Abstract
Autophagy is a regulated lysosomal degradation process that involves autophagosome formation and transport. Although recent evidence indicates that basal levels of autophagy protect against neurodegeneration, the exact mechanism whereby this occurs is not known. By using conditional knockout mutant mice, we report that neuronal autophagy is particularly important for the maintenance of local homeostasis of axon terminals and protection against axonal degeneration. We show that specific ablation of an essential autophagy gene, Atg7, in Purkinje cells initially causes cell-autonomous, progressive dystrophy (manifested by axonal swellings) and degeneration of the axon terminals. Consistent with suppression of autophagy, no autophagosomes are observed in these dystrophic swellings, which is in contrast to accumulation of autophagosomes in the axonal dystrophic swellings under pathological conditions. Axonal dystrophy of mutant Purkinje cells proceeds with little sign of dendritic or spine atrophy, indicating that axon terminals are much more vulnerable to autophagy impairment than dendrites. This early pathological event in the axons is followed by cell-autonomous Purkinje cell death and mouse behavioral deficits. Furthermore, ultrastructural analyses of mutant Purkinje cells reveal an accumulation of aberrant membrane structures in the axonal dystrophic swellings. Finally, we observe double-membrane vacuole-like structures in wild-type Purkinje cell axons, whereas these structures are abolished in mutant Purkinje cell axons. Thus, we conclude that the autophagy protein Atg7 is required for membrane trafficking and turnover in the axons. Our study implicates impairment of axonal autophagy as a possible mechanism for axonopathy associated with neurodegeneration.
Keywords: axon, axonopathy, neurodegeneration, autophagosome, Purkinje cell
Macroautophagy is characterized by dynamic membrane rearrangements, involving the formation, trafficking, and degradation of double-membrane autophagic vacuoles (autophagosomes) in the cytoplasm. Macroautophagy (hereafter referred to as autophagy) is a highly regulated process, which can be induced by nutrient starvation, trophic factors, and stress (1). Despite recent advances in characterizing autophagy in several model systems, autophagic processes in the nervous system remain poorly understood. On one hand, nutrient deprivation has not been observed to induce autophagy in the mammalian brain (2), thus suggesting a specific regulatory system for autophagy that is not typically activated by starvation. On the other hand, a variety of conditions that cause neuronal stress or degeneration can lead to the accumulation of autophagosomes in neurons, thus implicating autophagy in these neuropathogenic processes (3, 4).
The axon is a highly specialized neuronal compartment that performs many functions independently from the cell body. After axotomy or excitotoxicity, double-membrane vacuoles resembling autophagosomes were originally observed to accumulate in dilated axon terminals that result from the injury (5, 6), a local phenomenon that is not observed in undisturbed axons. Autophagosome-like vacuoles have also been shown to be present in the dysfunctional or degenerating axons associated with a range of chronic neurodegenerative conditions, including Alzheimer's (7, 8), Parkinson's (9), Huntington's (10), and Creutzfeldt–Jakob (11) diseases and their animal models (12–14). These observations suggest a link between locally altered autophagy and axonopathy, which is one of the underlying mechanisms in neurodegeneration (15).
Although the biological significance of these autophagosomes-like vacuoles in degenerating axons is unclear, recent studies have shown that genetic inactivation of autophagy in the mouse CNS causes neurodegeneration accompanied by axonal dystrophy and the formation of intracellular ubiquitin-associated inclusions (16, 17). These studies suggest a role for basal levels of autophagy in neuronal protection and in protein quality control. However, the connection between the inactivation of autophagy and the observed axonal dystrophy and neurodegeneration remains to be determined. In addition, because autophagy was suppressed in all cell types in the CNS (including neurons and nonneuronal cells) (16, 17), it is not known whether the observed axonal dystrophy and neurodegeneration is cell-autonomous.
Here, we seek to further elucidate the physiological function of neuronal autophagy by generating conditional knockout mice with Purkinje cell-specific deletion of Atg7, an autophagy gene encoding E1-like enzyme in the two ubiquitin-like conjugation systems that are essential for the autophagosome biogenesis (18). We show that ablation of Atg7 leads to abnormal swellings and dystrophy of Purkinje cell axon terminals in the deep cerebellar nuclei (DCN). Subsequently, these Atg7-deletion mice develop cell-autonomous neurodegeneration of Purkinje cells, dendritic atrophy, and behavioral deficits. Moreover, double-membrane vacuole-like structures are formed in the distal ends of wild-type Purkinje cell axons, whereas they are absent in Atg7-deletion Purkinje cell axons. Instead, the mutant Purkinje cell axon terminal swellings accumulate aberrant membranous structures. Our results suggest that autophagy is required for normal axon terminal membrane trafficking and turnover, and indicate an essential role of local autophagy in the maintenance of axonal homeostasis and prevention of axonal degeneration.
Results
Specific Depletion of Atg7 in Purkinje Cells Caused Cell-Autonomous Dystrophy and Degeneration of Axon Terminals.
