Abstract
Site-specific recombination of the bacteriophage λ genome into and out of the host bacterial genome is postulated to involve the formation of Holliday structure intermediates by reciprocal single-strand exchanges. Synthetic analogues of the predicted recombination intermediates are resolved in vitro by the protein product of the λ int gene. Some of the structural features and reaction conditions for this genetic recombination can now be defined.
Integration of the bacteriophage λ chromosome into a bacterial chromosome occurs by a reciprocal site-specific recombination between the phage attP site and the bacterial attB site. The resulting prophage att sites, attL and attR, are themselves able to recombine to regenerate attP and attB during excision of the viral genome (see refs 1, 2 for reviews). Both integrative and excisive recombination require the phage-encoded protein Int and the host-encoded protein IHF (integration host factor)3,4. Additionally, efficient excision requires the phage-encoded protein Xis5,6. Although purified Int protein is a type I topoisomerase that nicks and reseals pBR322 supercoiled DNA one strand at a time3, no activity other than specific DNA binding has been found for purified IHF1,4.
The attP site has 240 base pairs (bp)7; it contains a 15bp ‘common core’ sequence (occurring in all four att sites)8, three binding sites for IHF1 and two classes of Int binding sites9,10. Essential DNA sequences to the left or right of the central ‘core region’ comprise the P arm and P’ arm respectively. (Similarly, in attB the essential left and right sequences are labelled B and B’ respectively.) The attB site is much smaller, extending approximately 5 bp to either side of the 15 bp common core11; it does not have any IHF recognition or arm-type Int binding sites. attB and attP do, however, have a similar junction-type Int binding site at each core-arm junction10.
Int-dependent recombination does not involve any degradation or synthesis of DNA nor does it require any high-energy cofactors4. During recombination, the DNA strands are cut within the common core at position −3/−2 (from the centre of the core) in the ‘top’ strand and at position +4/+5 in the ‘bottom’ strand, to generate a 3′-protruding 7 bp stagger or overlap region12, (referred to as O in POP’ and BOB’). Within the overlap region, DNA: DNA homology between the att sites is necessary for efficient recombination13. Genetic evidence suggests that at least some fraction of Int-dependent recombinants proceed via a single-strand exchange intermediate or Holliday structure14,15.
Holliday structures, also referred to as χ-forms, are formed by the reciprocal exchange of single strands between two DNA duplexes16–18. They are thought to be intermediates in several recombination pathways, and would be resolved to recombinant products by a second cycle of nicking and re-ligating19,20. χ-forms have been observed in Escherichia coli with phage21–24 and plasmid DNAs25, in yeast with 2 μ circle DNA26 and in HeLa cells with adenovirus DNA27. In the Int-dependent pathway of λ it has not yet been possible to detect χ-form recombination intermediates. We report here an investigation of this aspect of the recombination reaction by construction of ‘synthetic’ Holliday structures that are structurally analogous to a reciprocal single-strand exchange intermediate in site-specific recombination.
Generation of Holliday structures
In forming Holliday structures (Fig. 1), the two DNA helices are first aligned with respect to their common core sequences. The cutting and reciprocal exchange of the two ‘bottom’ strands results in form ExI, and of the ‘top’ strands, form ExII. (We identify the individual DNA strands in each helix as either ‘top’ or ‘bottom’ in accordance with the convention of writing the four att sites as POP’, BOB’, POB’ and BOP’; see Fig. 1.) In site-specific recombination, forms ExI and ExII are not conformational isomers—they cannot be interconverted without breakage and resealing of the DNA strands. This contrasts with Holliday structures generated in homologous recombination, where forms ExI and ExII are indistinguishable because both partners have the same DNA sequence.
Fig. 1.
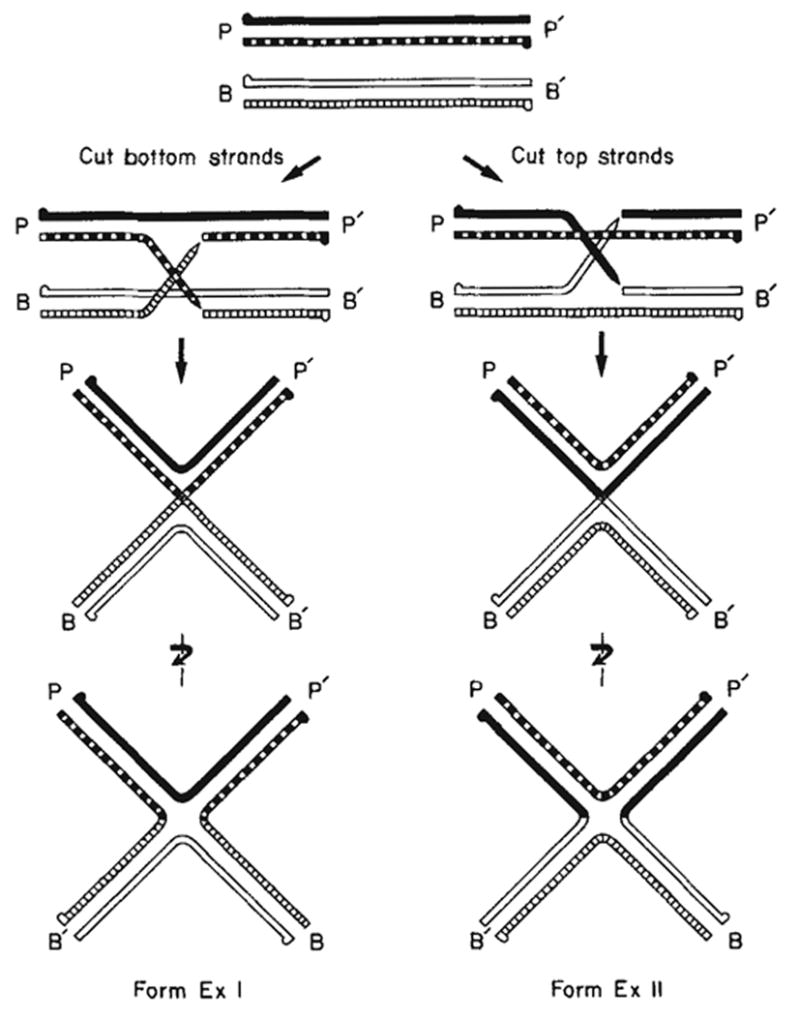
Formation of Holliday structures (χ-forms) during λ site-specific recombination. The attP top (
 ) and bottom (
) and bottom (
 ) strands and the attB top (
) strands and the attB top (
 ) and bottom (
) and bottom (
 ) strands have their 5′ termini indicated by a knob. The cutting and exchange of two bottom strands (left) results in form ExI, whereas the cutting and exchange of two top strands (right) results in form ExII. In the case where attL and attR are the parental DNAs (not shown here), the cutting and exchange of the two top strands would result in form ExI and the cutting and exchange of the two bottom strands would result in form ExII.
