Abstract
Degradation of the major alkamides in E. purpurea extracts was monitored under four different accelerated storage conditions, phenolic-depleted and phenolic-rich dry E. purpurea extracts and phenolic-depleted and phenolic-rich DMSO E. purpurea extracts at 70, 80, and 90 °C. Degradation of alkamides followed apparent first-order reaction rate kinetics. Alkamides degraded faster in dry films than in DMSO solution. The phenolic acids acted as antioxidants by limiting the loss of the alkamides in dry E. purpurea extracts. In contrast, E. purpurea alkamides in DMSO degraded faster when the phenolic fraction was absent. The overall order of degradation rate constants was alkamides 1 ≈ 2 ≈ 6 > 9 ≈ 8 > 3 ≈ 5 ≈ 7. The energy of activation (Ea) predicted for alkamide degradation averaged 101 ± 12 kJ/mol in dry films ± phenolic acids, suggesting the oxidation mechanism was the same under both conditions. In DMSO solutions, Ea values were about one-half of those in dry films (61 ± 14 kJ/mol), suggesting a different mechanism for alkamide oxidation in solution compared to dry. Predicted half-lives for alkamides in extracts suggested very good stability.
Keywords: Alkamide stability, Echinacea purpurea, phenolic acids
INTRODUCTION
Echinacea preparations are one of the best selling medicinal herbal medicines in both Europe and North America (1). A recent survey conducted by the National Center for Health Statistics showed that Echinacea was the most common herb taken by adults in the United States in 2002 (2). Echinacea purpurea, a member of the Compositae family, is commonly known as purple coneflower. Echinacea species are native to North America and grow naturally from eastern to western United States and southern Canada. Native Americans have used Echinacea for centuries to cure infected wounds, snake bites, insect stings, headaches, and common colds (3). Aqueous alcoholic extracts of E. angustifolia and E. pallida roots are reported to treat the common cold and flu (1, 5). However, Turner et al. (6) reported that Echinacea was not effective in treating or preventing the common cold. Echinacea contains a number of unique chemical constituents that may be responsible for its medicinal activities. The caffeic acid derivatives, mainly of cichoric acid, alkamides, glycoproteins, and polysaccharides, may contribute to the immunostimulatory activity of Echinacea’s (3). A number of factors, including the species, growing season, plant part, and harvest methods, may lead the variation of concentration of components in Echinacea products (7).
The alkamides of Echinacea species are highly unsaturated compounds, and therefore, they may be prone to oxidation (Figure 1). The alkamides, which may contain one or two alkyne moieties, seem to be susceptible to degradation at higher temperature and oxygen levels. Bauer et al. (8) suggested that the polyacetylenes in E. pallida extract were easily oxidized. They recommended that Echinacea roots remain intact and/or the extracts be preferably kept in solution to prevent the oxidative degradation of the diene structures. Rogers et al. (9) evaluated the stability of alkamides for the ground E. angustifolia roots and found a 13% reduction of the alkamides over 2 months. Livesey et al. (10) found that the total alkamide concentrations were reduced by 88% at 25 °C and by 95% at 40 °C in ground E. purpurea roots over 7 months. However, the alkamides were stable at −25, 25, and 40 °C for 7 months in alcohol extracts. A 40% and 80% loss in total alkamide in ground E. purpurea roots was observed at −18 and 24 °C over 64 weeks, respectively (11). Wills and Stuart (12) reported no change in alkamide concentrations at 60% relative humidity and 20 °C for the fresh Echinacea stored 1 month. Significant alkamide reduction was observed in the dried, crushed Echinacea stored in the light at 20 °C or in the dark at 30 °C over 60 days. No changes in alkamide concentrations were observed in the dark at either 5 or 20 °C over 60 days.
Figure 1.
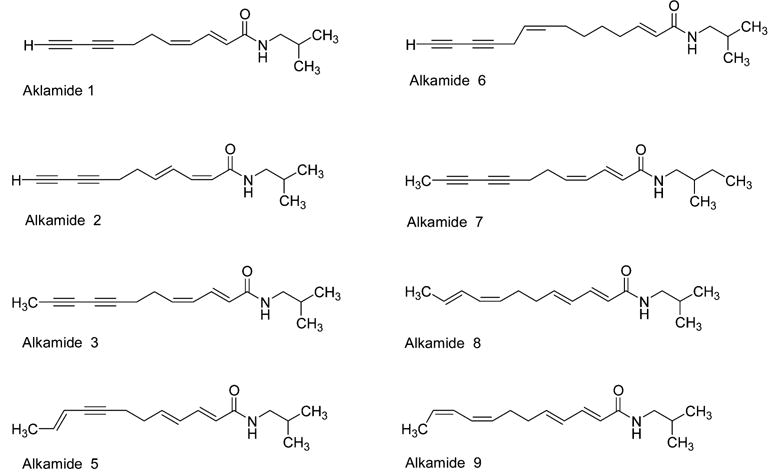
Chemical structures of the predominant alkamides in the extract of E. purpurea.
