Abstract
By using a functional genomics approach, we have identified a honey bee [Apis mellifera (Am)] odorant receptor (Or) for the queen substance 9-oxo-2-decenoic acid (9-ODA). Honey bees live in large eusocial colonies in which a single queen is responsible for reproduction, several thousand sterile female worker bees complete a myriad of tasks to maintain the colony, and several hundred male drones exist only to mate. The “queen substance” [also termed the queen retinue pheromone (QRP)] is an eight-component pheromone that maintains the queen's dominance in the colony. The main component, 9-ODA, acts as a releaser pheromone by attracting workers to the queen and as a primer pheromone by physiologically inhibiting worker ovary development; it also acts as a sex pheromone, attracting drones during mating flights. However, the extent to which social and sexual chemical messages are shared remains unresolved. By using a custom chemosensory-specific microarray and qPCR, we identified four candidate sex pheromone Ors (AmOr10, -11, -18, and -170) from the honey bee genome based on their biased expression in drone antennae. We assayed the pheromone responsiveness of these receptors by using Xenopus oocytes and electrophysiology. AmOr11 responded specifically to 9-ODA (EC50 = 280 ± 31 nM) and not to any of the other seven QRP components, other social pheromones, or floral odors. We did not observe any responses of the other three Ors to any of the eight QRP pheromone components, suggesting 9-ODA is the only QRP component that also acts as a long-distance sex pheromone.
Keywords: queen pheromone, olfaction, functional genomics
For several millennia, people have been intrigued by the honey bee Apis mellifera (Am), to exploit their honey production and pollination efficiency and simply to observe their complex social behavior. Honey bees live in colonies averaging 10,000–20,000 individuals that cooperatively care for their offspring by a division of labor between reproductive and nonreproductive biological castes (1, 2). A single female queen bee is responsible for reproduction, several hundred male drone bees exist only to mate, and thousands of sterile female workers maintain the colony (3). To regulate the complex social interactions, honey bees have evolved an intricate system of chemical communication that includes numerous glands that produce complex pheromone blends (4). The queen retinue pheromone (QRP), composed of at least eight components (5), maintains the queen's dominance in the colony. The main QRP component 9-oxo-2-decenoic acid (9-ODA), originally termed the queen substance (6), is a remarkable pheromone. 9-ODA acts as a releaser pheromone by attracting workers to the queen, as a primer pheromone by physiologically inhibiting worker ovary development, and as a sex pheromone attracting drones during mating flights (3, 4, 7, 8). More than 45 years after its discovery, 9-ODA remains the only long-distance sex pheromone that has been identified from honey bees, and the degree to which social and sexual chemical signals are shared remains unknown. Despite the fundamental importance of sexual reproduction and mating behavior, very few sex pheromones have been identified from the social Hymenoptera (bees, ants, and wasps) because of their sophisticated behavior and the difficulty of behavioral assays (9).
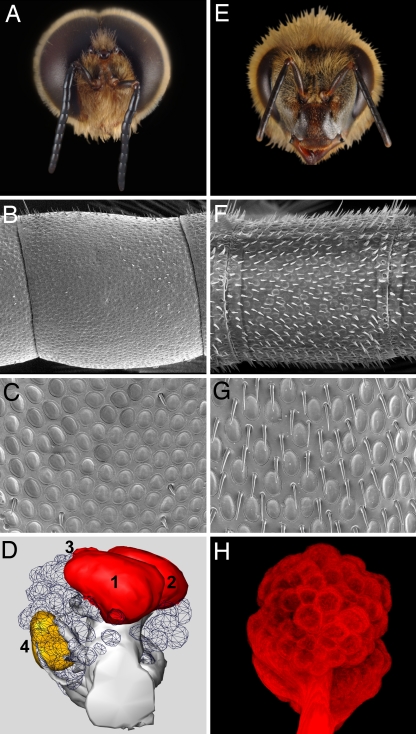
Honey bee mating behavior is striking. During summertime afternoons, sexually mature drones and virgin queens fly to discrete aerial congregation sites, typically 30–200 m in diameter and 10–40 m above ground (8). When a virgin queen enters the congregation area, drones (from a few hundred to thousands) begin pursuit, first attracted to chemical cues but also using vision at closer range. Drones mount virgin queens during flight, and copulation is complete within a few seconds, after which the drone dies (3). The role of olfaction in honey bee mating behavior was established in the early 1900s by using caged virgin queens to attract drone bees (10). The queen substance, 9-ODA, attracts drones on mating flights from distances up to 60 m (7). Drones exist only to mate with virgin queens, and their olfactory system has become specialized toward successful mating (3, 11, 12). Compared with worker bees, drones have larger antennae and about seven times as many placoid sensilla (≈18,000 compared with ≈2,700) (11, 12) (Fig. 1B, C, F, and G). Electrophysiological recordings have demonstrated that olfactory neurons within the placoid sensilla respond to 9-ODA (13, 14). Consequently, drone antennae have more olfactory neurons tuned to 9-ODA, and their antennae as a whole are more sensitive to 9-ODA as measured by electroantennography (15, 16). In addition, the drone antennal lobe (the first level of olfactory processing) has four enlarged male-specific macroglomeruli (17, 18) (Fig. 1 D and H). The number of male-specific moth macroglomeruli typically corresponds to the number of sex pheromone components (19).
