Abstract
A rat model of transient suture occlusion of one middle cerebral artery (MCA) was used to create an unilateral reperfused cerebral ischemic infarct with blood-brain barrier (BBB) opening. Opening of the BBB was visualized and quantitated by magnetic resonance (MR) contrast enhancement with a Look-Locker T1-weighted sequence either following an i.v. bolus injection (n=7) or during a step-down infusion (n=7) of gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA). Blood levels of Gd-DTPA after either input were monitored via changes in sagittal sinus relaxation rate. Blood-to-brain influx constants (Ki) were calculated by Patlak plots. On the basis of the MRI parameters and lesion size, the ischemic injury was determined to be similar in the two groups. The bolus injection input produced a sharp rise in blood levels of Gd-DTPA that declined quickly, whereas the step-down infusion led to a sharp rise that was maintained relatively constant for the period of imaging. Visual contrast enhancement and signal-to-noise ratios (S/N) were better with the step-down method (S/N=1.8) than with bolus injection (S/N=1.3). The Ki values were not significantly different between the two groups (p>0.05) and were around 0.005 ml g−1 min−1. The major reason for the better imaging of BBB opening by the step-down infusion was the higher amounts of Gd-DTPA in plasma and tissue during most of the experimental period. These results suggest that step-down MR contrast agent (MRCA) administration schedule may be advantageous for detection and delineation of acute BBB injury than the usually employed bolus injections.
Keywords: Arterial input functions, Cerebral ischemia, Magnetic resonance contrast agents, Middle cerebral artery
1. Introduction
Gadolinium-based MRCA such as Gd-DTPA are used routinely to detect BBB opening that often accompanies cerebral disorders such as stroke, tumors, and multiple sclerosis [1–6]. If such opening occurs, the extravasation of MRCA and the linked MR image enhancement can be used not only to detect and localize the lesion but also to quantify the increase in BBB permeability [7,8]. During the acute phase of experimental stroke in animals, a relatively modest increase in BBB permeability appears to precede parenchymal hemorrhage [9–11]. Early detection and accurate measurement of such opening may, therefore, be useful for screening patients for thrombolytic therapy and minimizing the incidence of parenchymal hemorrhage, the major drawback of this treatment.
When imaging MR contrast and assessing BBB permeability, the MRCA can be administered by either an intravenous (i.v.) bolus injection or some type of i.v. infusion or a combination of the two. In animal experiments, the bolus usually takes several seconds to administer, and the concentration of the MRCA in systemic arteries begins to rise some 5–6 sec later, reaches a peak after another 5 sec, falls sharply over the next several minutes, and declines slowly thereafter. Intravenous infusions may be made at either a constant rate (often referred to as a continuous infusion), an increasing rate (achieved by stepping up the infusion rate over time according to a prescribed schedule), or a decreasing rate (obtained by stepping down the infusion rate over time). The constant rate infusion leads to a gradual rise and subsequent flattening in blood concentration after many minutes or even several hours. The step-up infusion is usually done in such a way that the blood concentration rises linearly and rapidly; it is, thus, often referred to as a ramp infusion. In its most common usage, the step-down infusion is designed to produce a very quick rise in blood concentration and then maintain that level relatively constant for the duration of the experiment; this is sometimes called a steady-state or constant-concentration infusion. Lastly, the combined administration procedure starts with a bolus injection that is followed quickly thereafter by a constant rate infusion; it has been referred to as a hybrid injection by Tofts and Berkowitz [12].
In the past, a majority of MR imaging (MRI) studies have employed the bolus injection method, mostly because it is easy and seems to work. This choice has been examined in one study in which Early Enhancement Theory (EEF) was used to model three MRCA administration modes – bolus injection, constant rate infusion, and hybrid injection – for the case of a simulated blood-retinal barrier lesion [12]. The conclusion of this exercise was that bolus injections were more effective than constant rate infusions and should always be used. This modeling also predicted that a hybrid injection will produce a constant blood MRCA level after about 15 min but have considerable overshoot before that point. A human study with Gd-DTPA reported that during the subchronic and chronic phases of stroke the constant rate of infusion yielded more detailed enhancement and better evidence of BBB damage than does a bolus injection; moreover, subjects who did not show enhancement with a bolus Gd-DTPA input were seen to have BBB damage with a continuous infusion [13]. The conclusions from these two studies obviously conflict over the choice of an MRCA administration schedule for at least the subchronic and chronic stages of stroke. Further investigation of this issue, therefore, seemed in order, and an examination of it during the acute phase of transient focal cerebral ischemia appeared timely.
This study tests the hypothesis that a step-down infusion of Gd-DTPA produces better contrast enhancement and lesion visualization than does a bolus injection. For this testing, a rat model of transient cerebral ischemia was used to perturb BBB function and promote MRCA leakage. Bolus injections were made in one group of rats; step-down infusions in the second. The MRI data yielded blood concentration over time curves, plots of the number of pixels vs. intensity per pixel, contrast enhancement (apparent signal-to-noise ratio derived from the preceding graphs) and Gd-DTPA influx constants (Ki).
2. Materials and methods
2.1. Experimental model
Male Wistar rats (Charles River Laboratories, Wilmington, MA; 275–300 g; n=14) were used in the study. All animal handling and surgical procedures were done in an Association for Accreditation of Laboratory Animal Care (AALAC) certified facility under a protocol approved by the institutional animal care and use committee. One femoral artery and vein were cannulated for physiological monitoring and MRCA injection, respectively. The rats underwent suture MCA occlusion for 3 hr according to methods described in detail elsewhere [8,11,14,15]. After 3 hr, the suture was withdrawn to restore blood flow. Physiological parameters such as blood pressure, blood gases, pH, glucose, osmolality and hematocrit were measured during and after MR imaging. At ~2.5 hr post-reperfusion, Gd-DTPA-based contrast-enhanced MR imaging was performed to localize extravascular enhancement and quantify Ki.
