Abstract
Mitogen-activated protein kinases are crucial regulators of various cell fate decisions including proliferation, differentiation, and apoptosis. Depending on the cellular context, the Raf-Mek-Erk mitogen-activated protein kinase cascade responds to extracellular stimuli in an all-or-none manner, most likely due to bistable behavior. Here, we describe a previously unrecognized positive-feedback mechanism that emerges from experimentally observed sequestration effects in the core Raf-Mek-Erk cascade. Unphosphorylated/monophosphorylated Erk sequesters Mek into Raf-inaccessible complexes upon weak stimulation, and thereby inhibits cascade activation. Mek, once phosphorylated by Raf, triggers Erk phosphorylation, which in turn induces dissociation of Raf-inaccessible Mek-Erk heterodimers, and thus further amplifies Mek phosphorylation. We show that this positive circuit can bring about bistability for parameter values measured experimentally in living cells. Previous studies revealed that bistability can also arise from enzyme depletion effects in the Erk double (de)phosphorylation cycle. We demonstrate that the feedback mechanism proposed in this article synergizes with such enzyme depletion effects to bring about a much larger bistable range than either mechanism alone. Our results show that stable docking interactions and competition effects, which are common in protein kinase cascades, can result in sequestration-based feedback, and thus can have profound effects on the qualitative behavior of signaling pathways.
INTRODUCTION
The three-tiered mitogen-activated protein kinase (MAPK) pathways are known to be crucial regulators of various physiological processes such as proliferation, differentiation, senescence, and apoptosis (1). Cell-fate decisions such as differentiation are thought to occur in an all-or-none fashion and, once initiated, should be stably maintained in an irreversible manner. Theoretical and experimental work (2) suggest that such switchlike and irreversible signal transduction could arise due to bistability at the level of MAPK activation.
Single-cell measurements confirm that both the Raf-Mek-Erk pathway and the JNK cascade are indeed activated in an all-or-none manner in Xenopus oocytes (3,4). Additionally, switchlike activation was recently shown to occur in the yeast mating MAPK signaling module (5,6). In mammalian systems, all-or-none activation of the Raf-Mek-Erk pathway was observed in T cells (7), in BHK cells (8), in PC12-D2R cells (9), in dopaminergic SN4741 neurons (9), and in Hek 293 cells (Boris Kholodenko, Thomas Jefferson University, personal communication, 2007). In contrast, gradual MAPK activation at the single-cell level was seen in growth-factor-stimulated Swiss 3T3 fibroblasts (10), in HeLa cells (11), and in human foreskin fibroblasts (11). The qualitative behavior of Erk activation can also depend on the stimulus strength, with all-or-none activation at weak stimulation, but gradual activation upon strong stimulation in LβT2 gonadotrope cells (12). Finally, the opposite, i.e., a gradual response upon weak stimulation and all-or-none activation at high stimulus levels, was reported to occur in PC12 cells (13).
These single-cell measurements reveal that MAPK cascades frequently exhibit all-or-none behavior over a broad range of stimulus concentrations, which suggests that these pathways can indeed be bistable under physiological conditions. Bistable systems display hysteresis, meaning that different stimulus-response curves are obtained depending upon whether the system began in the off or on state (14,15). Experimental studies confirmed hysteresis for the JNK cascade in Xenopus oocytes (3) and for the Raf-Mek-Erk pathway in PC12 cells (13).
Bistability is thought to require a positive signaling circuit, which may be established either by feedback activation of upstream pathway intermediates or by relief from upstream pathway inhibition (14,15). All-or-none activation in the Mos-Mek-Erk MAPK cascade was indeed shown to depend on feedback pathway activation in Xenopus oocytes: Active phospho-Erk stimulates transcription and thereby upregulation of the constitutively active Raf homolog Mos, which is the uppermost member of the cascade (4). It has been proposed that similar positive feedback loops, which rely on Erk-dependent Raf activation, exist in mammalian cells (2,16).
However, direct experimental evidence for functionally significant positive feedback loops is scarce. Transfection with constitutively active Mek seemed to activate Raf-1 in NIH3T3 cells (17) and in Hek 293 cells (18). In contrast, constitutively active mutants of Raf, Mek, or Erk failed to activate their endogenous counterparts when exogenously expressed in C7 3T3 cells (Raf (19)), in BHK cells (Mek (8)), in Hek 293 cells (Erk (20)), in PC12 cells (Erk (20), and in Cos7 cells (Erk (21)). These data suggest that functionally relevant positive-feedback activation is the exception rather than the rule in the mammalian Raf-Mek-Erk pathway. Additionally, positive feedback activation does not seem to correlate with all-or-none Erk activation at the single-cell level, and is therefore unlikely to account for bistable behavior in the mammalian MAPK cascade.
Instead, these overexpression data support a model where bistability arises from a positive-feedback circuit that relies on elimination of upstream cascade inhibition, and not on upstream cascade activation. Such relief from inhibition is expected to be insufficient for full pathway activation in the absence of upstream input signals, and would thereby explain why overexpressed constitutively active mutants of Raf, Mek, or Erk failed to activate their endogenous counterparts. A recent study suggests that such relief from upstream inhibition occurs downstream of Raf kinase, i.e., within the core MAPK cascade: All-or-none activation of the MAPK cascade was observed even if cascade activation was triggered by an exogenously expressed Raf construct, which would most likely overcome endogenous feedback mechanisms acting upstream of Raf (8).
Recent theoretical studies indicate that implicit feedback and bistability can indeed arise in the core MAPK signaling module. Markevich et al. (22) described how relief from inhibition and hysteresis emerge in the basic motif of MAPK cascades, the double phosphorylation cycle, if realistic kinetic parameters are assumed. Additionally, we reported (23) that bistability due to relief from inhibition can be observed if two consecutive cascade members (e.g., Mek and Erk) are deactivated by the same phosphatase. This “shared phosphatase motif” applies for the mammalian Erk-MAPK signaling module, as PP2A was reported to dephosphorylate both Mek and Erk (24). However, these implicit mechanisms exhibit a relatively narrow range of bistability, and might thereby require amplification to bring about robust hysteresis in vivo.
