Abstract
Opioids remain the most effective analgesics despite their potential adverse effects such as tolerance and addiction. Mechanisms underlying these opiate-mediated processes remain the subject of much debate. Here we describe opioid-induced behaviors of Lmx1b conditional knockout mice (Lmx1bf/f/p), which lack central serotonergic neurons, and we report that opioid analgesia is differentially dependent on the central serotonergic system. Analgesia induced by a κ opioid receptor agonist administered at the supraspinal level was abolished in Lmx1bf/f/p mice compared with their wild-type littermates. Furthermore, compared with their wild-type littermates Lmx1bf/f/p mice exhibited significantly reduced analgesic effects of μ and δ opioid receptor agonists at both spinal and supraspinal sites. In contrast to the attenuation in opioid analgesia, Lmx1bf/f/p mice developed tolerance to morphine analgesia and displayed normal morphine reward behavior as measured by conditioned place preference. Our results provide genetic evidence supporting the view that the central serotonergic system is a key component of supraspinal pain modulatory circuitry mediating opioid analgesia. Furthermore, our data suggest that the mechanisms of morphine tolerance and morphine reward are independent of the central serotonergic system.
Keywords: serotonin, pain, conditioned place preference, behavior, mouse
It has been well established that neurons producing serotonin (5-hydroxytryptamine, 5-HT) contribute to descending pain control pathways (1). However, the role of the central 5-HT system in opioid analgesia is less clear. For the past several decades numerous pharmacological and behavioral studies have indicated that morphine-induced descending inhibition of nociceptive transmission depends on supraspinal 5-HT neurons (2–4). Despite these studies, many contradictory results concerning the role of central 5-HT neurons in morphine analgesia have also been reported, making it a long-standing controversial issue in pain research (5). More recently, the notion that central 5-HT neurons are involved in morphine analgesia has further been challenged by electrophysiological studies in rats that indicated a lack of firing responses of 5-HT neurons to morphine treatment (6, 7). In contrast to the “textbook” explanation, these findings have strengthened the view that 5-HT neurons are neither necessary nor sufficient for the analgesic effect of opioids and thus are not an integral part of opioid-mediated pain-modulatory circuits (8–10). Not surprisingly, these opposing results are difficult to reconcile, probably because of technical issues such as the difficulties of identifying 5-HT neurons in vivo, incomplete depletion of 5-HT or 5-HT neurons, nonspecific effects of drugs or surgical treatments, and the use of different species across the studies (5, 11, 12). The varying experimental paradigms that have been used in different studies make it difficult to compare the results between laboratories, further confounding the issue.
Although opioid analgesics are still the primary treatment for moderate to severe pain, prolonged use of opioids is known to result in an attenuated analgesia, also known as tolerance (13). There is evidence showing a correlation between the synthesis rate or release of 5-HT in the brain and the development of tolerance, supporting a role of 5-HT in morphine tolerance (14–16). On the other hand, some studies suggest that 5-HT might not be involved in morphine tolerance (17). Repeated use of opioids may also result in their abuse. Pharmacological studies regarding whether a 5-HT mechanism is involved in morphine-induced reward are inconsistent (18). The aforementioned discrepancies are difficult to reconcile based solely on the use of pharmacological experimental manipulations.
We have generated a line of conditional knockout mice called Lmx1bf/f/p mice in which the transcription factor Lmx1b, which is essential for the development of 5-HT neurons in the hindbrain, is selectively deleted in cells expressing Pet1 (19–21). Because 5-HT neurons lacking Lmx1b fail to survive, the deletion of Lmx1b results in the specific loss of the central 5-HT neurons in Lmx1bf/f/p mice (21). While the central 5-HT system is ablated, Lmx1bf/f/p mice have normal expression levels of peripheral 5-HT, dopamine, and norepinephrine (21). These mutant mice display normal locomotor activity, acute thermal pain, and enhanced inflammatory pain (22) and thus provide a unique model for assessing the involvement of central 5-HT neurons in opioid-mediated processes. In the present study we evaluated the role of the central 5-HT system in opioid-mediated analgesic effects and investigated the contribution of this neurotransmitter to morphine tolerance and reward using Lmx1bf/f/p mice.
Results
Opioid Receptor Agonist's Analgesia Differentially Relied on the Central 5-HT System.
