Abstract
Here, we report generation and characterization of Disrupted-In-Schizophrenia-1 (DISC1) genetically engineered mice as a potential model for major mental illnesses, such as schizophrenia. DISC1 is a promising genetic risk factor for major mental illnesses. In this transgenic model, a dominant-negative form of DISC1 (DN-DISC1) is expressed under the αCaMKII promoter. In vivo MRI of the DN-DISC1 mice detected enlarged lateral ventricles particularly on the left side, suggesting a link to the asymmetrical change in anatomy found in brains of patients with schizophrenia. Furthermore, selective reduction in the immunoreactivity of parvalbumin in the cortex, a marker for an interneuron deficit that may underlie cortical asynchrony, is observed in the DN-DISC1 mice. These results suggest that these transgenic mice may be used as a model for schizophrenia. DN-DISC1 mice also display several behavioral abnormalities, including hyperactivity, disturbance in sensorimotor gating and olfactory-associated behavior, and an anhedonia/depression-like deficit.
Keywords: model, MRI, translational, parvalbumin, depression
Rodents are frequently used to provide models for human disease. Especially, mice are used for genetically engineered models when causal or risk genes for specific diseases are known. In physical conditions, such as metabolic syndrome or cardiovascular diseases, information may be translated between mouse models and human disease, because disease-specific biochemical and physiological markers are available. In contrast, it has been very difficult to generate models that mimic psychiatric disorders, such as schizophrenia (SZ) and mood disorders (1).
Although mice treated with psychomimetic compounds, such as amphetamine or phencyclidine, are currently used as models for SZ (2), they may not reflect any disease etiology. It has been suggested that susceptibility genes for SZ that have recently become available may be advantageous in producing more etiology-relevant models (3). Disrupted-In-Schizophrenia-1 (DISC1) is one promising susceptibility factor for major mental illnesses, including SZ, bipolar disorder, and major depression (4, 5). Moreover, a chromosomal translocation disrupting the DISC1 gene in the middle of its ORF segregates with psychiatric disorders in a large Scottish pedigree (4, 5).
Two independent studies have addressed mutations in the mouse DISC1 gene and the resulting impact on central nervous system function. Gogos and colleagues (6) reported a 25-bp deletion in exon 6 of the DISC1 gene in the 129S6/SvEv mouse strain. When this spontaneous mutation is transferred to a C57BL/6 genetic background, together with an artificial stop codon at the end of exon 8 and an artificial polyadenylation signal after exon 8, the mice display deficits in working memory. At the protein level, 129S6/SvEv mice retain the majority of DISC1 isoforms in comparison with C57BL/6 mice (7). Clapcote and colleagues (8) reported on two independent mutant lines with missense mutations that were introduced by N-nitroso-N-ethylurea (ENU) mutagen. The amino acid changes at Q31 and L100 lead to anatomical and behavioral changes in mice. Interestingly, these two mutations result in different types of behavioral deficits and pharmacological responses. Because these two amino acids are not conserved between rodents and humans, their direct involvement in human conditions is unclear.
In contrast, in humans, several studies have suggested that partial loss of DISC1 may be associated with mental illness. Expression of DISC1 in lymphoblasts from subjects with bipolar disorder bearing a risk genetic haplotype is markedly decreased in comparison with that from control subjects who have a protective haplotype (9). The significance of the disruption of the DISC1 gene in the Scottish family is still controversial (10). Millar et al. (11) reported that there is no immunoreactivity for mutant DISC1 in lymphoblasts from patients in the Scottish family, suggesting that the familial mutation might lead to haploinsufficiency. Because protein isoforms of DISC1 are substantially different between lymphoblasts and brain, the disruption of the DISC1 gene could lead to production of the C-terminal truncated DISC1 protein in the patient brains. Two groups, including ours, reported that the putative truncated protein functions as a dominant-negative (12, 13). Our group published that in cell models the truncated protein forms a dimer with wild-type (wt) protein, disturbing the normal function and subcellular distribution of wt protein, particularly with respect to microtubular dynamics. Furthermore, both introduction of the C-terminal-truncated dominant-negative mutant and suppression of endogenous DISC1 with RNAi result in similar cellular effects in cultures as well as the developing cerebral cortex in vivo (12). Taken together, a partial loss of DISC1 may be involved in the pathology of major mental conditions. Although it would be desirable to generate DISC1 knockout or knockdown mice to mimic the disease conditions, the complexity of the exon usage in this gene, which results in complicated patterns of isoforms, has so far precluded such efforts.
