Abstract
The koala is the quintessential specialist herbivore, feeding almost exclusively on Eucalyptus foliage. Consequently, the limitations imposed on the koala's diet by plant defences indicate the extent to which evolutionary adaptations allow mammalian herbivores to circumvent such defences. We tested whether a recently discovered group of plant secondary metabolites, the formylated phloroglucinol compounds (FPCs), deters koalas from feeding on some eucalypt foliage. We found that captive koalas ate less foliage in a single night from trees with high FPC concentrations. Individual trees also differ in the types of FPC they possess, but for a given eucalypt species, most FPCs were similarly effective deterrents. Two closely related and sympatric eucalypt species could be clearly separated by the amounts that koalas ate from each; however, this difference could not be explained by total FPC concentrations alone. We suggest, that in this case, the presence of a distinct type of FPC deters koala herbivory on the less palatable species, and may have facilitated the evolutionary divergence of these species. We conclude that plant defences probably play an important role in determining the distribution and abundance of koalas.
Keywords: Phascolarctos cinereus, FPC, herbivore deterrence, specialist, herbivory, Eucalyptus
1. Introduction
The koala, Phascolarctos cinereus, is the quintessential specialist folivore, concentrating its feeding on a few species of Eucalyptus at any given locality. Dietary specialization generally allows herbivores to limit the range of potentially toxic plant secondary metabolites (PSMs) encountered, but also requires the ability to detoxify large concentrations of one or a few PSMs (Freeland & Janzen 1974). The chemical defences in eucalypt foliage, however, are chemically complex and their concentrations vary dramatically both within and between species. This chemical complexity has confounded previous attempts by ecologists to understand koala diet selection. Other studies have considered nutritional (e.g. nitrogen, lipids, sugars and leaf moisture) and anti-nutritional, or toxic factors, including condensed and hydrolysable tannins, total phenolics, fibre, terpenes and cyanogenic glycosides in Eucalyptus. Nonetheless, the chemical basis of koala diet selection (reviewed by Moore & Foley (2000)) has remained enigmatic.
Recently, a group of lipophilic phenolic compounds, known as the formylated phloroglucinol compounds (FPCs) has been shown to determine the quantity of Eucalyptus foliage less specialized folivores eat (Lawler et al. 2000; Wallis et al. 2002). As FPCs occur widely among the eucalypt species favoured by the koala, we asked whether these compounds also influence its diet selection. Diet selection studies involving other marsupials considered either the concentration of a single FPC (Lawler et al. 2000), or used crude measures of total FPC concentration (e.g. Lawler et al. 1998). Likewise, we first studied the effect on koala feeding of a single FPC, sideroxylonal, which is the only FPC found in Eucalyptus melliodora. However, recent analytical advances also allowed us to consider the complex mixtures of FPCs present in other eucalypt species. Accordingly, we conducted feeding experiments with koalas, using several eucalypt species (blue gum, Eucalyptus globulus; manna gum, Eucalyptus viminalis; swamp gum, Eucalyptus ovata; and Eucalyptus strzeleckii) that possess many types of FPC, and tested whether different FPCs have different effects on feeding by koalas. With the probable exception of E. strzeleckii, these are all important food species for wild koalas.
2. Methods
(a) Koala feeding experiments
We captured five adult male koalas (6.15–9.2 kg) from a wild population at Sandy Point, Victoria, in November 1997 and held them under standard conditions (Lawler et al. 1998). Over 12 nights, we fed the koalas only mature (fully expanded) adult foliage from 12 E. melliodora trees using a standardized no-choice protocol (Lawler et al. 2000) in a randomized block design. We dried subsamples of experimental foliage to a constant mass at 60 °C and used the mass ratio to calculate koala feeding rates from the mass difference between offered and uneaten foliage. Additional samples were frozen at −20 °C for subsequent chemical analysis.
We conducted further experiments in April–July 1998 and January–February 2002. On both occasions, we captured six different male koalas (5.1–11.25 kg) from French Island, Victoria, which we maintained under identical conditions to those used earlier. These experiments comprised a series of fully orthogonal, incomplete randomized blocks, where six trees were each fed to four koalas (but not necessarily the same four koalas) over the course of four-night experimental blocks. In all other respects, experimental protocol matched the previous experiment. In total, we fed koalas foliage from 50 E. globulus, 29 E. ovata, 8 E. strzeleckii and 51 E. viminalis trees.