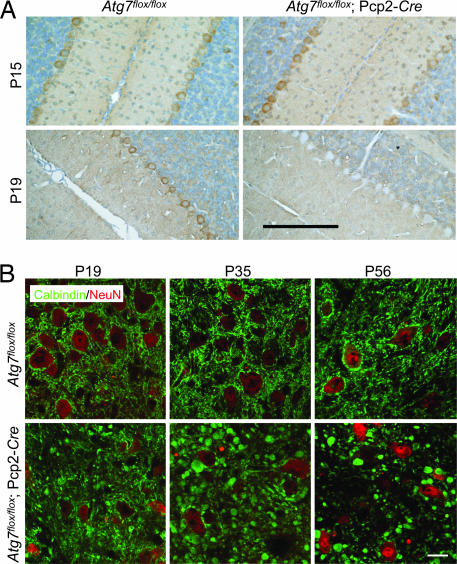
To generate Purkinje cell-specific deletion of Atg7 in mice, we crossed mice harboring the floxed Atg7 alleles (19) with transgenic mice expressing Cre recombinase under control of the Pcp2 (L7) promoter (20) to establish the mouse line Atg7flox/flox;Pcp2-Cre. To determine when loss of the endogenous Atg7 protein occurred, we examined Atg7 expression in Atg7flox/flox;Pcp2-Cre mice at postnatal day 15 (P15) and P19. At P15, Atg7 was expressed at similar levels in Purkinje cells of both mutant Atg7flox/flox;Pcp2-Cre and the control Atg7flox/flox mice, whereas at P19, despite residual expression in a small number of Purkinje cells (<12%), Atg7 immunostaining was largely diminished in Atg7flox/flox;Pcp2-Cre Purkinje cells (>88%), but unchanged in the Atg7flox/flox Purkinje cells (Fig. 1A). At P35, >95% Purkinje cells in Atg7flox/flox;Pcp2-Cre showed no detectable Atg7 expression (data not shown). In addition, Atg7 deficiency in Atg7flox/flox;Pcp2-Cre mice was specific for Purkinje cells because Atg7 is clearly present in the other cell types (Fig. 1A). Thus, the specific loss of Atg7 in Purkinje cells occurred largely between P15 and P19 in Atg7flox/flox;Pcp2-Cre mice.
Fig. 1.
Deletion of Atg7 specifically in Purkinje cells caused progressive dystrophic swelling of axon terminals. (A) Immunohistochemistry of Atg7 protein expression in Purkinje cells of Atg7flox/flox and Atg7flox/flox;Pcp2-Cre mice at P15 and P19. The endogenous Atg7 protein was present at P15 but absent at P19 in the Atg7flox/flox;Pcp2-Cre Purkinje cells. (Scale bar: 100 μm.) (B) Progression of the abnormal Purkinje cell axon terminal swellings in the DCN of Atg7flox/flox;Pcp2-Cre mice (anti-calbindin immunofluorescent staining in green with anti-NeuN counterstained in red) at P19, P35, and P56. Atg7flox/flox was used as control. (Scale bar: 20 μm.) n = 3–5.
Next, we examined Purkinje cell axons in the DCN of Atg7flox/flox;Pcp2-Cre mice by immunofluorescent staining using an antibody against calbindin, a Purkinje cell marker. At P15, no morphological alteration was observed in the axons of Atg7flox/flox;Pcp2-Cre Purkinje cells compared with those of Atg7flox/flox Purkinje cells [supporting information (SI) Fig. 7], consistent with the presence of normal levels of Atg7 in Atg7flox/flox;Pcp2-Cre Purkinje cells at this stage (Fig. 1A). However, at P19, Atg7flox/flox;Pcp2-Cre Purkinje cell axons were abnormally dilated, as visualized by green fluorescence-labeled “endbulbs” (Fig. 1B). In addition, these Purkinje cell axonal swellings were labeled with the antibody raised against synaptophysin, the presynaptic terminal marker (SI Fig. 8), suggesting that they were terminals of Purkinje cell axons. The number and size of the swollen axon terminals in the DCN of the Atg7flox/flox;Pcp2-Cre were markedly increased at P35 in comparison with those at P19 (Fig. 1B). At P56, the number of such axonal dystrophic swellings of the mutant Purkinje cells was noticeably decreased in comparison with that at P35, suggesting that many of these swollen axons had degenerated by this age (Fig. 1B). These data demonstrated that deletion of Atg7 caused cell-autonomous axonal dystrophy and degeneration in Purkinje cells.
Axonal Dystrophic Swellings of Atg7flox/flox;Pcp2-Cre Purkinje Cells Were Devoid of GFP-Light Chain 3 (LC3)-Labeled Puncta and Exhibited Increased Levels of p62/SQSTM1.