Fig. 1.
The drone olfactory system is sexually dimorphic. Morphology of drone (A–D) and worker (E–H) honey bee olfactory systems. (A) Frontal view of the drone head illustrating the enlarged eyes and antennae and reduced mandibles compared with the worker in E. SEM photograph of a single antennal segment from drone (B) and worker (F) antennae illustrating the higher density of poreplate sensilla and reduced number of trichoid sensilla on the surface of drone antennae. (C and G) Enlarged view of the drone and worker antennal surface (×1,950 magnification). Photographs of drone and worker antennal surfaces were taken with a Philips XL30 ESEM-FEG microscope (FEI, Hillsboro, OR; Imaging Technology Group, Beckman Institute at UIUC). (D) A reconstructed drone antennal lobe (frontal view) illustrating four enlarged macroglomeruli, MG1–4. MG2 responds to antennal stimulation with 9-ODA (36). 3D reconstruction of the drone antennal lobe was created with AMIRA for Windows 3.0 software (TGS, San Diego CA). (H) Frontal view (2D transparent projection) of a worker antennal lobe (tetramethylrhodamine dextran-stained) illustrating isomorphic glomeruli. Microscopy and image analysis were performed with a Leica TCS SP2 confocal microscope (Imaging Technology Group, Beckman Institute at UIUC) with green (543-nm) and red (633-nm) helium/neon laser lines. Images (1,024 × 1,024 pixels) were acquired by using a ×10 or ×20 objective.
Recent whole-genome sequencing has propelled forward the knowledge of the molecular mechanisms of insect chemoreception (reviewed in refs. 20–22). The chemoreceptor (Cr) superfamily [odorant receptor (Or) and gustatory receptor families] has been identified as the primary receptors responsible for sensory detection of chemical stimuli in the environment, including pheromones. The odorant detection characteristics of a wild-type sensory neuron can generally be replicated by transferring its Cr into an empty neuron created within the sensilla of transgenic flies (22, 23). The honey bee genome has been sequenced (24) and 180 Crs annotated (25). We hypothesized that the sexual dimorphism of the drone olfactory system would include Or genes that detect sex pheromones produced by virgin queens. A similar strategy was used to identify the first moth sex pheromone receptors (26–28).
Results and Discussion
Four Candidate Sex Pheromone Receptors Expressed at Higher Levels in Drone Antennae.
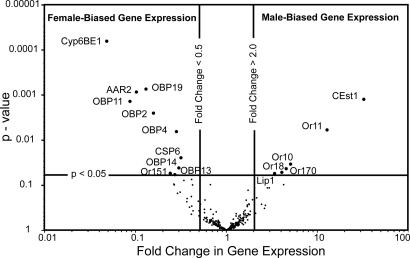
To screen Or gene expression levels, we constructed a custom chemosensory microarray with probes for the 170 Or and 10 gustatory receptor genes, as well as 133 other chemosensory-related genes [a similar approach was used with mosquitoes (29)]. These included odorant-binding protein (OBP) and chemosensory protein (CSP) genes that might be involved in odor transport, and P450, GST, and esterase genes that may be involved in odor degradation. Or gene expression in antennae collected from foraging workers returning to the hive with pollen loads and mature drones within hives was compared by using a dye-swap design with four replicated microarrays. Sixteen genes were differentially expressed (a >2-fold difference) in drone and worker honey bee antennae with statistical significance (P value <0.05) [Fig. 2; see SI Table 1]. Six were expressed at higher levels in drone antennae, whereas 10 genes were higher in worker antennae. Sex-biased expression of six OBP genes and one carboxyl esterase gene were also detected (AmOBP2, -4, -11, -13, -14, and -19 and AmCEst1; Fig. 2; SI Table 1). AmCEst1 protein is much more abundant in drone antennae compared with workers (30), and worker-biased expression of AmOBP2, -4, -11, and -19 has been reported (31), validating our microarray analysis. A dot-blot assay (31) and our microarray analysis (Fig. 2; SI Table 1) both show that AmOBP11 and -19 have the greatest worker-biased expression. We also detected worker-biased expression of genes encoding a CytP450 (AmCyp6BE1), an arrestin (AmAAR2), and a chemosensory protein (AmCSP6). Most significantly, the sex-biased expression of five Or genes was detected at P values <0.05, and four (AmOr10, -11, -18, and -170) were higher in drone antennae and therefore represent putative sex pheromone receptors. Similar to previous studies (ref. 29 and our unpublished results), microarrays are suited to detecting highly expressed Or genes.