2.2. MR Imaging System and Protocol
All studies were performed using a 7 Tesla, 20 cm bore superconducting Magnex magnet (Magnex Scientific Inc., Abingdon, UK) interfaced to a Bruker console (Bruker Biospin MRI, Inc. Billerica, MA, USA) and equipped with a 12 cm self-shielded gradient set capable of producing 25 gauss/cm gradients with 100 μsec rise times. T2-weighted imaging (T2WI) and CBF estimates by the arterial spin-tagging technique were acquired from 45 to 120 min after MCA occlusion as described earlier [11,15].
After 3 hr of occlusion, the animal was removed from the magnet, and the occluding suture withdrawn to begin reperfusion The rat was returned to the magnet immediately afterwards. Post-reperfusion T2WI and CBF data were acquired from 30–120 min thereafter. Some 15–20 min later, the blood-to-brain transfer of Gd-DTPA was measured.
2.3. Gd-DTPA administration
The Gd-DTPA was prepared in-house following published methods [16]; the concentration of the MRCA in the stock solution was about 400 mmol. For bolus injections, 60 μl of the stock solution was diluted in 0.1 ml of saline and injected through the femoral vein in 4–5 sec. The step-down infusion was done with a syringe pump (Model 944, Harvard Apparatus, South Natick, MA) and a prescribed infusion schedule (Table 1) that immediately raised blood Gd-DTPA to a concentration that was subsequently maintained nearly constant thereafter. For the infusate, 240 μl of the stock solution was diluted in 4.0 ml of saline. The osmolality of the Gd-DTPA preparation was very high (>1000 mOsm/kg), and this dilution brought it into the physiological range. Approximately 3.5–3.6 ml of infusate was used per step-down experiment.
Table 1.
Step-down infusion schedule derived from the data presented in Fig. 1A.
| Rate (ml/min) | Pump speed (%) | Time | Duration (min) | Volume (ml) |
|---|---|---|---|---|
| 0.68 | 100 | 0–30 sec | 0.5 | 0.34 |
| 0.53 | 77.7 | 30–60 sec | 0.5 | 0.26 |
| 0.39 | 57.4 | 1–2 min | 1.0 | 0.39 |
| 0.28 | 41.6 | 2–3 min | 1.0 | 0.28 |
| 0.22 | 33.3 | 3–4 min | 1.0 | 0.22 |
| 0.19 | 28.7 | 4–5 min | 1.0 | 0.19 |
| 0.17 | 25.0 | 5–7 min | 2.0 | 0.34 |
| 0.14 | 20.4 | 7–10 min | 3.0 | 0.42 |
| 0.12 | 17.6 | 10–15 min | 5.0 | 0.60 |
| 0.10 | 14.8 | 15–20 min | 5.0 | 0.50 |
Total volume infused = 3.54 ml
The rates of the step-down infusion were set by a procedure based on the plasma kinetics of 14C-sucrose [17]. Sucrose was chosen as a model compound because its biodistribution resembles that of Gd-DTPA In brief, an i.v. bolus injection of 14C-sucrose was made in three control rats, and a series of arterial blood samples drawn at 5, 10, 20, 30, 45, and 60 sec and at 2, 3, 5, 10, 30, and 60 min. One such curve is shown in Figure 1A. Then the transform that linked an idealized bolus (Dirac delta) input to the arterial blood curve was obtained. With this transform and a desired blood concentration time-course of an instant rise followed by constant level thereafter, a step-down infusion schedule was derived by means of the convolution integral. The step-down infusion schedule thus obtained and used in the present study is given in Table 1; an example of a blood curve of 14C-sucrose produced by the step-down procedure used in the present study is shown in Figure 1B.
Figure 1.
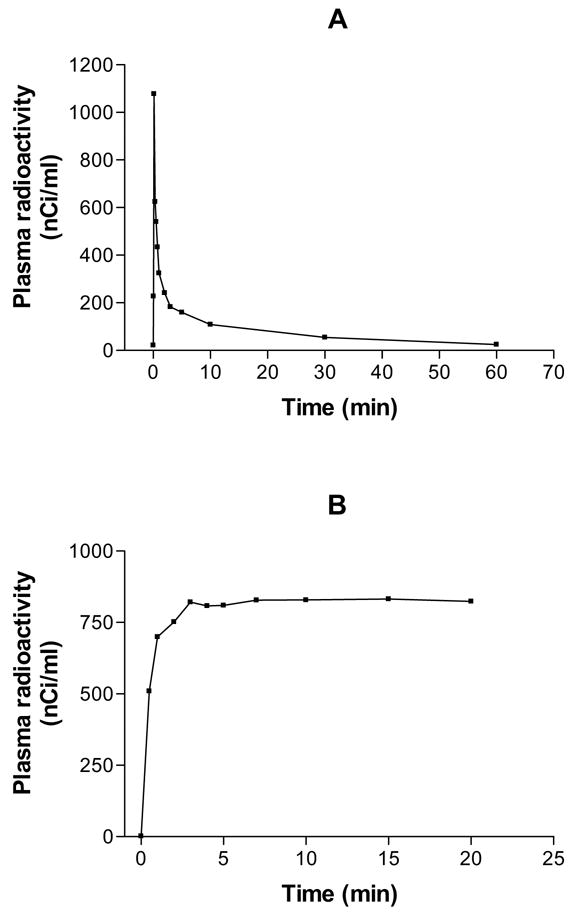
Examples of 14C-labeled sucrose plasma profiles after intravenous bolus injection (A) and step-down infusion (B). Notice the sharp rise and the steep fall in plasma levels in A and the sharp rise, but relatively constant plasma levels thereafter in B. Identical administration protocols were employed for MRCA injection in the present work.