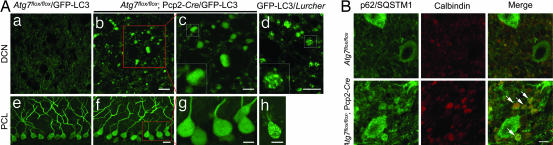
Transgenic mice producing GFP fused with microtubule-associated protein 1 light chain 3 (LC3), a specific marker for autophagosomes (21), were previously generated to monitor autophagosomes in vivo (2). By expressing GFP-LC3 in Lurcher mice (GFP-LC3/Lurcher), we showed that a large number of autophagosomes were formed in the dystrophic axon terminals of Lurcher Purkinje cells (Fig. 2Ad), providing in vivo evidence for the induction of autophagy in response to Lurcher-induced excitotoxicity (22). To assess autophagic activity in the dystrophic axons of Atg7flox/flox;Pcp2-Cre Purkinje cells, we crossed transgenic GFP-LC3 with Atg7flox/flox;Pcp2-Cre mice (Atg7flox/flox;Pcp2-Cre/GFP-LC3). Despite intense GFP-LC3 accumulation in the axonal dystrophic swellings of Purkinje cells in Atg7flox/flox;Pcp2-Cre/GFP-LC3 mice at P35, no GFP-LC3 fluorescent puncta characteristic of autophagosomes were observed in these swellings (Fig. 2A b and c). We contrasted this finding to our observation of GFP-LC3 puncta in Purkinje cell axons GFP-LC3/Lurcher mice (Fig. 2Ad) (22). In addition, no GFP-LC3 puncta were observed in the somata or dendrites of Atg7flox/flox;Pcp2-Cre/GFP-LC3 Purkinje cells (Fig. 2A f and g), again in contrast to the observation of GFP-LC3 puncta in the somata and dendrites of GFP-LC3/Lurcher Purkinje cells (Fig. 2Ah) (22).
Fig. 2.
The axonal dystrophic swellings of the Atg7-deficient Purkinje cells contained no GFP-LC3 labeled autophagosomes but accumulated p62/SQSTM1. (A) The absence of GFP-LC3 puncta in Purkinje cell axonal dystrophic swellings (b and c) and somata (f and g) of Atg7flox/flox;Pcp2-Cre/GFP-LC3 mice (P35). GFP-LC3 puncta were found in GFP-LC3/Lurcher Purkinje cell axonal dystrophic swellings (d) and somata (h) (P12). DCN (a) and Purkinje cell layer (PCL) (e) of control mice Atg7flox/flox/GFP-LC3 are shown. (Scale bars: a, b, e, and f, 20 μm; c, d, g, and h, 10 μm.) (B) Anti-p62/SQSTM1 immunofluorescent staining (in green) showed accumulation of p62/SQSTM1 in Purkinje cell axonal dystrophic swellings (calbindin labeling in red) (white arrows) in the DCN of Atg7flox/flox;Pcp2-Cre mice at P56. Atg7flox/flox was used as control. (Scale bar: 10 μm.)
It has been shown that inhibition of autophagy is correlated with increased levels of the polyubiquitin binding protein p62/SQSTM1 (22, 23). We thus examined the levels of p62/SQSTM1 in Atg7flox/flox;Pcp2-Cre Purkinje cells. As detected with anti-p62/SQSTM1 immunofluorescent staining, p62/SQSTM1 was markedly accumulated in the axonal dystrophic swellings (Fig. 2B, arrows) and somata (SI Fig. 9) of Atg7flox/flox;Pcp2-Cre Purkinje cells in comparison with Atg7flox/flox Purkinje cells. It is also noteworthy that the dystrophic axonal swellings in Lurcher Purkinje cells did not have detectable p62/SQSTM1 immunofluorescent staining (SI Fig. 10). These results provided molecular evidence for impaired autophagic activity in the dystrophic axons of Atg7flox/flox;Pcp2-Cre Purkinje cells, but not in the dystrophic axons of Lurcher Purkinje cells.
Atg7flox/flox;Pcp2-Cre Purkinje Cells Exhibited Normal Dendritic Tree and Spine Morphology at P56.