Here, we report the generation and initial characterization of DISC1 genetically engineered mice. In this model, we exogenously express the dominant-negative truncated DISC1 (DN-DISC1) under the αCaMKII promoter. We chose the αCaMKII promoter because endogenous DISC1 is preferentially expressed in the pyramidal neurons of the cortex and hippocampus (14). DN-DISC1 mice display phenotypic changes in measures that are translatable as objective markers for SZ.
Results
Generation of DN-DISC1 Transgenic (tg) Mice.
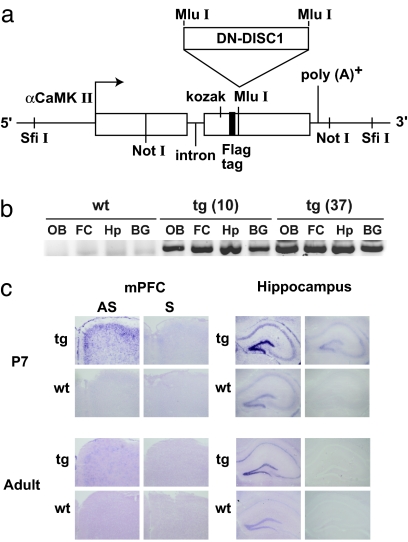
Expression of dominant-negative proteins has been frequently used in animal models to achieve partial loss of function for other proteins (15, 16). Therefore, we generated mice expressing the dominant-negative C-terminal truncated DISC1 (DN-DISC1) under control of the promoter for αCaMKII (Fig. 1a). This promoter drives gene expression after birth in forebrain neurons, mainly in the pyramidal neurons of the cortex and hippocampus, and the granule neurons in the dentate gyrus of the hippocampus (17). This expression pattern is similar to that of endogenous DISC1 (14). We generated the tg mice in the C57BL/6 background to avoid complicated interpretations because of strain differences. We confirmed that exogenous DN-DISC1 is expressed in the olfactory bulb, frontal cortex, hippocampus, and basal ganglia of two independent tg lines (lines 10 and 37) at 2 months old (Fig. 1b), consistent with the results of the αCaMKII-driven exogenous molecules in other tg mice (18, 19). To define the expression pattern of exogenous DN-DISC1 in more detail, we used in situ hybridization and observed that the exogenous DN-DISC1 is preferentially expressed in neonatal stages rather than in adulthood, especially in the pyramidal neurons in layers II-III of the medial prefrontal cortex and in the granule neurons in the dentate gyrus of the hippocampus (Fig. 1c). There is no antibody available that can selectively detect exogenous human DISC1 in mice. Consistent with the previous study (12), DN-DISC1 did not affect expression levels of endogenous DISC1 [supporting information (SI) Fig. 5]. Heterozygous tg and wt littermate males from the two lines (10 and 37) were compared in further experiments and produced similar results.
Fig. 1.
Production of DN-DISC1 tg mice. (a) Transgene construct. cDNA of DN-DISC1 was inserted into a modified pMM403 vector under the αCaMKII promoter. (b) DN-DISC1 transgene mRNA expression at the age of 2 months was assessed by RT-PCR. It was expressed in both tg lines in the following areas: OB, olfactory bulbs; FC, frontal cortex; Hp, hippocampus; BG, basal ganglia. (c) In situ hybridization on medial prefrontal cortex (mPFC) and hippocampus of tg line 37 at postnatal day 7 (P7) and at 3 months old (adult). AS, antisense probe; S, sense probe.
Disturbance of SZ-Associated Pathophysiological Markers in DN-DISC1 tg Mice.
As mentioned above, the main obstacle in translating between rodent models and humans is the lack of disease-specific and objective biomarkers relevant to SZ. SZ is a syndrome, that is, a mixture of etiologically heterogeneous but clinically similar conditions. Biomarkers applicable to all of the cases of SZ may not be available. Nevertheless, a few physiological markers, although not disease-specific, have been repeatedly found in many SZ patients. Enlargement of the lateral ventricles was found in 80% of 55 studies, making it the most robust MRI finding in SZ (20). Asymmetrical changes, such as left-dominated ventricular enlargement and hemispheric shrinkage, are frequently reported in SZ brains (21–23).