(b) Analysis of foliar chemistry
We analysed total sideroxylonal concentrations in E. melliodora using the high performance liquid chromatography (HPLC) method of Wallis et al. (2003). The HPLC analysis of the complex mixtures of FPCs found in the other species, and a description of their chemistry are provided by Moore et al. (2004a). These FPCs fall into four groups: (i) ‘Group 1’ macrocarpals (macrocarpals G, A, B, eucalyptone, and four unidentified macrocarpals); (ii) ‘Group 2’ macrocarpals (macrocarpals I, J and two unidentified macrocarpals); (iii) sideroxylonals (sideroxylonals A and C); (iv) jensenal and grandinal. The compounds within each of the first three groups always occur in the same proportions to each other, but the total concentrations of each group vary independently. We considered the effect of variation in concentration of each of these groups on koala feeding rates.
(c) Data analyses
We analysed the koalas' feeding rates from E. melliodora using a linear mixed model, fitted using restricted maximum likelihood. We included koala, tree and a koala×night interaction as random effects, and sideroxylonal concentration as a fixed effect. A linear mixed model describing the koalas' feeding from the remaining species included koala, experimental block, tree and a koala×block interaction as random terms. The full model included fixed terms for each tree species, for concentrations of FPC groups, and for interactions between these concentrations and tree species. In the design and analysis of this experiment, we have considered the swamp gums (E. ovata and E. strzeleckii) as a single taxon. These are closely related, sympatric species, and our interest lay in understanding whether FPCs caused koalas to discriminate within the swamp gum group. We deleted terms that were not significant at p=0.05 to obtain the final model. We performed all statistical analyses in Genstat v. 6.1.
3. Results
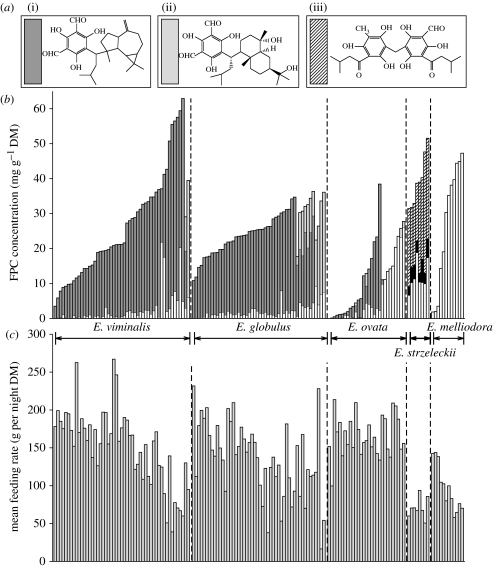
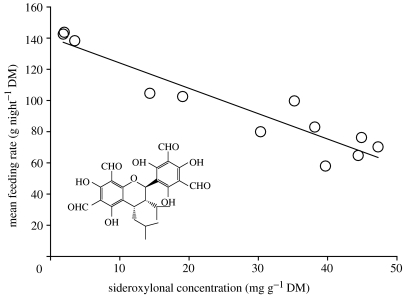
Koalas ate highly variable amounts of foliage from individual E. viminalis, E. globulus and E. melliodora trees, but consistently ate large amounts of E. ovata and small amounts of E. strzeleckii swamp gums (figure 1). The feeding rate on E. melliodora was negatively affected by sideroxylonal concentration (Wald statistic=20.63, d.f.=1, p<0.001; figure 2). A model describing the effect of FPC concentrations on koala feeding rates from the other species is summarized in table 1. Koalas ate less E. globulus and E. viminalis as concentrations of ‘Group 1’ and ‘Group 2’ macrocarpals increased. For E. viminalis, the concentration of sideroxylonals explained further variation still. Koalas also ate less foliage from the swamp gums containing jensenal and grandinal (E. strzeleckii) than from those without (E. ovata). However, variation in the concentrations of other FPCs, which were generally low, did not influence feeding from the swamp gums.
Figure 1.
(a(i)) Macrocarpal G; (ii) macrocarpal I; and (iii) jensenal. (b) Stacked bar charts indicating the concentrations of sideroxylonals (open bars), ‘Group 1’ macrocarpals (dark grey), ‘Group 2’ macrocarpals (light grey), grandinal (black) and jensenal (diagonally hatched) in 150 trees of five species fed to koalas. (c) Bar chart indicating mean amounts of foliage eaten by koalas from the trees indicated above.
Figure 2.
Effect of sideroxylonal concentrations on feeding rates of koalas fed E. melliodora foliage, from a linear mixed model (Wald statistic=20.63, d.f.=1, p<0.001). Open circles indicate mean koala intakes for 12 trees, adjusted for random effects (koala, tree, koala×night). Inset: sideroxylonal A.
Table 1.
