Abstract
Background
AMP-activated protein kinase (AMPK) is a heterotrimeric enzyme that is evolutionarily conserved from yeast to mammals and functions to maintain cellular and whole body energy homeostasis. Studies in experimental animals demonstrate that activation of AMPK in skeletal muscle protects against insulin resistance, type 2 diabetes and obesity. The regulatory γ3 subunit of AMPK is expressed exclusively in skeletal muscle; however, its importance in controlling overall AMPK activity is unknown. While evidence is emerging that gamma subunit mutations interfere specifically with AMP activation, there remains some controversy regarding the impact of gamma subunit mutations [1]–[3]. Here we report the first gain-of-function mutation in the muscle-specific regulatory γ3 subunit in humans.
Methods and Findings
We sequenced the exons and splice junctions of the AMPK γ3 gene (PRKAG3) in 761 obese and 759 lean individuals, identifying 87 sequence variants including a novel R225W mutation in subjects from two unrelated families. The γ3 R225W mutation is homologous in location to the γ2R302Q mutation in patients with Wolf-Parkinson-White syndrome and to the γ3R225Q mutation originally linked to an increase in muscle glycogen content in purebred Hampshire Rendement Napole (RN-) pigs. We demonstrate in differentiated muscle satellite cells obtained from the vastus lateralis of R225W carriers that the mutation is associated with an approximate doubling of both basal and AMP-activated AMPK activities. Moreover, subjects bearing the R225W mutation exhibit a ∼90% increase of skeletal muscle glycogen content and a ∼30% decrease in intramuscular triglyceride (IMTG).
Conclusions
We have identified for the first time a mutation in the skeletal muscle-specific regulatory γ3 subunit of AMPK in humans. The γ3R225W mutation has significant functional effects as demonstrated by increases in basal and AMP-activated AMPK activities, increased muscle glycogen and decreased IMTG. Overall, these findings are consistent with an important regulatory role for AMPK γ3 in human muscle energy metabolism.
Introduction
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is evolutionarily conserved from yeast to mammals and functions as a highly responsive energy sensor in cells [4]–[7]. It acts both centrally and peripherally to coordinate multiple inputs from various hormones, peptides and nutrients to maintain overall energy homeostasis. In response to cellular stress in the periphery, AMPK inhibits anabolic pathways and stimulates catabolic pathways to restore cellular energy charge. In vitro and experimental animal studies have demonstrated that altered activity of AMPK has significant implications for a number of cardiovascular risk factors, including type 2 diabetes [4]–[6]. In skeletal muscle, AMPK activation stimulates glucose uptake, fatty acid uptake and oxidation, and mitochondrial biogenesis [5]. Thus AMPK activation in skeletal muscle protects against the development of insulin resistance, type 2 diabetes and obesity [8]–[11].
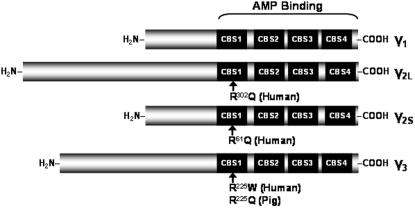
AMPK has a heterotrimeric structure and is composed of an α-catalytic, a β-regulatory and a γ-regulatory subunit. The α-catalytic subunit has two isoforms (α1, α2), the β-regulatory subunit has two isoforms (β1, β2), and the γ-regulatory subunit has three isoforms (γ1, γ2, γ3) [12], [13]. Figure 1 depicts the three γ isoforms and their structural characteristics. The various isoforms of each subunit are encoded by distinct genes, with alternative promoters and splice variants, adding to genetic and post-translational complexities [5]. Most isoforms are expressed to some extent in all tissues, although mRNA expression data indicate that the γ3 subunit is more abundant in skeletal muscle than in any other tissue [14]. The relative proportions of the γ subunits in human skeletal muscles are as yet unknown. Several exercise studies in humans have measured γ subunit protein levels in the vastus lateralis muscle. In these studies, protein levels were expressed as percentage of sedentary controls, pretraining values, or untrained muscles as opposed to absolute relative values [15]–[17]. A more recent study provided evidence that only 3 out of the 12 possible AMPK complex combinations are expressed in human vastus lateralis: α1/β2/γ1, α2/β2/γ1 and α2/β2/γ3 [18] . The only heterotrimer shown to be phosphorylated and activated during high intensity exercise was the complex containing the γ3 isoform. The activity associated with the other two heterotrimers either remained unchanged or decreased during exercise [18]. The γ2 long and short isoforms (γ2L and γ2S, respectively) result from alternative splicing of a common transcript in heart and other tissues [19]–[21]. While both long and short isoforms are expressed in heart, γ2S is predominant in skeletal muscle [19].
Figure 1. γ subunit isoforms showing the 4 tandem repeats of CBS domains responsible for AMP and ATP binding.
Locations of the human R225W and the porcine R225Q mutations in the γ3 subunit as well as the R302Q and R61Q mutations in the γ2 long and short forms, respectively, are indicated. Figure adapted from [12].
To date the only published mutations in the AMPK genes in humans are in the γ2-regulatory subunit, the predominant γ isoform in cardiac muscle. While AMPK activity stimulates fatty acid oxidation, glucose uptake and glycolysis in the heart, its regulation is complex, and still not well understood [2], [3], [22]. At least six different missense mutations within the CBS domains of γ2 have been shown to cause the excess storage of glycogen in pronounced glycogen-associated vacuoles[23]. This excess storage of glycogen results in a complex phenotype, including Wolf-Parkinson-White (WPW) syndrome, conduction system disease and cardiac hypertrophy [21], [24], [25]. In purebred Hampshire Rendement Napole (RN) pigs, a naturally occurring R225Q mutation in the γ3 subunit of AMPK was identified and linked to increased glycogen storage in skeletal muscle [26] and to increased resynthesis of glycogen after exercise when R225Q AMPKγ3 is overexpressed in transgenic mice [7].