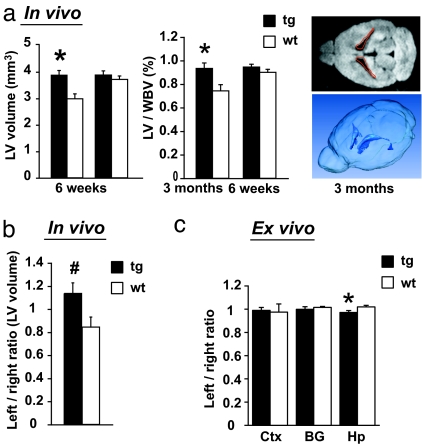
To determine whether such translatable and pathophysiological markers are disturbed in the DN-DISC1 tg mice, we first examined the volume of the lateral ventricles by in vivo MRI scans (Fig. 2a). In tg line 10, there was significant enlargement of the volume of the lateral ventricles at 6 weeks old; similar changes were observed also in line 37. This enlargement may not be due to progressive neurodegeneration, because the difference is compensated with unknown mechanisms in the same mice when they reach 3 months old. The total brain volume in DN-DISC1 tg mice was unchanged at both ages in comparison with that in wt mice.
Fig. 2.
Anatomical changes of DN-DISC1 tg mice detected by MRI scans. (a) In vivo MRI scans. (Left) Lateral ventricles (LV) are enlarged in tg (n = 8) in comparison with wt (n = 7) (line 10, 6 weeks old) (*, F(1,13) = 7.70, P = 0.016). (Center) The ratio of the LV to the whole brain volume (WBV) is increased in tg (*, F(1,13) = 6.83, P = 0.022). (Right) A representative two-dimensional image (Upper) and a three-dimensional construction (Lower). (b) Left/right lateral ventricle volume ratio between tg (n = 7) and wt (n = 4) (#, suggestive at P = 0.057). (c) Ex vivo imaging. Ratio of left/right areas is significantly changed in hippocampus (Hp), but not in lateral cortex (Ctx) and basal ganglia (BG) between tg (n = 6) and wt (n = 6) (*, P < 0.05).
When looking more carefully at the results of 6-week-old mice, we also found that asymmetry between the left and right lateral ventricles in the wt littermates of our cohort was lost or even reversed in the DN-DISC1 tg mice (Fig. 2b). In line 10 tg mice enlargement of the left side was augmented in the in vivo MRI scans, as shown by left-right ratio. To further characterize the anatomical changes at 6 weeks old, we conducted ex vivo MRI scans and found a significant change in the left/right ratio in the hippocampus, but not in the cortex and the basal ganglia, of the tg mice compared with wt littermates (Fig. 2c). The relative enlargement of the left lateral ventricle in the tg mice can reasonably be correlated with relative shrinkage of brain structures on the same side. There is suggestive evidence that may further support asymmetrical changes in DN-DISC1 tg mice, as the left/right ratio of T2 signal is significantly different in the thalamus of DN-DISC1 mice compared with that of wt littermates (SI Fig. 6).
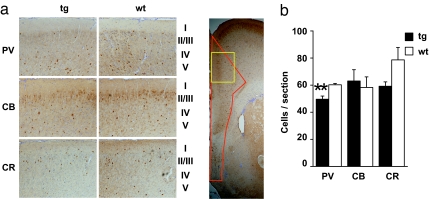
Abnormal neural synchrony has been reported in patients with SZ. Selective reduction of immunoreactivity in a specific type of interneurons, parvalbumin-positive neurons, in the cortex, especially in layers III and IV, is known to correlate with this physiological abnormality (24–26). Thus, we examined parvalbumin, calbindin, and calretinin in sections of medial prefrontal cortex from 3-month-old line 37 mice by immunohistochemistry (Fig. 3). We observed a significant, although modest, reduction in parvalbumin, but not calbindin immunoreactivity, as well as suggestive reduction of calretinin (27), in DN-DISC1 mice.
