Abstract
It is generally assumed that light has no effect on the physiology of oocytes, zygotes, or early embryos. Therefore, little or no attention has been paid to lighting conditions during the handling of these cells in vitro. Here we show that cool white fluorescent light, rich in short-wavelength visible light and commonly used in research and clinical laboratories, produces more reactive oxygen species in mouse and hamster zygotes than does warm white fluorescent light. Mouse blastocysts that developed from zygotes shielded from light best developed to term fetuses followed by those exposed to warm white fluorescent light and then by those exposed to cool white fluorescent light. We hypothesized that light is one of the physical factors affecting embryonic environment and that its effects on cultured mammalian zygotes and embryos should not be overlooked.
Keywords: apoptosis, fetal development, hamster, mouse
Under normal conditions, mammalian oocytes are fertilized, and the zygotes develop into blastocysts in the protective environment of the female reproductive tract. Although techniques for culturing mammalian embryos in vitro have improved greatly during the past 3 decades, it is still unlikely that the “best” culture conditions used today can replace all of the benefits of embryo development within the female tract (1). Although mammalian embryos exhibit remarkable plasticity and will form blastocysts under a wide range of culture conditions (2), suboptimal environments might disturb not only gene expression patterns of preimplantation embryos (3, 4) but also their postnatal development and even the growth and physiology of offspring (5).
During assisted fertilization procedures such as in vitro fertilization and intracytoplasmic sperm injection, oocytes, zygotes, and preimplantation embryos are kept in artificial medium and exposed to light from time to time before transfer to mothers. This process is also true for transgenesis, cloning, and other procedures applied during the in vitro manipulation of oocytes, zygotes, or preimplantation embryos. Although great attention has been paid for many years to the role of medium constituents on embryo development (6, 7), the effects of light have not been studied as extensively. According to Bedford and Dobrenis (8), rabbit oocytes or zygotes exposed to strong “cool white” fluorescent light develop into apparently normal near-term fetuses. However, this observation does not imply that light is harmless to the oocytes and embryos of all mammalian species. In fact, golden hamster eggs are very vulnerable to light, and their meiosis is affected severely by the short-wavelength visible light emitted from the cool white fluorescent lamps that are commonly used in laboratories (9). Cleavage rates of hamster embryos are reduced by the same type of light (10, 11). Therefore, the use of a darkened room with carefully controlled light is the key to the success of in vitro fertilization of hamster oocytes (11).
Retardation in cleavage of mouse and rabbit embryos by light has also been reported (12–14). It is important to realize that under natural conditions, mammalian oocytes and embryos are never exposed to sunlight or strong artificial light. This lack of exposure contrasts to the experience of oocytes of many oviparous animals, such as fish and amphibians, which can be exposed to direct sunlight containing UV light during normal fertilization and development. These oocytes and embryos have mechanisms to protect themselves from high levels of UV light (15). We report here that development of mouse zygotes to blastocysts is apparently unaffected by exposure to cool white fluorescent light, but they develop to term fetuses less efficiently than those exposed to warm white fluorescent light or those that are not exposed to light at all. We repeated our previous studies and confirmed that hamster zygotes are very vulnerable to light. Although the hamster and mouse may not be the best models for the effects of light on human oocytes, embryos and oocytes of many other mammalian species could be more sensitive to light than we presume.
Results
In Vitro Development of Zygotes After Exposure to Cool White Fluorescent Light.
Hamster and mouse zygotes were collected from oviducts of naturally mated golden hamsters and mice ≈8 h after the estimated time of the start of fertilization. They were handled under a microscope incandescent light covered by a piece of red cellophane. Zygotes were maintained in hamster embryo culture medium-9 (HECM-9) or potassium simplex optimized medium with nonessential and essential amino acids (KSOMaa) (mouse), exposed to 1,200 lx of cool white fluorescent light for 15 min at 37°C under 5% CO2/5% O2/90% N2, and cultured in the dark for 96 h before blastocysts were transferred to surrogate mothers (for details, see Materials and Methods). Zygotes collected and handled in the same manner but shielded from light served as controls. Of 37 hamster zygotes exposed to cool white light, 36 (97%) underwent the first cleavage but did not divide further; all died without developing into blastocysts. In contrast, 100% (40/40) of mouse zygotes exposed to the same light developed into blastocysts (Table 1).