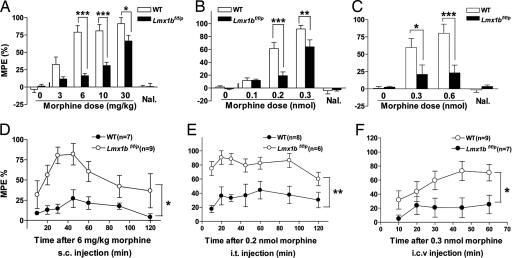
We first examined the analgesic effect of morphine [a μ opioid receptor (MOR) agonist] using the tail-flick test. Consistent with our previous work (22), no significant difference in the baselines of tail-flick latencies were found between Lmx1bf/f/p mice and their wild-type littermates (mean ± SEM: 4.15 ± 0.53 sec for wild-type and 4.23 ± 0.32 sec for Lmx1bf/f/p mice; P > 0.05, two-tailed t test). The analgesic effect presented as the percentage of maximum possible effect (%MPE) after vehicle administration was similar between wild-type and Lmx1bf/f/p mice (Figs. 1 A–C and 2A). Wild-type mice displayed a dose-dependent, naloxone-reversible analgesia after systemic administration of morphine, but this analgesic effect was significantly attenuated in Lmx1bf/f/p mice (Fig. 1 A and D). The strong attenuation of analgesia in the mutant mice reveals an important role for 5-HT neurons in morphine analgesia. We further examined the sites of action (spinal vs. supraspinal) of 5-HT neurons in morphine analgesia. Both intrathecal (i.t.) and intracerebroventricular (i.c.v.) injection of morphine evoked a dose-dependent, naloxone-reversible analgesic effect in wild-type mice, but the effects were much less pronounced in the mutants (Fig. 1 B, C, E, and F). In particular, supraspinal administration of morphine had only mild or weak effect in the mutants (Fig. 1 C and F). Thus, the central 5-HT system mediates morphine analgesia at both spinal and supraspinal sites.
Fig. 1.
Morphine-induced analgesia in Lmx1bf/f/p and wild-type mice. (A–C) Saline and escalating doses of morphine (3, 6, 10, and 30 mg/kg, multiple s.c. injections, n = 10–12 per genotype) or multiple doses of morphine (0.1, 0.2, and 0.3 nmol i.t. or 0.3 and 0.6 nmol i.c.v., n = 8–12 every dose per genotype) were administered to wild-type and Lmx1bf/f/p mice, and morphine analgesia was measured with the tail-flick assay. Immediately after the withdrawal latency measurement after 30 mg/kg s.c., 0.3 nmol i.t., or 0.6 nmol i.c.v. morphine injections, mice were injected with naloxone (1 mg/kg i.p.), and antinociception was assessed again after 15 min. The analgesic effect was presented as the percentage of maximum possible effect [%MPE = (drug latency − baseline latency) × 100/(cutoff latency − baseline latency)]. (D–F) Time course of morphine-induced analgesia of wild-type and Lmx1bf/f/p mice in the tail-flick test after a single morphine dose of 6 mg/kg s.c. (D), 0.2 nmol i.t. (E), or 0.3 nmol i.c.v. (F). All data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (repeated-measures ANOVA compared with wild type). Nal, naloxone.
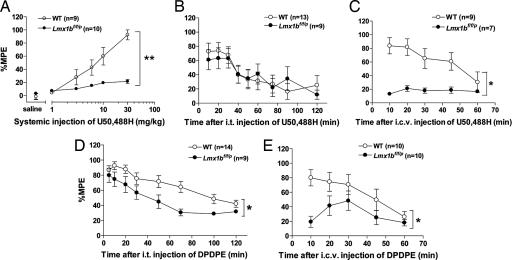
Fig. 2.
Analgesia evoked by KOR or DOR agonists in Lmx1bf/f/p and wild-type mice. (A) Saline and escalating doses of the KOR agonist U50,488H (1, 3, 6, 10, and 30 mg/kg s.c.) were administered to wild-type and Lmx1bf/f/p mice, and the analgesic effect was measured with the tail-flick assay. (B and C) The analgesic effect of the i.t. injection of U50,488H (60 μg) in Lmx1bf/f/p mice was comparable to wild-type mice (B), whereas effect of the i.c.v. injection of U50,488H (60 μg) was almost absent in Lmx1bf/f/p mice compared with that in wild type (C). (D and E) The analgesic effect of i.t. (D) and i.c.v. (E) injections of the DOR agonist [D-Pen2, D-Pen5]enkephalin (5 μg) in wild-type and Lmx1bf/f/p mice. All data are mean ± SEM. *, P < 0.05; **, P < 0.01 (repeated-measures ANOVA compared with wild type).
We next investigated analgesia induced by a selective κ opioid receptor (KOR) agonist, U50,488H. Systemic administration of U50,488H evoked a robust dose-dependent analgesia in wild-type mice but only weak analgesia in Lmx1bf/f/p mice (Fig. 2A). Even at the highest dose (30 mg/kg), Lmx1bf/f/p mice only showed weak analgesia relative to controls (Fig. 2A). Remarkably, the analgesic effect of i.t. U50,488H (60 μg) was indistinguishable between wild-type and Lmx1bf/f/p mice (Fig. 2B). In wild-type mice, the i.c.v. injection of U50,488H showed long-lasting analgesic effect, whereas this effect did not occur in Lmx1bf/f/p mice. These data suggest that 5-HT neurons contribute to KOR analgesia at a supraspinal site (Fig. 2C).