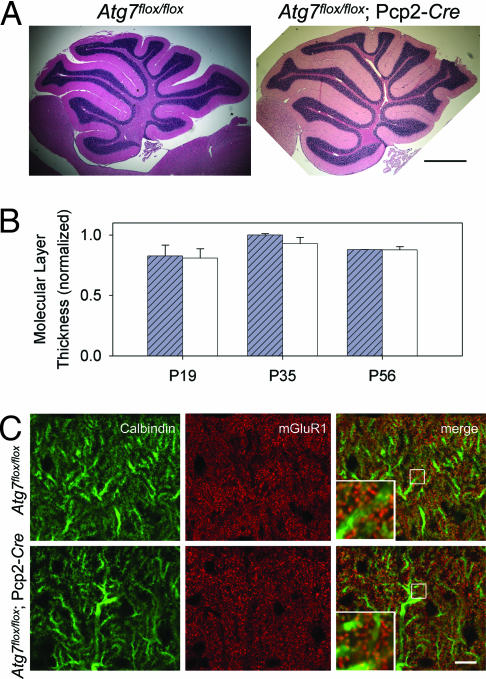
Despite the remarkable dystrophy and degeneration of Purkinje cell axon terminals in the DCN of Atg7flox/flox;Pcp2-Cre mice at P35 and P56, the cerebellar cortex displayed little change in its overall size and organization (Fig. 3A). For example, at P35 and P56, Atg7flox/flox;Pcp2-Cre mice and Atg7flox/flox mice did not exhibit significant difference in their cerebellar molecular layer thickness (Fig. 3 A and B). To evaluate changes in dendritic tree and spine morphology, we examined the expression pattern of metabotropic glutamate receptor 1α (mGluR1α) protein, a marker for parallel fiber–Purkinje cell synapses. No difference in localization and intensity of the anti-mGluR1α immunofluorescent staining was observed between the Atg7flox/flox;Pcp2-Cre and Atg7flox/flox cerebellar molecular layers at either P35 (data not shown) or P56 (Fig. 3C). Thus, Atg7 deletion had little effect on Purkinje cell dendritic tree and spine morphology up to at least P56. We conclude that Atg7 deletion in Purkinje cells elicit differential effects on the dendritic and axonal compartments, suggesting that the axon terminals are particularly vulnerable to autophagy deficiency.
Fig. 3.
Deletion of Atg7 in Purkinje cells had little effect on the morphology of cerebellar cortex, Purkinje cell dendritic tree and spines in Atg7flox/flox;Pcp2-Cre mice at P56. (A) H&E-stained images of midsagittal sections from Atg7flox/flox and Atg7flox/flox;Pcp2-Cre cerebella at P56. (Scale bar: 0.5 mm.) n = 3–5. (B) Quantification of the molecular layer thickness (as the distance between lobules V and VI of the Purkinje cell layer divided by 2) from the cerebellar midsagittal sections of Atg7flox/flox and Atg7flox/flox;Pcp2-Cre mice at P19, P35, and P56. n = 3–5. (C) Immunofluorescent staining of cerebellar midsagittal sections shows normal localization and appearance of mGluR1α (in red) in Atg7flox/flox;Pcp2-Cre mice compared with Atg7flox/flox mice at P56. Green indicates anti-calbindin. (Scale bar: 10 μm.) n = 3.
Axonal Dystrophy Preceded Cell-Autonomous Degeneration of Purkinje Cells and Behavioral Deficits in Atg7flox/flox;Pcp2-Cre Mice.
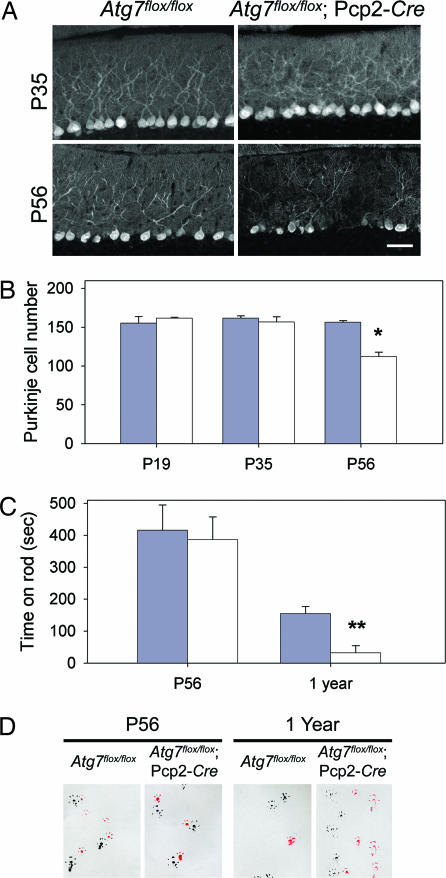
To further evaluate the effects of Atg7 deletion, we assayed for Purkinje cell degeneration and mouse behavioral deficits. Whereas the Purkinje cell axonal dystrophic swellings in the DCN of Atg7flox/flox;Pcp2-Cre mice first became apparent at P19 and grew severe at P35 (Fig. 2A), no significant difference in Purkinje cell numbers was observed between the Atg7flox/flox;Pcp2-Cre and Atg7flox/flox mice at both ages (Fig. 4 A and B). However, at P56, the number of Purkinje cells in Atg7flox/flox;Pcp2-Cre mice was reduced by 28.4% (P < 0.0005) compared with Atg7flox/flox mice (Fig. 4 A and B). Thus, loss of Purkinje cells in Atg7flox/flox;Pcp2-Cre mice occurred between P35 and P56. In comparison, the onset of axonal dystrophy began as early as P19. In addition, levels of GluRδ2 (a Purkinje cell-specific glutamate receptor subtype) in Atg7flox/flox;Pcp2-Cre cerebellar extract were not reduced until P56 (SI Fig. 11), further supporting that the onset of axon dystrophy was earlier than Purkinje cell degeneration in Atg7flox/flox;Pcp2-Cre mice.