In the context of our ongoing goal of identifying genes that underlie predisposition for diabetes and obesity, we searched for genetic variation in human PRKAG3. Of the 24 novel coding variants identified, we focused on the R225W mutation due to the established importance of the mutation in porcine AMPK γ3 and the homologous mutations in the γ2 subunit of human AMPK. We determined the functional significance of this mutation in differentiated muscle cells from the vastus lateralis of R225W carriers compared to matched controls.
Here we describe a previously unreported R225W mutation in the human AMPK γ3 gene, PRKAG3, found in two independent kindreds. We show that the R225W mutation causes increased basal and AMP-activated AMPK activity, as well as increased muscle glycogen storage and decreased intramuscular triglyceride (IMTG).
Methods
Subjects and Sequence Analysis
The coding exons and splice junctions of the human AMPK γ3 gene (NCBI accession no. NM_017431) were resequenced in obese Caucasian subjects from the Ottawa Hospital Weight Management Clinic and in lean healthy Caucasian individuals who participated in a study of leanness at the University of Ottawa Heart Institute. The cohorts consisted of 761 obese subjects with a mean BMI of 39.9 kg/m2 and>90th percentile adjusted for age and sex, and 759 lean subjects with a mean BMI of 20.1 kg/m2 and<25th percentile adjusted for age and sex. The study was approved by the institutional review boards of the University of Ottawa Heart Institute and the Ottawa Hospital. Informed written consent was obtained from all participants. A total of 58 genes were sequenced as part of this study and detailed findings have been presented elsewhere [27]. Selection criteria for our patient population as well as methods for DNA isolation, sequencing and data analysis have been described previously [28].
Predictions of the possible functional implications of the variants were made using PolyPhen (http://genetics.bwh.harvard.edu/pph/) and SIFT (http://blocks.fhcrc.org/sift/SIFT.html) programs [29], [30]. The R225W variant, which is found in the second CBS domain (Figure 1), was identified to be functionally damaging by both programs. This variant was intriguing since it is analogous to the R225Q mutation causing increased muscle glycogen content in pigs, as well as having the same amino acid location as the mutations in the human γ2 subunit that are known to alter AMPK protein function and cause Wolf-Parkinson-White syndrome. We thus contacted subjects that were identified as carrying the R225W mutation in the initial study cohorts, and screened their available family members for further study (Figure 2). The region of the AMPKG3 gene with the R225W variation was sequenced in DNA samples from family members to determine whether they carried the same variation. Subject characteristics are described in Table 1.
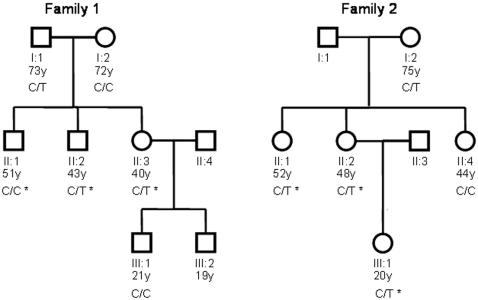
Figure 2. Families of the two probands with the PRKAG3-R225W mutation.
*Subjects who underwent muscle biopsy.
Table 1. Characteristics of PRKAG3-R225W subjects and controls who underwent muscle biopsy.
| R225W (n = 5) | Controls (n = 5) | |
| Age (years) | 40.6±12.4 | 42.8±12.9 |
| Weight (kg) | 82.5±23.2 | 80.9±27.1 |
| BMI (kg/m2) | 29.1±9.0 | 29.2±10.3 |
| Total Cholesterol (mmol/L) | 5.54±0.70 | 4.52±0.66 |
| Body Fat (%) | 34.6±10.4 | 35.6±8.7 |
| Basal Metabolic Rate (kJ) | 6773.8±1201.1 | 5380.4±2134.9 |
| HDL-C (mmol/L) | 1.29±0.26 | 1.52±0.70 |
| LDL-C (mmol/L) | 3.22±0.47 | 2.55±0.35 |
| TC:HDL | 4.40±0.71 | 3.44±1.24 |
| Triglycerides (mmol/L) | 2.27±1.00 | 1.11±0.61 |
| Glucose (mmol/L) | 4.94±0.61 | 5.16±0.63 |
| Insulin (pmol/L) | 54.2±10.1 | 47.4±35.8 |
| HbA1C | 0.0522±0.0031 | 0.0544±0.0013 |
Blood chemistry measurements were conducted following an overnight fast. Means +/− SD. n = 5, Student's t-test, no significant differences between R225W and control subjects.
Skeletal Muscle Biopsies and Cell Culture
Five subjects with the R225W mutation and five control subjects were matched for gender, age, weight, BMI, as well as amount and intensity of self-reported weekly physical activity. These subjects refrained from physical activity for three days prior to undergoing additional studies in the post-absorptive state after an overnight fast. Percutaneous skeletal muscle biopsies of the vastus lateralis muscle were obtained from R225W carriers and their matched controls using a 5 mm Bergstrom needle (Opitek) under local anaesthesia with 1% lidocaine. Vastus lateralis is commonly selected for studies of human muscle metabolism and was chosen for biopsy in the current study due to the demonstrated importance of the γ3 subunit in this muscle [18]. A 10 mg sample of the biopsy was flash frozen in O.C.T. compound for muscle fiber typing, glycogen content determination and IMTG determination. A 25 mg sample of tissue was subjected to trypsin digestion and cells were grown to ∼70% confluency in Ham's F10 media (supplemented with 1% antibiotic-antimycotic, 2.5 µg/mL gentamycin, 12.4% FBS, 0.41 mg/mL BSA, 826 nM dexamethazone, 8.3 ng/mL epidermal growth factor, 0.41 mg/mL fetuin, and 25 pmol insulin). Isolation of satellite cells was achieved via immuno-sorting using primary anti-5.1H11 (Developmental Studies Hybridoma Bank) and secondary MACS goat anti-mouse IgG microbeads. After the suspension was passed through a magnetic field, labelled cells were isolated, eluted and then grown up in culture. Upon reaching 80% confluency, cells were differentiated for ∼10 days in Dulbecco's modified Eagle's medium (supplemented with 2% horse serum, 1% antibiotic-antimycotic and 2.5 µg/mL gentamycin).