In this article, we identify a previously unrecognized relief-from-inhibition feedback mechanism in the core Raf-Mek-Erk cascade, which might mediate such amplification. It is proposed that unphosphorylated/monophosphorylated Erk sequesters Mek into Raf-inaccessible complexes upon weak stimulation, and thereby inhibits the cascade. Mek, once phosphorylated by Raf, triggers Erk phosphorylation, which in turn induces dissociation of Raf-inaccessible Mek-Erk heterodimers (relief from inhibition), and thus further stimulates Mek phosphorylation. The suggested mechanism is in accord with experimental studies, which showed that Mek and Erk form a stable complex under resting conditions, and dissociate upon Erk phosphorylation (see, e.g., Adachi et al. (25)). We show that this positive circuit can bring about bistability for parameter values that were experimentally measured in living cells (26). Additionally, we demonstrate that the feedback mechanism proposed in this article synergizes with that implicit in double phosphorylation (22) to bring about a much larger bistable range than either mechanism achieves alone.
RESULTS AND DISCUSSION
Rationale
Experimental studies revealed that unphosphorylated Mek and Erk form a stable complex in unstimulated cells, which dissociates upon stimulation with growth factors (see, e.g., Adachi et al. (25)). Such basal Mek-Erk association has been neglected in most mathematical models of the MAPK cascade. Therefore, we study the impact of Mek-Erk complex formation in more detail.
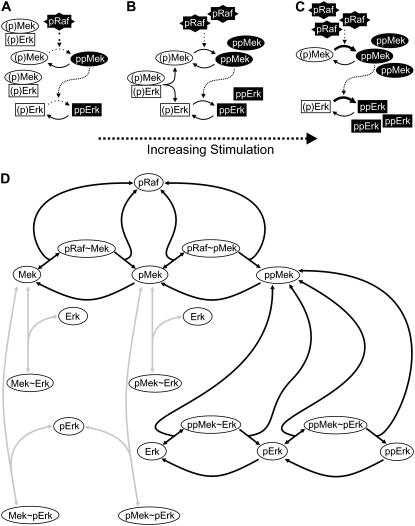
Fig. 1, A–C, shows how a positive-feedback circuit can arise in the core Raf-Mek-Erk cascade due to basal Mek-Erk association. At low levels of the phospho-Raf stimulus, Mek and Erk are mostly nonphosphorylated or monophosphorylated (i.e., inactive), as indicated by the white boxes ((p)Mek and (p)Erk) in Fig. 1 A. Stable heterodimer formation between inactive Mek and Erk molecules efficiently inhibits Raf-mediated Mek phosphorylation, and thereby suppresses pathway activation. Stronger stimulation results in the formation of some fully phosphorylated Mek and Erk, as indicated by the black boxes (ppMek and ppErk) in Fig. 1 B. This depletes nonphosphorylated/monophosphorylated Erk pools, and thereby promotes the dissociation of the Raf-inaccessible (p)Mek-(p)Erk complexes (relief from inhibition). In other words, Mek, once fully phosphorylated, triggers the release of monomeric, Raf-accessible Mek and hence further Mek phosphorylation in a positive-feedback circuit (Fig. 1 B). Upon sufficiently strong stimulation, all (p)Mek-(p)Erk are dissociated and almost all Mek and Erk molecules are fully phosphorylated (Fig. 1 C).
FIGURE 1.
Proposed bistability mechanism and model structure. (A–C) Schematic representation of the proposed bistability mechanism. Upon weak stimulation (i.e., at low pRaf levels), Erk and Mek are mostly unphosphorylated/monophosphorylated (indicated by white boxes), and pathway activation is suppressed by Mek sequestration into Raf-inaccessible (p)Mek-(p)Erk heterodimers (A). Stronger stimulation increases the amount of double phosphorylated ppMek, which then triggers Erk double phosphorylation (B). Erk double phosphorylation in turn induces dissociation of Raf-inaccessible Mek-Erk heterodimers (relief from inhibition), and thus amplifies Mek phosphorylation in a positive-feedback circuit (B), so that finally the pathway is completely activated (C). (D) Schematic representation of model topology (see Supplementary Material for differential equations). The black arrows indicate the previously described “basic model” that includes Raf-mediated Mek phosphorylation, Mek-mediated Erk phosphorylation, and the antagonizing phosphatase reactions (27). The “sequestration model” analyzed in this article additionally includes association of various Mek species with unphosphorylated/monophosphorylated Erk (gray arrows), and the resulting Mek sequestration complexes (i.e., Mek-Erk, Mek-pErk, pMek-Erk, and pMek-pErk).
Based on previous studies (22,23), we reasoned that this relief-from-inhibition feedback mechanism could result in bistability, and asked whether hysteresis can be observed in physiologically relevant parameter ranges.
Model implementation
We implemented a mathematical model of the core MAPK signaling module, which is schematically depicted in Fig. 1 D (see Supplementary Material for differential equations). Here, the black arrows indicate the previously described MAPK model (referred to as “basic model” hereafter) that includes Raf-mediated Mek phosphorylation, Mek-mediated Erk phosphorylation, and the antagonizing phosphatase reactions (27). For simplicity, we assumed that the phosphatases for Mek and Erk are less abundant than their substrates, and thus modeled the corresponding phosphatase reactions using the Michaelis-Menten approximation. In contrast, kinase catalysis was modeled using the elementary step description to take possible sequestration and back-propagation effects into account (28–30). Experimental studies revealed that Mek and Erk form stable heterodimers under basal conditions, and that these heterodimers dissociate upon stimulus-induced Erk phosphorylation (25). Therefore, we also considered association of unphosphorylated/monophosphorylated Erk (but not of bisphosphorylated Erk) with various Mek species, as indicated by the gray arrows in Fig. 1 D, and termed this the “sequestration model”.
To demonstrate that the proposed feedback mechanism is in fact responsible for bistability in the sequestration model, we excluded other possible sources of hysteresis in our simulations (except for the results shown below (see Fig. 5)). First, we excluded positive feedback that could arise if both the Mek and the Erk cycles were deactivated by the same phosphatase (23). This was accomplished by assuming that Mek and Erk proteins are dephosphorylated by different phosphatases. Second, we excluded that bistability implicitly arises in double phosphorylation (22,31) by assuming that in our model similar reaction steps are characterized by the same kinetics. For example, the same kinetic parameters were assumed for the phosphorylation of the first and second modification sites in Mek (“noncooperative phosphorylation”). Similar noncooperativity was also assumed for Mek dephosphorylation, Erk phosphorylation, and Erk dephosphorylation. Finally, we assumed the same association and dissociation rate constants for all Mek-Erk complexes (i.e., Mek-Erk, pMek-Erk, ppMek-Erk, Mek-pErk, pMek-pErk, ppMek-pErk). The resulting model comprised 10 kinetic parameters and three total protein concentrations (pRaf, Mek, and Erk), all of which could be taken from a recent quantitative study by Fujioka et al. (26) (see Table 1).