) strands have their 5′ termini indicated by a knob. The cutting and exchange of two bottom strands (left) results in form ExI, whereas the cutting and exchange of two top strands (right) results in form ExII. In the case where attL and attR are the parental DNAs (not shown here), the cutting and exchange of the two top strands would result in form ExI and the cutting and exchange of the two bottom strands would result in form ExII.
We constructed ‘artificial’ Holliday structures (that is, without recombination) from four restriction fragments containing the POP’, BOB’, BOP’ and POB’ DNA sequences. A mixture of the four restriction fragments was denatured and reannealed in a manner analogous to that first used by Bell and Byers26. During renaturation, some of the single strands anneal with their original complement to regenerate the original restriction fragment. Alternatively, annealing between two strands originating from different restriction fragments (such as POP’ and POB’) generates a ‘Y’ structure comprising one duplex arm (such as P) and two single-stranded tails (such as P’ and B’). Each of the single-stranded tails can further anneal with complementary sequences originating from a different restriction fragment, and this leads by analogous steps to formation of the four-armed Holliday structure (Fig. 2a).
Fig. 2.

Construction of forms ExI and ExII Holliday structures with a branch point in the common core region of the att site. Equivalent amounts of the four parental fragments, containing the POP’, BOP’, POB’ and BOB’ DNA sequences, were denatured and reannealed as described here, a, Schematic illustration of the stepwise formation of χ-structures during the reannealing process. Designations for the attP top (
 ) and bottom (
) and bottom (
 ) strands and the attB top (
) strands and the attB top (
 ) and bottom (
) and bottom (
 ) strands are also used to indicate the corresponding DNA strands in the prophage att sites BOP’ and POB’. The 5′ termini are marked with a knob. b, The ethidium bromide-stained agarose gel shows the four DNA restriction fragments before (lane 1) and after (lane 2) denaturation and after reannealing (lane 3). Electron micrograph shows DNA eluted from the gel band marked χ-form.
) strands are also used to indicate the corresponding DNA strands in the prophage att sites BOP’ and POB’. The 5′ termini are marked with a knob. b, The ethidium bromide-stained agarose gel shows the four DNA restriction fragments before (lane 1) and after (lane 2) denaturation and after reannealing (lane 3). Electron micrograph shows DNA eluted from the gel band marked χ-form.
Methods: 40-120 pmol of restriction fragments in 0.8-1.0 ml were denatured and reannealed by one of the following two procedures: (1), the DNAs were denatured by boiling at 100 °C for 2 min in 1 mM Tris-HCl (pH 7.9), 0.1 mM EDTA, followed by rapid chilling in ice water. One-tenth volume of 20 × SSC (3 M NaCl, 0.3 M Na citrate) was added and the mixture was placed in a 65 °C water bath that was then allowed to equilibrate to room temperature overnight. (2), DNA restriction fragments in low melting agarose were heated at 68 °C until the agarose was melted and then placed at 37 °C. They were then denatured in 0.1 M NaOH for 5 min; after adding ½ vol of deionized formamide and neutralizing with acetic acid, they were incubated at 37 °C overnight. The χ-forms were purified by gel electrophoresis in 1% agarose at 1.8 V cm−1 in E* buffer (18 mM NaCl, 2mM EDTA, 40 mM Tris, 20mM sodium acetate, adjusted to pH 8.0 with acetic acid). DNA was electroeluted from the excised gel band and purified by elution from a BND cellulose column as described previously42.
Forms ExI and ExII comprise two different sets of DNA strands; from each restriction fragment one DNA strand is found only in ExI, for example, the top strand of POP’, and the other strand is found only in ExII, for example, the bottom strand of POP’ (see Fig. 2a). Thus, by starting with a POP’ restriction fragment containing 32P in only the top strand or the bottom strand, it is possible to label specifically either ExI or ExII respectively. In an alternative procedure it is possible to start with the isolated strands of each restriction fragment; then the appropriate combination of four (out of the total of eight) can be annealed to generate either ExI or ExII.