Fig. 3.
Immunostaining of interneurons in the medial prefrontal cortex. (a) (Left) High magnification representative pictures of staining patterns in the area marked in yellow in Right. (Right) Low magnification image demonstrating the area for the quantitative analyses, outlined in red. (b) Quantification of the staining. There was a significant reduction (**, P = 0.0059) in the number of parvalbumin (PV) cells in DN-DISC1 line 37 mice and no difference in calbindin (CB) or calretinin (CR) immunoreactivity.
Behavioral Alterations in DN-DISC1 tg Mice.
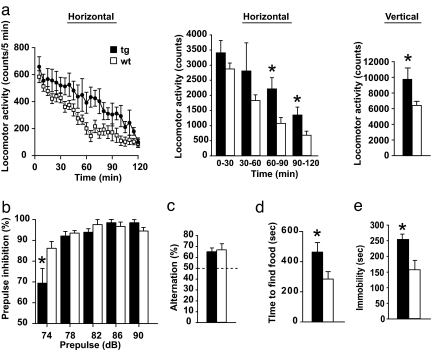
Next, we explored whether these disturbances detected by SZ-associated pathophysiological markers result in behavioral alterations in DN-DISC1 tg mice. The tg mice did not display abnormalities in the rotarod task, suggesting intact motor coordination (SI Fig. 7). DN-DISC1 mice placed in an open field for 2 h were hyperactive (increase in general and rearing activities) (Fig. 4a). Because the hyperactivity was more prominent during the second hour, it may not be directly induced by placement in a novel field, but by a more intrinsic disturbance. There was no difference in the percent of time spent in the center in comparison with the periphery between tg and wt mice, (SI Fig. 8a), suggesting no anxiety traits in DN-DISC1 mice. Consistent results were obtained from elevated plus maze studies (SI Fig. 8b). We also assessed prepulse inhibition, a measure of sensorimotor gating involving the cerebral cortex that is often impaired in patients with SZ (28). DN-DISC1 mice displayed no robust difference compared with wt littermates in prepulse inhibition, except significant reduction when the prepulse was 74 dB (Fig. 4b). Cortical disturbance may also lead to deficits in working memory. Nonetheless, at least in the Y-maze, we did not observe any difference in spontaneous alternation between DN-DISC1 tg and wt mice (Fig. 4c). In this model, spatial learning and memory in the Morris water maze were intact (SI Fig. 9). DN-DISC1 mice took significantly more time than the wt mice to find hidden food (Fig. 4d), which may reflect a problem in olfaction, but may also be influenced by poor motivation. Because no difference in body weight was observed between two groups, it is unlikely that the results reflect differences in hunger or metabolism. We did not observe difference between DN-DISC1 and wt in a paradigm testing social interaction (data not shown). This paradigm may be potentially affected by olfaction. DN-DISC1 mice displayed increased immobility in the forced swim test (Fig. 4e), which is frequently used as an indicator for depression but may parallel anhedonia found in patients with SZ (29).
Fig. 4.
Behavior assays. (a) tg mice are hyperactive. Results are shown for line 10 (line 37 behaved similarly). (Left) F(1,17) = 4.94, P = 0.04. (Center) Divided to 4 × 30 min intervals. DN-DISC1 mice were significantly hyperactive during the second hour of the test (*, 60–90 min: F(1,17) = 7.74, P = 0.013; 90–120 min: F(1,17) = 5.4, P = 0.033) (Right) tg mice reared more than wt mice. (b) tg display impaired prepulse inhibition when the 120-dB pulse was preceded by a prepulse of 74 dB. There is a significant interaction between genotype and prepulse intensity [F(4,68) = 5.18, P = 0.001]. (c) No significant difference between tg and wt in spatial working memory in a Y-maze paradigm, [F(1,24) = 0.087, P = 0.77]. The dotted line indicates chance performance level. (d) It takes longer for tg to find hidden food pellet (*, P = 0.038). (e) tg mice were immobile significantly longer than their wt littermates. ANOVA: F(1,20) = 8.66, P = 0.008.