Fig. 2.
Four candidate sex pheromone receptors are expressed at higher levels in drone antennae. Sex-biased expression of olfactory-related genes in drone and worker antennae determined by microarray analysis (two-way dye-swap design, n = four replicates). The volcano plot indicates that the majority of the genes were not differentially expressed (fold change in expression was >0.5 and <2.0, and the P values were >0.05). Ten genes were expressed at higher levels in worker antennae (upper left quadrant, P < 0.05, and fold change in expression <0.5), and six were expressed at higher levels in drone antennae (upper right quadrant, P < 0.05, and fold change in expression >2.0). GenBank accession nos.: AAR2 (XM394372), CEst1 (AY647436), CSP6 (NM1077819), Cyp6BE1 (XM624792), Lip1 (NM1011630), OBP2, -4, -11, -13, -14, and -19 (NM1011591, NM1011589, NM1040226, NM1040224, NM1040223, and NM1040209); Ors 10, 11, 18, 151, and 170 are published (25).
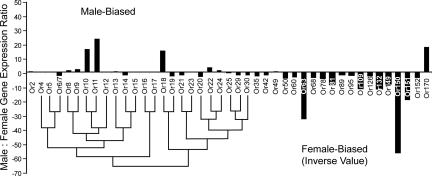
To validate the microarray results, differential expression of the AmOr genes in Fig. 2 was assayed by quantitative real-time PCR (qPCR) (Fig. 3). Because moth pheromone receptors form a conserved phylogenetic subfamily (26–28, 32), we included AmOrs with sequence similarity to the drone-biased Ors detected in the microarray analysis, as well as several randomly selected Ors to represent other lineages in their phylogenetic tree (25). Supporting our microarray results, of 43 AmOrs tested, only AmOr10, -11, -18, and -170 were expressed at higher levels in drone antennae (15- to 25-fold difference). Similarly, the worker-biased expression of AmOr151 was confirmed. The qPCR screen also detected two additional Ors with worker-biased expression (AmOr63 and -150) that were not detected by the microarray analysis, presumably because they were below the microarray threshold of detection. The drone-biased expression of AmOr10, -11, -18, and -170 compared with worker and queen bees was further confirmed with biological replication (SI Fig. 6). AmOr11 was the most highly expressed.
Fig. 3.
Three of the four candidate sex pheromone receptors group closely together in the honey bee phylogenetic tree. Drone-to-worker ratio of AmOr genes (n = 43) expressed in antennae, determined by qPCR. AmOr2, -4, -5, -6/-7, -8 to -25, -29, -30, -35, -42, -49, -50, -60, -63, -68, -78, -81, -89, -95, -109, -126, -132, -149 to -152, and -170 are depicted sequentially on the x axis. Cycle threshold (CT) values for each gene expressed in drone and worker antennae were normalized to the control gene AmRPS8 before calculating the drone-to-worker ratio (y axis). The phylogenetic relationships of AmOr4–30 are represented by a neighbor-joining tree as described in ref. 25.
AmOr11 Responds to 9-ODA, the Main Component of the Queen Substance.
In a few cases, the functional characterization of insect Ors using Xenopus oocytes and two-electrode voltage–clamp electrophysiology has been reported (28, 33). The mechanism by which insect Or activation results in a current response remains unknown (28, 34). Insect Ors may be ion channels, or they may couple to a transduction pathway that results in channel activation, in this case a transduction pathway and channel endogenous to Xenopus oocytes. To assess the accuracy of this expression system, we first examined the Drosophila receptor DmOr35a. The ligand specificity of this receptor has been characterized in an in vivo context (23, 35), offering an ideal test of the oocyte expression approach. We found that functional responses of DmOr35a could be observed only when DmOr83b was coexpressed, consistent with reports that the widely expressed DmOr83b serves as a dimerization, chaperone, or stabilization partner for many different DmOrs (28, 34). We also found a close correspondence between the functional properties of DmOr35a expressed in Xenopus oocytes (SI Fig. 7), and this receptor expressed in an in vivo context (23, 35).