Fig. 4.
Time course of Purkinje cell degeneration and locomotive behavioral deficits in Atg7flox/flox;Pcp2-Cre mice. (A) Anti-calbindin immunofluorescent staining of the cerebellar midsagittal sections of Atg7flox/flox and Atg7flox/flox;Pcp2-Cre mice at P35 and P56. (B) Quantitation of Purkinje cells at lobules IV–V of the midsagittal sections of Atg7flox/flox and Atg7flox/flox;Pcp2-Cre mice at P19, P35, and P56 based on H&E-stained images. n = 3, 3, and 3 for Atg7flox/flox at P19, P35, and P56, respectively. n = 2, 3, and 5 for Atg7flox/flox;Pcp2-Cre at P19, P35, and P56, respectively (*, P < 0.0005). (C) At P56, Atg7flox/flox and Atg7flox/flox;Pcp2-Cre mice showed no significant difference in the time spent on the rod in rotarod assay. At 1 year, Atg7flox/flox mice spent much longer time on the rod than Atg7flox/flox;Pcp2-Cre mice (**, P < 0.05). (D) In gait analyses at P56, Atg7flox/flox and Atg7flox/flox;Pcp2-Cre mice showed similar step width and overlapping of forefeet and hindfeet (forefeet, red; hindfeet, black). At 1 year old, Atg7flox/flox;Pcp2-Cre mice showed shorter step width than Atg7flox/flox mice as well as nonoverlapping forefeet and hindfeet (left feet, black; right feet, red). For both C and D, n = 5 at P56; n = 3 and 4 at 1 year.
Next, we assessed the locomotive behaviors in Atg7flox/flox;Pcp2-Cre mice by limb-clasping, rotarod, and gait analyses at different postnatal ages. At P19 and P35, Atg7flox/flox;Pcp2-Cre mice appeared normal and did not show any difference in performance compared with their control Atg7flox/flox littermates (data not shown). At P56, Atg7flox/flox;Pcp2-Cre and Atg7flox/flox mice performed equally well on the rotarod and gait analyses (Fig. 4 C and D). However, 5 of 13 Atg7flox/flox;Pcp2-Cre mice (38.5%) displayed limb-clasping reflexes on tail suspension, in comparison with 0 of 10 of their control littermates (P < 0.01). Thus, at P56, despite the 28.4% loss of Purkinje cells (Fig. 4B), Atg7flox/flox;Pcp2-Cre mice displayed only mild behavioral impairment. In contrast, at 1 year, these mice demonstrated severe behavioral disorders in locomotion and motor coordination when evaluated in all three behavioral tests (Fig. 4 C and D; data not shown).
We summarize the temporal relationship of the morphological alterations, differential pathology in different compartments of Purkinje cells, and behavioral changes in Atg7flox/flox;Pcp2-Cre mice in SI Table 1. These results demonstrate that axonal dystrophic swelling is an early pathogenic event and is likely to be a direct result of impairment of local autophagy in axon terminals.
Aberrant Membrane Structures Accumulated in the Dystrophic Axon Terminals of Atg7flox/flox;Pcp2-Cre Purkinje Cells.
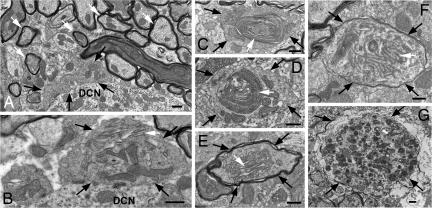
To further assess the effect of impairment of autophagy on axon terminals, we characterized the axonal dystrophic swellings of Atg7flox/flox;Pcp2-Cre Purkinje cells by transmission electron microscopy. The cross-sections of the Purkinje cell axon terminals in the DCN of the Atg7flox/flox mice were normally 0.5–2 μm in diameter (Fig. 5A, white arrows). Remarkably, the swollen Purkinje cell axon terminals in the DCN of the Atg7flox/flox;Pcp2-Cre mice often spanned 1–6 μm in diameter (Fig. 5 B–F, black arrows) and differed profoundly in their morphology from the axonal dystrophic swellings observed in Lurcher Purkinje cells (Fig. 5G, black arrows). The Purkinje cell axonal dystrophic swellings of Lurcher mice contained a large number of autophagosomes/autolysosomes (Fig. 5G), whereas those of the Atg7flox/flox;Pcp2-Cre mice were devoid of autophagosomes (Fig. 2 and 6Af). However, these autophagasome-free swellings of the Atg7flox/flox;Pcp2-Cre Purkinje cell axons often contained abnormal organelles or membrane structures (Fig. 5 B–F, white arrows), including stacks of cisternal membranes that formed lamellar bodies (Fig. 5 B and E, white arrows) (24), large and elaborate cisternal arrays and filaments (Fig. 5F, white arrow), and highly convoluted double-membrane whorls that occupied 1.5–2 μm of the swollen terminal (Fig. 5 C and D, white arrows). The exact nature of these aberrant structures was not clear; they were rarely seen in the somata of Atg7flox/flox;Pcp2-Cre Purkinje cells (data not shown) or in the Atg7flox/flox Purkinje cells axons (Fig. 5A). However, it is noteworthy that the formation of convoluted membrane whorls was previously described in hepatocytes with Atg7 deletion and was attributed to a failure in autophagic degradation (19). Our observations suggest a conserved function for autophagy in the clearance of cellular membranes and/or lipids in both axon terminals and hepatocytes.