FIGURE 5.
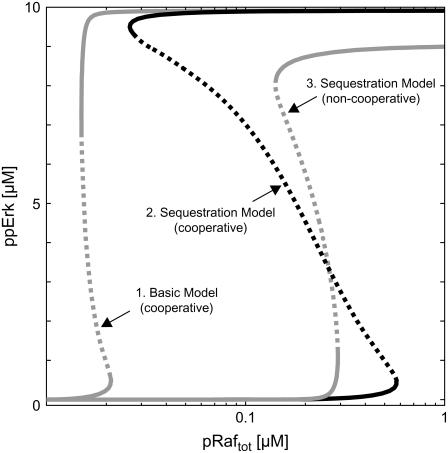
Synergism of bistability mechanisms. A broad range of bistability is observed in the stimulus-response (curve 2) of the sequestration model (Fig. 1 D, black and gray arrows) if the second step of Mek-mediated Erk phosphorylation proceeds faster than the first (“positive cooperativity”). Such pronounced hysteresis can be explained by synergism of the feedback mechanism discussed in this article with that described by Markevich et al. (22), which arises from enzyme depletion effects in the Erk cycle. The gray lines correspond to the stimulus-response curves of reduced models, where one of the two feedback mechanisms was eliminated, and thereby directly demonstrate such synergism. Curve 1 depicts the stimulus response of the basic model (Fig. 1 D, black arrows), which is devoid of Mek sequestration into Raf-inaccessible complexes. Curve 3 corresponds to a sequestration model (Fig. 1 D, black and gray arrows), where positive cooperativity and enzyme-depletion effects in the Erk cycle are eliminated. See Supplementary Material for differential equations and kinetic parameters.
TABLE 1.
Kinetic parameters
| Parameter | Notes | Fujioka et al. | This study |
|---|---|---|---|
| Mektot | Total cellular Mek concentration | 1.4 μM | 1 μM |
| Erktot | Total cellular Erk concentration | 0.96 μM | 10 μM |
| kon,Raf-Mek | Association rate constant of Raf-Mek complex | 0.65 μM−1 s−1 | 0.65 μM−1 s−1 |
| koff,Raf-Mek | Dissociation rate constant of Raf-Mek complex | 0.065 s−1 | 0.065 s−1 |
| kcat,Raf-Mek | Catalytic turnover constant of Raf-Mek complex | 0.18 s−1 | 0.18 s−1 |
| (Vmax/KM)Mek-PPase | First-order rate constant of Mek-phosphatase | 0.01 s−1 | (0.01 s−1) |
| Vmax,Mek-PPase | Maximal velocity of Mek-phosphatase | — | 0.001 μM s−1 |
| KM,Mek-PPase | Michaelis-Menten constant of Mek-phosphatase | — | 0.1 μM |
| kon,Mek-Erk | Association rate constant of Mek-Erk complex | 0.88 μM−1 s−1 | 0.88 μM−1 s−1 |
| koff,Mek-Erk | Dissociation rate constant of Mek-Erk complex | 0.088 s−1 | 0.088 s−1 |
| kcat,Mek-Erk | Catalytic turnover constant of Mek-Erk complex | 0.22 s−1 | 0.22 s−1 |
| (Vmax/KM)Erk-PPase | First-order rate constant of Erk-phosphatase | 0.014 s−1 | (0.08 s−1) |
| Vmax,Erk-PPase | Maximal velocity of Erk-phosphatase | — | 0.04 μM s−1 |
| KM,Erk-PPase | Michaelis-Menten constant of Erk-phosphatase | — | 0.5 μM |
The total protein concentrations and kinetic parameters of the model depicted in Fig. 1 D are listed under the heading “This study”, and compared to the values measured by Fujioka et al. (26). Fujioka et al. estimated the apparent first-order rate constant (Vmax/KM) of Mek and Erk dephosphorylation only. We assumed saturated Michaelis-Menten kinetics in the model, because 1), the time course data in Fujioka et al. (26) indicates saturation in the dephosphorylation reactions; and 2), the KM values of phosphatases toward full-length substrates are frequently in the submicromolar range (52,53). See Supplementary Material for differential equations of the model.
Bistability due to Mek sequestration
Single-cell analyses revealed that the MAPK cascade can respond to extracellular stimuli in an all-or-none manner, most likely due to bistable behavior of the system (see Introduction). We simulated such extracellular stimulation by varying the total concentration of active Raf protein (pRaf), and analyzed the amount of doubly phosphorylated, active Erk (ppErk) as the response. The system showed a simple, monostable stimulus response (not shown) when simulations were run using the kinetic parameters and the protein concentrations, which were measured by Fujioka et al. (26) in HeLa cells (Table 1). This result is in accordance with experimental measurements, as gradual Erk activation at the single-cell level was demonstrated in EGF-treated HeLa cells (11).
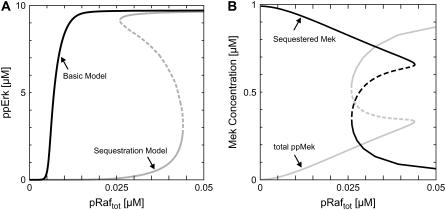
The intracellular concentrations of Mek and Erk depend on the cellular context, and have been reported to be 0.6–40 μM (7,32) and 0.8–30 μM (27,33) in mammalian cells. Thus, bistability might still be observed in other cell types than HeLa cells, especially because phosphatase activity in the MAPK signaling module is known to be intensely regulated as well (34). One important observation was bistability within the physiologically relevant kinase/phosphatase concentration ranges. Fig. 2 A (gray line) shows a representative stimulus-response curve. Here, the total Erk concentration and the Erk-phosphatase activity were modified compared to the default values measured in HeLa cells, whereas the total Mek concentration and the Mek-phosphatase activity were kept essentially unchanged (Table 1).
FIGURE 2.