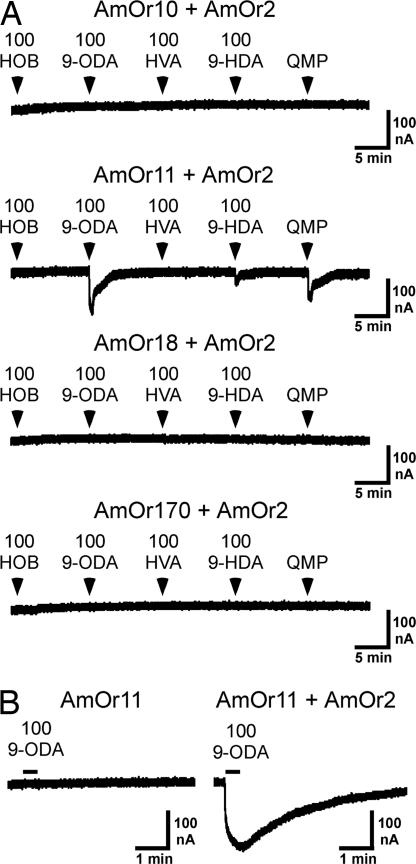
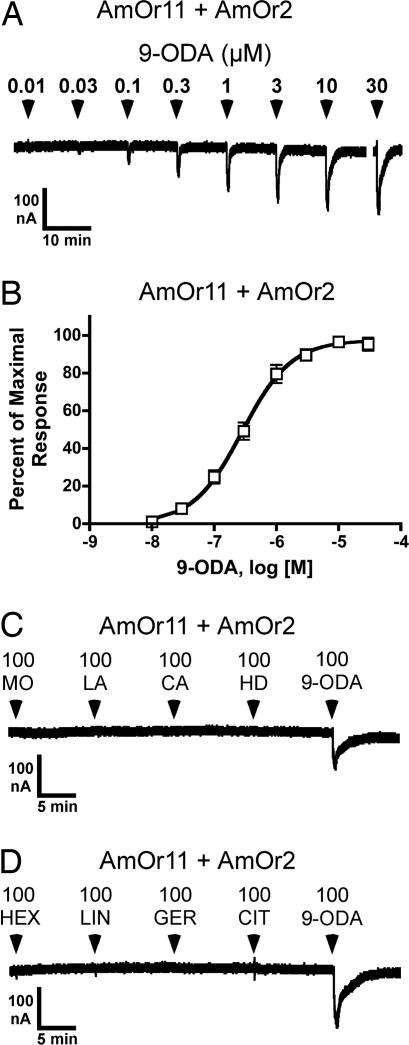
Next we used this Xenopus oocyte assay to examine the sensitivity of the four candidate pheromone receptors to components of the queen substance (QRP): 9-ODA, 9-hydroxy-2-decenoic acid (9-HDA), methyl p-hydroxybenzoate (HOB), 4-hydroxy-3-methyoxyphenylethanol (HVA), methyl oleate (MO), coniferyl alcohol (CA), 1-hexadecanol (HD), and linolenic acid (LA). The first four components have been termed the queen mandibular pheromone (QMP), because they are produced by the mandibular gland (3, 4). Oocytes injected with cRNA encoding AmOr10, -11, -18, or -170, in combination with mRNA encoding AmOr2 (the honey bee ortholog of DmOr83b), were screened with each of the four QMP components (HOB, 9-ODA, HVA, and 9-HDA), as well as a commercial QMP blend (the concentration of 9-ODA presented as a component of the blend was 100 μM). The AmOr11 + AmOr2 receptor responded to 100 μM 9-ODA and to the QMP preparation (Fig. 4A, n = 24). The response of AmOr11 to 9-ODA could be observed only when AmOr2 was coexpressed (Fig. 4B, n = 10). Similar to what we observed for the DmOr35a receptor (SI Fig. 7A), AmOr11 + AmOr2 activity reached a plateau during the 25-s 9-ODA application and began to decay upon washout of the 9-ODA (Fig. 4B). Dose–response analysis showed the AmOr11 + AmOr2 receptor to be highly sensitive to 9-ODA, with an EC50 of 280 ± 31 nM (Fig. 5 A and B). This is similar to the sensitivities reported for the silkworm moth pheromone receptors BmOr1 and -3, which, when expressed in Xenopus oocytes, respond to their cognate pheromone ligands bombykol and bombykal with EC50s of 1,500 and 260 nM, respectively (28). The AmOr11 + AmOr2 receptor did not respond to any of four additional minor components of QRP that have been described more recently: MO, LA, CA, and HD (5) (Fig. 5C, n = 5). We further examined the specificity of the AmOr11 + AmOr2 receptor by screening floral odorants (linalool and hexanol) and social pheromones (geraniol and citral) shown to activate ordinary glomeruli, but not macroglomerulus 2 (MG2) (36). AmOr11 + AmOr2 did not respond to any of these compounds (Fig. 5D, n = 8). Our results are similar to those of Sandoz (36), who found that the enlarged MG2 in the drone antennal lobe was activated when the antennae were specifically stimulated with 9-ODA, suggesting that olfactory neurons projecting to MG2 express AmOr11. With these results, we have identified AmOr11 + AmOr2 as a receptor for 9-ODA, the main component of the queen substance (also termed QMP/QRP). This receptor responds specifically to 9-ODA and not to other pheromone components or floral odors, thus detecting a critical chemical messenger that mediates sexual and social behavior via both primer and releaser effects.