Linear mixed model of koala feeding rates on Eucalyptus globulus (Eg), Eucalyptus viminalis (Ev), Eucalyptus ovata and Eucalyptus strzeleckii (combined as Eos). (Effect size±1 s.e.; estimated variance components for random effects; Wald statistics for fixed effects.) The analysis includes 552 observations from 138 trees.
| effect size (g DM) | estimated variance component | Wald statistic | d.f. | p | |
|---|---|---|---|---|---|
| random effects | |||||
| koala | 438 | ||||
| block | 82 | ||||
| koala×block | 27 | ||||
| Tree | 550 | ||||
| residual variance component | 1425 | ||||
| fixed effects | |||||
| Eg | 241.1±20.2 | 142.4 | 1 | <0.001 | |
| Eos | 168.0±9.0 | 349.2 | 1 | <0.001 | |
| Ev | 220.7±11.9 | 345.3 | 1 | <0.001 | |
| Eos×(jensenal & grandinal, mg g−1) | −1.54±0.21 | 51.6 | 1 | <0.001 | |
| Eg×(Group 1 macrocarpals, mg g−1) | −2.00±0.39 | 26.1 | 1 | <0.001 | |
| Ev×(Group 1 macrocarpals, mg g−1) | −1.20±0.18 | 43.8 | 1 | <0.001 | |
| Eg×(Group 2 macrocarpals, mg g−1) | −2.82±0.45 | 39.6 | 1 | <0.001 | |
| Ev×(Group 2 macrocarpals, mg g−1) | −1.56±0.47 | 11.1 | 1 | <0.001 | |
| Ev×(sideroxylonals, mg g−1) | −1.55±0.51 | 9.4 | 1 | 0.002 |
Each of the groups of FPCs was associated with similar reductions in koala feeding on E. globulus and on E. viminalis, when the species were considered separately. However, both groups of macrocarpals tended to cause greater reductions in koala feeding from E. globulus than from E. viminalis. The 95% confidence intervals surrounding the estimated effects for the E. viminalisבGroup 1’ macrocarpals term and the E. globulusבGroup 2’ macrocarpals term did not overlap.
4. Discussion
The koala is an extreme example of evolutionary adaptation to plant anti-herbivore defences. Nonetheless, many eucalypts contain sufficient concentrations of FPCs to deter koalas from feeding. FPCs stimulate the emetic (nausea) system and herbivores probably learn to recognize and avoid these compounds in foliage (Lawler et al. 1999). As FPC concentrations change along ecological gradients (Moore et al. 2004b), we expect that FPCs should also affect the abundance and distribution of koalas and other folivores across the landscape. Common ringtail possums (Pseudocheirus peregrinus) and common brushtail possums (Trichosurus vulpecula) fed E. melliodora also respond to sideroxylonal by decreasing the rates at which they feed, but their responses are more acute than the koala's. Relative to feeding from trees with near-zero sideroxylonal concentrations, koala feeding was halved by concentrations of 45 mg g−1. In contrast, feeding by brushtail and ringtail possums was halved by about 26 mg g−1 and 7 mg g−1 of sideroxylonal, respectively (Wallis et al. 2002; Moore et al. 2004c).
Herbivores' decisions of whether or not to feed on an individual plant, and when to terminate a feeding bout, are informed both by the plant's defences and by its nutritional value (Villalba et al. 2002). Consequently, while differences in total FPC concentrations account for most between-tree variation in the amount that koalas eat of a particular species, variation in the concentration and types of other PSMs and nutrients may also be influential. This is particularly true for the differences between tree species. Our observation that equivalent FPC concentrations were more deterrent when koalas were fed E. globulus than when they were fed E. viminalis, could be explained if koalas stand to gain a greater nutritional benefit from E. viminalis. In support of this hypothesis, E. viminalis typically possesses greater nitrogen concentrations and a lower proportion of fibre than E. globulus (B. D. Moore, unpublished data).
Koala feeding on each species, considered separately, was unaffected by variation in the types of FPCs present. Within E. viminalis and within E. globulus, the two macrocarpal groups and sideroxylonal caused similar decreases in koala feeding, and in trees where multiple FPC groups were present, their effect was cumulative. We conclude that the complex mixtures of FPCs found in many trees provide an equivalent level of defence to that afforded by the same concentrations of single FPCs.
Koala feeding on the swamp gums, E. ovata and E. strzeleckii, was strongly dichotomous. However, total FPC concentrations in these species were contiguous (figure 1), suggesting that a qualitative difference caused koalas to prefer E. ovata. It is possible that we did not measure the foliar attribute underlying this preference. However, we suggest that koalas may have rejected E. strzeleckii because jensenal and/or grandinal are more potent antifeedants than macrocarpals and sideroxylonals. Koalas impose extreme selective pressure on eucalypts, particularly swamp gums and manna gum, because repeated defoliation by dense populations causes tree death on a massive scale (e.g. Martin 1985). In such situations, the possession of a more effective chemical defence would provide a significant evolutionary advantage over neighbouring trees. We suggest that the acquisition of such a defence provides E. strzeleckii with an advantage over E. ovata, and consequently, that foliar chemistry may have facilitated the ecological and evolutionary divergence of these closely related and sympatric species.