AMPK Isolation
Approximately 10 days after differentiation, myotubes were incubated on ice while shaking in the presence of lysis buffer (30 mM HEPES, 2.5 mM EGTA, 3 mM EDTA, 70 mM KCl, 0.1% Nonidet P-40, 20 mM β-glycerophosphate, 20 mM NaF, 2 mM NaPPi, 1 mM Na3VO4, 200 µM PMSF and 1 µM pepstatin A). Partial purification of AMPK from the cell lysate was carried out as detailed in [31], [32]. The cell lysate was incubated at a 1∶1 ratio with 30% polyethylene glycol 6000 for 1 hour. Resulting purified protein was dissolved in assay buffer (62.5 mM Na HEPES, 62.5 mM NaCl, 62.5 mM NaF, 6.25 mM Na pyrophosphate, 1.25 mM EDTA, 1.25 mM EGTA, 1 mM dithiothreitol, 1 mM PMSF, 5 µg/mL SBTI and 1 µM pepstatin A) and then quantified using the bicinchoninic assay.
AMPK Activity Determination
The SAMS peptide assay was carried out as detailed in [32], [33] with minor modifications: 13 µL reaction buffer (20 mM HEPES-NaOH, 0.4 mM dithiothreitol, 0.01% Brij-35 with or without 300 µM AMP), 10 µL of 562 µM SAMS substrate peptide, 10 µCi [32P]ATP (3000 Ci/mmol), and 6 µg of partially purified AMPK were incubated with constant shaking at 30°C for 15 minutes. Negative controls consisted of all reagents except SAMS substrate peptide and AMP. All reactions were carried out in triplicate. AMPK activity was expressed as pmol of 32P-radiolabeled phosphate incorporated into the SAMS peptide per minute per mg of protein.
Quantitative RT-PCR Analysis
RNA from muscle biopsy samples was extracted using Trizol (Invitrogen) and treated with DNaseI (Ambion). cDNA was generated from 0.5 µg of RNA using random decamer primers (Ambion) with MMLV reverse transcriptase (Invitrogen). Amplicons of AMPKG3 and GAPDH genes were cloned into pCR 2.1-TOPO (Invitrogen). Serial dilutions of these constructs were used to generate standard curves, following assessment of primer efficiency and analysis of melting curve profiles. Quantitative real-time PCR analysis was performed in a LightCycler system (Roche) using FastStart DNA MasterPLUS SYBR Green I kit (Roche). The primers for real-time PCR were designed using LightCycler Probe Design Software v1.0 (Roche). Following 30 cycles of amplification with 1.0 µl of cDNA and 0.5 µM of F and R primers per reaction, expression of AMPKG3 was normalized to expression of GAPDH. Primer sequences: F: 5′-CAGAGGACACTATGTCTGG-3′ and R: 5′-GCTTGGGATTGAGGACT-3′ (AMPKG3), F: 5′-GATTTGGCCGTATTGGG-3′ and R: 5′-TCCACGACATACTCAGC-3′ (GAPDH).
Western Blotting and Densitometry
To determine protein levels of the γ3 isoform, the same purified protein extracts that were used for the AMPK activity assay were subjected to immunoblotting. Electrophoresis was carried out using a 12% polyacrylamide gel. Following transfer to nitrocellulose membranes, primary antibodies were incubated at a 1∶1000 dilution overnight at 4°C (AMPKγ1, AMPKγ3 and β-actin antibodies were from Cell Signaling Technology, Danvers, MA). The secondary antibody was a peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Inc., Santa Cruz, CA) and was incubated at a 1∶500 dilution for one hour at room temperature. For detection, blots were processed using enhanced chemiluminescence kits (Amersham Pharmacia; Baie d'Urfe, QC, Canada). β-actin was used as the loading control and densitometry was carried out to determine relative protein levels (values expressed as γ isoform integrated densitometry value (IDV) divided by β-actin IDV).
Muscle Glycogen Content Determination
Glycogen content was determined using Periodic Acid Schiff (PAS) staining. Frozen tissues were cut as 10 µm cross sections and mounted on Superfrost slides. After Schiff staining, sections were counterstained with Harris's hematoxylin. Imaging software (Northern Eclipse) was used to analyze tissues at 200× magnification with an Axiophot digital light microscope (Axiophot 2, Zeiss, Oberkochen, Germany). 2–3 fields of view in each of 3 sections per patient were analyzed for glycogen content as a proportion of surface area of each field.
Intramuscular Triglyceride (IMTG) Content
lMTG content was determined using osmium tetroxide (OsO4) staining. Frozen tissues were cut as 10 µm cross sections and mounted on Superfrost slides and fixed in 10% neutral buffered formalin. IMTG was stained black with 1% OsO4 and then cells were counterstained with hematoxylin and eosin (H&E). Imaging software (Northern Eclipse) was used to analyze tissues at 200× magnification with an Axiophot digital light microscope (Axiophot 2, Zeiss, Oberkochen, Germany). 2–3 fields of view in each of 3 sections per patient were analyzed for IMTG content as a proportion of surface area of each field.