Fig. 4.
9-ODA activates AmOr11 + AmOr2. (A) Oocytes injected with RNA encoding AmOr10 + AmOr2, AmOr11 + AmOr2, AmOr18 + AmOr2, or AmOr170 + AmOr2 are challenged with 100 μM HOB, 9-ODA, HVA, and 9-HDA. Each oocyte is also challenged with QMP prepared such that the concentration of 9-ODA is ≈100 μM. (B Left) An oocyte injected with RNA encoding AmOr11 fails to respond to 100 μM 9-ODA. (Right) A different oocyte expressing AmOr11 + AmOr2 responds to 100 μM 9-ODA. All applications were 25 s and are indicated by arrowheads (A) or bars (B). AmOr11 + AmOr2 also appeared to respond to a high concentration (100 μM) of 9-HDA (A). However, 9-HDA is synthesized from 9-ODA, and a slight contamination of 9-HDA with 9-ODA is possible.
Fig. 5.
Characterization of the 9-ODA receptor, AmOr11 + AmOr2. (A) An oocyte expressing AmOr11 + AmOr2 is challenged with a range of 9-ODA concentrations. (B) Dose–response relationship for 9-ODA activation of AmOr11 + AmOr2 (EC50 = 280 ± 31 nM, mean ± SEM, n = 10). (C) An oocyte expressing AmOr11 + AmOr2 responds to 100 μM 9-ODA but not to 100 μM methyl oleate (MO), linolenic acid (LA), coniferyl alcohol (CA), or 1-hexadecanol (HD). (D) An oocyte expressing AmOr11 + AmOr2 responds to 100 μM 9-ODA but not to 100 μM hexanol (HEX), linalool (LIN), geraniol (GER), or citral (CIT). All applications were 25 s and are indicated by arrowheads (A, C, and D).
A single Or gene is typically expressed in each insect olfactory sensory neuron, and the axons of all neurons expressing the same Or project to the same glomerulus in the antennal lobe (21, 22, 37). The antennae of male insects commonly have large numbers of olfactory sensory neurons tuned to female-produced sex pheromones, and their axons project to enlarged macroglomeruli. The macroglomeruli of male moths respond specifically to the female-produced sex pheromones, and the number of macroglomeruli generally corresponds to the number of sex pheromone components (19). Therefore, it is significant that we have identified four putative sex pheromone receptors, and that the antennal lobe of drone bees has four macroglomeruli absent in workers. AmOr11, the most highly expressed of our candidate sex pheromone receptors (SI Fig. 6), responds specifically to 9-ODA (Figs. 4 and 5), as does MG2, the largest of the four macroglomeruli (36). In contrast, we found that AmOr10, -18, and -170 did not respond to any of the eight QRP components (it remains a possibility that AmOr10, -18, and -170 simply failed to express in the oocyte system). On their own, QRP components such as 9-HDA, HOB, and HVA do not attract drones (38, 39). However, in a blend with 9-ODA, they increase the frequency of drones that make contact with the baited dummies, suggesting that these components may act as close-range pheromones stimulating courtship and copulation (39, 40). These components may exert their effects by activating ordinary glomeruli (rather than macroglomeruli) in the drone antennal lobe (36), and additional sex pheromones may exist. Two chemical fractions isolated from virgin queens were attractive to drones, one containing 9-ODA and the other unidentified (7). Furthermore, several behavioral studies suggest that the queen tergite glands located on the abdomen also secrete substances that promote courtship and copulation behavior (8, 41). Another interesting possibility is the existence of a drone aggregation pheromone that may help establish aerial congregation sites (behavioral observations supporting this hypothesis are reviewed in ref. 8). Male aggregation pheromones have been identified from the mandibular gland of some hymenopteran species (9), and one study suggests a similar gland exists in drone honey bees (42). In future studies, the availability of the Xenopus oocyte assay holds promise for identifying the missing ligands of AmOr10, -18, and -170, thereby illuminating novel aspects of honey bee mating biology and chemical communication.