Bistability due to Mek sequestration. (A) Bistable stimulus response of the core MAPK cascade. Extracellular stimulation was simulated by varying the total concentration of active Raf (pRaftot = pRaf + pRaf-Mek + pRaf-pMek), and bisphosphorylated Erk (ppErk) was taken as the response. The black curve corresponds to the previously analyzed basic model (Fig. 1 D, black arrows), whereas the gray stimulus-response was obtained for the sequestration model, which additionally takes Mek sequestration by Erk into account (Fig. 1 D, black and gray arrows). Kinetic parameters are given in Table 1. (B) Mek release from inactive sequestration complexes upon cascade activation. The amount of sequestered Mek (i.e., Mek-Erk + Mek-pErk + pMek-Erk + pMek-pErk) and the total amount of bisphosphorylated Mek (i.e., ppMek + ppMek-Erk + ppMek-pErk) is shown as a function of total active Raf for the kinetic parameters given in Table 1.
Markevich et al. (22) reported that hysteresis can implicitly arise in double (de)phosphorylation cycles even in the absence of allosteric feedback. To exclude that their mechanism is responsible for the observed bistability, we eliminated Mek sequestration from the model, and analyzed whether bistability was retained. The resulting basic model (Fig. 1 D, black arrows) exhibits a simple, monostable response, which demonstrates that Mek sequestration into Raf-inaccessible complexes (Fig. 1 D, gray arrows) is responsible for bistability. This conclusion also holds in general, i.e., regardless of the parameters chosen, because bistability in double phosphorylation cannot arise with noncooperative protein (de)phosphorylation reactions (31), which we assumed in the default sequestration model (see Model implementation).
We next analyzed the amount of sequestered Mek (i.e., Mek-Erk + Mek-pErk + pMek-Erk + pMek-pErk) to further corroborate that the positive circuit described in Rationale is responsible for the hysteresis. The corresponding simulations confirm a pronounced Mek release from Raf-inaccessible sequestration complexes upon switching from the lower to the upper steady-state branch (Fig. 2 B, black line). This Mek release relieves the cascade from strong inhibition (Fig. 2 A, black versus gray lines) and allows for coordinated activation of Mek and Erk (Fig. 2, A and B, gray lines). Taken together, these data reveal that Mek sequestration into Raf-inaccessible complexes and its subsequent ppMek-dependent release are responsible for hysteresis in Fig. 2.
Kinetic requirements for bistability
MAPK activation was shown to proceed in an all-or-none manner in some, but not all, cells (see Introduction). To get insights into such cell-type specific behavior, we sought to investigate the requirements for bistability in terms of protein expression and kinetic parameters.
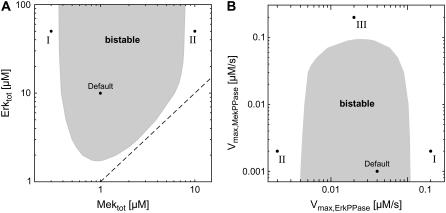
The impact of alterations in kinase expression was analyzed by classifying the stimulus-response curves as monostable and bistable (similar to those in Fig. 2 A) for varying total Mek and Erk concentrations. Fig. 3 A (gray area) shows that the stimulus-response is bistable over a relatively broad range of Mek and Erk expression levels, which match those previously measured experimentally (see above). Hysteresis seems to require that the Erk concentration exceeds that of Mek, as the bistable range is bounded by the dashed line in Fig. 3 A, which corresponds to equal Mek and Erk expression. Excess of Erk ensures efficient Mek sequestration into Raf-inaccessible complexes upon weak stimulation, and thereby strengthens the relief-from-inhibition feedback mechanism discussed in Rationale. Experimental studies confirmed that Erk is indeed more abundant than Mek in a variety of mammalian cell lines, including CHO cells (Erk/Mek = 2.15 (32)), Cos-1 cells (Erk/Mek = 2.03 (35)), Cos7 cells (Erk/Mek ≫ 1 (21)), 208F cells (Erk/Mek = 2.5 (35)), NIH3T3 cells (Erk/Mek = 12.86 (35)), and Rat1 cells (Erk/Mek = 1.5 (35)). Additionally, the yeast Erk homologs Kss1p and Fus3p were reported to significantly exceed their shared upstream activator, the Mek homolog Ste7p ((Kss1p + Fus3p)/Ste7p > 5.71 (32)).
FIGURE 3.
Kinetic requirements for bistability. (A) Bifurcation diagram for alterations in kinase expression. The stimulus-response curves of the sequestration model were calculated for varying total Mek and Erk concentrations, and were then classified into monostable (white area) and bistable (gray area). The dashed line corresponds to equal Mek and Erk expression. Default indicates the parameter set given in Table 1. Point I indicates the situation where the Mek concentration is low relative to that of the Erk phosphatase, so that Erk activation is completely abolished. Point II corresponds to a cell that expresses high levels of Mek relative to Erk phosphatase. This provokes strong Erk activation before the Mek cycle is switched on, and therefore excludes coordinated activation of both kinases in a positive-feedback circuit. (B) Bifurcation diagram for alterations in phosphatase expression. Similar to A, but bistable behavior was analyzed for varying maximal velocities (i.e., varying expression) of the phosphatases that dephosphorylate Mek and Erk. See A legend above for explanation of points I and II. Point III indicates the situation where strong Mek-phosphatase expression necessitates high levels of active Raf to elicit Mek phosphorylation. Under these conditions, Mek is strongly sequestered by active Raf, and this abolishes hysteresis.
We have experimentally measured the intracellular Erk concentration in Rat1 cells, where Erk > Mek, to demonstrate the physiological relevance of the proposed feedback mechanism. We found 2.3 × 106 molecules per Rat1 cell using Western blotting and a calibration curve of recombinant GST-Erk fusion proteins, as described previously (27). Assuming a cell volume of 1 pl (32) and an Erk/Mek ratio of 1.5 in these cells (35), we arrive at Mektot = 2.56 μM and Erktot = 3.83 μM. These values lie within the range of bistability (Fig. 3 A), and therefore further corroborate the physiological relevance of the implicit feedback mechanism discussed in this article.
We also analyzed how altered Mek- and Erk-phosphatase expression (i.e., changes in the corresponding Vmax values) affect the qualitative behavior of the stimulus-response curve, and it turned out that bistability is retained over a relatively broad range of phosphatase concentrations (Fig. 3 B).