Fiber Typing
Frozen tissues were cut as 10 µm cross sections and mounted on Superfrost slides. Sections were stained using the Vectastain ABC-AP kit (Vector Laboratories). Type 1 fibers were identified by using A4.840 as the primary antibody (Developmental Studies Hybridoma Bank), which is specific to the C-terminal part of the beta slow myosin heavy chain in type I skeletal muscle fibers. Biotinylated goat anti-mouse IgG (BA-9200, Vector Laboratories) was used as the secondary antibody, and then cells were stained red using SK-5100 (Vector Laboratories). Type 2a fibers were identified by using N2.261 as the primary antibody (Developmental Studies Hybridoma Bank), which is specific to the N-terminal part of the beta slow myosin heavy chain in adult skeletal myosin heavy chain type IIA. BA-9200 was used as the secondary antibody, and then cells were stained blue using SK-5300 (Vector Laboratories). Type 2b/x fibers were identified as unstained cells. Fiber type ratios were determined by counting numbers of each fiber type in 2–3 fields of view in 3 sections per patient.
Statistical Analysis
The management of clinical and genotypic data was carried out with SAS statistical software (Version 9.1, SAS Institute Inc., Cary, NC). A one-way ANOVA with Tukey post-test was used to determine differences in AMPK activity (Figure 3). Student's t tests were used to assess mRNA levels, protein levels, glycogen content, intramuscular triglyceride and fiber type ratio (Figures 4– 7).
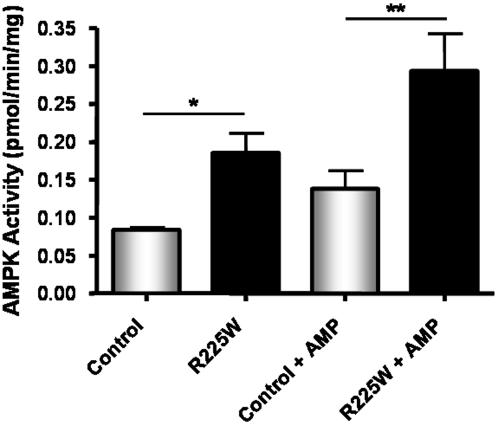
Figure 3. Basal and AMP-activated AMPK activities in differentiated skeletal muscle cells from R225W carriers and matched control subjects.
Means +/− SD. n = 3, One-way ANOVA, Tukey post-test, *p = 0.015, **p = 0.001.
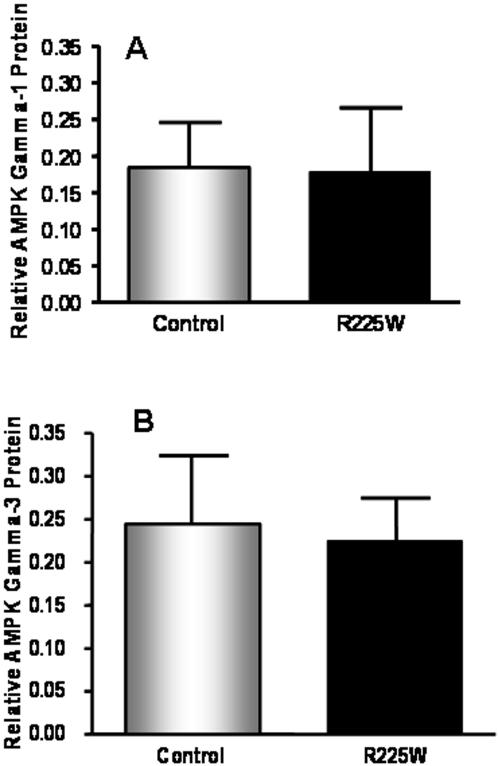
Figure 4. Relative protein levels of the γ1 (A) and γ3 (B) isoforms in differentiated skeletal muscle cells from R225W carriers and matched control subjects.
The integrated densitometry value (IDV) for each γ subunit was divided by the IDV of the loading control, β-actin. Means +/− SD. n = 2–4, Student's t-test, p = 0.389 (A), p = 0.229 (B).
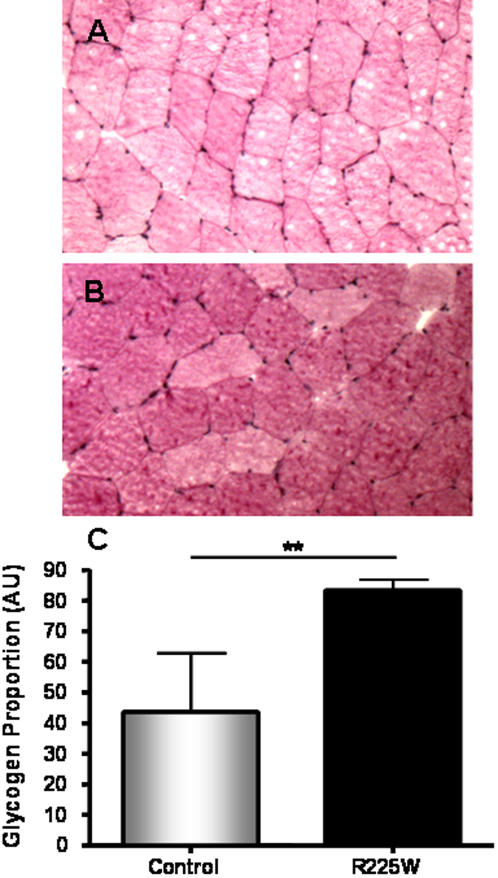
Figure 5. Glycogen content of vastus lateralis for R225W and control subjects.