Conclusions
By using a functional genomics approach, we have identified AmOr11 as a receptor for 9-ODA, the major queen substance component, and have demonstrated the high specificity of this receptor for its pheromone ligand. This specificity might be enhanced, because its cognate ligand mediates not one but several critical behaviors and physiological effects, communicating both sexual and social information. Our results indicate that of four candidate sex pheromone receptors, only AmOr11 responds to any of the eight QRP components. Thus, additional sex pheromones may remain to be identified. Functional genomic approaches, such as the one used in this study, hold promise for continued progress in this field. For example, deciphering the chemicals that mediate the great range of social behaviors of bees, wasps, and ants has been difficult because of their complex ethology and their use of complex chemical blends. Despite the fundamental importance of sexual reproduction and mating behavior, few sex pheromones have been identified from the social Hymenoptera because of their sophisticated behavior and the difficulty of behavioral assays (9).
Materials and Methods
QMP (a commercial blend of 9-ODA, 9-HDA, HOB, and HVA), 9-ODA, and 9-HDA were purchased from Pherotech, Delta, BC, Canada. Collagenase B was from Boehringer–Mannheim (Indianapolis, IN). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO), typically at purities >98%.
Microarray Analysis and qPCR Analysis.
A custom microarray containing 313 olfactory-related honey bee genes was constructed to screen Or gene expression levels in honey bee antennae. Oligonucleotides (70-mer sense-strand) were designed for 168 Or and 145 olfactory-related genes with ArrayOligoSelector3.5 software (43) (http://sourceforge.net) using default settings and the AM genome Assembly 2 (24). The Functional Genomics Unit at the W. M. Keck Center [University of Illinois at Urbana–Champaign (UIUC)] printed the oligonucleotides (IDT, Coralville, IA) onto glass slides using a GeneMachines (Genomic Solutions, Inc., Ann Arbor, MI) OmniGrid 100 microarray printer. Four housekeeping genes (positive controls) and ArrayControl sense 70-mer oligonucleotides (Ambion, Austin, TX; negative controls) were distributed throughout the 16-block array so that each block contained at least two positive and one negative control spot. Spot quality was assessed by using a Cy3-labeled 9-mer (Operon, Huntsville, AL) hybridized to a test microarray.
Foraging worker bees returning to the hive with pollen loads and mature drones bees within the hive were collected from colonies maintained by the UIUC Bee Research Facility. The bees were collected over several days in the summer months of 2005 and 2006 from several different colonies, in pools of n = 50 bees per sample. The antennae were dissected on dry ice and stored at −80°C until the total RNA was isolated from the pooled samples by using an RNeasy Mini Kit (Qiagen, Valencia, CA), quantified by absorption at a wavelength of 260 nm, and assessed for quality on a 1% agarose gel. First-strand cDNA was synthesized from 20 μg of total RNA by using an amino-allyl dNTP mix and, after purification, the cDNA was labeled with single-use CyDye (Cy5/Cy3; GE Healthcare, BioSciences Corp., Piscataway, NJ) by using standard protocols and further purified. Four microarray hybridizations were performed by using mature drone antennal total RNA vs. worker antennal total RNA with an even dye swap. Analysis was performed by using SAS software Ver. 9.1 (SAS Institute, Cary, NC).
The relative method of qPCR (44) and SYBR green dye (SYBR Green PCR Master Mix; Applied Biosystems, Foster City, CA) was used to assay Or gene expression levels in mature drone, worker, and queen antennae as published (25, 32). Complete details of the microarray and qPCR analysis are provided in SI Text.
Preparation of Oocytes and cRNA Injection.
Xenopus laevis frogs were purchased from Nasco (Fort Atkinson, WI). The care and use of X. laevis frogs in this study were approved by the University of Miami Animal Research Committee and meet the guidelines of the National Institutes of Health. Frogs were anesthetized by submersion in 0.1% 3-aminobenzoic acid ethyl ester, and oocytes were surgically removed. Oocytes were freed from the follicle cells by treatment with collagenase B for 2 h at room temperature. DmOr35a (GenBank accession no. NM_165117) and DmOr83b (GenBank accession no. NM_079511) cDNA clones were provided by John Carlson (23) and Leslie Vosshall (45), respectively. The complete ORFs of AmOr2, -10, -11, -18, and -170 were cloned from honey bee antennal cDNA. Ors were cloned into the pGEMHE vector (46) and confirmed by sequencing. Capped cRNA was synthesized in vitro from linearized template DNA encoding Ors by using mMessage mMachine kits (Ambion). Stage V oocytes were injected with cRNA in 25 nl of water. Oocytes were incubated in Barth's saline (88 mM NaCl/1 mM KCl/2.4 mM NaHCO3/0.3 mM CaNO3/0.41 mM CaCl2/0.82 mM MgSO4/15 mM Hepes, pH 7.6/12 μg/ml tetracycline) for 3–7 days before electrophysiological recording.