The bifurcation analysis with respect to kinase and phosphatase expression (Fig. 3, A and B) reveals several kinetic constraints for the existence of a bistable stimulus-response: 1), Mek concentrations that are too low or Erk-phosphatase concentrations that are too high abolish any significant Erk activation and, thereby, also hysteresis (Fig. 3, A and B, I). 2), Mek levels that are too high or Erk-phosphatase levels that are too low provoke strong Erk activation before the Mek cycle is switched on, and therefore exclude coordinated activation of both kinases in a positive circuit (Fig. 3, A and B, II). 3), Strong Mek-phosphatase expression necessitates high levels of active Raf to elicit Mek phosphorylation. Under these conditions, Mek sequestration by active Raf becomes significant and this abolishes bistability, because Mek activation is both subsensitive (29) and submaximal (28) (Fig. 3 B, III). 4), Bistability requires that the Mek and the Erk concentrations exceed the dissociation constant (Kd) of the Raf-inaccessible (p)Mek-(p)Erk sequestration complexes, since otherwise Mek sequestration is relatively inefficient. Experimental evidence suggests that this requirement holds in living cells, as the measured dissociation constant (Kd = 30–300 nM) (26,36,37) is indeed lower than typical intracellular Mek and Erk levels.
Structural requirements for bistability
We made two key topological assumptions when deriving the model depicted in Fig. 1 D. First, we assumed that Mek and Erk no longer associate once Erk has been fully phosphorylated by Mek; that is, we neglected product inhibition of ppMek-mediated Erk phosphorylation. This seems justified, since it has been shown that Mek and Erk form stable heterodimers under basal conditions, and that these heterodimers dissociate almost completely upon stimulus-induced Erk phosphorylation (25). Second, we assumed that Erk and Raf bind to Mek in a mutually exclusive manner (i.e., competitively). Experimental studies revealed that Raf associates with a C-terminal domain in Mek (38), whereas Erk is recruited to the N-terminus of Mek (39). Importantly, the C- and N-terminal domains adjoin to each other in the Mek crystal structure (40), which suggests competitive binding, especially because both Raf (74 kD) and Erk (44 kD) are relatively bulky and are known to homodimerize. Competition of Raf and Erk for Mek is further suggested by the fact that the Mek proline-rich domain, which adjoins to the Mek C-terminus (40), has been implicated in both Raf and Erk recruitment (41,42). Finally, mutually exclusive binding is also supported by biochemical analyses of the JNK MAPK pathway, which showed that the Raf homolog, Mekk-1, competes with JNK for binding to the Mek homolog, JNKK1 (43).
Taken together, these data suggest that the scheme depicted in Fig. 1 D applies for the core MAPK cascade. However, we wanted to characterize the topological requirements of the proposed bistability mechanism more generally. Additionally, scaffold proteins, which bring kinases and their substrates into close proximity, allow for cascade activation even if otherwise essential docking interactions are absent (39), and might thus alleviate competition effects. Therefore, we implemented an “extended sequestration model, which included Raf-mediated phosphorylation of Erk-bound Mek (i.e., noncompetitive Raf and Erk binding to Mek), as well as ppErk binding to Mek, pMek, and ppMek (see Supplement). Nine additional molecular species (compared to Fig. 1 D) are considered in the extended model: six ternary Raf-Mek-Erk complexes (i.e., pRaf-Mek-Erk, pRaf-pMek-Erk, pRaf-Mek-pErk, pRaf-pMek-pErk, pRaf-Mek-ppErk, and pRaf-pMek-ppErk) arising from noncompetitive Raf and Erk binding to Mek, and three Mek-ppErk heterodimers (i.e., Mek-ppErk, pMek-ppErk, and ppMek-ppErk), which can be considered as product inhibition complexes in Erk phosphorylation. Raf is assumed to catalyze Mek phosphorylation within the ternary Raf-Mek-Erk complexes (e.g., pRaf-Mek-Erk → pRaf + pMek-Erk), so that Mek sequestration by inactive Erk no longer prevents cascade activation.
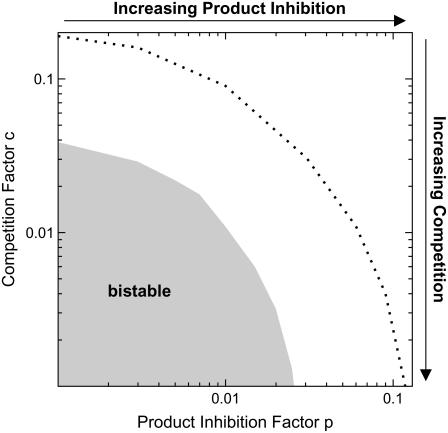
Fig. 4 shows the qualitative behavior of the extended sequestration model for varying degrees of competition and product inhibition. It can be seen that bistability is abolished in the extended sequestration model if competition between Raf and Erk for Mek is too weak, and if product inhibition in the Erk phosphorylation becomes significant. The competition factor, c, equals the fold change in Raf's affinity for Mek brought about by Erk binding to Mek (and vice versa). Thus, hysteresis requires that Erk binding to Mek decreases the affinity between Raf and Mek (and vice versa) at least by a factor of 25 (c < 0.04). Likewise, the product inhibition factor, p, quantifies how the affinity between Erk and Mek is altered by Erk double phosphorylation (relative to unphosphorylated/monophosphorylated Erk). According to Fig. 4, ppErk must have a 40-fold (p < 0.025) lower affinity for Mek than its unphosphorylated/monophosphorylated precursors.
FIGURE 4.
Structural requirements for bistability. The sequestration model depicted in Fig. 1 D was extended to study the topological constraints for bistability. More specifically, Raf-mediated phosphorylation of Erk-bound Mek (i.e., noncompetitive binding of Raf and Erk to Mek) was taken into account. Additionally, we considered ppErk binding to Mek, pMek, and ppMek (i.e., by product inhibition in Erk phosphorylation). The stimulus-response curves of the resulting “extended sequestration model” (see Supplementary Material for differential equations) were classified as monostable and bistable for varying degrees of competition and product inhibition. The competition factor, c, equals the fold change in Raf's affinity for Mek brought about by Erk binding to Mek (and vice versa). Likewise, the product inhibition factor, p, quantifies how the affinity between Erk and Mek is altered by Erk double phosphorylation (relative to unphosphorylated/monophosphorylated Erk). The gray bistability range was calculated using the default parameters given in Table 1. The dashed black line indicates the bistable-to-monostable transition for a 10-fold lower Michaelis-Menten constant of the Mek phosphatase (KM,Mek-PPase = 0.01 μM).
