Abstract
The short-tailed fruit bat, Carollia perspicillata, lives in groups in tree hollows and caves. To investigate whether these roosts might serve as information centres, we tested whether individuals' preferences for novel foods could be enhanced through social learning at the roost. We also determined whether socially learned preferences for novel foods were reversed through interaction with other roost mates by simulating changes in available food resources such as those associated with variations in timing of fruit production in different plant species. Bats exhibited socially induced preferences that were readily reversible. We suggest that for frugivorous bats, roosts can serve as centres for information exchange about novel and familiar, ephemeral foods without requiring conspecific recruitment to these resources.
Keywords: social learning, roosts, bats, information centres
1. Introduction
Bats are among the most gregarious and long-lived of mammals and are, therefore, likely to learn socially. However, there have been relatively few tests of social learning in bats (Wilkinson & Boughman 1999). Neotropical short-tailed fruit bats, Carollia perspicillata (family Phyllostomidae), live in groups in tree hollows and caves and have been proposed as candidates for social learning about foraging opportunities (Fleming 1982). Wilkinson (1987) found no evidence of information sharing outside the roost for this species, but suggested that information about foods may be exchanged by individuals in the roost (Wilkinson & Boughman 1999). Here we tested the hypothesis that social interactions among roosting short-tailed fruit bats result in socially induced preferences for novel foods and determined whether these socially influenced preferences are readily reversed through further social interaction. For detailed information on the social organization of short-tailed fruits bats, we direct the interested reader to Fleming (1988), Wilkinson (1987) and Williams (1986).
2. Methods
(a) Bats and housing conditions
Subjects were 10 randomly caught adult male short-tailed fruit bats of unknown relatedness from a mixed species colony kept under semi-naturalistic conditions at the Biodôme de Montréal (Quebec, Canada). Before experimentation, the two bats serving as demonstrators were maintained on a novel flavoured diet before interacting with another bat. The eight bats serving as observers were not (Galef & Wigmore 1983). These same eight bats acted as observers in both experiments 1 and 2. After we had collected all data for observers 1 and 2, these two bats then served as demonstrators for observers 5–8 to show that individual bats could act as both senders and receivers of information (Galef 1991).
Bats were housed individually in 25 cm3 wooden boxes with a Plexiglas front wall. To allow perches for roosting, the back and roof of the interior of each box was lined with a fine synthetic mesh. The floor was lined with clear plastic shelf-liner. Bats were kept at 30 °C (relative humidity 80%) with a light regime of 12 h low light, 12 h dark, and were provided with water ad libitum. The base diet was a blend of apple sauce, banana and marmoset chow (Ratcliffe et al. 2003). Bats were fed daily and for both experiments feeding bouts were 1.5 h in duration.
(b) Experiment 1
The purpose of this experiment was to simulate a successful forager's (i.e. a demonstrator's) return to the roost after having eaten a novel food. Demonstrators were presented with a modified version of the base diet (flavoured with either 0.3% w/w cinnamon or 0.6% w/w cocoa) 2 h after the onset of the dark cycle for at least 3 days before interacting with an observer. Each observer was presented with the base diet 4 h after the onset of the dark cycle for 4 days (days 1–4). On day 5, 3.5 h after the onset of the dark cycle, either a cinnamon-fed or a cocoa-fed demonstrator was placed in the cage of each observer. Demonstrators had finished feeding, on average, 45 min before interaction. Bats interacted for half an hour. We then removed the demonstrator to its cage. Observers began their first of four two-choice feeding bouts 5–10 min after removal of demonstrators.
On each of days 5–8, 4 h into the dark cycle, we offered each observer a choice between the modified diet of their demonstrator and the alternative modified diet. We switched the positions of the cinnamon and cocoa flavoured diets between days, measured daily intake of both diets, and calculated each observer's intake of its demonstrator's diet as a percentage of the total amount of both diets eaten (Ratcliffe et al. 2003).
(c) Experiment 2
The purpose of this experiment was to mimic a successful forager's return to the roost after having eaten a familiar, though ephemeral, resource that had again become available. After completing experiment 1, observers were kept on the same feeding schedule and returned to the base diet for 4 days (days 9–12). On day 13 (30 min prior to feeding), each observer that had interacted with a cinnamon-fed demonstrator in experiment 1 interacted with a cocoa-fed demonstrator, and each observer that had interacted with a cocoa-fed demonstrator in experiment 1 interacted with a cinnamon-fed demonstrator. On days 13–16, we offered each observer a choice between cinnamon- and cocoa-modified diets.