Accelerated shelf life testing has been used widely in the food industry to monitor losses of nutrients, chemical reactions in foods, and microbial changes (13–16). The Arrhenius relationship allows estimation of stability at lower temperatures and energy of activation (Ea) to provide insight on the reaction mechanism. The objective of this work was to estimate the rate of degradation of the predominant alkamides in E. purpurea root extracts in an accelerated shelf life testing model in both dry and solution formats. The antioxidant effect of the E. purpurea phenolic acids on alkamide stability was evaluated using extracts with phenolic acids and phenolic-acid-depleted extracts.
MATERIALS AND METHODS
Plant Materials
Ground dry roots of E. purpurea, 2004 harvest, PI 63137, were provided by Dr. Mark Widrlechner, Iowa Botanical Supplements Research Center and USDA Plant Introduction Station, Ames, IA. These materials were stored at −20 °C under nitrogen in double zip-lock plastic bags until use.
Chemical Reagents
HPLC-grade acetonitrile, methanol, chloroform, hexane, and acetic acid, regent-grade trifluoroacetic acid and phosphoric acid (85%), and certificated-grade dimethyl sulfoxide (DMSO) were purchased from Fisher Scientific (Fair Lawn, NJ). Undenatured ethanol (100%) was purchased from Chemistry Stores, Iowa State University. Milli-Q water (Millipore Co., Bedford, MA) was used to prepare all mobile phases for HPLC analyses.
Six Echinacea phenolic compounds, chlorogenic acid and caffeic acid (Sigma-Aldrich, Inc, St. Louis, MO), caftaric acid, echinacoside, cynarin, and cichoric acid (American Herbal Pharmacopoeia, Scotts Valley, CA), were used to make the standard curves for Echinacea phenolic HPLC gradient. Four Echinacea alkamides, undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide (alkamide 2), dodeca-2E,4E,8Z-trienoic acid isobutylamide (alkamide 10), dodeca-2E-ene-8,10-diynoic acid isobutylamide (alkamide 14), and 9-hydroxytrideca-2E-ene-10,12-diynoic acid isobutylamide, synthesized by Dr. Kraus’ group, Department of Chemistry, Iowa State University, were used as external standards for Echinacea alkamide HPLC gradient. Chlorogenic acid and caffeic acid were stored in desiccators at −20 °C, and alkamides were kept under nitrogen at −80 °C. Stock solutions of all of the standards were stored under nitrogen at −80 °C.
High-Performance Liquid Chromatography (HPLC) Analyses of Echinacea Extracts
The Echinacea extracts were analyzed by HPLC which consisted of a Beckman System Gold 126 solvent module, a Beckman model 508 autosampler, a Beckman model 168 detector (Beckman Coulter, Inc., Fullerton, CA), and a 250 × 10 mm i.d., 5 μm ODC-AM-303 RP–C18 column (YMC, Inc., Wilmington, NC). All Echinacea extracts were filtered through 0.45 μm polytetrafluoroethylene filters (Alltech Associates Inc., Deerfield, IL) before injecting into the HPLC.
Two HPLC methods were developed to analyze the lipophilic and phenolic acid constituents of Echinacea extracts. The Echinacea phenolic acid gradient was developed to separate the phenolic compounds of Echinacea extracts similar to the procedure reported by Senchina et al. (18) and Kraus et al. (19) The HPLC method for alkamide analysis was the same as that reported by Senchina et al. (18). The mobile phases for the phenolic acid gradient were as follows: (A) degassed Milli-Q water with 0.1% phosphoric acid (85%) and (B) acetonitrile. The gradient elution was modified from Senchina et al. (18): 0–13 min, 10–22% B; 13–14 min, 22–40% B; 14–17, 40% B; 17–17.5 min, 40–10% B; 17.5–30 min, 10% B. The injection volumes were 10 μL. The flow rate was 1.5 mL/min. The UV absorbance was monitored from 200 to 600 nm with a Beckman 32 KaratTM software (version 5.0) Phenolic acids were identified based on the retention time of authentic standards and the UV absorbance profile.
The mobile phases for the alkamide gradient were as follows: (A) degassed Milli-Q water and (B) acetonitrile. A linear gradient of increasing 40% B to 80% B was developed within 45 min at a flow rate of 1.0 mL/min with UV detection from 200 to 600 nm. The injection volume was 15 μL. Alkamides were identified in collaboration with Senchina et al. (18), Kraus et al. (19), and Wu et al. (20), our colleagues in the Iowa Botanical Research Center at Iowa State University, based on retention times and UV absorbance spectra.
The repeatability (average of the coefficient of variation) for the phenolic acid standards ranged from 0.88% to 3.32%. The repeatability for the alkamide standards ranged from 1.67% to 2.16%.
Accelerated Shelf Life Testing of Alkamide Stability in E. purpurea Extracts
We evaluated the stability of alkamides in four different types of extracts: dry phenolic-rich extracts, dry phenolic-poor extracts, phenolic-rich extracts in DMSO, and phenolic-poor extracts in DMSO. Loss of the predominant alkamides in E. purpurea was evaluated during storage in 70, 80, and 90 °C forced air ovens for 10-day incubation time.
