and associated limbic structures.29,32,33,64-66 Indeed in our assessment of anxiety, the unpaired group, with visceral hypersensitivity as adults, showed delayed emergence from the dark box as compared to the unshocked odour-only controls and predictable shock groups. The data showing that unpaired conditioned rats display higher levels of anxiety are also consistent with the clinical literature where emotional stressors and increased anxiety often exacerbate symptoms in IBS. Psychosocial problems that increase anxiety, such as a history of physical, emotional and sexual abuse are also more frequent in IBS patients than in those that have organic disease of the gastrointestinal tract.6,16,17,27,28,52 These findings are also consistent with the maternal deprivation/separation model of early life trauma that has already demonstrated effects on emotionality/anxiety probably mediated through the CORT stress system/CRF and the amygdala.35,35 Furthermore, our previous work and that of others has clearly demonstrated that the amygdala is involved in visceral hypersensitivity perhaps mediated through CORT and CRF targeting the amygdala.24-26 However, increased anxiety was not seen in the paired odour-shock (predictable) treatments, which showed some reduction in the VMR compared to the control group at the lowest distension pressure of 20 mmHg. A second potential mechanism to explain different visceromotor responses to CRD between the infant experience groups is that the colonic mucosal function in response to stress may itself have been altered differentially by predictable and unpredictable shock. Indeed, a low-grade colonic inflammation may play a role in the pathogenesis of IBS.67 Moreover, the maternal deprivation/separation model of infant trauma, known to induce colonic hypersensitivity, has been associated with intestinal epithelial barrier disruption increasing permeability for macromolecules in response to stress and mucosal immune changes such as increases in mast cell density.41,68 Furthermore, in support of a link between stress and mast cell function, Pavlovian conditioning has been shown to induce nasal tryptase release by mast cells in humans.70 and rat mast cell protease II.70 A third potential explanation for the differences observe between the predictable and unpredictable shock is that differences in noradrenaline turnover in different brain regions may have occurred.71,72 Taken together these findings provide possible explanations concerning differences in unpredictable and predictable shock stress effects known to contribute to adult somatic function and the gastrointestinal tract.15 However, because early life stress and trauma produces profound and enduring effects on both brain and behaviour, further experiments are required to delineate causation. An interesting and possibly important observation was the decrease in visceral sensitivity, characterized as a reduction in the VMR to CRD at the lowest distension pressure (20 mmHg) observed in adult rats exposed to predictable shock compared to odour-only controls. This finding suggests that at levels of luminal distension considered non-nociceptive, predictable early life trauma may also affect colorectal sensitivity.
In summary, the current study demonstrates in a rodent model that unpredictable, but not predictable early life trauma produces colonic hypersensitivity in adulthood. Taken together, this study has possible implications for the long-term effects of infant trauma and suggests a potentially important role for the context of trauma in somatic health outcome.
ACKNOWLEDGMENTS
This work was supported by grants NIH-NICHD-HD33402, NSF-IOB-0544406 and OCAST HR05-114 to RS.