Table 1.
In vitro development of mouse and hamster zygotes exposed to cool white fluorescent light
| Species | Exposure of zygote to light* | No. of zygotes cultured | No. (%) of zygotes developed to |
||
|---|---|---|---|---|---|
| Two-cell | Morulae | Blastocysts | |||
| Hamster | − | 37 | 36 (97) | 34 (92) | 25 (68) |
| + | 37 | 36 (97) | 0 (0)† | 0 (0)† | |
| Mouse | − | 41 | 41 (100) | 41 (100) | 40 (98) |
| + | 40 | 40 (100) | 40 (100) | 40 (100) | |
*Zygotes were exposed to cool white fluorescent light (1,200 lx, 15 min) and then cultured in the dark until they reached the blastocyst stage. Zygotes handled in the same manner but shielded from light by wrapping the dishes with aluminum foil served as controls. In mouse, almost all (≈100%) zygotes developed to blastocysts whether or not the microscope incandescent lamp was covered with a piece of red cellophane.
†Significant difference within the same column in hamster (P < 0.05; χ2 test).
Production of Reactive Oxygen Species (ROS) of Zygotes After Exposure to Cool White or Warm White Fluorescent Lights.
In the next experiment, we assessed the level of ROS production in hamster and mouse zygotes after exposure to cool white or warm white fluorescent light for 15 min at 37°C under 5% CO2/5% O2/90% N2 by measuring hydrogen peroxide concentration (see Materials and Methods). As seen in Fig. 1, we found that fluorescent light, in particular cool white light, increased ROS in zygotes. Furthermore, much more ROS was produced in hamster zygotes than in mouse zygotes.
Fig. 1.
Production of ROS in hamster and mouse zygotes after 15-min exposure to fluorescent light (1,200 lx). The level of hydrogen peroxide is expressed as the relative fluorescein intensity of 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate di(acetoxymethyl ester). Each column shows the mean ± SEM. *, P < 0.05; **, P < 0.001; one-way ANOVA and Bonferroni's multiple comparison test (10 zygotes for each group in hamster and 6 zygotes for each group in mouse were analyzed).
Apoptosis in Blastocysts After Exposure of Zygotes to Various Lights.
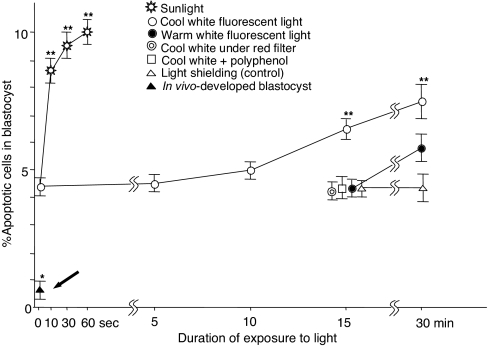
We examined apoptosis in mouse blastocysts developed from zygotes exposed to cool white or warm white fluorescent light for 5–30 min or direct midday sunlight (>20,000 lx) for 1–60 s (see Materials and Methods). Fig. 2 shows the incidence of apoptotic cells in blastocysts after exposure of zygotes to these lights. Sunlight was most effective in producing apoptosis, followed by cool white fluorescent light (1,200 lx) (Fig. 3). Warm white light (1,200 lx) produced many fewer apoptotic cells than cool white light. The incidence of apoptotic cells in blastocysts was low when the cool white fluorescent lamp was covered with red cellophane or when zygote-containing medium was supplemented with 10 μg/ml polyphenol (an antioxidant) (16) during exposure for 15 min to cool white fluorescent light. Warm white fluorescent light for 15 min also produced fewer apoptotic cells in blastocysts. It should be emphasized that blastocysts developed in vivo (the bold black arrow in Fig. 2) had many fewer apoptotic cells than any other blastocysts developed in vitro (in KSMOaa) with or without exposure to light.