2.4. Look-Locker MRI quantitation of T1 after Gd-DTPA infusion
Baseline T1-weighted spin-echo (TR/TE=1000 ms/20 ms) and Look-Locker (L-L) scans were collected prior to the Gd-DTPA administration. After obtaining one or two baseline estimates, Gd-DTPA was given using either bolus injection or step-down infusion. Estimates of T1 were acquired using the L-L T1 procedure to generate maps of the longitudinal relaxation rate R1 (R1=1/T1) at approximately 3-minute intervals for the next 21 min. Data was obtained for five interleaved 2 mm thick slices. At the conclusion of the L-L series, a final post-contrast T1-weighted multislice spin-echo image set was obtained [15].
2.5. MR Data analysis
The MR images were processed using a SUN workstation (Sun Microsystems, Santa Clara, CA) to generate pre- and post-contrast T1-weighted images (T1WI). The pre-contrast images were subtracted from the post-contrast images to identify regions of Gd-DTPA enhancement (T1-subtraction maps). Temporal changes in R1 (ΔR1) in the superior sagittal sinus were used to generate the arterial concentration curve. Ischemia-damaged areas with BBB opening were identified and segmented by applying the iterative self-organizing data analysis technique algorithm (ISODATA) to the Gd-DTPA L-L T1 data series [15]. For these regions of interest (ROIs), Gd-DTPA blood-to-brain influx constants (Ki) were estimated with the plasma and tissue MRI data via Patlak plots [8].
To obtain a quantitative index of signal-to-noise ratio (S/N), a 3-pixel-wide prectangle was drawn horizontally across the entire T1-subtracted map at the level of the widest part of the ROI. Intensity or brightness in arbitrary units was measured for each pixel within the ROI and the matching region on the contralateral side of the brain. Graphs of number of pixels vs. brightness were made from these data, the area under the curves assessed for the ipsilateral and contralateral ROI’s, and the former divided by the latter to get S/N for each mode of MRCA administration.
2.6. Histopathology
After MR imaging, the animals were removed from the magnet and killed by decapitation under deep anesthesia. The brains were rapidly frozen in situ and carefully dissected from the head. Frozen coronal sections (20 μm thick) were taken at 400 μm intervals spanning the complete brain. The sections were stained with cresyl violet (Nissl). The difference in the degree of Nissl staining was used to delineate the border between normal and ischemia damaged tissue. Due to a reduction in nuclear staining, ischemia damaged tissue takes up less stain and appears noticeably paler than normal tissue. This reduced staining and the presence of many fluid filled microvesicles or vacuoles (vacuolation) and swollen astrocytes result in an area of pallor that defines tissue injury.
The Nissl stained sections were viewed and digitized using an image analysis system (AIS, Imaging Research Inc., St. Catharines, Canada). The digitized histological data were imported into a free image analysis program (Eigentool, developed by the Dept. of Radiology, Henry Ford Health System) and were intensity reversed. Gray scale intensity per pixel was measured in three random contralateral (normal) regions. The mean plus 3 standard deviations (SD) of these data was taken to define the upper boundary of normal gray scale intensity. The number of pixels darker (inverted intensity scale) than this upper boundary were counted and summed to yield the area of pallor, i.e., ischemic injury, in units of pixels. To normalize the values between experiments, the areas of pallor were expressed as percentage of the total brain area in pixels. The area of pallor data was used to compare the size of the ischemic injury between the bolus injection and step-down infusion groups.
Results from the two different Gd-DTPA administration schedules were compared using a Student’s t-test and significance was inferred at p≤0.05.
3. Results
For both groups, the measured physiological parameters were within the normal range for halothane anesthetized rats (Table 2). The differences between the two groups were not significant for any of these values (p=0.5). At both times of measurement, the ipsilateral/contralateral CBF ratios within the ROI’s were similar in the two groups (Table 2; p=0.5). The measured T2 and T1 relaxation times were virtually identical for the two groups (Table 3; p=0.5). Further evidence of the similarity of the ischemic injury was obtained from the area of pallor data. The mean areas of the two groups were fairly similar (Table 3), although the variability (SD) was sizable. The difference between the areas of pallor between them was not statistically significant (p=0.45). All of the preceding results indicate that the severity of the ischemic injury was comparable in both groups, and any significant difference in contrast enhancement between them was mainly due to the Gd-DTPA administration protocol.
Table 2.
Physiological status of the rats from the bolus injection (n=7) and step-down infusion (n=7) groups. Values are given as mean ± SD.
| Input | pH | pCO2 | pO2 | Blood glucosea | Osmolalityb | % Hematocrit | Tc |
|---|---|---|---|---|---|---|---|
| Bolus | 7.4±0.04 | 55.5±6.7 | 116.3±9.0 | 148.7±7.4 | 305.5±4.7 | 0.42±0.02 | 37.1±0.2 |
| Infusion | 7.3±0.1 | 49.2±5.6 | 102.6±21 | 167.4±12.1 | 294.7±12 | 0.44±0.02 | 37.1±0.3 |
Measured in amg/100 ml plasma;
mOsm/L plasma;
T, °C rectal temperature
Table 3.