Three grams of accurately weighed E. purpurea were extracted with 95% ethanol for 6 h using a Soxhlet apparatus. After extraction, the ethanol was rotoevaporated at 30 °C to obtain the dry residue. These dry Echinacea extracts were rich with phenolic compounds. To prepare the phenolic-poor alkamide fraction, the dry Echinacea extracts with phenolics were redissolved in 10 mL of chloroform. A Maxiclean silica gel separation cartridge (Alltech Associates Inc., Deerfield, IL) was used for separation of alkamides from phenolic compounds. The cartridge was prewashed with 10.0 mL of hexane, the chloroform Echinacea extract was passed through the cartridge, and the eluent was saved. The cartridge was then washed with another 10.0 mL of chloroform and the eluent saved. The two eluents were combined, and the chloroform was evaporated at < 30 °C. The dry residues were used immediately in the stability studies.
For dry films, the dry residue was dissolved in 10.0 mL of methanol and 0.7 mL of solution was filtered with 0.45 μm filters into HPLC vials (12 × 32 mm standard mouth) (Alltech Associates, Inc., State College, PA). The methanol was blown off by a N-EVAP 111 nitrogen evaporator (Organomation Associates, Inc., Berlin, MA). These dry extracts, either as is or depleted in phenolic acids, were used to evaluate alkamides stability in dry films.
For DMSO stability, the dry residue was dissolved in 10.0 mL of DMSO and 0.7 mL of solution was filtered with 0.45 μm filters into HPLC vials (12 × 32 mm standard mouth) (Alltech Associates, Inc., State College, PA). These extracts in DMSO, either as is or depleted in phenolic acids, were used to evaluate the stability of alkamides in DMSO solution. To correct for evaporation, the volume was marked on the vials prior to start of storage and remade to volume prior to HPLC analysis.
All accelerated shelf life stability treatments were conducted in duplicate at 70, 80, and 90 °C in forced air ovens. At each time point over a 10 days the alkamides were analyzed using the Echinacea alkamide HPLC gradient. The dry Echinacea extracts were redissolved in the 0.7 mL of DMSO.
The apparent rate constants for degradation (k) of individual alkamides were determined by a linear regression plot of ln (peak area) versus time (day), where the negative of the slope is the apparent rate constant (k), using SAS procedure REG (SAS 9.1.2 Qualification Tools, Cary, NC), and standard errors of k were calculated. The Arrhenius equation was used to determine the energy of activation, Ea. Rate constants at lower temperatures were estimated by extrapolation of a linear regression of log(k) versus the reciprocal of absolute temperature (K) using SAS procedure REG, and half-lives were calculated by t1/2 = 0.693/k. The degradation rate constant and half-lives of the alkamides in phenolic acid-rich and phenolic acid-poor E. purpurea extracts were compared by analysis of variance (ANOVA) models. The SAS procedure MIXED was used for this analysis. The differences of least-squares (LS) means between two treatments were compared. The effects of storage conditions (dry vs DMSO) on the degradation rate constant and half-lives of alkamides in E. purpurea extracts were compared using the similar ANOVA model. The between treatment differences were calculated from the ANOVA model. The degradation rate constants of different alkamides were compared by Tukey’s multicomparison method.
RESULTS AND DISCUSSION
The stability of the constituents in extracts of Echinacea is very important because the medicinal properties are determined by the biological evaluation of these extracts. However, little published research has appeared on the stability of the individual constituents in Echinacea. The alkamides are unique components in the roots of E. angustifolia and E. purpurea, while the polyacetylenes are the major lipophilic compounds in the root of E. pallida (4). The objective of this study was to determine the stability of these alkamides. The alkamides are compounds that are similar to unsaturated fatty acids but are isobutyl amides (Figure 1). The medium-chained hydrocarbon portion of these alkamides contains one or more double bonds, and some alkamides have one or two acetylene bonds. None of the alkamides are saturated (20). As a result, alkamides should be prone to oxidation, especially in an environment rich in oxygen. Therefore, the stability of this class of compounds may be very important for the biological evaluation of Echinacea species.
Accelerated shelf life testing of alkamides in the extract of E. purpurea root was selected to determine their stabilities according to Taokis and Labuza (21). The E. purpurea extracts were incubated in accelerated storage temperatures of 70, 80, and 90 °C. The eight predominant alkamides in E. purpurea extracts, undeca-2E,4Z-diene-8,10-diynoic acid isobutylamide (alkamide 1, according to Bauer’s nomenclature), undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide (alkamide 2), dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide (alkamide 3), dodeca-2E,4E,10E-trien-8-ynoic acid isobutylamide (alkamide 5), trideca-2E,7Z-diene-10,12-diynoic acid isobutylamide (alkamide 6), dodeca-2E,4E-diene-8,10-diynoic acid 2-methylbutylamide (alkamide 7), dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide (alkamide 8), and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide (alkamide 9), were monitored over the three storage temperatures (Figure 1). The changes of peak areas for the alkamides were measured using the Echinacea alkamide HPLC gradient. Peak areas, which were directly proportional to the concentration of specific constituents, were obtained for major alkamides in E. purpurea.