Muscle sections were prepared and stained with PAS, as described in the methods section (A: control, B: R225W). C: quantification of glycogen content via imaging software. Means +/− SD. n = 5, Student's t-test, **p = 0.002.
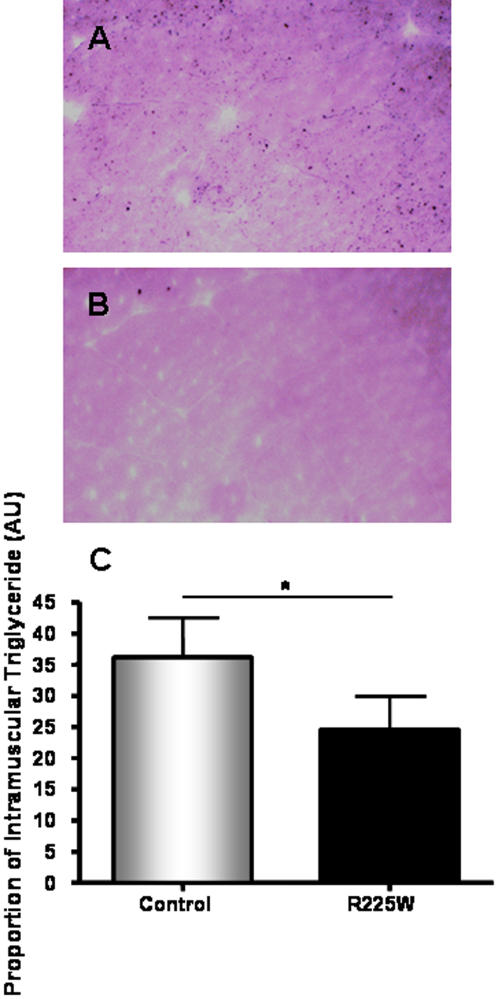
Figure 6. Intramuscular triglyceride content of vastus lateralis for R225W and control subjects.
Muscle sections were prepared and stained with OsO4, as described in the methods section (A: control, B: R225W). C: quantification of IMTG via imaging software. Means +/− SD. n = 4, Student's t-test, *p = 0.032.
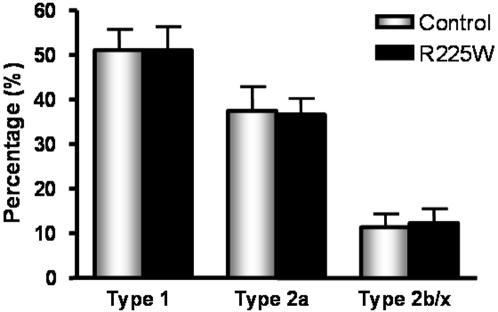
Figure 7. Fiber type ratios of vastus lateralis for R225W versus control subjects, expressed as percentage of each fiber type.
. Means +/− SD. n = 5, Student's t-test for each fiber type, no significant differences between R225W and control subjects (Type 1: p = 0.992, Type 2a: p = 0.896, Type 2b/x: p = 0.834).
Results
AMPK γ3 Sequence Variants
We sequenced the exons and intron/exon boundaries of PRKAG3, which codes for the subunit of AMPK that is specific to skeletal muscle. We studied 761 obese subjects with a mean BMI of 39.9 kg/m2 and>90th percentile adjusted for age and gender, and 759 lean subjects with a mean BMI of 20.1 kg/m2 and<25th percentile adjusted for age and gender. We identified 87 genetic variants in PRKAG3 and its flanking regions. Of the six common (minor allele frequency>0.05) variants, three were intronic polymorphisms (IVS2+45, IVS6+31 and IVS10+85), one was a synonymous variant (G180G), and two were non-synonymous variants (P71A and R340W). The 81 rare variants included one SNP in the upstream region, one in the 5′ UTR, 50 intronic SNPs, 10 synonymous and 16 nonsynonymous coding variants, one frameshift mutation that leads to a stop codon and two SNPs in the 3′ UTR. The frequency and functional prediction for the coding variants are outlined in Table 2. Of the 30 coding PRKAG3 variants observed in this study, 24 were not listed in the current SNP database (dbSNPbuild126). Computational predictions of the severity of missense substitutions were made using PolyPhen [29] and SIFT [30].
Table 2. Coding variants in the PRKAG3 gene observed in this study.