Fig. 5.
Deletion of Atg7 in Purkinje cells led to aberrant membrane structures in the axonal dystrophic swellings. Ultrastructural image of normal myelinated Purkinje cell axons (white arrows) and axon terminals (black arrows) in the DCN of Atg7flox/flox mice (A) and Purkinje cell axonal dystrophic swellings (black arrows) in the DCN of Atg7flox/flox;Pcp2-Cre mice (B–F). (B and E) Stacks of cisternal membranes (white arrows). (C and D) Convoluted double-membrane whorls (white arrows). (F) The arrays of abnormal filaments (white arrows). (G) A dystrophic axon (black arrows) of Lurcher Purkinje cells containing numerous autophagosomes. (Scale bars: 500 nm.)
Fig. 6.
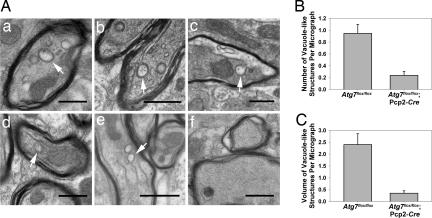
Deletion of Atg7 in Purkinje cells abolished double-membrane vacuole-like structures in their axon terminals in the DCN. (A) Ultrastructural images show the presence of vacuole-like structures with double membranes in Purkinje cell preterminal axons of Atg7flox/flox mice (a–e, white arrows) and the abolishment of these structures in Atg7flox/flox;Pcp2-Cre mice (f) at P35. (Scale bars: 0.5 μm.) (B) Comparison of the numbers of these vacuole-like structures per transmission electron microscopy micrograph (50 μm2) in the DCN of Atg7flox/flox;Pcp2-Cre versus Atg7flox/flox mice (ratio, 4.0; P = 0.00003). (C) Comparison of the volume fraction of double-membrane vacuole-like structures by point counting of transmission electron microscopy micrographs in the DCN of Atg7flox/flox;Pcp2-Cre versus Atg7flox/flox mice (ratio, 6.9; P = 0.00002).
Vacuole-Like Structures with Double Membranes Were Formed in Normal Purkinje Cell Axons but Were Absent in the Dystrophic Axon Terminals of Atg7flox/flox;Pcp2-Cre Purkinje Cells.
Interestingly, through further ultrastructural analysis, we observed vacuole-like structures with double membranes in the myelinated Purkinje cell axons in the DCN of control Atg7flox/flox mice (Fig. 6Aa and SI Fig. 12). These structures typically appeared to be closed and had diameters of 0.1–0.5 μm (Fig. 6Aa). We occasionally observed these double-membrane vacuole-like structures in the process of formation (Fig. 6A b–e and SI Fig. 12A). Many of these developing double-membrane vacuole-like structures appeared to be formed through invagination of the axolemma along the myelinated layers (Fig. 6A b and c, SI Fig. 12A), reminiscent of invasion by oligodendrocytic processes (25, 26). Some of them appear to be continuous with the axonal plasma membrane (Fig. 6A d and e), which are enwrapping portions of axoplasm (27). The average number of these distinct double-membrane vacuole-like structures (both closed and in the process of formation) in Purkinje cell axons of Atg7flox/flox mice was ≈0.9/50 μm2 (Fig. 6B). In contrast, these structures were virtually absent in the Atg7flox/flox;Pcp2-Cre mice (Fig. 6 Af, B, and C). Although we have yet to determine the exact nature of the vacuole-like structures with double membranes and their relationship with autophagosomes, our results suggested that Atg7 was required for the formation of these distinct structures in axon terminals of normal Purkinje cells.