Discussion
We have generated a mouse model with genetic disturbance of DISC1, a promising susceptibility factor for major mental illnesses such as SZ. The DN-DISC1 mice display several abnormalities, including enlargement of the lateral ventricles in juveniles, deficits in a specific subset of interneurons in cortex that underlie abnormal neural synchrony, and impaired sensorimotor gating. Disturbance of these objective markers, although not disease-specific, is commonly reported in SZ patients (20, 24, 28). Thus, consistent abnormalities of such SZ-associated changes can suggest that the present mouse model may mimic at least a subset of human SZ.
We believe that this model has two advantages: First, this DISC1 model is generated in a pure C57BL/6 background, which is a standard genetic background for behavioral assays (30). Second, the molecular mechanism of the C-terminal truncated DISC1 (DN-DISC1) has already been well characterized in cell models (12, 13). In utero injection of DN-DISC1 and DISC1 RNAi consistently results in dendritic changes in mice (12). These changes are postulated as the underlying reason of enlarged lateral ventricles in SZ patients (31). Therefore, this model is a promising tool for exploring the genetic-phenotypic relationships in the pathway(s) involving DISC1.
In MRI scans, we observed enlarged lateral ventricles in DN-DISC1 tg mice at 6 weeks old. We do not know why such significant enlargement may become undetectable at 3 months of age. Age-dependent change of ventricular enlargement is still a controversial issue in brain imaging of SZ patients. The consensus is that enlargement of lateral ventricles is seen at the onset of the disease in many cases of SZ. After the onset, some display further enlargement of the ventricles (20), but volume reduction has also been reported (32, 33). Our results indicate that the enlargement of DISC1-DN is associated with deficits in neurodevelopment, but not with the process of progressive neurodegeneration.
Asymmetrical alterations have been frequently reported in SZ brains (34). Consistent with observations that indicate greater enlargement of the lateral ventricle in the left hemisphere of SZ brains (21–23), relative enlargement of the left ventricle was observed in DN-DISC1 tg mice.
It is of interest that various types of disturbances of DISC1, such as deletion in a coding exon (6), missense mutation (8), and expression of a dominant-negative mutant in the present model, lead to common behavioral changes in some paradigms, but not in others (Table 1). Objective comparison between the DISC1 mouse models will provide us with a better understanding of the normal and pathological roles for DISC1 in the brain. Genetic polymorphisms in the DISC1 gene are associated with various types of neuropsychological alterations in humans (35–38). Possible correlations of altered behavioral traits between humans and DISC1 mouse models should be considered.
Table 1.
Comparison between the published DISC1 mouse models
| Behavioral domain | Test | Details | Reference |
|||
|---|---|---|---|---|---|---|
| Koike | Clapcote Q31L | Clapcote L100P | Hikida | |||
| Positive | ||||||
| Psychomotor agitation | Open field | Horizontal | 0 | 0 | ↑ | ↑ |
| Vertical | ND | 0 | ↑ | ↑ | ||
| Negative | ||||||
| Social interaction | Sociability | ND | ↓ | 0 | 0 | |
| Novelty | ND | ↓ | 0 | ND | ||
| Anhedonia/depression | Forced swim | Immobility | ND | ↓ | 0 | ↑ |
| Olfaction | Time to find hidden food | ND | 0 | 0 | ↓ | |
| Cognitive | ||||||
| Working memory | T-maze | Training | 0 | 0 | ↓ | ND |
| Test | ↓ | ↓ | ↓ | ND | ||
| Y-maze | Alternation | ND | ND | ND | 0 | |
| Spatial memory | Morris water maze | ND | 0 | 0 | 0 | |
| Sensorimotor gating | Startle response | 120 dB | ND | 0 | ↓ | ND |
| Prepulse inhibition | Prepulse | 0 | ↓ | ↓ | ↓ | |
| (dB) | (78–90) | (69–81) | (69–81) | (74) | ||
| Anxiety | Elevated plus maze | ND | 0 | 0 | 0 | |
Comparison of the behaviors of DISC1 mutant mice between the present and already reported models [see refs. 6 (Koike et al.) and 8 (Clapcote et al.)]. ↑, higher in tg than wt; 0, no difference; ↓, lower in tg than wt; ND, not reported. The (dB) under the prepulse inhibition depicts the prepulse range that showed significance.