Fig. 2.
Incidence of apoptotic cells in mouse blastocysts after exposure of zygotes to sunlight (>20,000 lx) and cool white and warm white fluorescent light (1,200 lx). Control zygotes were shielded from light by wrapping the dishes with aluminum foil. Zygotes were cultured in KSOMaa with 1 mg/ml albumin until they became blastocysts. The incidence of apoptosis in blastocyst developed in vivo throughout is indicated by a thick arrow. Each point represents the mean of 30 determinations from three replications. All error bars represent the S.E.M. *, P < 0.001 versus the development of all in vitro groups; **, P < 0.01 versus cool white fluorescent light exposure for 15 min; one-way ANOVA and Dunnett's test.
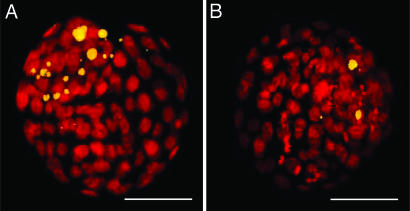
Fig. 3.
Apoptotic cells in mouse blastocysts. (A) Blastocyst developed from a zygote exposed to cool white fluorescent light (1,200 lx) for 15 min. Note the many fragmented, apoptotic cell nuclei shown by TUNEL assay (yellow). (B) Blastocyst developed from a zygote not exposed to light. (Scale bars: 30 μm.)
Full-Term Development of Mouse Blastocysts After Exposure of Zygotes to Various Lights.
We assessed the viability of blastocysts derived from zygotes exposed to cool white or warm white fluorescent light for 15 min or midday sunlight for 1 min. When we transferred normal-looking blastocysts to surrogate mothers (see Materials and Methods), those developed from the zygotes shielded from light (control) developed to term at the best rate (66 ± 13%) followed by those exposed for 15 min to warm white (58 ± 17%) and cool white fluorescent lights (42 ± 14%). Blastocysts that developed from zygotes exposed to sunlight developed most poorly (25 ± 14%) (Table 2). There were significant differences in live term fetus rates between cool white fluorescent light or sunlight and control (shielded from light) (P < 0.05; one-way ANOVA using Dunnett's test).
Table 2.
Postimplantation development of mouse zygotes exposed to fluorescent light or sunlight
| Zygotes exposed to* | Total no. blastocysts transferred (no. of replications) | No. of recipients† | Total no. (%, mean ± SEM) |
|
|---|---|---|---|---|
| Live term fetuses‡ | Resorbed fetuses‡ | |||
| No light (control) | 107 (4) | 10 | 73 (66 ± 13) | 18 (20 ± 7) |
| Warm white fluorescent light | 100 (4) | 10 | 58 (58 ± 17) | 19 (25 ± 16) |
| Cool white fluorescent light | 108 (3) | 10 | 44 (42 ± 14)§ | 20 (24 ± 23) |
| Sunlight | 100 (4) | 10 | 25 (25 ± 14)§ | 35 (57 ± 24)§ |
*Zygotes were exposed to warm or cool white fluorescent light (1,200 lx, 15 min) or sunlight (>20,000 lx, 1 min) and cultured in the dark until they reached the blastocyst stage. Zygotes handled in the same manner but shielded from light by wrapping the culture dishes with aluminum foil served as controls.
†ICR females (albino) mated with vasectomized ICR (albino) males. Blastocysts (5 or 6 embryos per uterine horn, or 10–12 per recipient) were transferred on day 3.5 of pseudopregnancy.
‡Live term and resorbed fetuses were examined on day 19 after coitus.
§Within the same column, significant difference (P < 0.01) versus control. Data were analyzed by one-way ANOVA and Dunnett's test.