δ opioid receptor (DOR) analgesia was also evaluated. The i.t. or i.c.v. injection of the selective DOR agonist [D-Pen2, D-Pen5]enkephalin produced strong analgesic effects in wild-type mice, but this effect was significantly attenuated in Lmx1bf/f/p mice (Fig. 2 D and E). Thus, the DOR mediates its analgesic effect through the central 5-HT system at both spinal and supraspinal levels. Given the known cross-talk between MOR and DOR and the possible interaction of [D-Pen2, D-Pen5]enkephalin with either of these receptors in the induction of thermal analgesia (23, 24), the finding that both MOR and DOR agonists require central 5-HT neurons for their analgesia in the spinal cord of the mutant mice may not be surprising.
Development of Morphine Tolerance in Lmx1bf/f/p Mice.
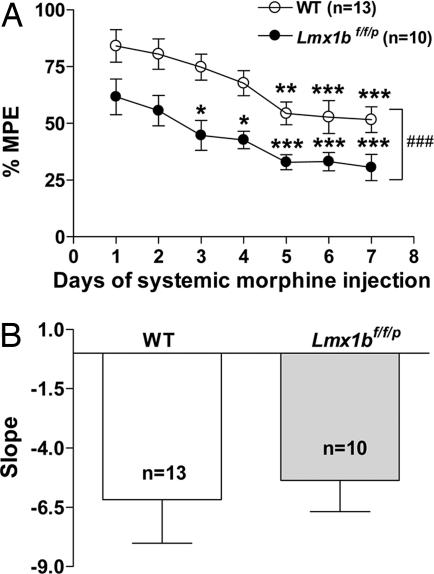
To determine whether the central 5-HT system is necessary for morphine tolerance, we assessed morphine analgesia after daily injection of morphine for 7 days at a dose of 15 mg/kg. In wild-type mice the analgesic effect was gradually reduced over days; analgesia was significantly lower after 7 days of morphine injection compared with the first day of treatment, indicating a development of morphine tolerance (Fig. 3A). In Lmx1bf/f/p mice, although the degree of initial morphine analgesia was reduced compared with wild-type mice, the analgesic effect of identical morphine injections also declined over the 7 days of repeated injections (Fig. 3A). The least-squares slope of the %MPE across 7 days showed no significant difference between the two groups (Fig. 3B). This suggests that morphine tolerance developed similarly in both Lmx1bf/f/p and wild-type mice. Thus, we conclude that the central 5-HT system is not required for the development of morphine tolerance (17).
Fig. 3.
Morphine tolerance developed in parallel in Lmx1bf/f/p and wild-type mice. (A) Wild-type and Lmx1bf/f/p mice were treated with morphine (15 mg/kg s.c.) daily for 7 days. The analgesic effect was assessed 30 min after the injection by water-immersion tail-flick assay. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (repeated-measures ANOVA followed by Newman–Keuls test compared with the first-day data of the same genotype). ###, P < 0.001 (repeated-measures ANOVA compared with wild type). (B) The rate of development of morphine tolerance denoted by the slope of the %MPE across 7 days (P > 0.05, two-tailed t test, genotype effect). All data are presented as means ± SEM.
Normal Morphine Reward in Lmx1bf/f/p Mice.