DISC1 can be a target for therapeutic strategies for major mental illnesses: DISC1 can regulate enzymatic activities of phosphodiesterase-4 (PDE4) (11) and endooligopeptidase A/NDEL1 (39). Genetic variations in DISC1 may alter these activities, which could affect key cellular signaling, such as the cAMP cascade, potentially involved in the pathophysiology of major mental illnesses. DN-DISC1 tg mice may be used to screen compounds that may normalize these cascades.
We note that, although we found significant alterations in several physiological and behavioral characteristics in DISC1 tg compared with wt mice, the differences are quite subtle. Because SZ is caused by a combination of several genetic and environmental factors (40), the subtle but significant changes caused by disturbance of a single genetic factor imply that additional genetic and/or environmental insults are required for full manifestation of the disease pathophysiology in mouse models. Thus, we suggest that the present model has advantages for testing genetic epistatic effects, as well as gene-environmental interactions for major mental illnesses. Cross-breeding of this mouse model with other genetically engineered mice or experiments involving viral infections may be proposed (41).
Materials and Methods
Generation of DN-DISC1 tg Mice.
A C-terminal truncated human DISC1 (amino acids 1–597) was inserted under the αCaMKII promoter in a modified pMM403 vector (42). The insert was injected into oocytes of C57BL/6 mice at the Transgenic Core Laboratory of The Johns Hopkins University. Integration of the transgenes into the mouse genome was confirmed by genomic PCR with primers containing a transgene specific sequence (sense human DISC1 nucleotides 1354–1373: 5′-GAATGGAGCCGAGGCTGTTG-3′) and a vector derived sequence (antisense, αCaMKII-R: CAGTGTGATGGATGGATATC). Lines 10 and 37 were established by mating with C57BL6 mice maintaining the purity of the genetic background. Heterozygous line 10 and 37 male tg and wt littermates were compared in further experiments. All procedures related to animals were performed according to the Johns Hopkins animal care and use guidelines.
RT-PCR.
Total RNA from olfactory bulbs, frontal cortex, hippocampus, basal ganglia, and cerebellum was purified by using the RNeasy Protect Mini Kit (Qiagen, Valencia, CA). RT-PCR was performed with the SuperScript III one-step RT-PCR system (Invitrogen, Carlsbad, CA) with an annealing temperature of 55°C. The primers were as follows: sense, αCaMKII 5′-ATTCGCTGTCTGCGAGGGCC-3′; antisense: human DISC1 5′-TCTCACAGAGGTCACAGTAGGGGCTGCTGCAC-3′.
In Situ Hybridization.
In situ hybridization was performed by using digoxygenin (DIG)-labeled cRNA probes with sequences corresponding to the SV40 polyA region in pBluescript. Sense and antisense probes were synthesized and DIG-labeled by in vitro transcription using T3 or T7 RNA polymerase (Roche, Basel, Switzerland). In situ hybridization was performed on 20-μm-thick coronal cryosections as described (15) with modifications as follows: for hybridization, 500 ng of probe cRNA at 56°C were used; anti-digoxygenin-alkaline phosphatase Fab fragments (Roche) were applied to sections at 1:1,000.
MRI.
In vivo MRI experiments were performed on the 11.7T Bruker Biospec small animal imaging system. A three-dimensional, fast-spin echo, diffusion weighted (DW) imaging sequence with twin navigation echoes was used (43, 44). For ex vivo MRI, three-dimensional multiple spin echo T2-weighted imaging was performed in 9.4 T Bruker scanners to fit the T2 map, with echo train length 2. For more details see SI Materials and Methods.
Immunohistochemistry.
Twenty-micrometer coronal cryosections were cut from brains of line 37 mice perfused at 3 months old. The following antibodies were used at 1:100 dilution: parvalbumin (Sigma, Poole, UK); calbindin (Sigma); and calretinin (Chemicon, Temecula, CA). Biotinylated anti-mouse antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used along with an ABC Elite kit (Vector Laboratories, Burlingame, CA) to amplify the signal and diaminobenzidine (Vector Laboratories) was used for development. Sections were counterstained with cresyl violet, and immunostained cells of the medial prefrontal cortex that includes prelimbic and infralimbic areas (the area outlined in red in Fig. 3a) were counted under ×10 magnification. This area was chosen as the initial evaluation, because the area can be objectively defined easily without costaining of layer markers.