The χ-structure has a higher molecular weight than any of the other DNAs in the annealing mixture and is readily purified by agarose gel electrophoresis on the basis of its unique mobility (Fig. 2b). To prove that the unique slow-migrating band is indeed χ-form DNA, we eluted it from the gel and examined it in the electron microscope. At least 90% of the DNA has the expected χ-structure and arm lengths (see Fig. 2c). Furthermore, if eluted DNA was subjected to a second cycle of denaturation and renaturation, the four initial restriction fragments were recovered as expected. Finally, in the resolution experiments described below we were able to convert 100% of the χ-form DNA preparation into those linear products expected from cutting at the branch points of a simple χ-form structure.
Resolution of Holliday structures
A critical test of whether the synthetic χ-structures do correspond to intermediates of site-specific recombination is the ability of Int protein to resolve them to the expected linear products. Theoretically a synthetic att site Holliday structure might be resolved in either the integrative mode, generating attL and attR (vertical cuts in ExI and ExII, Fig. 3) or in the excisive mode, generating attP and attB (horizontal cuts in ExI and ExII, Fig. 3). This resolution reaction is presumably equivalent to the second half of normal integrative or excisive recombination. In fact, in reaction conditions suitable for in vitro recombination, purified Int protein efficiently resolves both forms ExI and ExII into the expected linear products (Fig. 3).
Fig. 3.
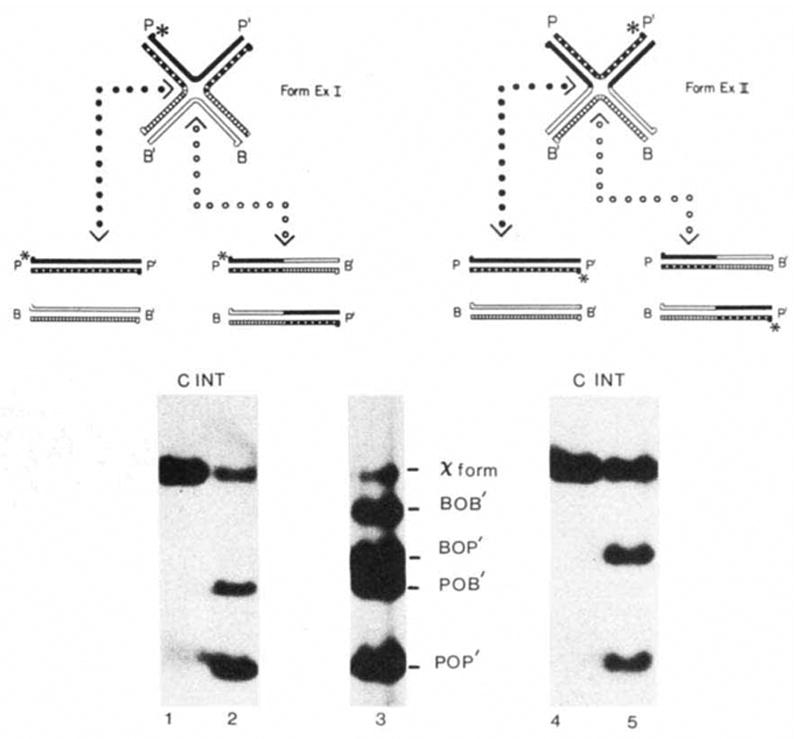
Resolution of forms ExI and ExII by Int protein. The strands of the four att sites and the 5 ′ termini are designated as in Fig. 2. The positions of the unique 32P label (*) in each χ-form that are expected after resolution are shown in the schematic diagram (see text). Lane 3 shows the positions of the χ-forms and the four restriction fragments used to make the χ-forms. Each labelled χ-form was incubated without (C) or with (INT) Int protein. Radioactive bands were visualized by autoradiography.
Methods: χ-Forms were constructed according to the first procedure described in Fig. 2 legend using the POP’ HmdIII-BamHI-492 fragment of pWR1, the BOB’ EcoRI-BamHI-1740 fragment of pWR101, the BOP’ EcoRI-BamHI-1210 fragment of pPH201 and the POB’ HindIII-BamHI-1020 fragment of pPH202 (Table 1). The POP’ restriction fragment was labelled before denaturation with [γ-32P]ATP and polynucleotide kinase at the HindIII site (for labelling form ExI) or at the Bam HI site (for labelling form ExII). Each reaction mixture (20 μl) contained 0.1 μg of 32P-labeIled form ExI or form ExII in 50 mM Tris-HCl (pH 7.9), 5 mM EDTA, 50 mM KC1 and 6.25 mM spermidine. Incubation with 1 U of Int protein (purified by N. Hasan) was carried out at 25 °C for 60 min. (In these reaction conditions 1 U of Int plus 1 U of purified IHF4,43 gives optimal recombination of 0.1 pmol supercoiled attP, pWRl, and 0.1 pmol of linear attB, pWR101.) The reactions were terminated with SDS (0.1% final) and electrophoresed on a 1% agarose gel as described for Fig. 2.