| Variant | Allele | MAF (Obese) | MAF (Lean) | S/NS | Functional Prediction | |
| PolyPhen | SIFT | |||||
| E70E | G>A | 0 | 0.001 | S | - | - |
| P71A (rs692243) | C>G | 0.179 | 0.160 | NS | possibly damaging | tolerated |
| E76Q | G>C | 0 | 0.001 | NS | benign | tolerated |
| E85E | G>A | 0.001 | 0 | S | - | - |
| T87T | C>T | 0.003 | 0.003 | S | - | - |
| D103G | A>G | 0.001 | 0 | NS | benign | tolerated |
| V107fs | indel | 0.001 | 0 | frameshift | stop codon | stop codon |
| G113V | G>T | 0 | 0.001 | NS | possibly damaging | tolerated |
| F139F (rs12328317) | C>T | 0.001 | 0.001 | S | - | - |
| L153V (rs35050588) | C>G | 0.001 | 0 | NS | benign | tolerated |
| L161P | T>C | 0.001 | 0 | NS | benign | tolerated |
| G171S | G>A | 0.001 | 0 | NS | benign | tolerated |
| P179P | C>T | 0.001 | 0 | S | - | - |
| G180G (rs35658907) | C>T | 0.026 | 0.034 | S | - | - |
| G180S | G>A | 0 | 0.001 | NS | benign | tolerated |
| Y194Y | C>T | 0.001 | 0 | S | - | - |
| M197T | T>C | 0.001 | 0 | NS | possibly damaging | affected |
| E211Q | G>C | 0.001 | 0 | NS | benign | tolerated |
| R225W | C>T | 0.001 | 0.001 | NS | probably damaging | affected |
| R225Q | G>A | 0 | 0.001 | NS | benign | tolerated |
| Q260R | A>G | 0.001 | 0.006 | NS | benign | tolerated |
| I269T | T>C | 0.001 | 0 | NS | possibly damaging | affected |
| R307C | C>T | 0.001 | 0 | NS | probably damaging | affected |
| P313P | G>A | 0.001 | 0.001 | S | - | - |
| R340W (rs33985460) | C>T | 0.053 | 0.056 | NS | probably damaging | affected |
| R340Q | G>A | 0.001 | 0 | NS | benign | tolerated |
| R446M | G>T | 0 | 0.001 | NS | possibly damaging | tolerated |
| A482V (rs34720726) | C>T | 0.002 | 0 | NS | benign | affected |
| D485N | G>A | 0.005 | 0.001 | NS | benign | tolerated |
| L487L | C>T | 0.001 | 0 | S | - | - |
(MAF: minor allele frequency; S/NS: synonymous/nonsynonymous)
Given the established importance of homologous mutations in Rendement Napole (RN-)pigs (γ3R225Q) [26], [34], [35] and in the γ2 subunit (R302Q/R61Q) in human patients [21], we pursued the functional significance of the novel R225W mutation identified in two unrelated subjects. A total of 11 subjects from the two families were screened for the R225W mutation. Two family members in the first family and three family members in the second family were observed to carry the mutation (Figure 2). Other sequence variants that are predicted to have functional implications according to PolyPhen and SIFT are shown in Table 2. Of interest are variants M197T, I269T, R307C and R340Q, all of which occur at similarly low frequencies in our cohorts.
Characteristics of R225W and Matched Control Subjects
The five carriers of the R225W variant were matched with control subjects on the basis of gender, age, weight, and BMI. The R225W carriers and control subjects were also well-matched for amount and intensity of self-reported weekly physical activity. Two brothers of the proband in the first family, and the sister and daughter of the proband in the second family consented to muscle biopsies and were included in the study (Figure 2). No significant differences were noted in fasting values for plasma lipids, glucose, insulin,or HbA1C in this small cohort (Table 1). During a hyperinsulinemic glucose clamp study, the rate of glucose infusion required to maintain euglycemia in the last hour of the clamp was somewhat higher in the 2 carriers of the R225W variant as compared to 2 closely matched controls but did not reach a nominal standard of significance (7.3±0.8 vs. 5.4±2.4 ml/kg/min, p = 0.16).
Basal and AMP-Activated AMPK Activity
We determined that the R225W mutation had an overall effect on AMPK activity. Myocytes derived from vastus lateralis biopsies were immunopurified and differentiated into myotubes, following which total AMPK activity was assayed using the ‘gold-standard’ SAMS peptide assay [32], [33] in purified protein extracts. Results demonstrated that the basal (p = 0.015) as well as the AMP-activated (p = 0.001) AMPK activities in subjects with the R225W mutation were two-fold higher compared to that found in controls (Figure 3). Control subjects exhibited a 0.054 pmol/min/mg mean increase in activity upon AMP-stimulation, whereas R225W carriers showed a mean increase of 0.108 pmol/min/mg, representing a 65% and a 58% increase from basal activity, respectively. Although R225W carriers demonstrate twice the AMP-stimulated AMPK activity of control subjects, the similar percent increases indicate that R225W AMPK is likely activated by AMP with a similar magnitude to control AMPK. R225W AMPK is therefore not constitutively active or AMP-independent, as it is able to be further activated by AMP.
Expression of AMPK γ Isoforms
AMPKγ3 mRNA levels in skeletal muscle biopsy samples were detected by RT-PCR analysis. There was no difference in AMPKγ3 mRNA levels between R225W carriers and control subjects (p = 0.083, 2-tailed t-test). AMPKγ1 and γ3 protein levels were detected by Western blotting in the same purified protein extracts as used for the AMPK activity assay. β-actin was used as the loading control and densitometry was carried out to determine relative protein levels. There were no differences in either AMPKγ1 or γ3 protein levels between R225W carriers and control subjects (p = 0.389 and p = 0.229, respectively) (Figure 4a and b). Importantly, the only three complexes that normally contribute to AMPK activity in human vastus lateralis are α1β2γ1, α2β2γ1 and α2β2γ3 [18]. We believe that the R225W variant is affecting AMPK activity without affecting the level of transcription or translation of the PRKAG3 gene.
Skeletal Muscle Glycogen Content
To determine the effect of the R225W mutation on skeletal muscle glycogen content, we used Periodic Acid Schiff (PAS) staining of cryo-sectioned vastus lateralis muscle. As shown in Figure 5, muscle of R225W carriers had approximately 90% more muscle glycogen content than controls (p = 0.002). This result was expected as the Hampshire RN- pigs carrying the R225Q γ3AMPK mutation have increased muscle glycogen content [26].
Intramuscular Triglyceride (IMTG) Content
We hypothesized that IMTG would be reduced in skeletal muscle of subjects with the R225W variant, given observations in transgenic mice expressing the homologous mutation in the AMPK γ3 gene and fed a high fat diet [7]. Osmium tetroxide (OsO4) staining of sections of the vastus lateralis muscle indicated lower IMTG levels in the R225W carriers as compared to matched controls (p = 0.032) (Figure 6).