The phenolic compounds in E. purpurea should act as anti-oxidants to prevent degradation of the alkamides. To determine the effect of phenolic compounds on the oxidation of the alkamides, the silica gel cartridge was used to remove the phenolic compounds to prepare the phenolic-poor E. purpurea extract. Perry et al. (18) indicated that cichoric acid was the major phenolic compound in the E. purpurea root. According to Pellati et al. (22), E. purpurea extracts had greater antioxidant activity than extracts of E. angustifolia and E. pallida and both echinacoside and cichoric acid had larger radical scavenging activities than other known phenolic compounds in Echinacea extracts. Dalby-Brown et al. (23) reported that cichoric acid had the highest antioxidant activity among the caffeic acid derivatives in an animal cell model. Therefore, our first hypothesis was that the phenolic compounds may prevent oxidation of the alkamides in E. purpurea extracts.
Figure 2 shows the HPLC profiles for the phenolic acid-rich E. purpurea extract and phenolic acid-depleted E. purpurea extract at time zero of the stability study. The HPLC chromatograms show that the phenolic acid-rich extract contains high levels of both phenolic acids and alkamides. The phenolic acid-depleted extract had similar levels of alkamides as the phenolic-rich extract but only a trace of phenolics.
Figure 2.
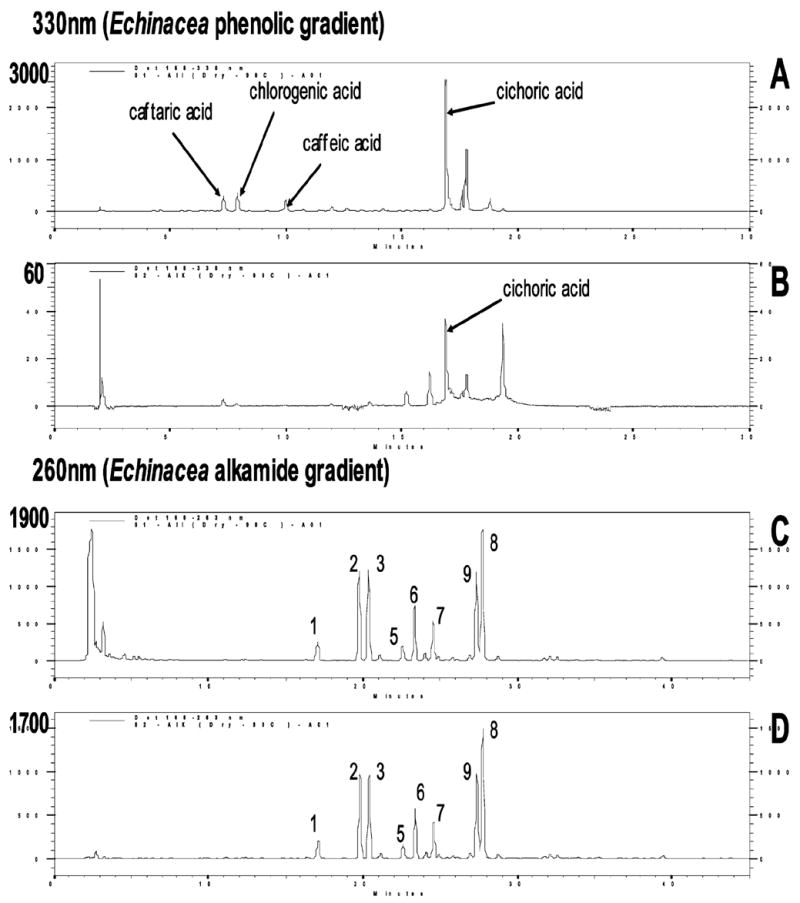
HPLC profile of E. purpurea extracts at time zero. (A and C) HPLC chromatograms of the phenolic-rich E. purpurea extracts. (B and D) HPLC chromatograms of the phenolic-depleted E. purpurea extracts. (A and B) Phenolic compounds in E. purpurea using the Echinacea phenolic HPLC gradient with the detection of 330 nm. The major known phenolic compounds are indicated. (C and D) Alkamides of E. purpurea using the Echinacea alkamide HPLC gradient.
Two storage conditions, dry extracts and extracts in DMSO, were used to evaluate the degradation of the alkamides in E. purpurea extracts. The thin film of dry extracts had more surface area and exposure to oxygen than the DMSO extracts. Therefore, the second hypothesis of this study was that the alkamides in dry E. purpurea extract would degrade faster than those in DMSO extract. The reason we chose DMSO was its high boiling point (189 °C) to minimize evaporation occurring during storage at high temperature.