Discussion
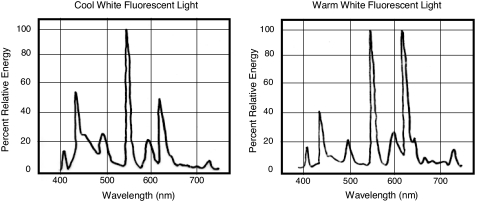
These results indicate that among the artificial lights we tested, cool white fluorescent light was the most “harmful” or “stressful” to mouse zygotes. Warm white fluorescent light and incandescent light, which have lesser amounts of short-wavelength visible light (Fig. 4), appear to be less stressful to oocytes and embryos than cool white light. Oocytes and embryos of different species must have different sensitivities to light, and the hamster could be an extreme example of animals whose eggs are particularly sensitive. The rabbit, whose oocytes and zygotes withstand strong light, could be an opposite extreme. Even though humans may be close to the rabbit in this respect (17), exposure of oocytes, zygotes, and embryos to visible light, in particular to short-wavelength (near-UV) light, should be avoided or minimized to make the physical environment of in vitro cultured embryos as close as possible to that of embryos in vivo. The use of a dark room with dim light is not practical and is unnecessary (17). Warm white fluorescent light containing little short-wavelength light is safe and more convenient to use. Incandescent light from the illuminators of ordinary optical microscopes would not produce any serious problems unless used excessively (9).
Fig. 4.
Spectral distribution of cool white and warm white fluorescent lamps (quoted Panasonic technical data).
The KSOMaa we used for the culture of mouse embryos in this work is one of the best media available, yet development was still retarded compared with that in vivo (18). We found that those blastocysts developed from oocytes fertilized and developed completely in vivo had many fewer apoptotic cells than those developed from zygotes cultured in vitro, regardless of exposure of zygotes to light (Fig. 2). Thus, this medium is still stressful to mouse zygotes and preimplantation embryos.
Apoptosis is a natural biological process to eliminate damaged cells or those expressing inappropriate phenotypes or with impaired developmental potential (19). In developing mouse embryos, it occurs predominantly in the inner cell mass of the blastocyst, which is the component that contributes to the embryo proper as distinct from the trophoblastic tissues (20). It is also known that apoptosis increases when embryos are cultured under suboptimal conditions (19–24). Increased apoptosis in blastocysts (and consequently fewer healthy inner-cell mass cells) impairs embryonic and fetal development (21, 23–25).
It is well known that UV light (300- to 400-nm wavelength) damages DNA and produces free radical oxygen species such as H2O2 within the cell (26), which in turn damage DNA, proteins, and lipids. Although short-wavelength visible light (400–450 nm) from the ordinary cool white fluorescent lamp would be far less damaging to cells than UV, prolonged exposure of mammalian oocytes and embryos to this light is inadvisable. Even warm white fluorescent light could be stressful to eggs if the exposure time is increased (Fig. 2).
Mammalian oocytes and embryos that are never exposed to direct sunlight under natural conditions may have lost the mechanism to protect themselves from such strong electromagnetic radiation. We expect that the in vitro handling of germ cells, zygotes, and embryos will become increasingly common with advancing biotechnologies. Because the in vitro culture of oocytes and embryos can modify genetic and epigenetic processes of embryos and produce long-term effects in offspring, every effort must be made to make the embryonic environment in vitro as close as possible to the natural embryonic environment in vivo. Light is one of the physical factors of the embryonic environment, and its effects should not be ignored.
Materials and Methods
Animals.
B6D2F1 (C57BL/6 × DBA/2) hybrid mice (8–12 weeks old) were each injected with 5 units of equine chorionic gonadotropin. Two days later, 5 units of human chorionic gonadotropin were injected, and the females were housed with fertile males. Zygotes (pronuclear oocytes) were collected from oviducts ≈10 h after midnight on the day vaginal plugs were found; animals were on a 14-h light/10-h dark cycle (27). Random-bred ICR (albino) female mice (8–12 weeks old), mated with vasectomized males of the same strain were used as embryo recipients on day 3.5 of pseudopregnancy. Hamster zygotes were obtained from female golden hamsters (Mesocricetus auratus) (2–3 months old) mated during their natural estrus, ≈13 h after midnight on the day spermatozoa were found in postestrus vaginal discharge; animals were on a 14-h light/10-h dark cycle (28). The experiments were approved by the committee for Ethics on Animal Experiments in the Prefectural University of Hiroshima, under the law (No. 105) and notification (No. 6) of the government of Japan.