Discussion
Axonal dystrophic swelling is a hallmark of CNS axonopathy, which can be triggered by neuronal injuries, excitotoxicity, and various neurodegenerative conditions. Despite the prevalence of this pathology, the molecular mechanisms underlying axonopathy as well as the connection between axonopathy and neurodegeneration remain poorly understood (28). A critical question is whether axonal dystrophy and degeneration precede neuronal cell death or are secondary to neurodegeneration. Here, we analyzed the time course of pathological events after the deletion of the autophagy gene Atg7 in cerebellar Purkinje cells. We showed that axonal dystrophy and degeneration caused by ablation of Atg7 occurred much earlier than the onset of neuronal death, indicating that axonal dystrophy was not secondary to neurodegeneration. In addition, axonal dystrophy and degeneration was a cell-autonomous event, which precluded the action of glia as the primary cause of the axonal dystrophy in Atg7flox/flox;Pcp2-Cre mice. Although this study was limited to Purkinje cells for the purpose of cell type-specific study of autophagy, axonal dystrophies occurred widely in many regions of the mutant mouse brain with Atg7 or Atg5 deletion (16, 17). We speculate that the axonal dystrophy associated with various types of neurons in these mutant mice are also cell-autonomous events caused by the absence of neuronal autophagy. Importantly, our results implied that interference in local autophagy would have a deleterious effect on axons, a potential mechanism of axonopathies involving impaired autophagy. Furthermore, we showed that deletion of Atg7 had little effect on the overall morphology of the Purkinje cell dendritic arbors at a stage when axon terminals displayed massive dystrophy and degeneration. These differential effects of Atg7 deletion suggested that basal levels of autophagy played a particularly important role in housekeeping functions in axon terminals and in protection against axonal degeneration.
We showed that Atg7 was indispensable for the formation of the distinctive vacuole-like structures with double membranes, which were normally present within wild-type Purkinje cell axons. These structures have not been described previously in the wild-type Purkinje cell axons. Although the majority of these structures were likely derived from invagination of neighboring oligodendrocytes (Fig. 6A b and c and SI Fig. 12A) (25, 26), some of them appeared to originate from axonal subsurface cisternae (27) or smooth endoplasmic reticulum (29) (Fig. 6A d and e). Although we have yet to determine the nature of these structures, we cannot exclude the possibility that some of these structures are autophagosome-related vacuoles. A previous study has shown the presence and transport of autophagosomes in the axons of cultured sympathetic neurons, despite that the physiological function of autophagy in the axons is unknown (30). Regardless of the nature of these vacuole-like structures, Atg7 deletion abolished their formation, and caused axonal swelling and accumulation of aberrant membrane structures in these swellings. These results established an important role of Atg7 in regulating local membrane trafficking and turnover. However, an important question arising from these results is whether the requirement of Atg7 for the formation of the double-membrane vacuole-like structures is somehow connected to autophagy or associated with a specific Atg7 function independent of autophagy. Current evidence suggests that Atg7 function is exclusively associated with autophagy. Consistent with this idea, the abolishment of the double-membrane vacuole-like structures caused by Atg7 deletion can be explained by the failure of autophagy, which would normally participate in the formation of these structures. Thus, we hypothesize that, in addition to the role in protein quality control (16, 17), neuronal autophagy regulates membrane homeostasis in the axon terminals. Furthermore, we showed that deletion of Atg7 caused the axonal terminal swelling, which was a reminiscence of hepatic cell swelling in Atg7-deleleted mouse liver (19). It is conceivable that axon terminals and hepatocytes may share a similar mechanism for cell (or cellular compartment) size control, which requires autophagy (31).
Previous morphological studies have consistently shown the presence of large numbers of autophagosome-like vacuoles in axonal dystrophic swellings of injured neurons (4–13). We have previously demonstrated that induction of autophagy in Lurcher Purkinje cells involved accumulation of autophagosomes in the dystrophic axons (22). In contrast, our present study shows that the axonal swellings of Purkinje cells in Atg7flox/flox;Pcp2-Cre mice were devoid of vacuoles that resembled the autophagosomes observed in Lurcher mice. Although our present study demonstrates a role for basal levels of autophagy in axonal protection and indicates that altered autophagy could serve as an adaptive response for remodeling the axon terminals for regeneration (3, 22), we cannot exclude the possibility that up-regulation of autophagy in dystrophic axons is actually destructive, causing overdegradation of axonal structures. This possibility can be tested, in principle, by genetic crossing of the autophagy-deficient mice with diseased mice containing the autophagosome-like vacuoles in dystrophic axons or cell bodies.
In summary, our study provides genetic and molecular evidence for the indispensable role of neuronal autophagy in the maintenance of axonal homeostasis, particularly in local membrane trafficking and turnover. Perturbation of local autophagy in the axons leads to axonopathy. We believe that it is important to study the connection between axonal autophagy impairment and human neuropathological conditions associated with axonal dystrophy.
Materials and Methods
Antibodies.
Antibodies used were mouse monoclonal anti-calbindin D-28K (Swant, Bellinzona, Switzerland), anti-calbindin (Sigma, St. Louis, MO), anti-GluRδ2 (BD Transduction Laboratories, San Diego, CA), anti-p62 (American Research Products, Belmont, MA), Cy3-conjugated anti-mouse and anti-rabbit IgG (Upstate Biotechnology, Lake Placid, NY), and NeuN, mGluR1α, and actin antibodies (Chemicon International, Temecula, CA). Anti-Atg7 is described in ref. 19.
Animals.