The data for the disappearance of alkamides from the extracts gave the best fit to an apparent first-order model. Therefore, degradation of alkamides followed apparent first-order reaction rate kinetics under the four storage conditions examined. The rate constant of each alkamide at three temperatures in dry E. purpurea extracts and DMSO extracts are shown in Figure 3. The degradation rate constants of all alkamides were compared by Tukey’s multicomparison method. In phenolic-poor dry E. purpurea extracts, the degradation rate constants of alkamide 1, alkamide 2, alkamide 6, and alkamide 9 were not significantly different from each other and the degradation rate constants of alkamide 3, alkamide 5, and alkamide 7 were not statistically different. In phenolic-rich dry E. purpurea extracts, alkamide 1, alkamide 2, alkamide 6, and alkamide 9 degraded significantly faster than alkamide 3, alkamide 5, and alkamide 7. If the effect of the phenolic acids was not considered, the rate constants of alkamide 1, alkamide 2, and alkamide 6 were not significantly different. Similarly, alkamide 3, alkamide 5, and alkamide 7 did not degrade significantly differently from each other. The degradation rate differences between alkamide 1, alkamide 2, and alkamide 6 with alkamide 3, alkamide 5, and alkamide 7 were significant. Alkamide 8 and alkamide 9 degradation rate constants were not significantly different from each other. Alkamide 8 degraded significantly slower than alkamide 1, alkamide 2, and alkamide 6, while alkamide 9 degraded significantly faster than alkamide 3 and alkamide 5. The differences in degradation rates of alkamides may be due to their different structures. The terminal end of the fatty acid chains for alkamides 1, 2, and 6 is a hydrogen atom, while the methyl group is the terminal group for alkamides 3, 5, 7, 8, and 9. Alkamides 8 and 9 contain four double bonds, which would degrade faster than the alkamides with fewer double bonds if they oxidized like unsaturated fatty acids. Alkamide 5 has three double bonds, whereas the other five alkamides have two double bonds in their medium-chained hydrocarbon portion. However, alkamide 1, alkamide 2, and alkamide 6 have larger degradation rate constants with only two double bonds compared to alkamides with more double bonds. The position of acetylene bonds may affect the alkamide degradation by withdrawing electrons, which would lead to easier initiation of alkene oxidation. However, the position of the acetylene bond is relatively distant for it to have an electron-withdrawing effect for the hydrogens of the carbons α to the alkene bonds.
Figure 3.
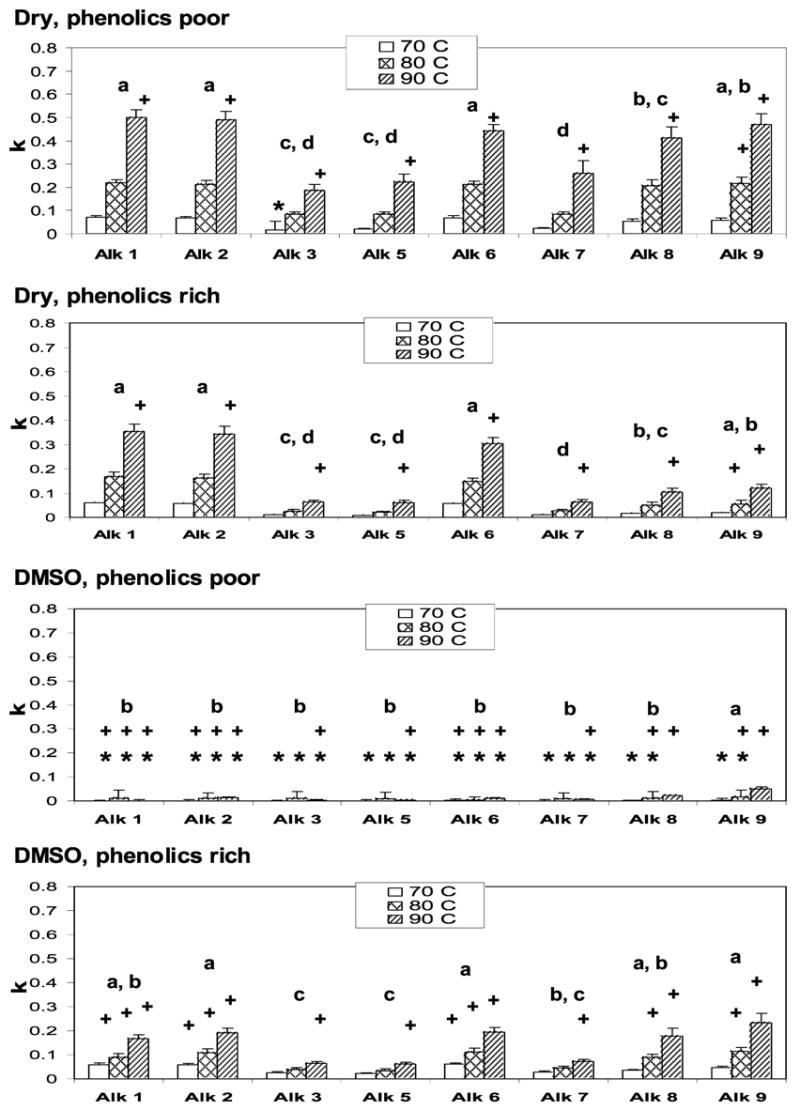
Apparent first-order degradation rate constants for E. purpurea alkamides in dry form and DMSO at 70, 80, and 90 °C. Asterisk (*) indicates that the degradation rate constant of alkamide was not different from zero at selected temperature (p < 0.05); plus (+) indicates that the degradation rate constant of alkamide without phenolics is different (p < 0.05) from that with phenolics at selected temperature; different small letter means that the alkamides degrade different from each other (p < 0.05), with a degraded fastest and d degraded slowest at the three temperatures.