Some MR parameters and areas of pallor measured during ischemia and reperfusion in bolus injection and step-down infusion groups. All MR values are given as mean ± SD of the ipsilateral/contralateral ratios. The differences between the two groups were not significant.
| Input group | During Ischemia | During Reperfusion | |||||
|---|---|---|---|---|---|---|---|
| T2a | T1a | CBFb | T2a | T1a | CBFb | Area of Pallorc | |
| Bolus | 1.2±0.1 | 1.00±0.1 | 0.3±0.2 | 1.3±0.1 | 1.3±0.1 | 0.6±0.3 | 18.9±7.1 |
| Infusion | 1.2±0.2 | 1.1±0.1 | 0.3±0.2 | 1.4±0.1 | 1.3±0.1 | 0.5±0.2 | 22.9±10.2 |
Measured in
milliseconds;
ml/g/min;
% total brain area
The earliest time of assaying Gd-DTPA blood concentrations was 3 min after starting i.v. administration (Fig 2). The 14C-sucrose plasma curves (Fig. 1) during the first 3 min approximate the early portion of Gd-DTPA ones that were missed by the imaging protocol. For the bolus injection, it is likely that the blood Gd-DTPA concentration peaked around 10 sec, quickly fell over the next several min (Fig. 1A), and then slowly dropped thereafter (Fig. 2). With a step-down infusion, the blood concentration of Gd DTPA probably rose quickly over the first minute and became relatively flat after 2–4 min (as modeled by the 14C-sucrose data in Fig. 1B) and then remained so for the remainder of the experiment (Fig. 2).
Figure 2.
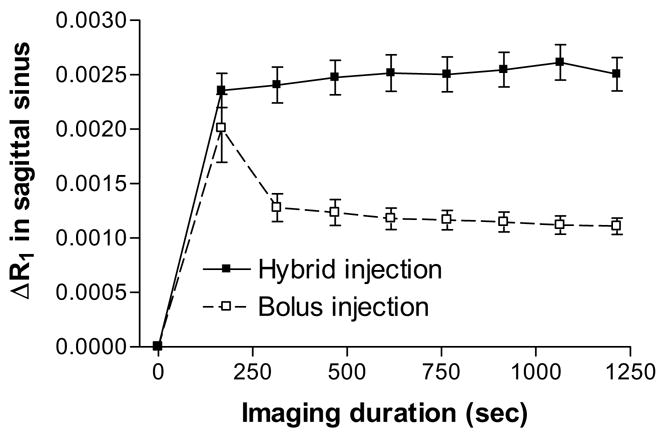
Examples of changes in Gd-DTPA levels with time in the sagittal sinus shown by MR imaging for two inputs. The imaging duration is indicate on the abscissa, and the changes in Gd-DTPA relaxation rates, ΔR1, at each time of measurement is plotted on the ordinate. The bolus input resulted in a quick rise in the blood levels that fell exponentially over the imaging period. The step-down infusion, however, resulted in an elevated blood level that remained more or less constant throughout the imaging interval. The similarity between the curves in Figure 1 from 3 min onward and those in Figure 2 is obvious. The Figure 1 curves from 0–180 min almost certainly indicate the data missing first 3 min of data in Figure 2. Values are mean ± SD of 7 experiments for each group.
Subtraction of pre-contrast T1WI from post-contrast T1WI yielded T1 subtraction maps that were rather noisy for bolus injection experiments (Figs. 3A, 3C, and 3E); those from the step-down infusion studies were cleaner and less noisy (Figs. 4A, 4C, and 4E). No contrast enhancement, indicative of BBB opening and Gd-DTPA leakage, was seen in one of the bolus injection studies (Fig. 3A) even though the area of pallor was appreciable (Fig. 3B). In this example, the area of ischemia injured tissue was the smallest among the animals studied and was located in the preoptic area and ventral striatum. It is possible that the BBB was intact in this ischemia-damaged tissue.
Figure 3.
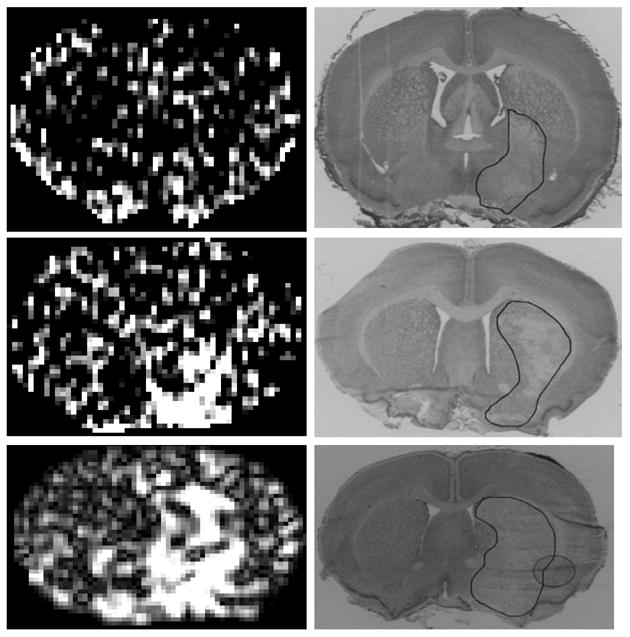
Three examples of MR Look-Locker T1 maps after Gd-DTPA enhancement observed after a typical bolus injection (left set of panels) and of the histologies obtained from within the MRI slices (right set of panels). The area of pallor is outlined in black on the histologies. The MR maps were generated by off-line analyses of the Look-Locker T1 data. The subtraction maps for this group of bolus injection experiments are more highly pixilated than those from the step-down infusion (Fig. 4) and no leakage of Gd-DTPA was evident in example 4A, although an appreciable sized ischemic lesion was apparent on the accompanying histology (4B). In this example and the other two, the S/N ratio appeared to be fairly low, and the identification of the enhancing regions was, accordingly, more difficult. Despite sizable ischemic lesions in all seven rats, Gd-DTPA enhancement was either relatively modest (most cases) or none existent (one case) for the bolus injection experiments.