Each χ-form is labelled with only one 32P atom and, therefore, in each pair of products, only one will be radioactively labelled. Under these experimental conditions the resolution of form ExI yields approximately three times more attP than attR product, thus indicating a preference for the ‘excisive mode’ (cutting the bottom strands). On the other hand, resolution of form ExII yields approximately twice as much attL as attP product, thus indicating a preference for the ‘integrative mode’ (cutting the bottom strands). This is a good example of how an overall preference for cutting the bottom strand has opposite consequences for ExI (generating excision-type products) and ExII (generating integration-type products). The patterns and degree of preferential strand-cutting are significantly influenced by several experimental parameters (currently under investigation); this is a particularly interesting aspect of the reaction because of its relevance to directionality in site-specific recombination.
Following resolution, there is an additional band on alkaline denaturing gels, corresponding to full-length attR. for ExI, and full-length attL for ExII (data not shown). This transfer of label from a POP’ strand to the corresponding strand of a prophage att site indicates the successful ligation of DNA strands during resolution.
Specificity and factors
The strand exchange in λ site-specific recombination resembles the relaxation of superhelical DNA by certain topoisomerases (including the type I topoisomerase activity of Int protein) in that the DNA strands are broken and resealed in the absence of DNA synthesis and energy cofactors (see refs 28, 29 for reviews). This raises two points of interest: as the topoisomerase I activity of Int works efficiently with a substrate such as supercoiled pBR322 DNA3, can it resolve a Holliday structure that does not contain an att site? Similarly, is the Holliday structure resolved by other DNA topoisomerases?
To address these questions, we constructed a Holliday structure that bears no att site DNA sequences (Fig. 4). This structure is not resolved into linear products by Int protein, either in conditions for in vitro recombination or conditions for the nicking-closing reaction. The addition of host factor also has no effect. We also tested the ability of three different topoisomerases to resolve the att site Holliday structure (these enzymes were a gift from Leroy Liu), HeLa cell topoisomerase I29, T4 topoisomerase II30,31 and calf thymus topoisomerase II, whose purification and properties (L. F. Liu, unpublished results) are identical to HeLa topoisomerase II32. In conditions where these enzymes relaxed approximately 30–70% of supercoiled DNA, we observed no resolution of Holliday structure (data not shown). We conclude that, in these experimental conditions, the ability of Int protein to resolve a Holliday structure is specific for the att site DNA sequences and is not merely a general property of DNA topoisomerases.
Fig. 4.

Construction of a non-att Holliday structure. The HindIII fragment of λ DNA (
 ), which extends from 57.0% to 52.4% on the λ map and does not contain an att site, was cloned as a tandem duplication into the HindIII site of pBR322 (—), to generate plasmid pPH910. Three HinfI fragments of pPH910 were isolated for the χ-form construction: fragment II consists of the left pBR322-λ junction, fragment III of the head-to-tail junction of the two λ fragments, and fragment IV of the right λ-pBR322 junction. Fragment I is the pBR322 HinfI fragment into which the λ DNA had been inserted. For each fragment, and for each arm of the χ-structure, the amount (base pairs) of pBR322 DNA or λ DNA is indicated.
), which extends from 57.0% to 52.4% on the λ map and does not contain an att site, was cloned as a tandem duplication into the HindIII site of pBR322 (—), to generate plasmid pPH910. Three HinfI fragments of pPH910 were isolated for the χ-form construction: fragment II consists of the left pBR322-λ junction, fragment III of the head-to-tail junction of the two λ fragments, and fragment IV of the right λ-pBR322 junction. Fragment I is the pBR322 HinfI fragment into which the λ DNA had been inserted. For each fragment, and for each arm of the χ-structure, the amount (base pairs) of pBR322 DNA or λ DNA is indicated.
Methods: The χ-form was generated by alkali denaturation and renaturation according to scheme (2) and purified by agarose gel electrophoresis as described in Fig. 2 legend.
In an in vitro reaction, the integrative and excisive recombination functions of Int require the E. coli-encoded IHF protein and are strongly stimulated by the presence of spermidine. In contrast, the topoisomerase activity of Int does not require IHF protein and is inhibited by the addition of spermidine3. Int resolution of the Holliday structures is also independent of IHF protein (Fig. 3), resolving 95–100% of the Holliday structure to linear products in the absence of IHF. Int purified from the himA deletion mutant K5242 (ref. 33 and H. Miller, in preparation) is also efficient in resolution (data not shown). However, when limiting amounts of Int are used, IHF does stimulate resolution in a dose-dependent manner (Fig. 5). Spermidine inhibits resolution; omission of spermidine results in a three-to fivefold enhancement (Fig. 5). Thus, as might be expected, the requirements for the overall recombination reaction are greater than the requirements for the resolution phase.
Fig. 5.