Reagents and Media.
Inorganic salts were purchased either from Sigma–Aldrich (St. Louis, MO) or from Nacalai Tesque, Inc. (Kyoto, Japan). All organic reagents were purchased from Sigma–Aldrich unless otherwise stated. The medium used for collection and manipulation of mouse embryos was a modified CZB with 20 mM Hepes/5 mM NaHCO3/0.1 mg/ml polyvinyl alcohol (cold water-soluble, Mr 30,000–50,000) instead of BSA (Hepes/CZB) (29). Mouse embryos were cultured in KSOMaa containing nonessential amino acids (30) (MEM nonessential amino acids solution, GIBCO, Grand Island, NY) and essential amino acids (MEM amino acids solution, GIBCO) and 1 mg/ml BSA (fraction V). KSOMaa was maintained in an atmosphere of 5% CO2 in air and Hepes/CZB in air. The medium used for collection and manipulation of hamster zygotes was TCM199 (with Earle's salts) containing 26 mM NaHCO3, 25 mM Hepes, 5% heat-inactivated FBS, 5 mM taurine, and 25 μM EDTA (11). TCM199 and FBS were purchased from GIBCO and ICN Biomedical, Inc. (Aurora, OH), respectively. Hamster zygotes were cultured in HECM-9 (31) supplemented with 0.5 mg/ml human serum albumin (Cohn fraction V, A-1653) at 37.5°C under 10% CO2/5% O2/85% N2.
Lighting Conditions.
Manipulation of embryos was performed in a windowless room [6 m (length) × 4 m (width) × 3 m (height)] with nine fluorescent ceiling lamps either all cool white or warm white (100 V, 40 W each). Spectral distributions of the light sources are shown in Fig. 4. Light intensity at the laboratory bench top was 1,200 lx. A dissecting microscope, used for harvesting zygotes and brief examinations of preimplantation embryos, had an incandescent lamp (6 V, 15 W). Light intensity at the specimen stage of the microscope was 1,500 lx. When all ceiling lamps were turned off and the room was illuminated with a single, small desktop incandescent lamp (100 V, 20 W), light intensities at the bench top and the specimen stage of the dissecting microscope were 8 lx and 250 lx, respectively. When the microscope lamp was covered with a red cellophane filter, light intensity at the microscope stage was 80 lx.
Collection and Light Exposure of Hamster and Mouse Zygotes.
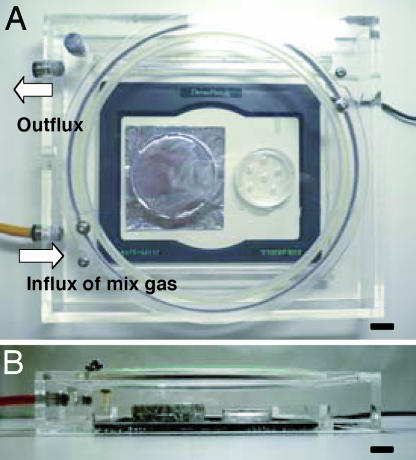
Zygotes flushed out of oviducts were rinsed with either gas-equilibrated HECM-9 (hamster) or KSOMaa (mouse) and transferred to 35- or 50-μl droplets of the same medium under paraffin oil (26137-85; Nacalai Tesque, Inc., Kyoto, Japan) in plastic Petri dishes (35 × 10 mm; Becton Dickinson, Franklin lake, NJ). The depth of paraffin oil in the dish was ≈5 mm. Each medium droplet contained 8–12 zygotes. Dishes were put on a heated plate (37°C) (Thermo plate MATS-505SF; TOKAI HIT, Shizuoka, Japan) and covered with a transparent plastic hood (clear polystyrene, 1 mm thick at the hood top) through which a gas mixture (5% CO2/5% O2/90% N2) was passed constantly (Fig. 5). After exposing them to cool white or warm white fluorescent light (1,200 lx) for 5–30 min or to direct midday sunlight (>20,000 lx) for 1–60 s, dishes were transferred to a CO2 incubator with gas phase of 5% CO2 in air. Zygotes in dishes covered with aluminum foil during exposure to room light (Fig. 5) or to sunlight served as controls. Zygotes in dishes not exposed to light (except for the microscope incandescent light covered with a red cellophane filter) also served as other controls. In one series of experiments, 10 μg/ml polyphenol (an antioxidant from green tea) (16) was added to KSOMaa to see whether it could protect zygotes from damage caused by cool white fluorescent light exposure for 15 min.