Vastus Lateralis Muscle Fiber Type Composition
Finally, we determined whether or not there were differences in muscle fiber type composition in the biopsied muscle. Immunohistochemistry of the sections revealed a similar proportional distribution of fiber types in subjects with the R225W mutation compared to controls (Figure 7). Fiber type ratio is an important measure due to its influence on muscle glycogen content and IMTG content. Since there are no differences in fiber type ratio between R225W subjects and controls, differences in glycogen and IMTG cannot be attributed to fiber type composition.
Discussion
AMPK is widely recognized as a key metabolic regulator of energy expenditure in peripheral tissues and of energy intake in the hypothalamus. In this study we focused on the functional importance of a rare mutation in PRKAG3 encoding the AMPK regulatory γ3 subunit that is expressed exclusively in skeletal muscle. While the functions of the three AMPK subunits have not been fully characterized, it is becoming increasingly clear that the γ subunit is a major regulator of holoenzyme activity [4]–[6], [22]. The γ subunit is thought to be involved in AMP binding, and in the regulation of glycogen content as variants in its CBS domains affect glycogen metabolism in skeletal and cardiac muscle [19], [23], [25], [26], [36]. This is the first report of functional effects of nonsynonymous sequence variants in the γ3 subunit in humans. We have demonstrated that the R225W mutation increases basal and AMP-activated AMPK activities in differentiated muscle cells obtained from the vastus lateralis of carriers (Figure 3), and that γ1 and γ3 protein levels are unchanged in R225W carriers (Figure 4). Normally in human vastus lateralis, the complexes that contribute to AMPK activity are α1β2γ1, α2β2γ1 and α2β2γ3 [18]. Future studies will need to assess the subunit combinations that contribute to AMPK activity in R225W carriers. We have also demonstrated that R225W results in increased skeletal muscle glycogen content (Figure 5) and decreased IMTG content (Figure 6), while there was no change in muscle fiber type composition (Figure 7).
Further studies are required to characterize the effects of R225W on metabolic responses to challenges such as acute and chronic exercise. However, given the literature on Hampshire RN- pigs with an analogous γ3 mutation [26], [34], [35], and the Tg-R225Qγ3 mice [37], [38], it is anticipated that the human R225W variant would result in improved capacity during chronic exercise challenges due to their increased muscle glycogen. The natural occurrence of mutations in AMPKγ3 was first discovered in Hampshire RN- pigs [26], [34]. The single missense mutation, arginine 225 to glutamine (R225Q) results in a dominant mutation that is homologous to the γ3 R225W variant described here. R225Q causes an approximate 70% increase in skeletal muscle glycogen, but no change in heart and liver glycogen content, resulting in poor meat quality. Other phenotypic effects include lower muscle fat content and increased oxidative capacity. Furthermore, Hampshire RN- pigs carrying the mutation showed normal resting levels of glucose and insulin as well as normal glucose tolerance tests. When challenged with exercise, it was found that R225Q pigs depleted their glycogen stores at normal rates, but re-synthesized glycogen at faster rates than control pigs due to increased muscle glucose uptake rates [34], [35]. This limited evidence indicated no functional impairments associated with the increased glycogen in skeletal muscle. Our findings that human R225W carriers have an approximate 90% increase in skeletal muscle glycogen, an approximate 30% decrease in intramuscular triglyceride, and normal levels of plasma lipids, glucose, insulin and HbA1C are consistent with the animal studies described above. Future studies will investigate the physiological response of these subjects to exercise challenges.
Recently mice bearing the homologous human AMPK γ3 transgene, Tg-R225Q, were developed to study the effects of the naturally occurring pig mutation. The R225Q mutation produced a fully dominant protein [7] with increased AMPK activity. ACC phosphorylation was increased in the basal state as well as after stimulation with AICAR (an artificial activator of AMPK), indicating that the mutated AMPK could be activated [7]. Glycogen content of various muscles in mice bearing the R225Q mutation have 1.5–2 times the glycogen content of controls [36], consistent with our findings in humans. Moreover, exercise increased ACC phosphorylation and decreased intramuscular triglyceride (IMTG) in the gastrocnemius muscle of transgenic, compared to wild-type, mice. Tg-R225Q mice thus oxidized more fat in the AMP-stimulated state (during exercise) compared to wild-type controls [38]. As demonstrated in the current study, the R225Wγ3 mutation in humans is a gain-of-function mutation that exhibits increased basal and AMP-activated activity of AMPK. It should be noted that total cellular AMPK activity was assayed in this study, not immuno-purified γ3 activity. Therefore, the R225W mutation in γ3 has an important effect overall on total AMPK activity in skeletal muscle. It will be of interest in future studies to determine whether the α1β2γ1, α2β2γ1 and α2β2γ3 subunit combinations normally found in human vastus lateralis are also formed in skeletal muscle of R225W carriers.
Interestingly, this novel mutation is analogous to the γ2 subunit mutation that causes inherited cardiac arrhythmias and cardiac hypertrophy [21], [24], [25]. While it is known that AMPK activity stimulates fatty acid oxidation, glucose uptake and glycolysis in the heart, its regulation is complex, and still not well understood [22]. Missense mutations within the CBS domains of γ2 have been shown to cause the excess storage of glycogen in pronounced glycogen-associated vacuoles [23]. This excess storage of glycogen is thought to result in myocyte hypertrophy, and gives rise to WPW syndrome and cardiac conduction disease [23]. The enhanced skeletal muscle glycogen deposition demonstrated in carriers of the analogous γ3 mutation is consistent with the above results in the heart. Subsequent introduction of some of the γ2 mutations into the AMPK homologue in yeast (SNF1/SNF4) produced a constitutively active enzyme, indicating that these heterozygous missense mutations were dominant in their effect [25]. Our findings indicate that the γ3 R225W mutation increases basal AMPK activity, but results in a protein that can be further activated by AMP, unlike the latter γ2 mutations.