Behavioral Analysis.
Behavioral analysis was done between 3 and 8 months of age, with intervals between the different behavioral tasks of ≈1 week. tg (n = 17) and wt littermates (n = 8) of line 37 were subjected to all of the tests except for rotarod. Only Y-maze was performed with another 37 cohort (tg, n = 15; wt littermates, n = 11). Tg (n = 9) and wt littermates (n = 10) of line 10 were tested in rotarod, open field, prepulse inhibition, and hidden food tests.
Open Field.
Each mouse was placed in a novel open field box (40 cm × 40 cm; San Diego Instruments, San Diego, CA) for 2 h. Horizontal and vertical locomotor activities in the periphery as well as the center area were automatically recorded by an infrared activity monitor (San Diego Instruments). Single beam breaks are reported as “counts.”
Hidden Food Test.
The mice were food-deprived for 24 h. After habituation to a new cage for 5 min, a food pellet was hidden under the bedding. The time it took for the mouse to find the food pellet was measured until a maximum of 10 min was reached.
Prepulse Inhibition.
Acoustic startle and prepulse inhibition responses were measured in a startle chamber (San Diego Instruments). Each mouse was subjected to six sets of seven trial types distributed pseudorandomly: pulse-alone trials, prepulse–pulse trials, and no-stimulus trials. The pulse used was 120 dB and the prepulses were as follows: 74, 78, 82, 86, and 90 dB. For more details see SI Materials and Methods.
Forced Swim Test.
Each mouse was put in a large plastic cylinder, which was half-filled with room temperature water. The test duration was 6 min, during which the swim/immobility times were recorded.
Statistical Analysis.
Statistical analysis was performed by ANOVA or repeated ANOVA. Values in graphs are expressed as mean plus standard error. Significance levels are marked as follows: *, P < 0.05; **, P < 0.01.
Supplementary Material
Acknowledgments
We thank Ms. Yukiko Lema for organizing figures and manuscript; Ms. Pamela Talalay for critical reading; and Drs. Masahiko Morita, Akira Yamamoto, Hao Huang, Jiangyang Zhang, and Dani Smith and Ms. Karen Lancaster for technical assistance. This work was supported by U.S. Public Health Service Grant MH-069853 (to A.S.); Neurogenetics and Behavior Center (The Johns Hopkins University) Grant P01-AG-017688 (to M.G.); foundation grants from the National Alliance for Research on Schizophrenia and Depression (to A.S.), The Stanley Medical Research Institute (to A.S.), and S-R Foundation (to A.S.). H.J.-P. is a fellow supported by The Stanley Medical Research Institute. T.H. is supported by fellowships from the Sankyou Foundation of Life Science and the Uehara Memorial Foundation.
Abbreviations
- DISC1
Disrupted-In-Schizophrenia-1
- DN
dominant-negative
- SZ
schizophrenia
- wt
wild-type
- tg
transgenic.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704774104/DC1.