REFERENCES
- 1.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–32. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–92. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 3.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–93. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 5.Gaynes BN, Drossman DA. The role of psychosocial factors in irritable bowel syndrome. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:437–52. doi: 10.1053/bega.1999.0038. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA. Do psychosocial factors define symptom severity and patient status in irritable bowel syndrome? Am J Med. 1999;107:41S–50S. doi: 10.1016/s0002-9343(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard EB, Scharff L. Psychosocial aspects of assessment and treatment of irritable bowel syndrome in adults and recurrent abdominal pain in children. J Consult Clin Psychol. 2002;70:725–38. doi: 10.1037//0022-006x.70.3.725. [DOI] [PubMed] [Google Scholar]
- 8.Kendall-Tackett KA. Physiological correlates of childhood abuse: chronic hyperarousal in PTSD, depression, and irritable bowel syndrome. Child Abuse Negl. 2000;24:799–810. doi: 10.1016/s0145-2134(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 9.Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology. 2000;122:1701–14. doi: 10.1053/gast.2002.33741. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 11.Mayer EA, Naliboff BD, Chang L, Countino SV. Stress and irritable bowel syndrome. Am J Physiol. 2001;280:G519–24. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 12.Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hyper-sensitivity in high-anxiety rats. Physiol Behav. 2000;69:379–82. doi: 10.1016/s0031-9384(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 13.Tache Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol. 1999;13:18A–25A. doi: 10.1155/1999/375916. [DOI] [PubMed] [Google Scholar]
- 14.Seligman ME, Meyer B. Chronic fear and ulcers in rats as a function of the unpredictability of safety. J Comp Physiol Psychol. 1970;73:202–20. doi: 10.1037/h0030219. [DOI] [PubMed] [Google Scholar]
- 15.Weiss JM. Somatic effects of predictable and unpredictable shock. Psychosom Med. 1970;32:397–408. doi: 10.1097/00006842-197007000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Lowman BC, Drossman DA, Cramer EM, McKee DC. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 1987;9:324–30. doi: 10.1097/00004836-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 18.Ader R. Social factors affecting emotionality and resistance to disease in animals: III. Early weaning and susceptibility to gastric ulcers in the rat. A control for nutritional factors. J Comp Physiol Psychol. 1962;55:600–2. doi: 10.1037/h0041164. [DOI] [PubMed] [Google Scholar]
- 19.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 20.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–8. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- 21.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotrophin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 22.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–95. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 23.Hatalski CH, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–9. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 2000;893:135–42. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- 25.Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol. 2003;89:1343–52. doi: 10.1152/jn.00834.2002. [DOI] [PubMed] [Google Scholar]
- 26.Myers DA, Gibson M, Schulkin J, Greenwood-Van Meerveld B. Corticosterone implants to the amygdala and type 1 CRH receptor regulation: effects on behavior and colonic sensitivity. Behav Brain Res. 2005;161:39–44. doi: 10.1016/j.bbr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Talley JN, Fett SL, Zinsmeister AR, Melton LJ. Gastrointestinal tract symptoms and self-reported abuse: A population-based study. Gastroenterology. 1994;107:1040–9. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 28.Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: Association with irritable bowel-type symptoms. Am J Gastroenterol. 1995;90:366–71. [PubMed] [Google Scholar]
- 29.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–11. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18:10171–9. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teicher MH, Anderson SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 33.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 35.Ackerman SH, Hofer MA, Weiner H. Early maternal separation increases gastric ulcer risk in rats by producing a latent thermoregulatory disturbance. Science. 1978;201:373–6. doi: 10.1126/science.566471. [DOI] [PubMed] [Google Scholar]
- 36.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–6. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol. 2002;282:G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 38.Milde AM, Enger O, Murison R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiol Behav. 2004;81:71–84. doi: 10.1016/j.physbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Rosztoczy A, Fioramonti J, Jarmay K, Barreau F, Wittmann T, Bueno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol Motil. 2003;15:679–86. doi: 10.1046/j.1350-1925.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwetz I, McRoberts JA, Coutinho SV, et al. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol. 2005;289:G704–12. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- 41.Soderholm JD, Yates DA, Gareau MG, Yang PC, Mac-Queen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol. 2002;283:G1257–63. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 42.Levine S, Chevalier JA, Korchin SJ. The effects of early shock and handling on later avoidance learning. J Pers. 1956;24:475. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 43.Fride E, Weinstock M. The effects of prenatal exposure to predictable or unpredictable stress on early development in the rat. Dev Psychobiol. 1984;17:651–60. doi: 10.1002/dev.420170607. [DOI] [PubMed] [Google Scholar]