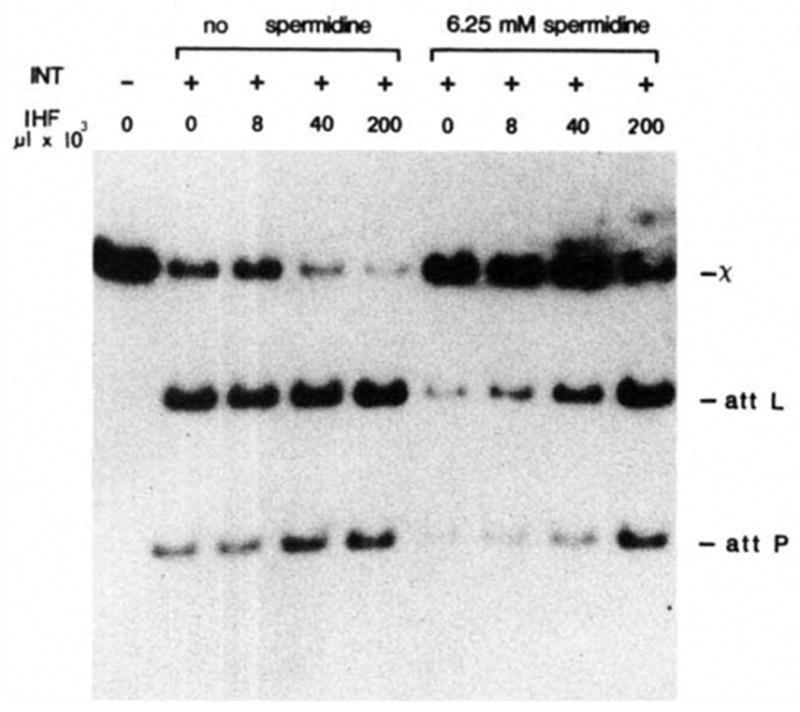
Effect of IHF protein and spermidine on resolution. 32P-labelled form ExII was constructed and isolated as described in Fig. 3 legend. The composition and processing of each resolution reaction was the same as described for Fig. 3 except that incubation with 0.2 U of purified Int protein was for 30 min. Purified IHF protein4 (a gift from H. Nash) at 1 U μl −1 and spermidine were present as indicated.
Structural requirements
We have shown that the arm-type Int binding sites are essential for integrative recombination. The role of these sites, which are quite distant from the cross-over locus, is unclear (see Fig. 6, bottom)7,9. Are they required throughout the reaction or only involved in some phases? If the latter, are they required for synapsis between the recombining partners and possibly the initial strand exchange, or are they required for resolution of the recombinational intermediate? To address these questions, we have constructed Holliday structures lacking the P arm and/or P’ arm Int binding sites and asked which, if any, of these is an acceptable substrate in the resolution reaction.
Fig. 6.

Int resolution of χ-forms lacking one or more arm-type Int binding sites. χ-forms composed of intact att sites (W.T.) or lacking the indicated arm-type Int binding site(s) (−P1, P2, etc.) were constructed from the appropriate HincII restriction fragments (Table 1, using the second procedure in Fig. 2). b, The attP coordinates for the 15 bp common core (
 ); the consensus recognition sequences (
); the consensus recognition sequences (
 ) and the approximate region of DNA covered (as determined by nuclease protection) for each of the following: the junction-type Int binding sites, C and C’ (
) and the approximate region of DNA covered (as determined by nuclease protection) for each of the following: the junction-type Int binding sites, C and C’ (
 ), the arm-type Int binding sites P1, P2, P’1, P’2, and P’3 (
), the arm-type Int binding sites P1, P2, P’1, P’2, and P’3 (
 )9,10, the IHF binding sites H1, H2 and H’ (
)9,10, the IHF binding sites H1, H2 and H’ (
 )1. The endpoints of att DNA in the three deletions used to construct the different χ-structures are indicated (−109, −89 and +46) (see Table 1). Each χ-form was incubated in the absence (−) or presence (+) of purified Int protein in a 20-μl reaction that contained ~0.01 μg of χ-forrn, 0.5 μg of supercoiled pBR322 DNA and 1 U of purified Int. After electrophoresis of the reaction products, gels were stained with ethidium bromide (0.5 μg ml−1) to visualize the nicking-closing activity of Int on the supercoiled pBR322 DNA. The expected levels of activity were observed in each case (data not shown). The 32P-labelled χ-forms and resolution products were visualized by autoradiography. The size (in base pairs) of each labelled resolution product is as follows: BB’, 940; BP’, 1,077; BΔ’, 886; PB’, 1,114; ΔB’, 972 (lane 4) and 952 (lanes 6 and 8). In this exposure of the autoradiogram, resolution products of similar size are not clearly resolved in several of the lanes; shorter exposures (and other gels) show that both resolution products are obtained in each reaction. The incomplete resolution observed in lane 6 of the upper gel is not typical; in other experiments in these conditions resolution is complete.
)1. The endpoints of att DNA in the three deletions used to construct the different χ-structures are indicated (−109, −89 and +46) (see Table 1). Each χ-form was incubated in the absence (−) or presence (+) of purified Int protein in a 20-μl reaction that contained ~0.01 μg of χ-forrn, 0.5 μg of supercoiled pBR322 DNA and 1 U of purified Int. After electrophoresis of the reaction products, gels were stained with ethidium bromide (0.5 μg ml−1) to visualize the nicking-closing activity of Int on the supercoiled pBR322 DNA. The expected levels of activity were observed in each case (data not shown). The 32P-labelled χ-forms and resolution products were visualized by autoradiography. The size (in base pairs) of each labelled resolution product is as follows: BB’, 940; BP’, 1,077; BΔ’, 886; PB’, 1,114; ΔB’, 972 (lane 4) and 952 (lanes 6 and 8). In this exposure of the autoradiogram, resolution products of similar size are not clearly resolved in several of the lanes; shorter exposures (and other gels) show that both resolution products are obtained in each reaction. The incomplete resolution observed in lane 6 of the upper gel is not typical; in other experiments in these conditions resolution is complete.