References
- 1.Arguello PA, Gogos JA. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Steinpresis RE. Behav Brain Res. 1996;74:45–55. doi: 10.1016/0166-4328(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Lipska BK, Weinberger DR. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Ishizuka K, Paek M, Kamiya A, Sawa A. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 5.Porteous DJ, Thomson P, Brandon NJ, Millar JK. Biol Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Proc Natl Acad Sci USA. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka K, Chen J, Taya S, Li W, Millar JK, Xu Y, Clapcote SJ, Hookway C, Morita M, Kamiya A, et al. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002024. in press. [DOI] [PubMed] [Google Scholar]
- 8.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Maeda K, Nwulia E, Chang J, Balkissoon R, Ishizuka K, Chen H, Zandi P, McInnis MG, Sawa A. Biol Psychiatry. 2006;60:929–935. doi: 10.1016/j.biopsych.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Sawa A, Snyder SH. Science. 2005;310:1128–1129. doi: 10.1126/science.1121114. [DOI] [PubMed] [Google Scholar]
- 11.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 12.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, et al. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 13.Pletnikov MV, Xu Y, Ovanesov MV, Kamiya A, Sawa A, Ross CA. Neurosci Res. 2007 doi: 10.1016/j.neures.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. Mol Psychiatry. 2004;9:1100–1110. doi: 10.1038/sj.mp.4001574. [DOI] [PubMed] [Google Scholar]
- 15.Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- 16.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O'Donnell P, et al. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Muller MR, Zheng F, Werner S, Alzheimer C. J Biol Chem. 2006;281:29076–29084. doi: 10.1074/jbc.M604959200. [DOI] [PubMed] [Google Scholar]
- 20.McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aso M, Kurachi M, Suzuki M, Yuasa S, Matsui M, Saitoh O. Eur Arch Psychiatry Clin Neurosci. 1995;245:142–144. doi: 10.1007/BF02193086. [DOI] [PubMed] [Google Scholar]
- 22.Buchsbaum MS, Yang S, Hazlett E, Siegel BV, Jr, Germans M, Haznedar M, O'Flaithbheartaigh S, Wei T, Silverman J, Siever LJ. Schizophr Res. 1997;27:45–53. doi: 10.1016/S0920-9964(97)00087-X. [DOI] [PubMed] [Google Scholar]
- 23.Narr KL, Thompson PM, Sharma T, Moussai J, Blanton R, Anvar B, Edris A, Krupp R, Rayman J, Khaledy M, et al. Biol Psychiatry. 2001;50:84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- 24.Lewis DA, Hashimoto T, Volk DW. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 25.Beasley CL, Reynolds GP. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braff DL, Geyer MA, Swerdlow NR. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 29.Cryan JF, Mombereau C. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 30.Seong E, Saunders TL, Stewart CL, Burmeister M. Trends Genet. 2004;20:59–62. doi: 10.1016/j.tig.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Harrison PJ. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 32.Puri BK, Hutton SB, Saeed N, Oatridge A, Hajnal JV, Duncan L, Chapman MJ, Barnes TR, Bydder GM, Joyce EM. Psychiatry Res. 2001;106:141–150. doi: 10.1016/s0925-4927(01)00072-5. [DOI] [PubMed] [Google Scholar]
- 33.Puri BK, Saeed N, Richardson AJ, Oatridge A, Hajnal JV, Bydder GM. Int J Clin Pract. 2005;59:399–402. doi: 10.1111/j.1368-5031.2005.00501.x. [DOI] [PubMed] [Google Scholar]
- 34.Shenton ME, Dickey CC, Frumin M, McCarley RW. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdick KE, Hodgkinson CA, Szeszko PR, Lencz T, Ekholm JM, Kane JM, Goldman D, Malhotra AK. NeuroReport. 2005;16:1399–1402. doi: 10.1097/01.wnr.0000175248.25535.f6. [DOI] [PubMed] [Google Scholar]
- 36.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, et al. Proc Natl Acad Sci USA. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, Gasperoni T, Tuulio-Henriksson A, Pirkola T, Toga AW, et al. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 38.Hennah W, Tuulio-Henriksson A, Paunio T, Ekelund J, Varilo T, Partonen T, Cannon TD, Lonnqvist J, Peltonen L. Mol Psychiatry. 2005;10:1097–1103. doi: 10.1038/sj.mp.4001731. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi MA, Portaro FC, Bastos MF, Guerreiro JR, Oliveira V, Gorrao SS, Tambourgi DV, Sant'Anna OA, Whiting PJ, Camargo LM, et al. Proc Natl Acad Sci USA. 2005;102:3828–3833. doi: 10.1073/pnas.0500330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuang MT. Am J Psychiatry. 2000;157:489–491. doi: 10.1176/appi.ajp.157.4.489. [DOI] [PubMed] [Google Scholar]
- 41.Sawa A, Pletnikov MV, Kamiya A. Trends Neurosci. 2004;27:294–297. doi: 10.1016/j.tins.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 43.Mori S, van Zijl PC. Magn Reson Med. 1998;40:511–516. doi: 10.1002/mrm.1910400403. [DOI] [PubMed] [Google Scholar]
- 44.Xue R, Sawada M, Goto S, Hurn PD, Traystman RJ, van Zijl PC, Mori S. Magn Reson Med. 2001;46:183–188. doi: 10.1002/mrm.1174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.