Functional Analysis of Insect Ors in Xenopus Oocytes.
Odorant-induced currents were measured 3–7 days after cRNA injection by using two-electrode voltage clamp in an automated parallel electrophysiology system (OpusExpress 6000A; Molecular Devices, Union City, CA). Detailed electrophysiological methods are provided in previous publications (47, 48). Briefly, micropipettes were filled with 3 M KCl and had resistances of 0.2–2.0 MΩ. The holding potential was −70 mV. Oocytes were continuously perfused with ND96 (96 mM NaCl/2 mM KCl/1 CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.5). High-concentration stock solutions of each odorant were freshly prepared in DMSO on the day of each experiment. Each odorant, diluted in ND96, was applied for 25 sec at 10-min intervals. Immediately after odorant application, the oocytes were perfused with ND96 to wash out the odorant. Current responses, filtered (4-pole, Bessel, low pass) at 20 Hz (−3 db) and sampled at 100 Hz, were captured and stored by using OpusXpress 1.1 software (Molecular Devices). Initial analysis was done by using Clampfit 9.1 software (Molecular Devices). Dose–response analysis was done by using PRISM 4 (GraphPad, San Diego, CA). Dose–response curves were fit according to the equation: I = Imax/(1+(EC50/X)n), where I represents the current response at a given concentration of odorant, X; Imax is the maximal response; EC50 is the concentration of odorant yielding a half-maximal response; and n is the apparent Hill coefficient.
SI.
SI includes detailed experimental procedures (SI Text), SI Figs. 6 and 7, and SI Table 1.
Supplementary Material
Acknowledgments
We thank Harland Patch for early work on AmOr2; Ana Mederos for technical assistance; Chris Dietrich, James Zahniser, and Roman Rakitov for assistance with pictures; John Carlson (Yale University, New Haven, CT) for the DmOr35a clone; and Leslie Vosshall (The Rockefeller University, New York, NY) for the DmOr83b clone. Karen Pruiett and the UIUC Bee Research Facility provided the bees. We also thank Robert Brandt and Giovanni Galizia for providing confocal scans of drone antennal lobes. We thank Gene Robinson for helpful comments and for the use of laboratory equipment. This work was funded by National Institutes of Health Grants AI56081 (to H.M.R.) and MH66038 (to C.W.L.).
Abbreviations
- Am
Apis mellifera
- Cr
chemoreceptor
- 9-HDA
9-hydroxy-2-decenoic acid
- HOB
methyl p-hydroxybenzoate
- HVA
4-hydroxy-3-methyoxyphenylethanol
- 9-ODA
9-oxo-2-decenoic acid
- Or
odorant receptor
- QMP
queen mandibular pheromone
- QRP
queen retinue pheromone
- MG2
macroglomerulus 2
- UIUC
University of Illinois at Urbana–Champaign
- OBP
odorant-binding protein
- qPCR
quantitative real-time PCR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE8519).
This article contains supporting information online at www.pnas.org/cgi/content/full/0705459104/DC1.
References
- 1.Crespi BJ, Yanega D. Behav Ecol. 1995;6:109–115. [Google Scholar]
- 2.Wilson EO, Holldobler B. Proc Natl Acad Sci USA. 2005;102:13367–13371. doi: 10.1073/pnas.0505858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winston ML. The Biology of the Honeybee. Cambridge, MA: Harvard Univ Press; 1987. [Google Scholar]
- 4.Slessor KN, Winston ML, Le Conte Y. J Chem Ecol. 2005;31:2731–2745. doi: 10.1007/s10886-005-7623-9. [DOI] [PubMed] [Google Scholar]
- 5.Keeling CI, Slessor KN, Higo HA, Winston ML. Proc Natl Acad Sci USA. 2003;100:4486–4491. doi: 10.1073/pnas.0836984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler CG, Gibbons DA. J Ins Physiol. 1958;2:61–64. [Google Scholar]
- 7.Gary NE. Science. 1962;136:773–774. doi: 10.1126/science.136.3518.773. [DOI] [PubMed] [Google Scholar]
- 8.Free JB. Pheromones of Social Bees. Ithaca, NY: Cornell Univ Press; 1987. [Google Scholar]
- 9.Ayasse M, Paxton RJ, Tengo J. Annu Rev Entomol. 2001;46:31–78. doi: 10.1146/annurev.ento.46.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Salmon AW. Br Bee J. 1938;66:62. [Google Scholar]
- 11.Esslen J, Kaissling KE. Zoomorphology. 1976;83:227–251. [Google Scholar]