Methods: Reaction conditions, processing and gel electrophoresis were as for Fig. 3, with no spermidine. For 32P labelling of forms ExI and ExII, the BOB’ HincII fragment was first labelled at the 5′ termini with polynucleotide kinase and then strand-separated by gel electrophoresis. The top strand of BOB’ was incorporated into form ExI and the bottom strand into form ExII (see Fig. 2) by annealing with the other three restriction fragments (Table 1). The specific activity is the same for the entire form ExI family (or the entire form ExII family), because the 32P label is always on the BOB’ strand which is common to all five χ-forms (see Table 1).
Holliday structures containing various deletions of the P and/or P’ arms were constructed by annealing a 32P-labelled separated strand of BOB’ with different combinations of the appropriate restriction fragments. Two families of χ-forms were constructed, representing forms ExI and ExII for each deletion analysed (Table 1). The restriction fragments and constructions were designed so that all of the arms in every χ-structure are perfect duplexes over their entire length.
Table 1.
Construction of different χ-form structures
| χ-Forms Arm sites deleted
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −P1 | ||||||||||
| −P1 | −P1 | −P2 | ||||||||
| Intact | −P2 | −P2 | −P’ | −P’ | Plasmid | att | P arm or Δ arm | P’ arm or Δ’ arm | B arm | B’ arm |
| + | pWR1 | PP’ | −251(734) | +242(517) | ||||||
| + | pPH55 | ΔP’ | −109(592) | +242(517) | ||||||
| + | pPH56 | ΔP’ | −89(572) | +242(517) | ||||||
| + | pPH310 | PΔ’ | −251(734) | +46(326) | ||||||
| + | pPH5610 | ΔΔ’ | −89(572) | +46(326) | ||||||
| + | + | + | pPH201 | BP’ | +242(517) | −560 | ||||
| + | + | pPH110 | BΔ’ | +46(326) | −560 | |||||
| + | + | pPH202 | PB’ | −251(734) | +380 | |||||
| + | pPH551 | ΔB’ | −109(592) | +380 | ||||||
| + | + | pPH561 | ΔB’ | −89(572) | +380 | |||||
| + | + | + | + | + | pWR101 | BB’ | −560 | +380 | ||
χ-Forms with intact att sites or lacking one or more of the arm-type Int binding sites (as labelled), were constructed using the indicated ( + ) restriction fragments, one from each of the four classes (see Fig. 6). For example, to make a Holliday structure lacking the P1 and P2 sites, an isolated BOB’ strand from pWR101 was annealed with the ΔOB’-containing fragment of pPH551, the BOP’-containing fragment of pPH201 and the ΔOP’-containing fragment of pPH55. The plasmids from which the att site restriction fragments (HincII) are isolated are shown. The construction att sites (pWR1, pWR101, pPH201 and pPH202), or attP with distal deletions of the P and P’ arms (pPH55, pPH56, pPH310), has been described previously7. Transfer of the attP deletions to the other att sites was as follows: Int-dependent in vitro recombination was carried out (with an extract containing Xis) on head-to-tail heterodimer plasmids containing ΔOP’ and POB’; to make pPH551, the dimer consisted of pPH55 and pPH202, to make pPH561, the dimer consisted of pPH56 and pPH202. After recombination the DNA was digested with EcoRI, circularized and transformed into HB101. Plasmids containing the recombinant ΔOB’ site were identified by their restriction digestion patterns. For plasmids pPH110 (BOΔ’) and pPH5610 (ΔOΔ’) the Δ’ arm is the Dde-BamHI fragment from pPH310. It was ligated through the Dde site in the core to the appropriate EcoRI-Dde fragment, from pPH201 for the B arm or from pPH56 for the Δ arm. The four att site arms are denoted P, P’, B and B’ as described in the text; any change (deletion) in one of the phage att site arms is denoted as Δ (corresponding to P) or Δ’ (corresponding to P’). The extent of phage att site sequence is indicated in base pairs from the centre of the core extending left (−) or right (+) (using the conventions of ref. 8) (see Fig. 6). The HincII restriction fragments chosen for these constructions also contain neighbouring pBR322 DNA, thus making it possible to generate physically longer (fully duplex) arms in the final χ-structure; numbers in parentheses indicate the total amount of DNA (phage DNA plus pBR322 DNA) in that arm of the χ-form. DNA from the region around the bacterial att site is indicated in base pairs (approximate) from the centre of the core extending left (−) or right (+)8; these numbers also indicate the total amount of DNA in that arm of the χ-form (that is, these arms do not have pBR322 sequences).
In any one series of experiments (see Fig. 6), all of the χ- forms have the same specific activity. This is because the single 32P atom in each χ-structure is donated by the same BOB’ restriction fragment which has been end-labelled and strand-separated before being distributed into the respective annealing mixtures. This protocol facilitates the comparison of resolution efficiencies under identical Int:DNA ratios. As an additional (internal) control, supercoiled pBR322 DNA was added to each reaction mixture to monitor the topoisomerase activity of Int.
In a 1-h incubation with χ-form, pBR322 supercoiled DNA and Int (present in a molar ratio of 1: 20: 30), approximately 70% of the supercoiled molecules are relaxed in all of the reaction mixtures. Concomitantly, Int protein resolves all of the Holliday structures with 95–100% efficiency, irrespective of the presence or absence of the arm-type Int binding sites (Fig. 6). For all Holliday structures in this form ExI family, resolution occurs in both the integrative and excisive modes. Similar results and efficiencies are obtained with the analogous family of form ExII Holliday structures. When the concentration of Int is lowered (χ-form: pBR322: Int at 1: 20: 10) so that χ-form resolution is reduced to ~30%, the resolution efficiency of Holliday structures with each of the deletions is still the same as the non-deleted form (data not shown). We conclude that the arm-type Int binding sites that are required for integrative recombination are dispensable for the resolution of Holliday structures resembling the cross-over region of recombining att sites.