Figure 4.
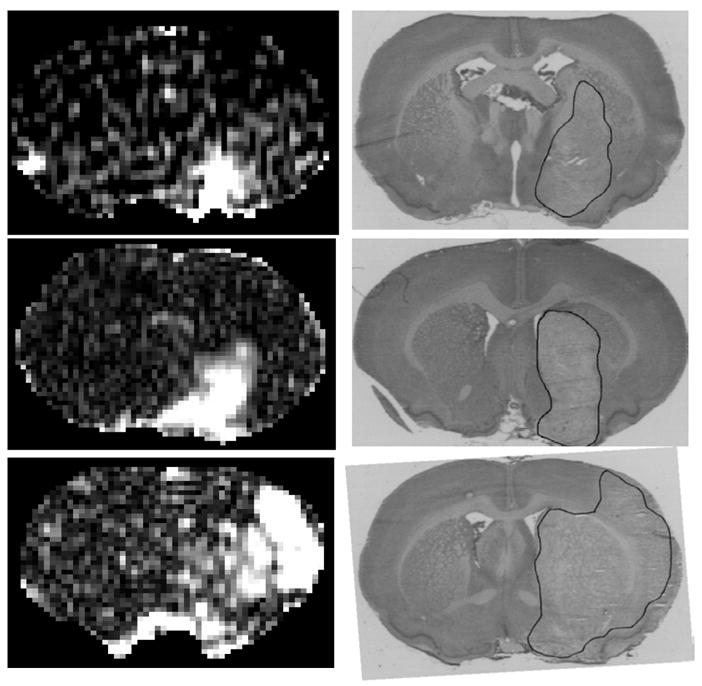
Three examples of MR images after Gd-DTPA enhancement observed after a typical step-down infusion (left set of panels) and of the histologies obtained from within the MRI slices (right set of panels). The pre-Gd-DTPA T1WI images were subtracted from the post-Gd-DTPA T1WI images to highlight the enhancing areas. The ischemic lesion seen on the Nissl stained sections from the respective experiments are outlined in black on the histological images on the right. It can be seen in the MR subtracted maps that the MR contrast images are relatively smooth. The S/N ratio appears to be quite high from the step-down infusion studies, and the identification of the enhancing regions is simple and straightforward from these images. Blood-brain barrier opening was, thus, readily detectable from the Gd-DTPA distribution data from the step-down infusion studies.
The areas of pallor were fairly similar in location (preoptic area and medial striatum) and size among two of the bolus injection experiments (Fig. 3D and 3F) and two of the step-down infusion studies (Fig. 4B and 4D). The smallest area of contrast enhancement among these four examples was found in the preoptic area of one of the step-down infusion animals (Fig. 4A). The area of BBB opening to Gd-DTPA was similar in Figures 3C (bolus) and 4C (infusion) but the latter subtraction map was less noisy.
Large areas of MRCA-enhancement were seen in one bolus injection example (Fig. 3E) and one step-down infusion case (Fig. 4E), but the location of these areas was not identical. In the latter, the area of pallor was extremely large and extended to the parietal cortex (Fig. 4F) as did the BBB lesion. In the other of these two examples (Fig. 3E; bolus injection), there appears to be leakage into not only the striatum but also the adjacent lateral ventricle plus the tissue around that ventricle; in this case, the ventricle has apparently been compressed by the swollen tissue around it. The level of noise seems to be less in Figure 4E (infusion) than in Figure 3E (bolus).
These examples cover the range of abnormalities found in the MR and histopathological data among the two groups. As noted in Table 3, the size of the area of pallor was slightly larger in the step-down group than the bolus injection one. However, this slight difference is not likely to be the cause of the lesser noise, better visual enhancement, and clearer definition of the BBB damaged region of the step-down infusion.
The graph of the number of pixels vs. degree of brightness (Fig. 5) demonstrates some differences in the enhancement between the two modes of Gd-DTPA administration. On the contralateral side, the highest (peak) number of pixels (around 15) comes at the same intensity for both, but more pixels with intensity greater than that (>1400 units) were found for the step-down infusion than the bolus injection. This modest difference in the contralateral data between the two groups could be mostly due to dissimilarity in the concentration of contrast agent in the vascular system (see Fig. 2 for relative blood MRCA levels).
Figure 5.
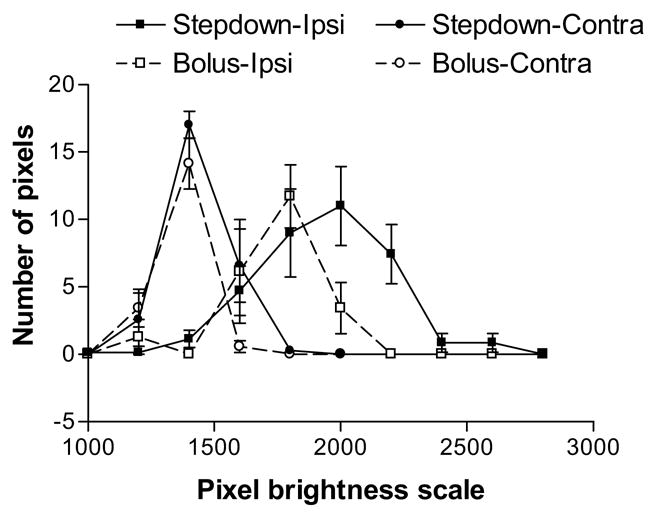
The distribution of ipsi- and contralateral pixel intensities are shown for the two modes of Gd-DTPA administration. In both groups, the range of pixel intensities on the contralateral side was lower than on the ipsilateral side. On both sides of the brain, the number of pixels on the higher end of the brightness scale was larger with the step-down infusion than with the bolus injection, and the range of brightness extended to higher values for the former than the latter. The data points are the mean ± SD (n=7 for both groups).