Pcp2-Cre transgenic mice (20) (The Jackson Laboratory, Bar Harbor, ME) and Atg7flox/flox mice (19) were crossed to produce Atg7flox/flox;Pcp2-Cre mice. Atg7flox/flox;Pcp2-Cre mice and GFP-LC3 transgenic mice (2) were crossed to produce Atg7flox/flox;Pcp2-Cre/GFP-LC3 mice.
Histological Examination.
Mice were fixed by cardiac perfusion with 0.1 M phosphate buffer containing 4% paraformaldehyde. The color images of the Meyer's H&E-stained midsagittal cryosections (10 μm) of cerebella were acquired with a 20× objective lens and a color CCD camera, and later assembled to full images in Photoshop for the quantification of Purkinje cells. Immunofluorescent stained cerebellar samples were prepared as described in ref. 22 and examined by using confocal microscopy.
Behavioral Analyses.
Motor function was assessed by the limb-clasping test, rotarod assay, and gait analysis (SI Materials and Methods).
Electron Microscopy.
Tissue samples were obtained from three Atg7flox/flox mice and three Atg7flox/flox;Pcp2-Cre mice. Details of the experiment are described in SI Materials and Methods. In brief, thin sections (70 nm) of the lateral cerebellar nucleus and cerebellar cortex were prepared and examined by transmission electron microscopy (H7500; Hitachi, Tokyo, Japan). A double-blind point-counting method was used to quantify double-membrane vacuole-like structures in 55 micrographs of either Atg7flox/flox or Atg7flox/flox;Pcp2-Cre mice. The total number of these structures overlying at least one intersection of a Photoshop-generated grid was counted to be 52 and 13 for Atg7flox/flox and Atg7flox/flox;Pcp2-Cre samples, respectively. The total number of intersections within these structures was 132 and 19 for Atg7flox/flox and Atg7flox/flox;Pcp2-Cre samples, respectively.
Statistical Analyses.
The equality of the variance was first tested by using the F test. Pair-wise comparisons were calculated by using one-tailed Student's t test. The standard error was calculated for each sample.
Supplementary Material
Acknowledgments
We thank X. Li, Y. Ding, T. Kouno, and K. Tatsumi for excellent technical assistance; A. North in the Rockefeller Bio-Imaging Resource Center for help with microscopy; and S. Waguri, T. Ueno, I. Tanida, J. Ezaki, and N. Heintz for helpful discussion. This study was supported by National Institutes of Health Grants RNS055683A (to Z.Y.), RR00862, and RR022220 (both to B.T.C.).
Abbreviations
- DCN
deep cerebellar nucleus
- Pn
postnatal day n
- LC3
light chain 3
- mGluR
metabotropic glutamate receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701311104/DC1.
References
- 1.Levine B, Klionsky DJ. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubinszstein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 4.Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JS. Nature. 1967;215:657–658. doi: 10.1038/215657a0. [DOI] [PubMed] [Google Scholar]
- 6.Matthews MR, Raisman G. Proc R Soc Lond Ser B. 1972;181:43–79. doi: 10.1098/rspb.1972.0040. [DOI] [PubMed] [Google Scholar]
- 7.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 8.Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. J Neurosci. 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 10.Roizin L, Stellar S, Willson N, Whittier J, Liu JC. Trans Am Neurol Assoc. 1974;99:240–243. [PubMed] [Google Scholar]
- 11.Sikorska B, Liberski PP, Giraud P, Kopp N, Brown P. Int J Biochem Cell Biol. 2004;36:2563–2573. doi: 10.1016/j.biocel.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Yu WH, Cuervo AM, Kumar A, Peternoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, et al. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. J Neurocytol. 2003;32:1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. J Neurosci. 2001;21:8473–8481. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman MP, Perry VH. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 17.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 18.Ohsumi Y, Mizushima N. Semin Cell Dev Biol. 2004;15:231–236. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barski JJ, Dethleffsen K, Meyer M. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- 21.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang QJ, Ding Y, Kohtz S, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banno T, Kohno K. J Comp Neurol. 1998;402:252–263. [PubMed] [Google Scholar]
- 25.Zhang P, Land W, Lee S, Juliani J, Lefman J, Smith SR, Germain D, Kessel M, Leapman R, Rouault TA, et al. J Struct Biol. 2005;150:144–153. doi: 10.1016/j.jsb.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddleman CS, Ballinger ML, Smyers ME, Fishman HM, Bittner GD. J Neurosci. 1998;18:4029–4041. doi: 10.1523/JNEUROSCI.18-11-04029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YC, Li YN, Cheng CX, Sakamoto H, Kawate T, Shimada O, Atsumi S. Neurosci Res. 2005;53:298–303. doi: 10.1016/j.neures.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Coleman M. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 29.Broadwell RD, Cataldo AM. J Comp Neurol. 1984;230:231–248. doi: 10.1002/cne.902300208. [DOI] [PubMed] [Google Scholar]
- 30.Hollenbeck PJ. J Cell Biol. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosokawa N, Hara Y, Mizushima N. FEBS Lett. 2006;580:2623–2629. doi: 10.1016/j.febslet.2006.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.