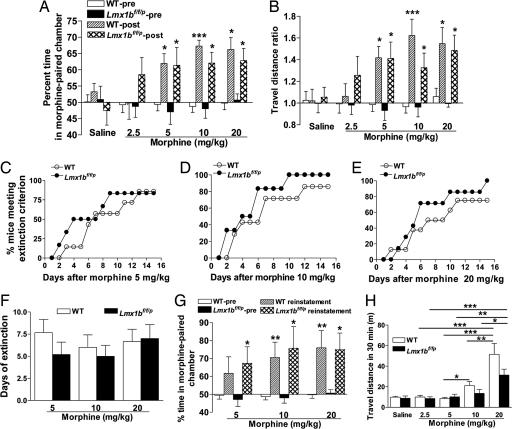
To evaluate whether the central 5-HT system contributes to the rewarding properties of morphine, we examined Lmx1bf/f/p and wild-type mice using a conditioned place preference (CPP) paradigm (18). Both Lmx1bf/f/p and wild-type mice spent significantly more time and traveled a significantly longer distance in the morphine-paired chamber on the posttest day than the pretest day at three doses tested (5, 10, and 20 mg/kg), but there was no significant difference between Lmx1bf/f/p and wild-type mice in place preference under any condition (saline or different doses of morphine) as measured in both time and travel distance (Fig. 4 A and B). Insofar as wild-type and Lmx1bf/f/p mice displayed similar preference for the chamber paired with morphine, our data are consistent with the hypothesis that the central 5-HT system is dispensable for the acquisition and expression of morphine-induced reward. We further examined the extinction of morphine CPP in the same group of animals used in the acquisition. No significant difference in the number of days required for extinction of the place preference was found between wild-type and Lmx1bf/f/p mice (Fig. 4 C–F). Similarly, subsequent reinstatement of the place preference was normal in the mutant mice (Fig. 4G). Because acute administration of morphine is known to facilitate locomotor activity in rodents by inducing dopamine release in the brain (25), and because 5-HT acts through several 5-HT receptors to modulate dopamine release (26), the role of the central 5-HT system in morphine-induced locomotion was examined by recording the travel distance of wild-type and Lmx1bf/f/p mice after acute morphine injection. Morphine increased the travel distance of wild-type mice in a dose-dependent manner (5, 10, and 20 mg/kg) (Fig. 4H). In Lmx1bf/f/p mice, the travel distance induced by 20 mg/kg morphine was significantly longer than all other doses (Fig. 4H). In all tests after vehicle or any doses of morphine administration, however, there was no significant difference between wild-type and Lmx1bf/f/p mice (Fig. 4H).
Fig. 4.
Morphine-induced CPP and locomotor activity in Lmx1bf/f/p and wild-type mice. (A and B) Dose–response study of morphine as measured by percentage of time spent (A) or travel distance ratio (B) comparing time or travel distance in the morphine-paired chamber before (pretest) and after (posttest) conditioning. (C–E) Extinction of morphine-induced CPP in Lmx1bf/f/p and wild-type mice at doses of 5 (C), 10 (D), and 20 (E) mg/kg. Data are described as the percentage of mice meeting the extinction criterion [see supporting information (SI) Methods] (P > 0.05, Fisher's exact test, genotype effect). (F) Days of extinction at doses of 5, 10, and 20 mg/kg morphine. Data are shown as mean ± SEM (P > 0.05, unpaired t test, genotype effect). n = 6–7 in each genotype per dose in C–F. (G) Reinstatement of CPP at different doses of morphine. (H) Locomotor activity reflected by travel distance during the 30-min period after injection of saline or different doses of morphine. In all experiments morphine was administered i.p. n = 9–14 in each genotype per dose in A, B, and H, and n = 6–7 in G. Except in C–E, all data are means ± SEM (P > 0.05, two-tailed paired or unpaired t test comparing genotypes within treatment in A, B, G, and H). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-tailed paired or unpaired t test, compared with pretest within the same genotype and dose in A, B, and G, or ANOVA followed by Newman–Keuls tests compared with different doses in H).
Expression of MOR in the Brains of Lmx1bf/f/p Mice.
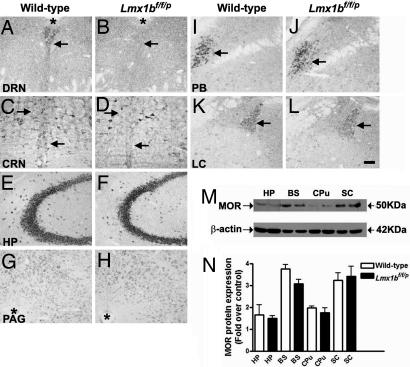
To assess whether a loss of central 5-HT neurons may result in an alteration of MOR expression in the brain that may subsequently contribute to the attenuated analgesia in Lmx1bf/f/p mice, we compared MOR expression between wild-type and Lmx1bf/f/p mice by in situ hybridization and Western blot studies. Consistent with previous anatomical evidence indicating the high levels of MOR expression in the midline raphe nuclei of rodents (27, 28), MOR mRNA distribution in wild-type mice exhibited a characteristic pattern along the midline of the hindbrain similar to 5-HT neuronal clusters (Fig. 5 A and C). In contrast, we found that MOR in Lmx1bf/f/p mice was lost mainly in the raphe nuclei (Fig. 5 B and D, and data not shown), presumably because of the loss of 5-HT neurons. However, MOR expression was maintained in areas surrounding the raphe nuclei in the mutants (Fig. 5D). In other brain regions of wild-type and Lmx1bf/f/p mice, including several pain-associated areas such as the hippocampus, the periaqueductal gray, the parabrachial nucleus, and the locus ceruleus (Fig. 5 E–L), no discernable differences in MOR expression were found between wild-type mice and Lmx1bf/f/p mice. Consistent with these findings, Western blot analysis showed comparable MOR protein levels in various regions of wild-type and Lmx1bf/f/p mice including caudate putamen and spinal cord to which 5-HT fibers heavily projected (Fig. 5 M and N). Thus, our results suggest that, with respect to either level of expression or distribution pattern, no gross alteration of MOR expression in pain-associated areas outside the raphe system occurred in Lmx1bf/f/p mice.