On the ipsilateral side (Fig. 5), the number of pixels with abnormally high intensities (≥1600 units) was larger in the step-down infusion than the bolus injection group. In addition, the brightness of these pixels tended to be sizably greater for the former than the latter. The areas under the curves in Figure 5 are approximately 32 × 103 pixel-intensity units for the bolus injection and 68 × 103 pixel-intensity units for the step-down infusion.
The area-under-the-curve on the contralateral side was about 25 × 103 pixel-intensity units for bolus infusions but 38 × 103 pixel-intensity units for the step-down infusions (Fig. 5). Assuming that the contralateral side integral represents noise, the S/N ratios are approximately 1.30 for the bolus injection and 1.80 for the step-down infusion; this difference in S/N was marginally significant (p=0.06). This comparison demonstrates quantitatively that the contrast enhancement on the ipsilateral side was greater with the step-down infusion for this model of transient cerebral ischemia.
For bolus injection and step-down infusion groups, the Gd-DTPA Ki’s (means ± SD) calculated from Patlak plots were 0.004 ±0.001 and 0.006±0.005 ml/g/min, respectively, for the BBB-damaged regions of interest. The difference in Ki for the two groups were not significant (p=0.5). It should be noted, however, that the full arterial concentration of Gd-DTPA over time integral that appears in the abscissa of the Patlak plot is underestimated by us in both cases because the time of the first data point is 3 min (Fig. 2). In addition, Figure 1 suggests that more of the total Gd-DTPA concentration-time integral falls within the first 3 min with a bolus injection than a step-down infusion and that more of this integral is missed with the former than the latter. Our previous analysis of this problem [18] suggests that our evaluation of Ki by Patlak plots in both groups was not significantly in error by this missing data, but that of the other Patlak-plot parameter, the y-intercept (the distribution space of the protons affected by plasma contained Gd-DTPA) was overestimated. Hence such values are not reported in the current work.
4. Discussion
The rat stroke model employed in this study was 3 hr of MCA occlusion and followed by 3 hr of reperfusion. As illustrated in these experiments, it produces ischemic injury to the preoptic area, striatum, and occasionally the parietal and insular cortex and to the microvasculature within these regions. All the rats studied except one showed contrast enhancement and some degree of BBB opening but no acute hemorrhages which is consistent with this model [11].
Contrast enhancement after Gd-DTPA injection is considered to be one of the signatures for BBB breakdown in cerebral ischemia. It has been used as a quantitative indicator of treatment and qualitative indices such as hyperintense acute reperfusion marker (HARM) have been suggested as reliable indicators of cerebrovascular pathology [19,20]. While most such studies employ a standard dose of a gadolinium-based MRCA, some have pointed out the usefulness of increased gadolinium doses [21,22]. Such studies have, however, been limited to brain tumor studies, and none are available for stroke.
Because of its established clinical role, absence of enhancement in stroke is taken as evidence of an intact BBB. This assumption could, however, be misleading for several reasons. For instance, such absence may be due to greatly lowered CBF in the lesion that leads to sub-optimal delivery of the contrast agent to the affected regions [13] or a rather small increase in microvascular permeability. In stroke as well as other cerebral pathologies, BBB opening can be missed by MRI with an inadequate mode of MRCA administration.
Contrast agents as well as radiotracers and fluorescent materials can be administered intravenously by bolus injection or by infusion for subsequent brain imaging. Bolus injections were tested in the present study, and the images obtained of Gd-DTPA leakage were noisier and less intense than with the step-down infusion. This difference implies that there more Gd-DTPA leaks into the tissue with a step-down infusion than a bolus injection.
There are several factors that could contribute to this discrepancy. First, more contrast agent was administered with the step-down method. Approximately four times more Gd-DTPA was infused over the 20 min duration of the experiment than was injected by bolus. High doses of MRCA are possible with infusion and can be done in a more physiological way than with a bolus injection. The latter involves a high pressure injection of a highly concentrated solution; the step-down infusion uses a much less concentrated infusion solution and a gentler rate of administration, thereby minimizing untoward cardiovascular effects. As suggested by others (21,22), increasing the dose as was done herein with a step-down infusion, led to better contrast enhancement and imaging. Although the difference in S/N ratio between the two inputs did not reach statistical significance (p=0.06), it is likely that it would in a larger series of experiments.
Second and related to the preceding, the blood levels of the contrast agent from 180 sec onward were approximately two-fold higher for the step-down infusion than the bolus injection (Fig. 2). Accordingly, the amount of Gd-DTPA in the intravascular compartment after 180 sec is greater for the step-down infusion than the bolus injection. This may explain most of the difference in intensity between the two modes for both sides of the brain.
Third and related to the preceding, there was also more contrast agent in the parenchyma with the step-down infusion than with the bolus injection. The driving force for uptake across the capillary wall is the plasma concentration, which was nearly two-fold higher for most of the experimental period for the step-down infusion. The nearly identical Ki’s from the two inputs testify to the greater amount of Gd-DTPA in the parenchyma and the better, stronger signal for the step-down infusion.