- 44.Moriceau S, Sullivan RM. Maternal presence serves to switch between attraction and fear in infancy. Nat Neurosci. 2006;9:1004–6. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–48. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive period learning. Behav Neurosci. 2004;118:274–81. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–69. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 48.Langlois A, Pacaud X, Junien JL, Dahl SG, Riviere PJ. Response heterogeneity of 5-HT3 receptor antagonists in a rat visceral hypersensitivity model. Eur J Pharmacol. 1996;318:141–4. doi: 10.1016/s0014-2999(96)00857-6. [DOI] [PubMed] [Google Scholar]
- 49.Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. 1997;273:G191–6. doi: 10.1152/ajpgi.1997.273.1.G191. [DOI] [PubMed] [Google Scholar]
- 50.Greenwood-Van Meerveld B, Campbell-Dittmeyer K, Johnson AC, Hicks GA. 5-HT2B receptors do not modulate sensitivity to colonic distension in rats with acute colorectal hypersensitivity. Neurogastroenterol Motil. 2006;18:343–5. doi: 10.1111/j.1365-2982.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 51.Greenwood-Van Meerveld B, Venkova K, Hicks G, Dennis E, Crowell MD. Activation of peripheral 5-HT receptors attenuates colonic sensitivity to intraluminal distension. Neurogastroenterol Motil. 2006;18:76–86. doi: 10.1111/j.1365-2982.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 52.Salmon P, Skaife K, Rhodes J. Abuse, dissociation, and somatization in irritable bowel syndrome: towards an explanatory model. J Behav Med. 2003;26:1–18. doi: 10.1023/a:1021718304633. [DOI] [PubMed] [Google Scholar]
- 53.Hofer MA, Sullivan RM. Toward a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 599–616. [Google Scholar]
- 54.Harlow HF, Harlow MK. The affectional systems. In: Schrier A, Harlow HF, Stollnitz F, editors. Behavior of Non-human Primates. Vol. 2. Academic Press; New York: 1965. Chapter in book. [Google Scholar]
- 55.Helfer ME, Kempe RS, Krugman RD. The Battered Child. University of Chicago Press; Chicago: 1997. [Google Scholar]
- 56.Salzen EA. Imprinting and environmental learning. In: Aronson LR, Tobach E, Lehrman DS, Rosensblatt J, editors. Development and Evolution of Behavior. W.H. Freeman; San Francisco: 1970. Chapter in book. [Google Scholar]
- 57.Rajecki DW, Lamb ME, Obmascher P. Towards a general theory of infantile attachment: A comparative review of aspects of the social bond. Behav Brain Sci. 1978;3:417–64. [Google Scholar]
- 58.Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev Psychobiol. 1999;34:29–35. [PubMed] [Google Scholar]
- 59.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 60.Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Dev Psychobiol. 1980;13:513–8. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- 61.Myslivecek J. Inhibitory learning and memory in newborn rats. Prog Neurobiol. 1997;53:399–430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 62.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 63.Scarinci IC, McDonald-Haile J, Bradley LA, Richter JE. Altered pain perception and psychosocial features among women with gastrointestinal disorders and history of abuse: a preliminary model. Am J Med. 1994;97:108–18. doi: 10.1016/0002-9343(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 64.Coplan JD, Andrews MW, Rosenblum LA, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the patho-physiology of mood and anxiety disorders. Proc Natl Acad Sci U S A. 1996;93:1619–23. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79:471–8. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 66.Tang AC, Reeb BC, Romeo RD, McEwen BS. Modification of social memory, hypothalamic-pituitary-adrenal axis, and brain asymmetry by neonatal novelty exposure. J Neurosci. 2003;23:8254–60. doi: 10.1523/JNEUROSCI.23-23-08254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins SM, Barbara G, Vallance B. Stress, inflammation and the irritable bowel syndrome. Can J Gastroenterol. 1999;13:47A–9A. doi: 10.1155/1999/916075. [DOI] [PubMed] [Google Scholar]
- 68.Barreau F, Cartier C, Leveque M, et al. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: CRF and NGF interplay. J Physiol. 2007;580:347–56. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gauci M, Husband AJ, Saxarra H, King MG. Pavlovian conditioning of nasal tryptase release in human subjects with allergic rhinitis. Physiol Behav. 1994;55:823–5. doi: 10.1016/0031-9384(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 70.MacQueen G, Marshall J, Perdue M, Siegel S, Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989;243:83–5. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka M. Emotional stress and characteristics of brain noradrenaline release in the rat. Ind Health. 1999;37:143–56. doi: 10.2486/indhealth.37.143. [DOI] [PubMed] [Google Scholar]
- 72.Tsuda A, Ida Y, Satoh H, Tsujimaru S, Tanaka M. Stressor predictability and rat brain noradrenaline metabolism. Pharmacol Biochem Behav. 1989;32:569–72. doi: 10.1016/0091-3057(89)90198-6. [DOI] [PubMed] [Google Scholar]