The above constraints for p and c can be relaxed if one assumes kinetic parameters that differ from those given in Table 1. The dotted line in Fig. 4 shows, for example, how the bistable-monostable border of the extended sequestration model is shifted if the Michaelis-Menten constant of the Mek-phosphatase is assumed to be 10-fold less than in Table 1 (i.e., KM,Mek-PPase = 0.01 μM). Such strong phosphatase saturation, which increases zero-order ultrasensitivity in the Mek cycle (44), allows the system to filter out leakage from Raf-inaccessible sequestration complexes, and thereby broadens the range of bistability.
In conclusion, we have shown in this section that significant competition between Raf and Erk for Mek, and pronounced release of doubly phosphorylated Erk from Mek is required for bistability to be observed.
Synergism of bistability mechanisms
Markevich et al. (22) reported that hysteresis can implicitly arise in double (de)phosphorylation cycles if the kinetic parameters for the first and second phosphorylation sites differ (“kinetic asymmetry”). More specifically, hysteresis is favored if the kinase (ppMek) has significantly higher affinity for the unphosphorylated substrate (Erk) than for the monophosphorylated substrate (tyrosine-phosphorylated Erk) (31). Experimental studies revealed that such kinetic asymmetry may occur in the Erk (de)phosphorylation cycle, as monophosphorylated Erk seems to have weaker affinity for ppMek than unphosphorylated Erk (25).
We therefore analyzed whether the bistability mechanism proposed in this article and that described by Markevich et al. (22) synergize to yield a larger bistable region than either mechanism alone. Simulations were done using the model structure depicted in Fig. 1 D. In contrast to the default model (see Model implementation), we now assume positive cooperativity in ppMek-mediated Erk phosphorylation. More specifically, the first phosphorylation step (Erk → pErk) is modeled with a low Michaelis-Menten constant, but with a slow catalytic rate constant. On the contrary, we assume a higher Michaelis-Menten constant and a much faster catalytic rate constant for the second phosphorylation step (pErk → ppErk), as this should favor bistability in the Erk cycle (31).
These parameters yield a narrow bistable range for the basic MAPK model, which neglects Mek sequestration (Fig. 5, curve 1). Hysteresis of curve 1 in Fig. 5 can be attributed to the mechanism described by Markevich et al. (22). Fig. 5 (curve 2) shows that this narrow bistable region is strongly enlarged if Mek sequestration into Raf-inaccessible complexes is additionally taken into account. Thus, both feedback mechanisms in combination bring about much more pronounced bistability than enzyme-depletion effects in double phosphorylation alone. We also analyzed how the bistable range of the sequestration model is affected if kinetic asymmetry in Erk phosphorylation is eliminated from the model. This was done by assuming equal catalytic rate constants for the first and second phosphorylation steps. As expected, the bistable range got significantly narrower once kinetic asymmetry was removed from the model (Fig. 5, curve 3).
Taken together, we have shown that the bistability mechanism proposed in this article and that described by Markevich et al. (22) synergize to yield a much larger bistable region than either mechanism alone. Generally, the bistable range due to Mek sequestration, which we analyzed for the noncooperative system in Figs. 2–4, can be enlarged if kinetic asymmetries in the Mek and/or Erk (de)phosphorylation cycles are taken into account. In this context, it has recently been discussed that strong positive cooperativity occurs in Raf-mediated Mek phosphorylation (31). Finally, Fig. 5 demonstrates that positive circuits, which are bistable in isolation, cooperate to bring about even more pronounced bistability when combined in a network of nested positive circuits. Thus, bistability due to Mek sequestration might be even further enhanced by outer positive-feedback circuits, which act at or upstream of Raf.
CONCLUDING REMARKS
In this article, we showed that bistability is caused by an implicit positive-feedback circuit that emerges from the network structure of the core MAPK cascade: unphosphorylated/monophosphorylated Erk sequesters Mek into Raf-inaccessible complexes upon weak stimulation, and thereby inhibits the cascade (see Fig. 1 A). Mek, once phosphorylated by Raf, triggers Erk phosphorylation, which in turn induces dissociation of Raf-inaccessible Mek-Erk heterodimers (relief from inhibition) and thus further Mek phosphorylation (Fig. 1, B and C). The suggested mechanism is in accord with experimental studies, which showed that Mek and Erk form a stable complex under resting conditions, and dissociate upon Erk phosphorylation (see, e.g., Adachi et al. (25)). Positive feedback due to Mek sequestration can bring about bistability for experimentally measured parameters (26), and is expected to enhance ultrasensitive behavior of the MAPK signaling module outside the bistable range (45).
Experimental studies suggest that the MAPK cascade is frequently bistable, even though overexpression of constitutively active Raf, Mek, or Erk mutants does not result in positive-feedback activation of their endogenous counterparts (see Introduction). The relief-from-inhibition mechanism discussed in this article resolves this apparent contradiction. Markevich et al. (22) demonstrated that relief from inhibition and bistability can arise in double phosphorylation cycles, but hysteresis was restricted to a relatively narrow parameter range. We show here that implicit feedback in double phosphorylation and feedback due to Mek sequestration synergize to yield a significantly larger bistable region than either mechanism alone.
We propose to test for feedback due to Mek sequestration by initial velocity analysis of Raf-mediated Mek phosphorylation in vitro. The proposed feedback mechanism requires that unphosphorylated/monophosphorylated Erk, but not bisphorylated Erk, acts as a competitive inhibitor of Raf-mediated Mek phosphorylation. Competitive inhibition can be shown by analyzing Lineweaver-Burk plots for varying Erk concentrations (see biophysical textbooks). Kinase-defective Mek and Erk mutants should be used in these assays to prevent Mek-mediated Erk phosphorylation and Erk-mediated feedback phosphorylation of Mek. Significant competitive inhibition of Raf-mediated Mek phosphorylation should be seen with unphosphorylated Erk as a competitor, but not with bisphosphorylated Erk.
Bistability due to Mek sequestration can be directly proven in an in vitro reconstitution system. Mek, a Mek-phosphatase (e.g., PP2A), Erk, and an Erk-phosphatase (e.g., MKP3) should be incubated with varying amounts of active Raf, and the stimulus-response is expected to exhibit true all-or-none behavior (Fig. 2 A). Hysteresis can be shown by varying the time of phosphatase addition to the system in the following ways: 1), a weak response is expected within the bistable range if the Mek/Erk-phosphatases are added simultaneously with Raf to Mek and Erk; and 2), a strong response will be observed if the Mek/Erk-phosphatases are added after Mek and Erk have been fully activated by Raf.