Fig. 5.
A simple incubator used during light exposure of zygotes. Dishes with zygotes were put on a heated plate (37°C) covered with a transparent plastic hood (clear polystyrene, 1 mm in thickness at the hood top) through which a mixed gas (5% CO2/5% O2/90% N2) was constantly passed. The left dish was a control covered with aluminum foil. (A) Top view. (B) Side view. (Scale bars: 1 cm.)
Examination of in Vitro Development of Zygotes.
Hamster zygotes with or without exposure to fluorescent light were cultured in 35-μl droplets of HECM-9 under a humidified atmosphere of 5% CO2/10% O2/85% N2 for up to 72 h in the dark before examination of their development to morulae/blastocysts. Mouse zygotes were cultured in 50-μl droplets of KSOMaa under a humidified atmosphere of 5% CO2 in air for up to 96 h in the dark before examination of their development to blastocysts and detection of apoptosis by TUNEL assay (see below).
Mouse Embryo Transfer to Surrogate Mothers and Examination of Fetuses at Term.
Mouse blastocysts selected from embryos cultured for 96 h in vitro were transferred into the uteri of day 3.5 pseudopregnant females (32). Implantation of blastocysts to the uterus was expected to occur 2 days later. Females were killed on day 19 of pregnancy, and the numbers of implantation sites and live fetuses were determined. Some live fetuses were selected randomly and raised by foster mothers.
Detection of Apoptosis by TUNEL Assay.
The numbers of apoptotic cells in mouse blastocysts were determined by the TUNEL method by using an in situ cell death detection kit (Roche Diagnostics, East Sussex, U.K.) according to the manufacturer's instructions. Some blastocysts were collected from uteri of naturally mated females on day 4 of pregnancy. Blastocysts were fixed in 4% paraformaldehyde in PBS (137 mM NaCl/2.7 mM KCl/10 mM Na2HPO4/1.8 mM KH2PO4) supplemented with 0.1% polyvinylpyrrolidone-40 (average molecular weight 40,000; PBS/PVP) for at least 1 h at room temperature, and permeabilized by immersing them in 0.5% Triton X-100 in PBS for 60 min at room temperature. Embryos were then washed twice in PBS/PVP and treated with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase for 1 h at 37°C in the dark. Cell nuclei were counterstained with 50 μg/ml propidium iodide. Embryos were washed and then mounted between a slide and coverslip by using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). The numbers, morphology, and DNA fragmentation of cell nuclei were determined by using an epifluorescence microscope equipped with filter sets for rhodamine and fluorescein. The percentages of cells with nuclear fragmentation (TUNEL labeling) were determined.
Measurement of ROS.