Fig. 5.
MOR expression in Lmx1bf/f/p and wild-type mice assessed by in situ hybridization and Western blotting assays. (A–L) MOR expression detected in various brain areas of wild-type and Lmx1bf/f/p animals by in situ hybridization. Except the raphe system including dorsal raphe nucleus (DRN) (A and B) and caudal raphe nuclei (CRN) (C and D) where 5-HT neurons are clustered along the midline, no major differences of MOR expression was detected (E–L) in various brain regions between wild-type and Lmx1bf/f/p mice. Asterisks in A, B, G, and H indicate the cerebral aqueduct. Arrows in A–D and I–L indicate the typical MOR expression areas. (Scale bar: 100 μm.) (M and N) Western blot analysis showing no significant difference in MOR protein levels found in four brain areas of wild-type (left sample in M and open bars in N) and Lmx1bf/f/p (right sample in M and black bars in N) mice (n = 4 per genotype). Data are expressed as the ratio of MOR band densities to band densities of the corresponding β-actin on the same film. Error bars represent mean ± SEM (P > 0.05, one-way ANOVA followed by Newman–Keuls post hoc test; the difference in the same areas between genotypes were compared). HP, hippocampus; PAG, periaqueductal gray; PB, parabrachial nucleus; LC, locus ceruleus; BS, brain stem; CPu, caudate putamen; SC, spinal cord.
Discussion
In this study we report opioid-mediated behaviors of Lmx1bf/f/p mice in which central 5-HT neurons fail to survive because of the genetic elimination of Lmx1b in Pet1-expressing neurons (21). By taking the advantage of this animal model, we provide genetic evidence indicating that central 5-HT neurons are necessary for the full expression of opioid analgesia. Compared with a large body of pharmacologic and lesion studies, which often showed poor selectivity and partial depletion of 5-HT when para-chlorophenylalanine or 5,7-dihydroxytryptamine was used (2, 5), our approach is unique because the deletion of central 5-HT neurons is highly specific and complete in Lmx1bf/f/p mice. Although the ablation of the central 5-HT system from early developmental stages may have resulted in compensatory changes in the brain, the findings that several major monoaminergic systems are not affected in any significant manner and that pain behaviors of Lmx1bf/f/p mice are largely in accordance with many pharmacological studies (21, 22) suggest that any developmental compensation in the Lmx1bf/f/p mice has less impact on pain behaviors than might be predicted.
Although a spinal mechanism has previously been implicated in the analgesic actions of all three classes of opioid receptors (29), whether spinally projecting 5-HT neurons are involved remains unclear. Our data delineate the role of spinal 5-HT in opioid analgesia and reveal that 5-HT neurons are required for MOR- and DOR-induced spinal analgesia, but not for KOR-induced spinal analgesia. Importantly, the absence of KOR analgesia in the mutant mice demonstrates that KOR analgesia completely depends on supraspinal 5-HT neurons. This striking result suggests that a central serotonergic mechanism is a key component in the neural circuits that mediate KOR analgesia and provide genetic evidence in support of an earlier pharmacological study (30).
Because all three classes of opioid receptors are expressed in 5-HT neurons (4, 31, 32), it remains to be determined to what degree and in what way the loss of each individual opioid receptor in 5-HT neurons may have contributed to the altered behavioral phenotype of Lmx1bf/f/p mice. Obviously, it is possible that the attenuation of morphine analgesia in Lmx1bf/f/p mice could be due to a secondary change of MOR expression in other regions of the brain. However, a general or global alteration of MOR expression and/or activity in Lmx1bf/f/p mice appears unlikely for several reasons. First, our data indicate that there is no obvious change in the levels of MOR mRNA and protein expression except in the raphe nuclei. Second, Lmx1bf/f/p mice develop morphine tolerance and exhibit normal morphine reward, which are known to be initiated or mediated by the MOR (33, 34). Last, our results showing the requirement of the central 5-HT system for morphine analgesia are in line with some pharmacological studies (1).
On the other hand, given that 5-HT neurons are known to produce many other neurotransmitters and peptides that may interact with the opioid system to mediate analgesia (8, 35), it is noteworthy that our findings reflect the net effect of a loss of central 5-HT neurons on opioid analgesia in a physiological context. Future targeted deletion of each opioid receptor in spinally projecting 5-HT neurons is required to delineate the involvement of opioid receptors in opioid analgesia. Nevertheless, by examining Lmx1bf/f/p mice in which central 5-HT neurons are genetically ablated, we are able to provide the genetic evidence suggesting that the central 5-HT neurons are an essential component of the supraspinal modulatory circuit that is required for the full expression of opioid analgesia.