(d) Video analysis
We recorded demonstrator–observer interactions on days 5 and 13 using an infrared-sensitive CCD camera, VCR and infrared light source, and measured: nose–nose/nose–body contacts, time (in seconds) spent in physical contact and in close proximity (within 5 cm) over the 30 min interaction period.
3. Results
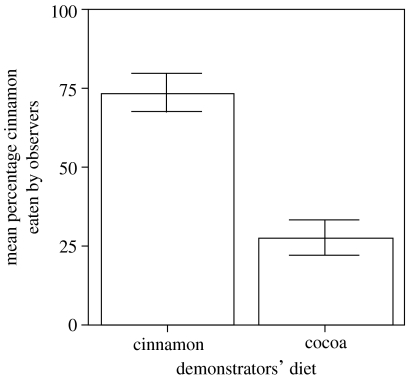
On days 5–8, all observers displayed a preference for the diet of their respective demonstrators (figure 1). The difference between cinnamon- and cocoa-demonstrated subjects' percentage intake of the cinnamon-flavoured diet was significant (two-tailed Mann–Whitney U-test, U=0, N1=N2=4, p<0.03).
Figure 1.
Mean (±s.e.m.) amount of cinnamon-flavoured diet ingested (expressed as a percentage of the total amount ingested) by observers assigned a demonstrator that had eaten either a cinnamon- or cocoa-flavoured diet.
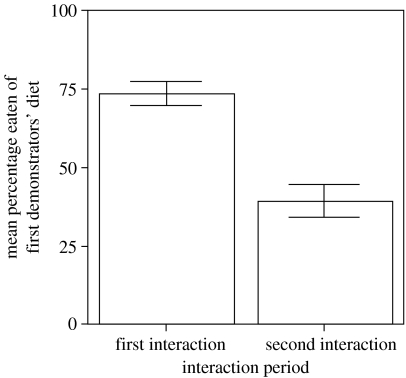
On days 13–16, after interacting with a second demonstrator, all observers displayed a reversal (7 of 8 individuals) or reduction (1 of 8 individuals) in their preference for the food eaten by their first demonstrator (figure 2). The difference between each subject's intake of the first demonstrator's diet after the first and second interaction periods was significant (two-tailed Wilcoxon signed-ranks test, t=0, N=8, p<0.03). Furthermore, observer intake of their second demonstrator's diet was significantly greater than expected by chance (sign test; x=1, N=8, p<0.04).
Figure 2.
Mean (±s.e.m.) amount of first demonstrator's diet ingested (expressed as percentage of total amount ingested) by observers after their first and second social interaction with a demonstrator (see text for details).
Observer percentage intake of demonstrator diet over the first and second days versus the third and fourth days of two-choice presentations was not significantly different in either experiment 1 or 2 (two-tailed Wilcoxon signed-ranks test; T=6, n=8, p>0.5).
On days 5 and 13, observers and demonstrators actively investigated each other during interaction periods by pushing their noses against the head, neck and ventral side of the other bat (mean±s.e.m.=15.4±2.4 times per period). Of these contacts, 21.0±4.2% were nose to nose. Bats spent 42.6±7.7% of the total time in contact and 73.9±7.5% in close proximity. There was no correlation between percentage of demonstrator's diet eaten by observer and three measures of interaction: number of contact events (R2<0.001, p=0.927), percentage of time in physical contact (R2<0.001, p=0.941), and percentage of time in close proximity (R2=0.094, p=0.248). These measures did not differ significantly between the first and second interaction period (two-tailed Wilcoxon signed-ranked tests; p>0.3). Aggression and allogrooming were never observed.
4. Discussion
The results of our experiments support the hypotheses that short-tailed fruit bats learn about novel foods through roosting interactions with conspecifics and that these socially induced preferences are reversible by further interaction (figures 1 and 2). The results also indicate that individual bats can act as both senders and receivers of information (Galef 1991). In a study of wild-caught short-tailed fruit bats foraging in an outdoor enclosure, bats rested for approximately 30 min between foraging bouts. Non-harem males usually remained together during these rest periods (Bonaccorso & Gush 1987). Our data show that during such periods of social interaction, bats could exchange information about recently consumed food.