The level of ROS production in hamster and mouse zygotes was assessed by the concentration of hydrogen peroxide by using 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate di(acetoxymethyl ester) (Molecular Probes, Eugene, OR) (33, 34). Hamster or mouse zygotes in HECM-9 or KSOMaa were washed thoroughly in Hepes/CZB supplemented with 0.1% PVP-40 and then loaded with the dye for 30 min. Zygotes were then washed with Hepes/CZB to remove surface-bound dye before being mounted between a slide and coverslip. Fluorescence emissions of zygotes were recorded by Image-Pro Plus version 4.0 (Media Cybernetics, Silver Spring, MD) using a stabilized mercury lamp and fluorescent filters (excitation at 480 nm and emission at 510 nm) in an inverted microscope (Optiphoto-2, Nikon, Tokyo, Japan) to determine emission values by using ImageJ 1.33u (National Institutes of Health, Bethesda, MD).
Statistical Analysis.
Differences in the percentages of embryos developed to morulae and blastocysts in different experiments were compared by χ2 test. Data for the value of hydrogen peroxide concentration, total cell numbers per blastocyst, proportions of TUNEL-labeled nuclei and apoptotic cells, and fetal development after embryo transfer were assessed by using one-way ANOVA. The experiments were replicated at least three times except the experiment for ROS measurement. In measurement of ROS, a total of 18 or 30 zygotes obtained from three mice or three hamsters were allocated randomly and equally into three light-exposed groups. Differences in the incidence of apoptosis in blastocysts and in the concentration of hydrogen peroxide in zygotes among different experimental groups were assessed by using Bonferroni's multiple comparison test. Differences in the fetal development and resorption after embryo transfer between control and experimental groups were assessed by using Dunnett's multiple comparison test. P < 0.05 was considered significant.
Acknowledgments
We thank Dr. George Seidel of Colorado State University for reading the original manuscript and giving us invaluable advice. This work was supported in part by Basic Research grants from the Prefectural University of Hiroshima (to T.H.).
Abbreviations
- CZB
Chatot–Ziomet–Bavister medium
- HECM-9
hamster embryo culture medium-9
- KSOMaa
potassium simplex optimized medium with nonessential and essential amino acids
- lx
lux
- ROS
reactive oxygen species.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bavister BD. Theriogenology. 2000;53:619–626. doi: 10.1016/s0093-691x(99)00262-9. [DOI] [PubMed] [Google Scholar]
- 2.Roberts M. Endocrinology. 2005;146:2140–2141. doi: 10.1210/en.2005-0221. [DOI] [PubMed] [Google Scholar]
- 3.Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, Bartolomei MS. Development (Cambridge, UK) 2004;131:3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- 4.Schultz RM. Reproduction. 2005;130:825–828. doi: 10.1530/rep.1.00902. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez De Fonseca F, Pintado B, Gutierrez-Adan A. Proc Natl Acad Sci USA. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitten WK, Biggers JD. J Reprod Fertil. 1968;17:399–401. doi: 10.1530/jrf.0.0170399. [DOI] [PubMed] [Google Scholar]
- 7.Brinster RL. J Anim Sci. 1968;27(Suppl 1):1–14. [PubMed] [Google Scholar]
- 8.Bedford JM, Dobrenis A. J Reprod Fertil. 1989;85:477–481. doi: 10.1530/jrf.0.0850477. [DOI] [PubMed] [Google Scholar]
- 9.Hirao Y, Yanagimachi R. J Exp Zool. 1978;206:365–369. doi: 10.1002/jez.1402060308. [DOI] [PubMed] [Google Scholar]