The mesolimbic dopamine system has been considered to be important in the reinforcing effects of drugs of abuse and in morphine-induced alterations in locomotion (36–39). In addition, norepinephrine has been implicated in morphine-related reward (40). In the present study we found no major difference in morphine reward between wild-type and Lmx1bf/f/p mice, suggesting that the central 5-HT system is not required for hedonic responses to morphine or morphine reward-related learning (41). Our finding that morphine-induced reinstatement is not altered in the mutant mice is unexpected because reinstatement has been tightly associated with the function of the forebrain to which 5-HT+ and dopamine+ fibers densely project (42). Previous studies have shown that morphine can increase extracellular 5-HT and dopamine transmission in the ventral tegmental area, nucleus accumbens, and the forebrain (38, 43); possible synergistic interactions between the 5-HT system and the dopamine system have also been reported (26, 44, 45). For example, 5-HT can regulate the activity of the ventral tegmental area dopaminergic neurons and dopamine release in the nucleus accumbens and the prefrontal cortex, which are important for drug-induced reinstatement (44, 46, 47). Our results suggest that central 5-HT neurons are not required for the development of the opioid responsiveness in the forebrain. The present findings that the morphine-induced reward and locomotor activity are not altered in Lmx1bf/f/p mice raise an intriguing possibility that the dopamine and norepinephrine systems are not significantly altered in Lmx1bf/f/p mice even after morphine treatment. Although we cannot rule out the possibility that an effect resulting from possible alterations of dopamine and/or norepinephrine after morphine treatment on reward-related behaviors may be developmentally compensated for in Lmx1bf/f/p mice, we consider this unlikely (22). Nevertheless, future conditional temporal knockout studies are required to distinguish these possibilities.
The observation that morphine tolerance develops independent of central 5-HT neurons may also be unexpected, given that synergistic interactions between spinal and supraspinal actions are believed to be important for spinal morphine potency (48). Furthermore, because morphine exerts its effect via MORs at multiple sites including 5-HT neurons of the central nervous system (24), our results suggest that supraspinal 5-HT neurons expressing MORs are not part of the neural circuit that mediates morphine tolerance and morphine reward despite their likely involvement in opioid analgesia. Collectively, these studies suggest that the role of the central 5-HT system in opioid analgesia can be disassociated from its role in morphine tolerance and morphine reward.
Materials and Methods
Animals.
Lmx1bf/f/p mice were generated by crossing homozygous floxed Lmx1b mice and ePet-cre mice maintained in a mixed genetic background (C57BL/6 and 129SvEv) and genotyped as described previously (21). Because our pilot studies indicated no significant molecular, anatomic, or behavioral differences between wild-type mice and Lmx1bf/+/p or Lmx1bf/+ mice, wild-type or Lmx1bf/+/p or Lmx1bf/+ littermate mice were used as the control group in the present study and were referred to as wild-type mice throughout the text. Male mice aged between 8 and 12 weeks were acclimated to the experimental room and were used for behavioral tests by observers blind to both the genotype and the treatment of the animals. All experiments were performed in accordance with experimental protocols approved by the Animal Studies Committee at Washington University School of Medicine.
Tail-Flick Assays.
For analgesia studies of different opioid receptor agonists, we used the radiant tail-flick assay. A noxious heat stimulus was applied via a focused, radiant heat source (IITC, Woodland Hills, CA) to the dorsal surface of the tail. The time from initiation of the light beam until the time at which the mouse flicked its tail was recorded. For the morphine tolerance study we performed the tail-flick assays using the 52°C water tail-immersion approach. All tail-flick results were expressed as %MPE = (post-drug latency − pre-drug latency) × 100/(cutoff time − pre-drug latency).
Agonists' Administration.
Systemic injection.
Morphine sulfate (Sigma, St. Louis, MO) and U50,488H (Sigma) were injected s.c. 30 min before the tail-flick assays in doses of 3, 6, 10, and 30 mg/kg and 1, 3, 6, 10, and 30 mg/kg, respectively. Because [D-Pen2, D-Pen5]enkephalin (Sigma) exhibits a poor blood–brain barrier permeability (49), no systemic injection was attempted. For morphine-induced tolerance and reward experiments, morphine was administrated s.c. and i.p., respectively.
i.t. and i.c.v. injection.