Recent theoretical studies revealed that sequestration-based feedback (i.e., feedback without explicit allosteric regulation) might be a common principle in signal transduction, and that it allows for bistable (22,23,46) or oscillatory behavior (6,47). Feedback emerges in these systems due to high-affinity protein-protein interactions, which appear to be an ubiquitous and robust means to achieve nonlinear behavior in biochemical networks (23,48,49). Sequestration-based feedback also requires that protein-protein interactions are competitive at least to some extent, because otherwise the bound protein can still participate in other cellular reactions (i.e., it cannot be sequestered).
We are convinced that sequestration-based feedback is a common feature of protein kinase cascades: Enzyme-substrate binding in these cascades is generally mediated by relatively stable docking/domain interactions in addition to transient recognition by the enzyme's active site (50). Additionally, cascade intermediates frequently engage a single binding site to recruit upstream kinases, phosphatases, and downstream substrates in a competitive manner (50,51). The results presented in this article demonstrate that such competition effects profoundly affect the qualitative behavior of protein kinase cascades. Scaffold proteins, which bring kinases and their substrates into close proximity, allow for cascade activation even if otherwise essential docking/domain interactions are absent (39). It is tempting to speculate that scaffold proteins might alleviate competition effects within the MAPK kinase cascade, and thereby regulate the qualitative behavior of the stimulus-response (monostable versus bistable).
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Nils Blüthgen's present address is Manchester Integrative Biocenter, University of Manchester, Manchester, UK.
Editor: Alexander van Oudenaarden.
References
- 1.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 2.Bhalla, U. S., P. T. Ram, and R. Iyengar. 2002. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 297:1018–1023. [DOI] [PubMed] [Google Scholar]
- 3.Bagowski, C. P., and J. E. Ferrell, Jr. 2001. Bistability in the JNK cascade. Curr. Biol. 11:1176–1182. [DOI] [PubMed] [Google Scholar]
- 4.Ferrell, J. E., Jr., and E. M. Machleder. 1998. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 280:895–898. [DOI] [PubMed] [Google Scholar]
- 5.Paliwal, S., P. A. Iglesias, K. Campbell, Z. Hilioti, A. Groisman, and A. Levchenko. 2007. MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature. 446:46–51. [DOI] [PubMed] [Google Scholar]
- 6.Wang, X., N. Hao, H. G. Dohlman, and T. C. Elston. 2006. Bistability, stochasticity, and oscillations in the mitogen-activated protein kinase cascade. Biophys. J. 90:1961–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altan-Bonnet, G., and R. N. Germain. 2005. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 3:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding, A., T. Tian, E. Westbury, E. Frische, and J. F. Hancock. 2005. Subcellular localization determines MAP kinase signal output. Curr. Biol. 15:869–873. [DOI] [PubMed] [Google Scholar]
- 9.Nair, V. D., T. Yuen, C. W. Olanow, and S. C. Sealfon. 2004. Early single cell bifurcation of pro- and antiapoptotic states during oxidative stress. J. Biol. Chem. 279:27494–27501. [DOI] [PubMed] [Google Scholar]
- 10.Mackeigan, J. P., L. O. Murphy, C. A. Dimitri, and J. Blenis. 2005. Graded mitogen-activated protein kinase activity precedes switch-like c-Fos induction in mammalian cells. Mol. Cell. Biol. 25:4676–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehurst, A., M. H. Cobb, and M. A. White. 2004. Stimulus-coupled spatial restriction of extracellular signal-regulated kinase 1/2 activity contributes to the specificity of signal-response pathways. Mol. Cell. Biol. 24:10145–10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruf, F., M. J. Park, F. Hayot, G. Lin, B. Roysam, Y. Ge, and S. C. Sealfon. 2006. Mixed analog/digital gonadotrope biosynthetic response to gonadotropin-releasing hormone. J. Biol. Chem. 281:30967–30978. [DOI] [PubMed] [Google Scholar]
- 13.Santos, S. D., P. J. Verveer, and P. I. Bastiaens. 2007. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 9:324–330. [DOI] [PubMed] [Google Scholar]
- 14.Ferrell, J. E., Jr. 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14:140–148. [DOI] [PubMed] [Google Scholar]
- 15.Tyson, J. J., K. C. Chen, and B. Novak. 2003. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15:221–231. [DOI] [PubMed] [Google Scholar]
- 16.Shvartsman, S. Y., M. P. Hagan, A. Yacoub, P. Dent, H. S. Wiley, and D. A. Lauffenburger. 2002. Autocrine loops with positive feedback enable context-dependent cell signaling. Am. J. Physiol. Cell Physiol. 282:C545–C559. [DOI] [PubMed] [Google Scholar]
- 17.Alessandrini, A., H. Greulich, W. Huang, and R. L. Erikson. 1996. Mek1 phosphorylation site mutants activate Raf-1 in NIH 3T3 cells. J. Biol. Chem. 271:31612–31618. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann, S., C. Rommel, A. Ziogas, J. Lovric, K. Moelling, and G. Radziwill. 1997. MEK1 mediates a positive feedback on Raf-1 activity independently of Ras and Src. Oncogene. 15:1503–1511. [DOI] [PubMed] [Google Scholar]
- 19.Samuels, M. L., M. J. Weber, J. M. Bishop, and M. McMahon. 1993. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol. Cell. Biol. 13:6241–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson, M. J., S. A. Stippec, E. Goldsmith, M. A. White, and M. H. Cobb. 1998. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr. Biol. 8:1141–1150. [DOI] [PubMed] [Google Scholar]
- 21.Miyata, Y., S. Adachi, H. Mizuno, and E. Nishida. 1999. A strategy to make constitutively active MAP kinase by fusing with constitutively active MAP kinase kinase. Biochim. Biophys. Acta. 1451:334–342. [DOI] [PubMed] [Google Scholar]
- 22.Markevich, N. I., J. B. Hoek, and B. N. Kholodenko. 2004. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J. Cell Biol. 164:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legewie, S., N. Bluthgen, and H. Herzel. 2006. Mathematical modeling identifies inhibitors of apoptosis as mediators of positive feedback and bistability. PLoS Comput. Biol. 2:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417–439.11171037 [Google Scholar]