- 12.Brockmann A, Bruckner D. Naturwissenschaften. 2001;88:78–81. doi: 10.1007/s001140000199. [DOI] [PubMed] [Google Scholar]
- 13.Kaissling KE, Renner M. Z Vergl Physiol. 1968;59:357–361. [Google Scholar]
- 14.Vareschi E. Z Vergl Physiol. 1971;75:143–173. [Google Scholar]
- 15.Vetter RS, Visscher PK. J Chem Ecol. 1997;23:1867–1880. [Google Scholar]
- 16.Brockmann A, Bruckner D, Crewe RM. Naturwissenschaften. 1998;85:283–285. [Google Scholar]
- 17.Arnold G, Masson C, Budharugsa S. Cell Tissue Res. 1985;242:593–605. [Google Scholar]
- 18.Brockmann A. Bremen, Germany: University of Bremen; 1999. PhD thesis. [Google Scholar]
- 19.Hansson BS, Anton S. Annu Rev Entomol. 2000;45:203–231. doi: 10.1146/annurev.ento.45.1.203. [DOI] [PubMed] [Google Scholar]
- 20.Dahanukar A, Hallem EA, Carlson JR. Curr Opin Neurobiol. 2005;15:423–430. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Rutzler M, Zwiebel LJ. J Comp Physiol A. 2005;191:777–790. doi: 10.1007/s00359-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 22.Hallem EA, Dahanukar A, Carlson JR. Annu Rev Entomol. 2006;51:113–135. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- 23.Hallem EA, Ho MG, Carlson JR. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Honeybee Genome Sequencing Consortium. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson HM, Wanner KW. Genome Res. 2006;16:1395–1403. doi: 10.1101/gr.5057506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger J, Grosse-Wilde E, Gohl T, Breer H. Eur J Neurosci. 2005;21:2167–2176. doi: 10.1111/j.1460-9568.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- 27.Krieger J, Grosse-Wilde E, Gohl T, Dewer YM, Raming K, Breer H. Proc Natl Acad Sci USA. 2004;101:11845–11850. doi: 10.1073/pnas.0403052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa T, Sakurai T, Nishioka T, Touhara K. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- 29.Biessmann H, Nguyen QK, Le D, Walter MF. Insect Mol Biol. 2005;14:575–589. doi: 10.1111/j.1365-2583.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 30.Kamikouchi A, Morioka M, Kubo T. Zoolog Sci. 2004;21:53–62. doi: 10.2108/0289-0003(2004)21[53:IOHAGE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Foret S, Maleszka R. Genome Res. 2006;16:1404–1413. doi: 10.1101/gr.5075706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner KW, Anderson AR, Trowell SC, Theilmann DA, Robertson HM, Newcomb RD. Insect Mol Biol. 2007;16:107–119. doi: 10.1111/j.1365-2583.2007.00708.x. [DOI] [PubMed] [Google Scholar]
- 33.Wetzel CH, Behrendt HJ, Gisselmann G, Stortkuhl KF, Hovemann B, Hatt H. Proc Natl Acad Sci USA. 2001;98:9377–9380. doi: 10.1073/pnas.151103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benton R, Sachse S, Michnick SW, Vosshall LB. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallem EA, Carlson JR. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 36.Sandoz JC. J Exp Biol. 2006;209:3587–3598. doi: 10.1242/jeb.02423. [DOI] [PubMed] [Google Scholar]
- 37.Vosshall LB, Wong AM, Axel R. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 38.Blum MS, Boch R, Dollittle RE, Trirrle MT, Traynham JG. J Insect Physiol. 1971;17:349–364. [Google Scholar]
- 39.Loper GM, Taylor OR, Foster LJ, Kochansky J. J Apic Res. 1996;35:122–123. [Google Scholar]
- 40.Brockmann A, Dietz D, Spaethe J, Tautz J. J Chem Ecol. 2006;32:657–667. doi: 10.1007/s10886-005-9027-2. [DOI] [PubMed] [Google Scholar]
- 41.Wossler TC, Crewe RM. J Apic Res. 1999;38:137–148. [Google Scholar]
- 42.Lensky Y, Cassier P, Notion M, Delorme-Joulie C, Levinsohn M. J Insect Physiol. 1985;31:265–276. [Google Scholar]
- 43.Bozdech Z, Zhu J, Joachimiak MP, Cohen FE, Pulliam B, DeRisi JL. Genome Biol. 2003;4:R9. doi: 10.1186/gb-2003-4-2-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Liman ER, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 47.Abaffy T, Malhotra A, Luetje CW. J Biol Chem. 2007;282:1216–1224. doi: 10.1074/jbc.M609355200. [DOI] [PubMed] [Google Scholar]
- 48.Abaffy T, Matsunami H, Luetje CW. J Neurochem. 2006;97:1506–1518. doi: 10.1111/j.1471-4159.2006.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.