Injections were performed as previously described (50). For ascertaining the areas in the brain ventricular system into which the drugs penetrated, the i.c.v. injection site was confirmed after the experiment by using a 10-μl injection of 0.1% Fast Green, and the brains were sectioned and studied histologically. Only those with correct injection sites were included in the analysis.
Tolerance Induction.
Morphine tolerance induction was performed according to methods described previously (51) with slight modification. Mice received daily morphine sulfate injections (15 mg/kg s.c. between 1500 hours and 1600 hours) for 7 days. For assessment of tolerance, the antinociceptive effect of morphine was determined daily 30 min after the morphine injection by warm water tail-immersion tests as described above, and the effect of morphine (%MPE) was compared.
CPP Experiment.
The same custom-made apparatus was used as previously described (36), and the acquisition, extinction, and reinstatement of morphine CPP (52) were examined (see details in SI Methods). The design of the CPP paradigm was as described (40). The animals' activities were recorded by a video camera (Creative Technology, Milpitas, CA), and the travel distance and time in each compartment were analyzed by using ANYMAZE software (Stoelting, Wood Dale, IL). The extinction of morphine CPP was determined in the same group of animals used in the acquisition of morphine CPP. Once the extinction criterion was met, on the following day the animals were injected with morphine (the same dose used in the training of expression of morphine CPP), and the time spent in each chamber was recorded. The horizontal locomotion of mice in the morphine-paired chamber was also measured after different doses of morphine injection during the conditioning procedure.
In Situ Hybridization.
In situ hybridization was performed as previously described (19). For the mouse MOR probe, the MOR plasmid was cut with HindIII and transcribed with SP6 RNA polymerase to produce the antisense cRNA probe (53).
Western Blot Analysis.
Western blot analysis was performed on protein samples isolated from brain regions of 2-month-old male wild-type and Lmx1bf/f/p animals using a modified protocol (54). After protein concentration was measured, aliquots of protein extract (150 μg of total protein for MOR) were separated by gel electrophoresis on 5–10% precast polyacrylamide gels (Bio-Rad, Oakland, CA). Bands were transferred to a nitrocellulose membrane for immunoblotting with a rabbit polyclonal antibody against the N-terminal domain of MOR (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-linked IgG (goat anti-rabbit, 1:2,000; Cell Signaling Technology, Beverly, MA) was applied as the secondary antibody. Bands were visualized by chemiluminescence detection as described (55), and band intensities were normalized to those for a mouse monoclonal antibody against β-actin (1:500,000; Chemicon, Temecula, CA) on the same film as a loading control. Band intensities were determined by densitometric analysis using a OneTouch 9220 USB scanner (Visioneer), MagnaFire software (version 2.1C; Olympus, Melville, NY), and NIH ImageJ version 1.34e.
Statistical Analysis.
All details for statistical methods and subjects for comparisons can be found in SI Methods. Statistical comparisons were performed by using Prism Software (GraphPad, San Diego, CA) and STATISTICA 7 (StatSoft, Tulsa, OK). Except for the mentioned exceptions, data are expressed as the mean ± SEM and error bars represent SEM. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank J. Yin for technical assistance, C. Evans (University of California, Los Angeles, CA) for the MOR probe, C. J. Coscia (St. Louis University, St. Louis, MO) for the MOR antibody, and R. L. Johnson (University of Texas, Houston, TX) and E. S. Deneris (Case Western Reserve University, Cleveland, OH) for floxed Lmx1b mice and ePet-cre mice. We also thank Z. Z. Pan (University of Texas, Houston, TX) and M. Jacquin (Washington University, St. Louis, MO) for comments on the manuscript. This work was supported by National Institutes of Health Grants R01 NS043968-05 (to Z.-F.C.) and R01 NS48602 (to R.W.G.) and National Natural Science Foundation of China 30500153 (to Y.-J.G.).
Abbreviations
- 5-HT
5-hydroxytryptamine
- MOR
μ opioid receptor
- DOR
δ opioid receptor
- KOR
κ opioid receptor
- CPP
conditioned place preference
- i.t.
intrathecal
- i.c.v.
intracerebroventricular
- %MPE
percentage of maximum possible effect.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705740104/DC1.