Finally, some of the lower MRCA level and poorer resolution of ischemic BBB damage by the bolus injection may be due to loss of Gd-DTPA from the imaging field. With a bolus injection, much of the uptake into tissue occurs during the first several passes when the blood concentration is very high; as the latter falls, backflux into the blood becomes more probable during the remaining time than with a step-down infusion. In addition to the preceding, there may be more loss of the influxed contrast agent from the field of observation by diffusion and solute drag (bulk flow) into adjacent brain and CSF with a bolus injection than a step-down infusion.
As mentioned in the Introduction, infusions have been done in at least three ways. Of them, the simplest, most commonly used one is a constant rate infusion. It produces a gradual rise and subsequent flattening in blood concentration after a long period of time. When this plateau is reached – usually after several hours – the rate of infusion is equal to the rate of elimination from the blood. As modeled by Tofts and Berkowitz [12] for MRI, such a continuous infusion schedule takes considerable time to reach a detectable level of contrast agent in the tissue and even longer to achieve a relatively constant plasma concentration. It was concluded that a constant rate infusion was not useful or effective for assessing increases in capillary permeability by MRI. Additionally, in acute stroke situations such long imaging durations are undesirable both in terms of rapidly evolving injury and expensive MRI time.
The other mode is the step-up infusion. Such infusions are usually designed to produce a steep, virtually linear rise in blood concentration of a radiotracer in 30–60 sec experiments and are often called ramp infusions. They are used mostly when measuring the rate of local cerebral blood flow with 14C-iodoantipyrine [14] or influx across the normal BBB of highly permeable material such as 3-O-[14C]methyl-D-glucose and 14C-antipyrine [23]. During such short-term step-up infusions, influx dominates tissue uptake, backflux is minimal, and tissue distribution space is of minor importance in the calculation of the blood-to-brain transfer constant. To the best of our knowledge, step-up infusions of contrast agents have not been done to date and may have little application to MRI studies.
The near-constant blood levels attained by step-down infusions have advantages other than just greater MR enhancement. The constant plasma concentration case is much easier to work with when doing data processing and modeling. With a bolus injection, the blood curve is usually resolved as a sum of exponentials and the plasma-to-tissue kinetics and models are more complicated. For the step-down infusion, the concentration gradients across the BBB – whether damaged or normal – favor influx over efflux for most of the duration of the experiment. The Patlak plot allows the selection of the data for the period over which efflux is negligible and influx can be calculated from the simplest blood-tissue exchange model. In contrast, backflux becomes appreciable fairly early on after a bolus injection and the linearity of the Patlak plot extends over only a few times of sampling, if at all, forcing the employment of a more complex model. The calculation of influx in this case becomes complicated because assumptions concerning tissue distribution space and equality of flux in both directions (i.e., diffusion but no bulk flow with coupled solute-solvent drag) come into play and the outcome is model dependent. This seems to be the case with, for example, experimental brain tumor models with highly leaky capillaries. In these instances, the extended Patlak plot or compartmental analysis is employed to estimate influx from the blood and tissue data [18].
The question remains whether a much higher concentration of Gd-DTPA given as a bolus will result in similar enhancement and better S/N ratios as the same concentration given using the step-down method. This was not attempted because the very high osmolalities of Gd-DTPA preparations, both commercial and lab-made, might result in untoward cardiovascular effects if given in high concentrations/volumes as a bolus.
To summarize, the higher signal-to-noise ratio attained by the step-down infusion provided superior visual appreciation of BBB injury and MRCA leakage. Of importance, the MRI detected abnormalities and lesion sizes in the two groups were comparable. This implicates the input function as the variable leading to the observed differences in contrast enhancement and signal-to-noise ratio. The lack of significant differences between the Ki from the two inputs suggests that the step-down infusion did not change the permeability characteristics of the BBB after stroke and, thus, had no apparent secondary effect on the ischemic injury to cerebral microvessels. Therefore, despite its perceived complexities, a step-down infusion protocol may be better than the bolus method for precise identification of BBB lesions in acute cerebral ischemia.