Of several frugivorous bat species that are found in Costa Rica, short-tailed fruit bats have the most diverse diet (Fleming et al. 1977; Fleming 1988). As dietary generalists, short-tailed fruit bats must either use familiar food resources, which may be of low quality or ephemeral, or sample unknown and possibly toxic foods (Day et al. 2003; Ratcliffe et al. 2003). More than this, short-tailed fruit bats operate on tight energy budgets (Delorme & Thomas 1996), so individuals have much to gain through social learning about both novel and familiar ephemeral food sources. We suggest two adaptive functions for social learning of food preferences.
(a) Social learning about novel food resources
Plant-produced olfactory cues can themselves serve as recruiting agents for bats (Mikich et al. 2003). Bats that experienced novel food cues only on the breath or body of a roost mate could use these odour plumes to find sources of food. We suggest that, if this is the case, following one's nose rather than a successful roost mate may be a hidden means by which bat roosts function as information centres (Richner & Heeb 1995). Short-tailed fruit bats have an extremely sensitive sense of smell (Laska 1990a) and are proficient at discriminating between similar odours (Laska 1990b). Field and laboratory experiments have shown that in this species olfaction is more important than either vision (Laska & Schmidt 1986; Mikich et al. 2003) or echolocation (Theis et al. 1998) for the detection and gross location of food.
(b) Social learning about familiar food resources
Social transmission of information about familiar, yet ephemeral, resources may be beneficial to individuals by reducing costs associated with home-range monitoring. If cues gleaned from roost mates can inform a bat that fruit A is available, then the bat may begin to search for the relevant odour plume or visit neglected patches that, until recently, had contained as yet unpalatable resources (Ratcliffe et al. 2003). Fleming et al. (1977) showed experimentally that short-tailed fruit bats are sensitive to spatio-temporal fluctuations in fruit availability and suggested the use of spatial memory to locate ephemeral resources. Among mammals, microchiropteran bats have well-developed spatial memories (Schnitzler et al. 2003). Among bats, the short-tailed fruit bat has a relatively large hippocampus (Hutcheon et al. 2003), providing anatomical evidence for adaptive specialization of spatial memory (Sherry et al. 1992).
5. Conclusion
Here we have shown that short-tailed fruit bats can learn from one another about food and suggest that there are both opportunities and potential benefits for the social learning of food preferences. As in honey bees and rats, adaptive information transfer in bats need not require that successful foragers be followed (Richner & Heeb 1995). The social transmission of food preferences through chemical cues carried on the breath and bodies of conspecifics, though well documented in Old World rats and mice, may be found in many bats and other mammals that live in groups.
Acknowledgments
We are grateful to Brock Fenton, James Fullard, Jeff Galef, Sara Shettleworth and three anonymous reviewers for helpful comments on earlier versions of the manuscript, and Michel Delorme for lending us the bats. This research was financed through operating and equipment grants from the Natural Science and Engineering Council of Canada (NSERC) to B. Fenton and J. Fullard and an NSERC postgraduate scholarship to J. Ratcliffe. Experiments were approved by York University's Animal Care Committee (protocol no. 2003-4) and conform to the guidelines of the Canadian Council on Animal Care.