References
- 1.Millan MJ. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 2.Le Bars D. In: Neuronal Serotonin. Osborne NN, Hamon M, editors. New York: Wiley; 1988. pp. 171–229. [Google Scholar]
- 3.Sawynok J. Can J Physiol Pharmacol. 1989;67:975–988. doi: 10.1139/y89-154. [DOI] [PubMed] [Google Scholar]
- 4.Marinelli S, Vaughan CW, Schnell SA, Wessendorf MW, Christie MJ. J Neurosci. 2002;22:10847–10855. doi: 10.1523/JNEUROSCI.22-24-10847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond DL. In: Serotonin and Pain. Besson JM, editor. Amsterdam: Elsevier Science; 1990. pp. 251–261. [Google Scholar]
- 6.Gao K, Chen DO, Genzen JR, Mason P. J Neurosci. 1998;18:1860–1868. doi: 10.1523/JNEUROSCI.18-05-01860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao K, Kim YH, Mason P. J Neurosci. 1997;17:3285–3292. doi: 10.1523/JNEUROSCI.17-09-03285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields H. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 9.Mason P. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- 10.Mason P. Curr Opin Neurobiol. 1999;9:436–441. doi: 10.1016/S0959-4388(99)80065-8. [DOI] [PubMed] [Google Scholar]
- 11.Christie MJ. Pain Forum. 1998;7:155–158. [Google Scholar]
- 12.Wessendorf MW. Pain Forum. 1998;7:159–162. [Google Scholar]
- 13.McQuay H. Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- 14.Way EL, Loh HH, Shen F. Science. 1968;162:1290–1292. doi: 10.1126/science.162.3859.1290. [DOI] [PubMed] [Google Scholar]
- 15.Tao R, Ma Z, Auerbach SB. J Pharmacol Exp Ther. 1998;286:481–488. [PubMed] [Google Scholar]
- 16.Li JY, Wong CH, Huang EY, Lin YC, Chen YL, Tan PP, Chen JC. Anesth Analg. 2001;92:1563–1568. doi: 10.1097/00000539-200106000-00043. [DOI] [PubMed] [Google Scholar]
- 17.Bhargava HN, Matwyshyn GA. Eur J Pharmacol. 1977;44:25–33. doi: 10.1016/0014-2999(77)90112-1. [DOI] [PubMed] [Google Scholar]
- 18.Tzschentke TM. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 19.Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 20.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. Proc Natl Acad Sci USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RW, Chen ZF. J Neurosci. 2007;27:6045–6053. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- 24.Kieffer BL, Gaveriaux-Ruff C. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 25.Cowan A. In: Opioids II: Handbook of Experimental Pharmacology. Hertz A, editor. Vol 104. Berlin: Springer; 1993. pp. 393–414. [Google Scholar]
- 26.Alex KD, Pehek EA. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. J Neurosci. 1996;16:6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 29.Dickenson AH. Br Med Bull. 1991;47:690–702. doi: 10.1093/oxfordjournals.bmb.a072501. [DOI] [PubMed] [Google Scholar]
- 30.Vonvoigtlander PF, Lewis RA, Neff GL. J Pharmacol Exp Ther. 1984;231:270–274. [PubMed] [Google Scholar]
- 31.Kalyuzhny AE, Wessendorf MW. Neuroscience. 1999;90:229–234. doi: 10.1016/s0306-4522(98)00376-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Wessendorf MW. J Comp Neurol. 1999;404:183–196. doi: 10.1002/(sici)1096-9861(19990208)404:2<183::aid-cne4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Contet C, Kieffer BL, Befort K. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Bailey CP, Connor M. Curr Opin Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Pan ZZ, Tershner SA, Fields HL. Nature. 1997;389:382–385. doi: 10.1038/38730. [DOI] [PubMed] [Google Scholar]
- 36.Hnasko TS, Sotak BN, Palmiter RD. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE, Berridge KC. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 38.Hyman SE, Malenka RC, Nestler EJ. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 39.Wise RA. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 40.Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- 41.Berridge KC, Robinson TE. Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 42.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. Psychopharmacology (Berlin) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 43.Tao R, Auerbach SB. Neuroscience. 1995;68:553–561. doi: 10.1016/0306-4522(95)00154-b. [DOI] [PubMed] [Google Scholar]
- 44.Iyer RN, Bradberry CW. J Pharmacol Exp Ther. 1996;277:40–47. [PubMed] [Google Scholar]
- 45.Benes FM, Taylor JB, Cunningham MC. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- 46.Van Bockstaele EJ, Cestari DM, Pickel VM. Brain Res. 1994;647:307–322. doi: 10.1016/0006-8993(94)91330-7. [DOI] [PubMed] [Google Scholar]
- 47.Broderick PA, Phelix CF. Neurosci Biobehav Rev. 1997;21:227–260. doi: 10.1016/s0149-7634(96)00048-6. [DOI] [PubMed] [Google Scholar]
- 48.Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. J Neurosci. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, Pollack GM. J Pharmacol Exp Ther. 1997;283:1151–1159. [PubMed] [Google Scholar]
- 50.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 51.Bohn LM, Lefkowitz RJ, Caron MG. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoblock JR, Wichmann J, Maidment NT. Neuropharmacology. 2005;49:439–446. doi: 10.1016/j.neuropharm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 54.Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. J Biol Chem. 2006;281:33749–33760. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.