Acknowledgments
We thank Jun Xu, Richard Croxen and Kelly Keenan for technical assistance. The results were presented in part as an abstract at the 13th scientific meeting of International Society for Magnetic Resonance in Medicine, Miami, FL. This study was supported by NIH grant 1RO1 NS38540 and American Heart Association-Bugher Foundation Award (0270176N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinmann H-J, Brasch RC, Press W-R, Wesbey GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. Am J Radiol. 1984;142:619–624. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- 2.Niendorf HP, Felix R, Laniado M, Schorner W, Claussen C, Wienmann H-J. Gadolinium-DTPA: a new contrast agent for magnetic resonance imaging. Radiation Med. 1985;3:7–12. [PubMed] [Google Scholar]
- 3.Virapongse C, Mancuso A, Quisling R. Human brain infarcts: Gd-DTPA-enhanced MR imaging. Radiology. 1986;161:785–794. doi: 10.1148/radiology.161.3.3786734. [DOI] [PubMed] [Google Scholar]
- 4.Elster AD, Moody DM. Early cerebral infarction: gadopentedate dimeglumine enhancement. Radiology. 1990;177:627–632. doi: 10.1148/radiology.177.3.2243961. [DOI] [PubMed] [Google Scholar]
- 5.Esser M, von Kummer R, Egelhof T, Winter R, Sartor K. Vascular MR contrast enhancement in cerebrovascular disease. Am J Neuro Radiol. 1996;17:887–894. [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson HBW, Stubgaard M, Frederiksen JL, Jensen M, Henriksen O, Paulson OB. Quantitation of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn Reson Med. 1990;16:117–131. doi: 10.1002/mrm.1910160111. [DOI] [PubMed] [Google Scholar]
- 7.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17:367–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 8.Ewing JR, Knight RA, Nagaraja TN, Yee JS, Nagesh V, Whitton PA, Li L, Fenstermacher JD. Patlak plots of Gd-DTPA MRI data yield blood-brain transfer constants concordant with those of 14C-sucrose in areas of blood-brain opening. Magn Reson Med. 2003:283–292. doi: 10.1002/mrm.10524. [DOI] [PubMed] [Google Scholar]
- 9.Knight RA, Barker PB, Fagan SC, Li Y, Jacobs MA, Welch KM. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke. 1998;29:144–151. doi: 10.1161/01.str.29.1.144. [DOI] [PubMed] [Google Scholar]
- 10.Hamann GF, del Zoppo GJ, von Kummer R. Hemorrhagic transformation of cerebral infarction--possible mechanisms. Thrombosis & Haemostasis. 1999;82 (Suppl 1):92–94. [PubMed] [Google Scholar]
- 11.Knight RA, Nagaraja TN, Ewing JR, Nagesh V, Whitton PA, Bershad E, Fagan SC, Fenstermacher JD. Quantitation and localization of blood-to-brain influx by MRI and quantitative autoradiography in a model of transient focal ischemia. Magn Reson Med. 2005;54:813–821. doi: 10.1002/mrm.20629. [DOI] [PubMed] [Google Scholar]
- 12.Tofts PS, Berkowitz BA. Measurement of capillary permeability from the Gd enhancement curve: a comparison of bolus and constant infusion injection methods. Magn Reson Imaging. 1994;12:81–91. doi: 10.1016/0730-725x(94)92355-8. [DOI] [PubMed] [Google Scholar]
- 13.Merten CL, Knitelius HO, Assheuer J, Bergmann-Kurz B, Hedde JP, Bewermeyer H. MRI of acute cerebral infarcts: increased contrast enhancement with continuous infusion of gadolinium. Neuroradiology. 1999;41:242–248. doi: 10.1007/s002340050740. [DOI] [PubMed] [Google Scholar]
- 14.Ewing JR, Wei L, Knight RA, Pawa S, Nagaraja TN, Brusca T, Divine GW, Fenstermacher JD. Direct comparison of local cerebral blood flow rates measured by MRI arterial spin-tagging and quantitative autoradiography in a rat model of experimental cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2003;23:198–209. doi: 10.1097/01.WCB.0000046147.31247.E8. [DOI] [PubMed] [Google Scholar]
- 15.Knight RA, Nagesh V, Nagaraja TN, Ewing JR, Whitton PA, Bershad E, Fagan SC, Fenstermacher JD. Acute BBB opening in experimentally induced focal cerebral ischemia is preferentially identified by quantitative magnetization transfer imaging. Magn Reson Med. 2005;52:822–832. doi: 10.1002/mrm.20630. [DOI] [PubMed] [Google Scholar]
- 16.Stritch G, Hagan PL, Gerber KH, Slutsky RA. Tissue distribution and magnetic resonance spin lattice relaxation effects of gadolinium-DTPA. Radiology. 1985;154:723–726. doi: 10.1148/radiology.154.3.3969477. [DOI] [PubMed] [Google Scholar]
- 17.Patlak CS, Pettigrew KD. A method to obtain infusion schedules for prescribed blood concentration time courses. Journal of Applied Physiology. 1976;40:458–463. doi: 10.1152/jappl.1976.40.3.458. [DOI] [PubMed] [Google Scholar]
- 18.Ewing JR, Brown SL, Lu M, Churchman JL, Panda S, Ding G, Knight RA, Cao Y, Jiang Q, Nagaraja T, Fenstermacher JD. Model selection in MRI measurements of vascular permeability: Gadomer in a 9L model of rat cerebral tumor. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600189. in press. [DOI] [PubMed] [Google Scholar]
- 19.Rydberg JN, Riederer SJ, Rydberg CH, Jack CR. Contrast optimization of fluid-attenuated inversion recovery (FLAIR) imaging. Magn Reson Med. 1995;34:868–877. doi: 10.1002/mrm.1910340612. [DOI] [PubMed] [Google Scholar]
- 20.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35 (Suppl 1):2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 21.Bruening R, Berchtenbreiter C, Holzknecht N, Essig M, Wu RH, Simmons A, Heuck A, Maschek A, Meusel M, Williams SC, Cox T, Knopp MV, Reiser M. Effects of three different doses of a bolus injection of gadodiamide: assessment of regional cerebral blood volume maps in a blinded reader study. Ajnr: American Journal of Neuroradiology. 2000;21:1603–1610. [see comment] [PMC free article] [PubMed] [Google Scholar]
- 22.Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, Schibany N, Prokesch R, Mehrain S, Mlynarik V, Fog A, Heimberger K, Trattnig S. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Investigative Radiology. 2003;38:415–422. doi: 10.1097/01.RLI.0000067488.57101.bd. [DOI] [PubMed] [Google Scholar]
- 23.Chen JL, Wei L, Bereczki D, Hans FJ, Otsuka T, Acuff V, Richardson G, Patlak C, Fenstermacher J. Virtually unaltered permeability-surface area products imply little capillary recruitment in brain with hypoxia. Microcirculation. 1994;1:35–47. doi: 10.3109/10739689409148260. [DOI] [PubMed] [Google Scholar]