Our first hypothesis was that phenolic compounds may prevent oxidation of the alkamides and, therefore, decrease the degradation rate of the alkamides. Analysis of the phenolic effect on rate constant was performed using an analysis of variance (ANOVA) model with main effects of alkamide species (alkamides 1–9), temperature (70, 80, and 90 °C), phenolic (yes or no), and their interactions. The differences of least-squares mean rate constants between phenolic-rich and phenolic-poor extracts were calculated from this model. For each alkamide at each temperature in dry E. purpurea extracts, the least-squares mean degradation rates of alkamides with phenolic acids are smaller than those without phenolic acids. The rate constants for alkamides 8 and 9 were significantly different at 80 °C. All alkamides degraded faster in dry phenolic-poor extracts than that of phenolic-rich extracts. If we only consider the effect of phenolic compounds and omit the temperature effect, the rate constants were significantly different for all alkamides. These results indicate that in dry E. purpurea extracts the alkamides degraded significantly slower with phenolic acids than without phenolic acids. These data supported the hypothesis that the phenolic compounds acted as antioxidants to prevent oxidation of alkamides in the dry E. purpurea extracts.
The apparent first-order rate constants of each alkamide at the three temperatures in DMSO E. purpurea extracts are presented in Figure 3. All degradation rate constants of alkamides were significantly different from zero in phenolic-rich DMSO E. purpurea extracts. However, most of the alkamide degradation rate constants were not significantly different from zero in phenolic-poor DMSO E. purpurea extracts, which means that the alkamides were very stable in this treatment. Tukey’s multicomparison method was used to compare the degradation rate constants of the alkamides in DMSO E. purpurea extract. In phenolic-poor DMSO extract, alkamide 9 degraded significantly faster than all other alkamides. In phenolic-rich DMSO extracts, the differences of the degradation rate constants for alkamide 1, alkamide 2, alkamide 6, alkamide 8, and alkamide 9 were not significant. The degradation rate constants for alkamide 3, alkamide 5, and alkamide 7 were different from the other alkamides. If the effect of the phenolic acids was not considered, the results from Tukey’s multicomparison showed that alkamide 6, alkamide 9, and alkamide 2 degraded significantly faster than alkamide 3, alkamide 5, and alkamide 7. Alkamide 1 and alkamide 8 rate constants were significantly different from alkamide 3 and alkamide 5, while rate constants for alkamide 1 and alkamide 8 were not significantly different from alkamide 7. The terminal hydrogen atom, in contrast to a terminal methyl group and the number of the double bonds in the hydrocarbon chains of alkamides, may accelerate degradation of the alkamides. As temperature increased, the number of double bonds became more dominant in affecting degradation rate than the terminal hydrogen atom in DMSO extracts.
A majority of the degradation rate constants of the alkamides was significantly affected by the phenolic compounds at these three temperatures in DMSO E. purpurea extracts. For each alkamide at each temperature in DMSO E. purpurea extracts, the least-squares mean degradation rates of the alkamides with phenolic acids were larger than those without phenolic acids, which means that alkamides degraded faster in phenolic-rich DMSO extracts than phenolic-poor DMSO extracts. These data contradict the antioxidant hypothesis that phenolic acids prevent oxidation of alkamides. The reason of this phenomenon is still unknown. Apparently, phenolic acids had different effects on the degradation of alkamides in dry extracts and DMSO solutions. The phenolic acids may have different mobility in dry and DMSO solutions. It is possible that pro-oxidants that are effective in solution but not dry were removed when phenolic acids were retained on the silica gel column.
The second hypothesis was that the alkamides in dry E. purpurea extracts would degrade faster than those in DMSO extract. This hypothesis was examined using an ANOVA model with terms for storage condition (dry, DMSO), alkamide species (alkamides 1–9), temperature (70, 80, and 90 °C), phenolic acids (+ or −), and their interactions. The alkamide degradations were significantly different between the dry E. purpurea extracts and DMSO extracts. The storage condition affected the alkamide degradation significantly for each alkamide. The least-squares mean rate constants of alkamides in dry extracts were significantly larger than in DMSO extracts. This analysis supported our second hypothesis that the alkamides degraded faster in dry extracts than in DMSO extracts, probably because of greater exposure to oxygen.
Ea predicted for alkamide degradation averaged 101 ± 12 kJ/mol in dry films whether or not phenolic acids were present, suggesting the oxidation mechanism was the same under both conditions. In DMSO solutions, Ea values were about one-half of those in dry films (61 ± 14 kJ/mol), suggesting a different mechanism for alkamide oxidation in solution compared to dry. The Ea for alkamide degradations was much larger than coffee lipids at 13kJ/mol (14) but similar to retinyl acetate degradation in model cereal systems (27). The activation energies of the alkamide degradation in phenolic acid-depleted DMSO E. purpurea extracts were quite different and variable. These activation energies for alkamides in phenolic acid-depleted DMSO would not be as accurate since less degradation occurred. Only alkamides 8 and 9 degraded to a significant extent in DMSO. The activation energy of each alkamide in dry E. purpurea extracts was almost one-half to two times larger than that in the phenolic-rich DMSO extracts, which suggests that the mechanism of oxidation of the alkamides is different in dry versus DMSO solution. Therefore, the temperature had a greater effect on the alkamide degradation in the phenolic-rich dry extracts because the activation energies predict the effect of temperature on the reaction rate.