Discussion
Does Int-dependent site-specific recombination proceed by a concerted mechanism in which both DNA strands on each partner are cut at the same time and then exchanged and re-ligated, or does it proceed in two distinct steps where first, only one strand on each partner is exchanged to generate a Holliday structure, and second, the single-strand exchange intermediate is resolved by a second cycle of nicking and re-ligation?
We have shown here that Holliday structures with their branch points in the common core region of the att site are efficiently resolved by purified Int protein. In a mixed recombination reaction containing both χ-form (0.01 pmol) and attP and attB (0.1 pmol each), 1 U of Int and IHF give ~30% recombination between the att sites and ~90% resolution of the χ-form (data not shown). It seems highly unlikely that this unique substrate would be utilized in such a specific and efficient manner if it were not part of the normal recombination pathway. We infer that Int-dependent recombination most probably proceeds through a single-strand exchange Holliday intermediate. Resolution of this intermediate is probably very closely coupled with its formation, as suggested by the efficiency of χ-form resolution, the low frequency of branch migration observed in in vivo crosses14,15 and the failure to find such forms accumulating during recombination. A mechanism that exchanges DNA strands one pair at a time is compatible with the type I topoisomerase activity of Int, and with the four-stranded DNA structure that has been proposed for synapsis3,34.
The finding that purified Int protein efficiently resolves the Holliday intermediate in the absence of IHF (or Xis) substantiates its role in executing the cutting and ligating reactions of recombination3,4,12,35. Because IHF does not participate directly in the strand exchange reaction (resolution), its obligatory participation in integrative recombination is probably due to a role in the formation of Int-att complexes or in the synapsis of two att sites.
A striking feature of the Int resolution reaction is its specificity for att site DNA—in particular for the Int recognition sites at the core-arm junctions. Holliday structures lacking att site sequences are not resolved by Int. Of the known enzymes, only the T4-encoded endonuclease VII has also been shown to nick specifically at the branch points of χ-structures36. In contrast to the Int reaction, however, endonuclease VII is not sequence specific and does not appear to reseal the nicked DNA without the aid of DNA ligase.
We have shown here that resolution is efficient even with a Holliday structure lacking all the Int binding sites in the P and P’ arms and containing only the four Int binding sites at the core-arm junctions (Fig. 6). Because a Holliday structure lacking the junction-type sites is not resolved by Int (Fig. 4), we conclude that this family of Int recognition sites (C, C’, B and B’) is both necessary and sufficient for cutting and re-ligating DNA. The corollary of this conclusion is that the Int binding sites in the arms, although required for attP recombination, are not required for the cutting and re-ligation reaction. This is consistent with the finding that a single core-type Int binding sequence, even in non-att DNA, is sufficient for nicking by Int protein (ref. 35 and W. Ross and A.L., unpublished results). We infer that the Int sites in the arms function in synapsis of the recombining sites and/or formation of a presynaptic multimeric complex involving attP and the necessary proteins. In this context it is interesting that higher order complexes involving attP and approximately 10 Int monomers have been observed in the electron microscope37,38 and nuclease digestion patterns7,9,39 and topological transformations during recombination40,41 suggest a wrapping of DNA around, or along the surface of, Int protein(s).
We have shown here that both forms ExI and ExII can be resolved in the integrative mode (producing attL and attR) or in the excisive mode (producing attP and attB). This suggests that in these experimental conditions either form ExI or form ExII could serve as an intermediate of either integrative or excisive recombination. Furthermore, as resolution can occur by cutting either pair of strands, there is the possibility that some attempts at recombination are abortive: if the same pair of strands is used for both the first and second cross-over, the original parents are restored. Thus, the overall efficiency of recombination between any two att sites could be determined not only by the formation of Holliday intermediates but also by the directionality of their resolution. In our experiments the relative efficiency of resolution was not the same in both directions, with either form ExI or form ExII. It will be interesting to determine how this directionality is influenced by reaction conditions and the various components of the recombination reaction.
Acknowledgments
We thank Noaman Hasan for purified Int protein, Howard Nash for purified IHF protein, Leroy Liu for the topoisomerases, David Straney for the electron microscopy and Breck Beyers for helpful advice. Lina Vargas and Shirley Rodrigues provided technical assistance. This work was supported by NIH grant AI13544.