Acknowledgments
We thank Phillip Island Nature Park, Parks Victoria, Gavin Moore, Miranda Ebbers and Karen Marsh for assistance with the koala experiments, and Tony Herlt, Bart Eschler, Midori Takasaki and Noel Davies for help and advice with chemical analyses. Jane DeGabriel, Jennifer Sorensen and Ivan Lawler provided comments on the manuscript. All experiments were approved by the Animal Experimentation and Ethics Committees of the Australian National University (F.BTZ.67.97) and Phillip Island Nature Park (PINP 1.98 and 2.01). Funding was provided to W.J.F. from the Australian Research Council.
Footnotes
Present address: School of Tropical Biology, James Cook University, Townsville, QLD 4811, Australia.
References
- Freeland W.J, Janzen D.H. Strategies in herbivory by mammals: the role of plant secondary compounds. Am. Nat. 1974;108:269–289. doi:10.1086/282907 [Google Scholar]
- Lawler I.R, Foley W.J, Eschler B.M, Pass D.M, Handasyde K. Intraspecific variation in Eucalyptus secondary metabolites determines food intake by folivorous marsupials. Oecologia. 1998;116:160–169. doi: 10.1007/s004420050575. doi:10.1007/s004420050575 [DOI] [PubMed] [Google Scholar]
- Lawler I.R, Stapley J, Foley W.J, Eschler B.M. Ecological example of conditioned flavor aversion in plant–herbivore interactions: effect of terpenes of Eucalyptus leaves on feeding by common ringtail and brushtail possums. J. Chem. Ecol. 1999;25:401–415. doi:10.1023/A:1020863216892 [Google Scholar]
- Lawler I.R, Foley W.J, Eschler B.M. Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology. 2000;81:1327–1338. [Google Scholar]
- Martin R.W. Overbrowsing, and decline of a population of the koala, Phascolarctos cinereus, in Victoria I. Food preference and food tree defoliation. Aust. Wildl. Res. 1985;12:355–365. doi:10.1071/WR9850355 [Google Scholar]
- Moore B.D, Foley W.J. A review of feeding and diet selection in koalas (Phascolarctos cinereus) Aust. J. Zool. 2000;48:317–333. doi:10.1071/ZO99034 [Google Scholar]
- Moore B.D, Wallis I.R, Palá Paúl J, Brophy J.J, Willis R.H, Foley W.J. Anti-herbivore chemistry of Eucalyptus—cues and deterrents for marsupial folivores. J. Chem. Ecol. 2004a;30:1743–1769. doi: 10.1023/b:joec.0000042399.06553.c6. doi:10.1023/B:JOEC.0000042399.06553.c6 [DOI] [PubMed] [Google Scholar]
- Moore B.D, Wallis I.R, Woods J.T, Foley W.J. Foliar nutrition, site quality and temperature affect foliar chemistry of tallowwood (Eucalyptus microcorys) Ecol. Monogr. 2004b;74:553–568. doi:10.1890/03-4038 [Google Scholar]
- Moore B.D, Wallis I.R, Marsh K.J, Foley W.J. The role of nutrition in the conservation of the marsupial folivores of eucalypt forests. In: Lunney D, editor. Conservation of Australia's forest fauna. Royal Zoological Society of New South Wales; Mosman, NSW: 2004a. pp. 549–575. [Google Scholar]
- Villalba J.J, Provenza F.D, Bryant J.P. Consequences of the interaction between nutrients and plant secondary metabolites on herbivore selectivity: benefits or detriments for plants? Oikos. 2002;97:282–292. doi:10.1034/j.1600-0706.2002.970214.x [Google Scholar]
- Wallis I.R, Watson M.L, Foley W.J. Secondary metabolites in Eucalyptus melliodora: field distribution and laboratory feeding choices by a generalist herbivore, the common brushtail possum. Aust. J. Zool. 2002;50:507–519. doi:10.1071/ZO02029 [Google Scholar]
- Wallis I.R, Herlt A.J, Eschler B.M, Takasaki M, Foley W.J. Quantification of sideroxylonals in Eucalyptus foliage by HPLC. Phytochem. Anal. 2003;14:360–365. doi: 10.1002/pca.728. doi:10.1002/pca.728 [DOI] [PubMed] [Google Scholar]