References
- Bonaccorso F.J, Gush T.J. Feeding behaviour and foraging strategies of captive phyllostomid fruit bats: an experimental study. J. Anim. Ecol. 1987;56:907–920. doi:10.1016 [Google Scholar]
- Day R.L, Coe R.L, Kendal J.R, Laland K.N. Neophilia, innovation and social learning: a study of intergenic differences in callitrichid monkeys. Anim. Behav. 2003;65:559–571. doi:10.1006/anbe.2003.2074 [Google Scholar]
- Delorme M, Thomas D.W. Nitrogen and energy requirements of the short-tailed fruit bat (Carollia perspicillata): fruit bats are not nitrogen constrained. J. Comp. Physiol. B. 1996;166:427–434. doi: 10.1007/BF02337887. [DOI] [PubMed] [Google Scholar]
- Galef B.G., Jr Information centres of Norway rats: sites for information exchange and information parasitism. Anim. Behav. 1991;41:295–301. doi:10.1016/S0003-3472(05)80481-6 [Google Scholar]
- Galef B.G, Jr, Wigmore S.W. Transfer of information concerning distant foods: a laboratory investigation of the information center hypothesis. Anim. Behav. 1983;31:748–758. doi:10.1016/S0003-3472(83)80232-2 [Google Scholar]
- Fleming T.H. Foraging strategies in plant-visiting bats. In: Kunz T.H, editor. Bat ecology. Plenum Press; New York: 1982. pp. 287–325. [Google Scholar]
- Fleming T.H. University of Chicago Press; Chicago: 1988. The short-tailed fruit bat: a study in plant–animal interactions. [Google Scholar]
- Fleming T.H, Heithaus E.R, Sawyer W.B. An experimental analysis of the food location behavior of frugivorous bats. Ecology. 1977;58:619–627. doi:10.2307/1939011 [Google Scholar]
- Hutcheon J.M, Kirsch J.A.W, Garland T., Jr A comparative analysis of brain size in relation to foraging ecology and phylogeny in the Chiroptera. Brain Behav. Evol. 2003;60:165–180. doi: 10.1159/000065938. doi:10.1159/000065938 [DOI] [PubMed] [Google Scholar]
- Laska M. Olfactory sensitivity to food odor components in the short-tailed fruit bat, Carollia perspicillata (Phyllostomidae, Chiroptera) J. Comp. Physiol. A. 1990a;166:395–399. doi:10.1007/BF00204812 [Google Scholar]
- Laska M. Olfactory discrimination ability in the short-tailed bat, Carollia perspicillata (Chiroptera: Phyllostomidae) J. Chem. Ecol. 1990b;16:3291–3299. doi: 10.1007/BF00982099. doi:10.1007/BF00982099 [DOI] [PubMed] [Google Scholar]
- Laska M, Schmidt U. Olfactory orientation in Carollia perspicillata (Chiroptera) Z. Saugetierkd. 1986;51:129–138. [Google Scholar]
- Mikich S.B, Bianconi G.V, Maia B.H.L.N.S, Teixeira S.I. Attraction of the fruit-eating bat Carollia perspicillata to Piper gaudichaudianum essential oil. J. Chem. Ecol. 2003;29:2379–2383. doi: 10.1023/a:1026290022642. doi:10.1023/A:1026290022642 [DOI] [PubMed] [Google Scholar]
- Ratcliffe J.M, Fenton M.B, Galef B.G., Jr An exception to the rule: common vampire bats do not learn taste aversions. Anim. Behav. 2003;65:385–389. doi:10.1006/anbe.2003.2059 [Google Scholar]
- Richner H, Heeb P. Is the information center hypothesis a flop? Adv. Stud. Behav. 1995;24:1–45. [Google Scholar]
- Schnitzler H.-U, Moss C.F, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 2003;18:386–394. doi:10.1016/SO169-5347(03)00185-X [Google Scholar]
- Sherry D.F, Jacobs L.F, Gaulin S.J.C. Spatial memory and the adaptive specialization of the hippocampus. Trends Neurosci. 1992;15:298–303. doi: 10.1016/0166-2236(92)90080-r. doi:10.1016/0166-2236(92)90080-R [DOI] [PubMed] [Google Scholar]
- Thies W, Kalko E.K.V, Schnitzler H.-U. The roles of echolocation and olfaction in two Neotropical fruit-eating bats, Carollia perspicillata and C. castanea, feeding on piper. Behav. Ecol. Sociobiol. 1998;42:397–409. doi:10.1007/s002650050454 [Google Scholar]
- Wilkinson G.S. Altruism and co-operation in bats. In: Fenton M.B, Racey P, Rayner J.M.V, editors. Recent advances in the study of bats. Cambridge University Press; Cambridge: 1987. pp. 299–323. [Google Scholar]
- Wilkinson G.S, Boughman J.W. Social influences on foraging in bats. In: Box H.O, Gibson K.R, editors. Mammalian social learning: comparative and ecological perspectives. Cambridge University Press; Cambridge: 1999. pp. 188–204. [Google Scholar]
- Williams C.F. Social organization of the bat Carollia perspicillata (Chiroptera: Phyllostomidae) Ethology. 1986;71:265–282. [Google Scholar]