- 25.Adachi, M., M. Fukuda, and E. Nishida. 1999. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 18:5347–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujioka, A., K. Terai, R. E. Itoh, K. Aoki, T. Nakamura, S. Kuroda, E. Nishida, and M. Matsuda. 2006. Dynamics of the Ras/ERK MAPK cascade as monitored by fluorescent probes. J. Biol. Chem. 281:8917–8926. [DOI] [PubMed] [Google Scholar]
- 27.Schoeberl, B., C. Eichler-Jonsson, E. D. Gilles, and G. Muller. 2002. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat. Biotechnol. 20:370–375. [DOI] [PubMed] [Google Scholar]
- 28.Legewie, S., N. Bluthgen, R. Schafer, and H. Herzel. 2005. Ultrasensitization: switch-like regulation of cellular signaling by transcriptional induction. PLoS Comput. Biol. 1:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bluthgen, N., F. J. Bruggeman, S. Legewie, H. Herzel, H. V. Westerhoff, and B. N. Kholodenko. 2006. Effects of sequestration on signal transduction cascades. FEBS J. 273:895–906. [DOI] [PubMed] [Google Scholar]
- 30.Asthagiri, A. R., and D. A. Lauffenburger. 2001. A computational study of feedback effects on signal dynamics in a mitogen-activated protein kinase (MAPK) pathway model. Biotechnol. Prog. 17:227–239. [DOI] [PubMed] [Google Scholar]
- 31.Ortega, F., J. L. Garces, F. Mas, B. N. Kholodenko, and M. Cascante. 2006. Bistability from double phosphorylation in signal transduction. Kinetic and structural requirements. FEBS J. 273:3915–3926. [DOI] [PubMed] [Google Scholar]
- 32.Ferrell, J. E., Jr. 1996. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21:460–466. [DOI] [PubMed] [Google Scholar]
- 33.Whitehurst, A. W., J. L. Wilsbacher, Y. You, K. Luby-Phelps, M. S. Moore, and M. H. Cobb. 2002. ERK2 enters the nucleus by a carrier-independent mechanism. Proc. Natl. Acad. Sci. USA. 99:7496–7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang, M., and I. B. Dawid. 2004. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci. STKE. 2004:pe17. [DOI] [PubMed] [Google Scholar]
- 35.Yeung, K., T. Seitz, S. Li, P. Janosch, B. McFerran, C. Kaiser, F. Fee, K. D. Katsanakis, D. W. Rose, H. Mischak, J. M. Sedivy, and W. Kolch. 1999. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 401:173–177. [DOI] [PubMed] [Google Scholar]
- 36.Mansour, S. J., J. M. Candia, J. E. Matsuura, M. C. Manning, and N. G. Ahn. 1996. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 35:15529–15536. [DOI] [PubMed] [Google Scholar]
- 37.Haystead, T. A., P. Dent, J. Wu, C. M. Haystead, and T. W. Sturgill. 1992. Ordered phosphorylation of p42mapk by MAP kinase kinase. FEBS Lett. 306:17–22. [DOI] [PubMed] [Google Scholar]
- 38.Takekawa, M., K. Tatebayashi, and H. Saito. 2005. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol. Cell. 18:295–306. [DOI] [PubMed] [Google Scholar]
- 39.Bardwell, A. J., L. J. Flatauer, K. Matsukuma, J. Thorner, and L. Bardwell. 2001. A conserved docking site in MEKs mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission. J. Biol. Chem. 276:10374–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohren, J. F., H. Chen, A. Pavlovsky, C. Whitehead, E. Zhang, P. Kuffa, C. Yan, P. McConnell, C. Spessard, C. Banotai, W. T. Mueller, A. Delaney, C. Omer, J. Sebolt-Leopold, D. T. Dudley, I. K. Leung, C. Flamme, J. Warmus, M. Kaufman, S. Barrett, H. Tecle, and C. A. Hasemann. 2004. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 11:1192–1197. [DOI] [PubMed] [Google Scholar]
- 41.Catling, A. D., H. J. Schaeffer, C. W. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dang, A., J. A. Frost, and M. H. Cobb. 1998. The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J. Biol. Chem. 273:19909–19913. [DOI] [PubMed] [Google Scholar]
- 43.Xia, Y., Z. Wu, B. Su, B. Murray, and M. Karin. 1998. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 12:3369–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldbeter, A., and D. E. Koshland, Jr. 1981. An amplified sensitivity arising from covalent modification in biological systems. Proc. Natl. Acad. Sci. USA. 78:6840–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Legewie, S., N. Bluthgen, and H. Herzel. 2005. Quantitative analysis of ultrasensitive responses. FEBS J. 272:4071–4079. [DOI] [PubMed] [Google Scholar]
- 46.Salazar, C., and T. Hofer. 2006. Competition effects shape the response sensitivity and kinetics of phosphorylation cycles in cell signaling. Ann. N. Y. Acad. Sci. 1091:517–530. [DOI] [PubMed] [Google Scholar]
- 47.Clodong, S., U. Duhring, L. Kronk, A. Wilde, I. Axmann, H. Herzel, and M. Kollmann. 2007. Functioning and robustness of a bacterial circadian clock. Mol. Syst. Biol. 3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eissing, T., H. Conzelmann, E. D. Gilles, F. Allgower, E. Bullinger, and P. Scheurich. 2004. Bistability analyses of a caspase activation model for receptor-induced apoptosis. J. Biol. Chem. 279:36892–36897. [DOI] [PubMed] [Google Scholar]
- 49.Eissing, T., S. Waldherr, F. Allgower, P. Scheurich, and E. Bullinger. 2007. Response to bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys. J. 92:3332–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remenyi, A., M. C. Good, and W. A. Lim. 2006. Docking interactions in protein kinase and phosphatase networks. Curr. Opin. Struct. Biol. 16:676–685. [DOI] [PubMed] [Google Scholar]
- 51.Bardwell, A. J., M. Abdollahi, and L. Bardwell. 2003. Docking sites on mitogen-activated protein kinase (MAPK) kinases, MAPK phosphatases and the Elk-1 transcription factor compete for MAPK binding and are crucial for enzymic activity. Biochem. J. 370:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown, G. C., and B. N. Kholodenko. 1999. Spatial gradients of cellular phospho-proteins. FEBS Lett. 457:452–454. [DOI] [PubMed] [Google Scholar]
- 53.Zhao, Y., and Z. Y. Zhang. 2001. The mechanism of dephosphorylation of extracellular signal-regulated kinase 2 by mitogen-activated protein kinase phosphatase 3. J. Biol. Chem. 276:32382–32391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.