- 10.Umaoka Y, Noda Y, Nakayama T, Narimoto K, Mori T, Iritani A. Theriogenology. 1992;38:1043–1054. doi: 10.1016/0093-691x(92)90118-b. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi Y, Yanagimachi R, Horiuchi T. Biol Reprod. 2002;67:534–539. doi: 10.1095/biolreprod67.2.534. [DOI] [PubMed] [Google Scholar]
- 12.Barlow P, Puissant F, Van der Zwalmen P, Vandromme J, Trigaux P, Leroy F. Mol Reprod Dev. 1992;33:297–302. doi: 10.1002/mrd.1080330310. [DOI] [PubMed] [Google Scholar]
- 13.Daniel JC., Jr Nature. 1964;201:316–317. doi: 10.1038/201316a0. [DOI] [PubMed] [Google Scholar]
- 14.Fischer B, Schumacher A, Hegele-Hartung C, Beier HM. Fertil Steril. 1988;50:938–944. doi: 10.1016/s0015-0282(16)60377-1. [DOI] [PubMed] [Google Scholar]
- 15.Hamdoun A, Epel D. Proc Natl Acad Sci USA. 2007;104:1745–1750. doi: 10.1073/pnas.0610108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra-Mohan K, Devaraj H, Prathiba D, Hara Y, Nagini S. Biochim Biophys Acta. 2006;1760:1536–1544. doi: 10.1016/j.bbagen.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Ottosen LD, Hindkjaer J, Ingerslev J. J Assist Reprod Genet. 2007;24:99–103. doi: 10.1007/s10815-006-9081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brison DR, Schultz RM. Biol Reprod. 1997;56:1088–1096. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- 19.Hardy K. Mol Hum Reprod. 1997;3:919–925. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- 20.Kamjoo M, Brison DR, Kimber SJ. Mol Reprod Dev. 2002;61:67–77. doi: 10.1002/mrd.1132. [DOI] [PubMed] [Google Scholar]
- 21.Lane M, Gardner DK. Biol Reprod. 2003;69:1109–1117. doi: 10.1095/biolreprod.103.018093. [DOI] [PubMed] [Google Scholar]
- 22.Byrne AT, Southgate J, Brison DR, Leese HJ. J Reprod Fertil. 1999;117:97–105. doi: 10.1530/jrf.0.1170097. [DOI] [PubMed] [Google Scholar]
- 23.Knijn HM, Gjorret JO, Vos PL, Hendriksen PJ, van der Weijden BC, Maddox-Hyttel P, Dieleman SJ. Biol Reprod. 2003;69:1371–1378. doi: 10.1095/biolreprod.103.017251. [DOI] [PubMed] [Google Scholar]
- 24.Gjorret JO, Knijn HM, Dieleman SJ, Avery B, Larsson LI, Maddox-Hyttel P. Biol Reprod. 2003;69:1193–1200. doi: 10.1095/biolreprod.102.013243. [DOI] [PubMed] [Google Scholar]
- 25.Pampfer S, de Hertogh R, Vanderheyden I, Michiels B, Vercheval M. Diabetes. 1990;39:471–476. doi: 10.2337/diab.39.4.471. [DOI] [PubMed] [Google Scholar]
- 26.Squirrell JM, Wokosin DL, White JG, Bavister BD. Nat Biotechnol. 1999;17:763–767. doi: 10.1038/11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards RG, Gates AH. J Endocrinol. 1959;18:292–304. doi: 10.1677/joe.0.0180292. [DOI] [PubMed] [Google Scholar]
- 28.Yanagimachi R. J Reprod Fertil. 1966;11:359–370. doi: 10.1530/jrf.0.0110359. [DOI] [PubMed] [Google Scholar]
- 29.Kimura Y, Yanagimachi R. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 30.Biggers JD, McGinnis LK, Raffin M. Biol Reprod. 2000;63:281–293. doi: 10.1095/biolreprod63.1.281. [DOI] [PubMed] [Google Scholar]
- 31.McKiernan SH, Bavister BD. Hum Reprod. 2000;15:157–164. doi: 10.1093/humrep/15.1.157. [DOI] [PubMed] [Google Scholar]
- 32.Lane M, Gardner DK. In: A Laboratory Guide to the Mammalian Embryo. Gardner DK, Lane M, Watson AJ, editors. Vol 1. New York: Oxford Univ Press; 2004. pp. 38–40. [Google Scholar]
- 33.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- 34.Nasr-Esfahani MH, Aitken JR, Johnson MH. Development (Cambridge, UK) 1990;109:501–507. doi: 10.1242/dev.109.2.501. [DOI] [PubMed] [Google Scholar]