In summary, we report the identification of human subjects bearing a R225W mutation in PRKAG3 which causes increased basal and AMP-stimulated AMPK activity, increased glycogen content and decreased IMTG in skeletal muscle. These findings are relevant to an improved understanding of the regulatory role that AMPK plays in skeletal muscle energy metabolism. The γ3 subunit represents an attractive pharmacological target due to its exclusive expression in skeletal muscle. It has already been demonstrated that Metformin, commonly prescribed for treatment of type 2 diabetes, acts via stimulation of AMPK [39]–[41]; and although it is not possible to draw conclusions regarding therapeutics from this study, it is tempting to hypothesize that the stimulation of AMPK activity in skeletal muscle alone, without affecting AMPK activity in the brain or heart, could be effective in the treatment of obesity and type 2 diabetes mellitus.
Acknowledgments
We would like to thank Linda Jui for histochemistry work, Heather Doelle, Brenda Bradley and Sybil Hébert for patient recruitment and assistance with clinical studies, Thet Naing for technical assistance, and Dr. Michael Gollob for critically reading the manuscript. The authors would also like to express their sincere thanks to the subjects who participated in this research.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a grant (#NA-5413) from the Heart and Stroke Foundation of Ontario (to R. McPherson and M-E Harper) and was partially conducted at the E.O. Lawrence Berkeley National Laboratory, and performed under Department of Energy Contract DE-AC02-05CH11231, University of California.
References
- 1.Hamilton SR, Stapleton D, O'Donnell JB, Jr, Kung JT, Dalal SR, et al. An activating mutation in the gamma1 subunit of the AMP-activated protein kinase. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- 2.Burwinkel B, Scott JW, Buhrer C, van Landeghem FK, Cox GF, et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 5.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, et al. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, 3rd, et al. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 9.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 10.Buhl ES, Jessen N, Schmitz O, Pedersen SB, Pedersen O, et al. Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes. 2001;50:12–17. doi: 10.2337/diabetes.50.1.12. [DOI] [PubMed] [Google Scholar]
- 11.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 12.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 14.Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, et al. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for gamma3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E194–200. doi: 10.1152/ajpendo.00147.2003. [DOI] [PubMed] [Google Scholar]
- 15.Wojtaszewski JF, Birk JB, Frosig C, Holten M, Pilegaard H, et al. 5′AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol. 2005;564:563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E411–417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, et al. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- 18.Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 346 Pt. 2000;3:659–669. [PMC free article] [PubMed] [Google Scholar]
- 20.Lang T, Yu L, Tu Q, Jiang J, Chen Z, et al. Molecular cloning, genomic organization, and mapping of PRKAG2, a heart abundant gamma2 subunit of 5′-AMP-activated protein kinase, to human chromosome 7q36. Genomics. 2000;70:258–263. doi: 10.1006/geno.2000.6376. [DOI] [PubMed] [Google Scholar]
- 21.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, et al. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 22.Dyck JRB, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or Ally? J Physiol (Lond): 2006:jphysiol.2006.109389. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel T, Carling D. Functional analysis of mutations in the gamma 2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Biol Chem. 2002;277:51017–51024. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- 24.Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 25.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milan D, Jeon JT, Looft C, Amarger V, Robic A, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 27.Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Martin J, et al. Medical sequencing at the extremes of human body mass. Am J Hum Genet. 2007;80:779–791. doi: 10.1086/513471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Collier JM, et al. A PYY Q62P variant linked to human obesity. Hum Mol Genet. 2006;15:387–391. doi: 10.1093/hmg/ddi455. [DOI] [PubMed] [Google Scholar]
- 29.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–446. doi: 10.1101/gr.212802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingham KC. Precipitation of proteins with polyethylene glycol. Methods Enzymol. 1990;182:301–306. doi: 10.1016/0076-6879(90)82025-w. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol. 2000;99:63–74. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- 33.Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 34.Andersson L. Identification and characterization of AMPK gamma 3 mutations in the pig. Biochem Soc Trans. 2003;31:232–235. doi: 10.1042/bst0310232. [DOI] [PubMed] [Google Scholar]
- 35.Hedegaard J, Horn P, Lametsch R, Sondergaard Moller H, Roepstorff P, et al. UDP-glucose pyrophosphorylase is upregulated in carriers of the porcine RN- mutation in the AMP-activated protein kinase. Proteomics. 2004;4:2448–2454. doi: 10.1002/pmic.200300761. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Hirshman MF, Fujii N, Pomerleau JM, Peter LE, et al. Muscle-specific overexpression of wild type and R225Q mutant AMP-activated protein kinase gamma3-subunit differentially regulates glycogen accumulation. Am J Physiol Endocrinol Metab. 2006;291:E557–565. doi: 10.1152/ajpendo.00073.2006. [DOI] [PubMed] [Google Scholar]
- 37.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867–877. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- 38.Barnes BR, Long YC, Steiler TL, Leng Y, Galuska D, et al. Changes in Exercise-Induced Gene Expression in 5′-AMP-Activated Protein Kinase {gamma}3-Null and {gamma}3 R225Q Transgenic Mice. Diabetes. 2005;54:3484–3489. doi: 10.2337/diabetes.54.12.3484. [DOI] [PubMed] [Google Scholar]
- 39.Zhou G, Myers R, Li Y, Chen Y, Shen X, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 41.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]