References
- 1.Nash HAA. Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg R, Landy A. In: Lambda II. Hendrix RW, Roberts JW, Stahl FW, Weisberg RW, editors. Cold Spring Harbor Laboratory; New York: 1983. pp. 211–250. [Google Scholar]
- 3.Kikuchi A, Nash HA. Proc natn Acad Sci USA. 1979;76:3760–3764. doi: 10.1073/pnas.76.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash HA, Robertson CA. J biol Chem. 1981;256:9246–9253. [PubMed] [Google Scholar]
- 5.Abremski K, Gottesman S. J biol Chem. 1982;257:9658–9710. [PubMed] [Google Scholar]
- 6.Better M, Wickner S, Auerback J, Williams R, Echols H. Cell. 1983;32:161–168. doi: 10.1016/0092-8674(83)90506-8. [DOI] [PubMed] [Google Scholar]
- 7.Hsu PL, Ross W, Landy A. Nature. 1980;285:85–91. doi: 10.1038/285085a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landy A, Ross W. Science. 1977;197:1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross W, Landy A. Proc natn Acad Sci USA. 1982;79:7724–7728. doi: 10.1073/pnas.79.24.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross W, Landy A. Cell. 1983;33:261–272. doi: 10.1016/0092-8674(83)90355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuuchi M, Mizuuchi K. Proc natn Acad Sci USA. 1980;77:3220–3224. doi: 10.1073/pnas.77.6.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuuchi K, et al. Cold Spring Harb Symp quant Biol. 1981;45:429–437. doi: 10.1101/sqb.1981.045.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg R, Enquist L, Foeller C, Landy A. J molec Biol. 1983;170:319–342. doi: 10.1016/s0022-2836(83)80151-x. [DOI] [PubMed] [Google Scholar]
- 14.Enquist LW, Nash HA, Weisberg RA. Proc natn Acad Sci USA. 1979;76:1363–1367. doi: 10.1073/pnas.76.3.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echols H, Green L. Genetics. 1979;93:297–307. doi: 10.1093/genetics/93.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holliday R. Genet Res. 1964;5:282–304. [Google Scholar]
- 17.Sigal N, Alberts B. J molec Biol. 1972;71:789–793. doi: 10.1016/s0022-2836(72)80039-1. [DOI] [PubMed] [Google Scholar]
- 18.Holliday R. Genetics. 1974;78:273–287. doi: 10.1093/genetics/78.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dressler E, Potter H. A Rev Biochem. 1982;51:727–761. doi: 10.1146/annurev.bi.51.070182.003455. [DOI] [PubMed] [Google Scholar]
- 20.Stahl F. Genetic Recombination: Thinking about it in Phage and Fungi. Freeman; San Francisco: 1979. [Google Scholar]
- 21.Thompson B, et al. J molec Biol. 1975;91:409–419. doi: 10.1016/0022-2836(75)90269-7. [DOI] [PubMed] [Google Scholar]
- 22.Benbow R, Zuccavelli A, Sinsheimer R. Proc natn Acad Sci USA. 1975;72:235–239. doi: 10.1073/pnas.72.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenzuela M, Inman R. Proc natn Acad Sci USA. 1975;72:3024–3028. doi: 10.1073/pnas.72.8.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson BJ, Camien MN, Warner RC. Proc natn Acad Sci USA. 1976;73:2299–2303. doi: 10.1073/pnas.73.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter H, Dressler D. Proc natn Acad Sci USA. 1976;73:3000–3004. doi: 10.1073/pnas.73.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell L, Byers B. Proc natn Acad Sci USA. 1979;76:3445–3449. doi: 10.1073/pnas.76.7.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolgemuth DJ, Hsu MT. Nature. 1980;287:168–171. doi: 10.1038/287168a0. [DOI] [PubMed] [Google Scholar]
- 28.Cozzarelli NR. Science. 1980;207:953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- 29.Gellert MA. Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- 30.Liu LF, Miller KG. Proc natn Acad Sci USA. 1981;78:3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu LF, Liu CC, Alberts BM. Nature. 1979;281:456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- 32.Miller KG, Liu LF, Englund PT. J biol Chem. 1981;256:9334–9339. [PubMed] [Google Scholar]
- 33.Miller HI, Friedman DI. Cell. 1980;20:711–719. doi: 10.1016/0092-8674(80)90317-7. [DOI] [PubMed] [Google Scholar]
- 34.Nash HA, Mizuuchi K, Enquist LW, Weisberg RA. Cold Spring Harb Symp quant Biol. 1981;45:417–428. doi: 10.1101/sqb.1981.045.01.056. [DOI] [PubMed] [Google Scholar]
- 35.Craig N, Nash H. Cell. 1983;35:795–803. doi: 10.1016/0092-8674(83)90112-5. [DOI] [PubMed] [Google Scholar]
- 36.Mizuuchi K, Kemper B, Hays J, Weisberg R. Cell. 1982;29:357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton P, Yuan R, Kikuchi Y. J molec Biol. 1981;152:163–170. doi: 10.1016/0022-2836(81)90100-5. [DOI] [PubMed] [Google Scholar]
- 38.Better M, Chi L, Williams RC, Echols H. Proc natn Acad Sci USA. 1982;79:5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies RW, Schreier PH, Kotewicz ML, Echols H. Nucleic Acids Res. 1979;7:2255–2273. doi: 10.1093/nar/7.8.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nash H, Pollock T. J molec Biol. 1983;170:19–38. doi: 10.1016/s0022-2836(83)80225-3. [DOI] [PubMed] [Google Scholar]
- 41.Pollock T, Nash H. J molec Biol. 1983;170:1–18. doi: 10.1016/s0022-2836(83)80224-1. [DOI] [PubMed] [Google Scholar]
- 42.Rossi JJ, Ross W, Egan J, Lipman DJ, Landy A. J molec Biol. 1979;128:21–47. doi: 10.1016/0022-2836(79)90307-3. [DOI] [PubMed] [Google Scholar]
- 43.Nash HA. Meth Enzym. 1983;100:210–216. doi: 10.1016/0076-6879(83)00057-9. [DOI] [PubMed] [Google Scholar]