The predicted degradation rate constants and half-lives for each alkamide at 20 °C in dry and DMSO E. purpurea extracts were estimated. The order of predicted degradation rates for the alkamides at 20 °C followed the same pattern observed at higher temperatures. Alkamides 1, 2, and 6 had the shortest half-lives at 20 °C, about 4000 days, in dry phenolic-poor E. purpurea extracts. The half-lives of alkamides 8 and 9 were about two times longer than alkamides 1, 2, and 6. Alkamides 3, 5, and 7 had the longest half-lives. The predicted degradation rate constants at 20 °C in phenolic acid-depleted DMSO extracts were all smaller than the corresponding ones in phenolic acid-rich extracts. We replicated these observations in an ongoing study that is being conducted over a longer storage period. Unfortunately, the storage period selected for this report was not long enough at higher temperatures to result in standard errors in the 20 °C predicted rate constants that would allow differences in the treatments to be discerned. According to Benson (28), the accuracy of the measured reaction rate of reactant is related to both the analytical precision of the method and the percentage of remaining reactant. The more reactant remaining, the higher the error of estimated reaction rate constant. The precision of our HPLC analysis was about 3%. Some alkamides degraded less than 5% within a 10-day incubation time at elevated temperatures. Our experimental results showed that alkamides are very stable even at accelerated temperatures, therefore making extrapolated values susceptible to large standard errors.
Perry et al. (12) reported on the alkamide degradation in chopped E. purpurea root. Using their data we determined that the reaction order for the loss of alkamides in chopped E. purpurea roots over 64-week stored time was an apparent first-order degradation. Perry’s alkamide degradation data at 24 °C was used to calculate the degradation rate constant for each observed alkamide (Figure 4). In the chopped E. purpurea roots, alkamides 1 and 2 degraded faster than the other alkamides, followed by alkamides 8 and 9, 3, and 7. Although the degradation rate constants were different from our extracts, Perry et al. (12) data had the same alkamide degradation pattern as our extracts.
Figure 4.
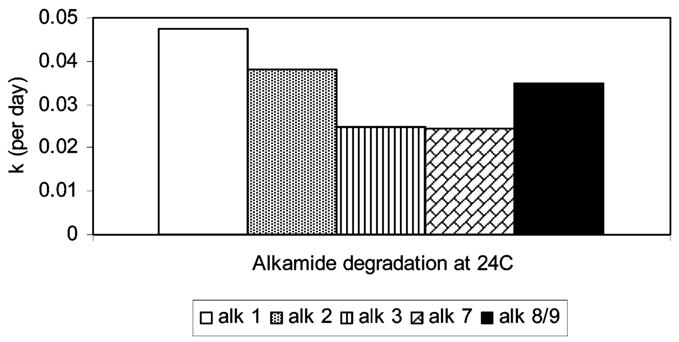
Predicted alkamide stability in ground E. purpurea roots.
Our study may be the first report to predict individual alkamide degradation in the E. purpurea extracts. The results supported the hypothesis that the degradation of alkamides followed apparent first-order reaction kinetics. Second, we found that greater surface area exposed to oxygen resulted in faster alkamides degradation. Finally, the phenolic compounds in E. purpurea had different effects on the degradation of alkamides in dry and DMSO extracts. In the dry E. purpurea extract, phenolics may act as antioxidant to decrease the degradation of the alkamides. However, the alkamides had a different degradation mechanism in DMSO extracts. Alkamides degraded faster in phenolic-rich DMSO extract than in phenolic-poor DMSO extract. Some unknown constituents may accelerate the alkamide degradation in DMSO extracts containing phenolic acids that were removed in the phenolic-poor DMSO treatments.
Acknowledgments
This publication was made possible by Grant P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS), the Office of Dietary Supplements (ODS), NIH, and the Iowa Agriculture and Home Economics Experiment Station. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
LITERATURE CITED
- 1.Bauer R. Echinacea: biological effects and active principles. In: Lawson LD, Bauer R, editors. ACS Symposium Series 691 (Phytomedicines of Europe: chemical and biological activity) American Chemical Society; Washington, DC: 1998. pp. 40–157. [Google Scholar]
- 2.Hall CI, Schwarz J. Phytochemicals from Echinacea. Functional Foods. 2002;2:239–262. [Google Scholar]
- 3.Kennedy J. Herb and supplement use in the US adult population. Clin Ther. 2005;27:1847–58. doi: 10.1016/j.clinthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Bauer R, Wagner H. Echinacea species as potential immunostimulatory drugs. Econ Med Plant Res. 1991;5:253–321. [Google Scholar]
- 5.Bauer R. Chemistry, pharmacology and clinical applications of Echinacea products. In: Mazza GO, editor. Herbs, Botanicals & Teas. Technomic; Lancaster, U.K.: 2000. pp. 45–73. [Google Scholar]
- 6.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi J. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 7.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Family Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 8.Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 9.Bauer R, Khan IA, Wagner H. TLC and HPLC analysis of Echinacea pallida and E. angustifolia roots. Planta Med. 1988;54:426–30. doi: 10.1055/s-2006-962489. [DOI] [PubMed] [Google Scholar]
- 10.Rogers KL, Grice ID, Mitchell CJ, Griffiths LR. High performance liquid chromatography determined alkamide levels in Australian-grown Echinacea spp. Aust J Exp Agric. 1998;38:403–408. [Google Scholar]
- 11.Livesey J, Awang DVC, Aranson JT, Letchamo W, Barrett M, Penyroyal G. Stability testing of marker compounds in Echinacea. Phytomedicine. 1999;6:347–349. doi: 10.1016/S0944-7113(99)80057-9. [DOI] [PubMed] [Google Scholar]
- 12.Perry NB, Van Klink JW, Burgess EJ, Parmenter Graeme A. Alkamide levels in Echinacea purpurea. Effects of processing drying and storage. Planta Med. 2000;66:54–56. doi: 10.1055/s-2000-11111. [DOI] [PubMed] [Google Scholar]
- 13.Wills RBH, Stuart DL. Effect of handling and storage on alkylamides and cichoric acid in Echinacea purpurea. J Sci Food Agric. 2000;80:1402–1406. [Google Scholar]
- 14.Cardelli C, Labuza TP. Applications of Weibull hazard analysis to the determination of the shelf life of roasted and ground coffee. Lebensm-Wiss Technol. 2001;34:273–278. [Google Scholar]
- 15.Labuza TP, Fu B, Taoukis PS. Prediction of shelf life and safety of minimally processed CAP/MAP chilled foods. J Food Prot. 1992;55:741–750. doi: 10.4315/0362-028X-55.9.741. [DOI] [PubMed] [Google Scholar]
- 16.Bell LN, Labuza TP. Aspartame stability of commercially sterilized flavored dairy beverages. J Dairy Sci. 1994;77:34–38. doi: 10.3168/jds.S0022-0302(94)76925-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Guinard JX, Krochta JM. Relating sensory and instrumental data to conduct an accelerated shelf-life testing of whey-protein-coated peanuts. In: Cadwallader K, Weenen H, editors. Freshness and Shelf Life of Foods. American Chemical Society; Washington, DC: 2003. pp. 175–187. [Google Scholar]
- 18.Senchina DS, Wu L, Flinn GN, Konopka DN, McCoy JA, Widrelechner MP, Wurtele ES, Kohut ML. Year-and-a-half old, dried Echinacea roots retain cytokine-modulating capabilities in an in vitro human older adult model of influenza vaccination. [accessed Oct 20, 2006];Planta Med. doi: 10.1055/s-2006-947254. http://www.thieme-connect.de/ejournals/html/plantamedica/doi/10.1055/s-2006–94725.4. [DOI] [PMC free article] [PubMed]
- 19.Kraus GA, Bae J, Wu L, Wurtele E. Synthesis and natural distribution of anti-inflammatory alkamides from Echinacea. Molecules. 2006;11:758–767. doi: 10.3390/11100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu L, Bae J, Kraus GA, Wurtele ES. Diacetylenic isobutylamides of Echinacea: Synthesis and natural distribution. Phytochemistry. 2004;65:2477–2495. doi: 10.1016/j.phytochem.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Perry NB, Burgess EJ, Glennie VL. Echinacea standardization: analytical methods for phenolic compounds and typical levels in medicinal species. J Agric Food Chem. 2001;49:1702–1706. doi: 10.1021/jf001331y. [DOI] [PubMed] [Google Scholar]
- 22.Bauer R, Remiger P, Wagner H. Alkamides from the roots of Echinacea purpurea. Phytochemistry. 1988;27:2239–2342. [Google Scholar]
- 23.Bauer R, Remiger P. TLC and HPLC analysis of alkamides in Echinacea drugs. Planta Med. 1989;55:367–71. doi: 10.1055/s-2006-962030. [DOI] [PubMed] [Google Scholar]
- 24.Taoukis P, Labuza TP. Summary: Integrative Concepts. In: Fennema O, editor. Food Chemistry. 3. Marcel Dekker; New York: 1996. pp. 1013–1042. [Google Scholar]
- 25.Pellati F, Benvenuti S, Melegari M, Lasseigne T. Variability in the composition of anti-oxidant compounds in Echinacea species by HPLC. Phytochem Anal. 2005;16:77–85. doi: 10.1002/pca.815. [DOI] [PubMed] [Google Scholar]
- 26.Dalby-Brown L, Barsett H, Landbo AR, Meyer AS, Molgaard P. Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions from Echinacea purpurea on in vitro oxidation of human low-density lipoproteins. J Agric Food Chem. 2005;53:9413–9423. doi: 10.1021/jf0502395. [DOI] [PubMed] [Google Scholar]
- 27.Kirk JR. Stability of vitamins in dehydrated foods. In: Rockland LB, Stewart GF, editors. Water Activity: Influences on Food Quality. Academic Press; New York: 1981. pp. 531–566. [Google Scholar]
- 28.Benson SW. Foundations of chemical kinetics. McGraw-Hill; New York: 1960. [Google Scholar]


