SUMMARY
Both small GTPase Rac and second messenger cGMP (guanosine 3′,5′-cyclic monophosphate) are critical regulators of cell functions. Here we report that Rac uses its effector PAK (p21-activated kinase) to directly activate transmembrane guanylyl cyclases (GCs) leading to increased cellular cGMP levels. Furthermore, we have shown that this new Rac/PAK/GC/cGMP pathway is involved in platelet-derived growth factor-induced fibroblast cell migration and lamellipodium formation. Our finding provides a general mechanism for diverse receptors to modulate physiological responses through elevating cellular cGMP levels.
INTRODUCTION
The small GTPase Rac plays critical roles in a wide variety of cell functions including cell cycle control, regulation of gene expression, activation of NADPH oxidase of phagocytic cells, actin cytoskeletal reorganization, axonal guidance and cell migration (Jaffe and Hall, 2005). Rac can be activated by extracellular signals through various types of membrane receptors including receptor tyrosine kinases such as the PDGF (platelet-derived growth factor) receptor. Rac executes its biological functions through activating downstream effectors. One of the best-characterized downstream effectors of Rac is PAK (p21-activated kinase). PAKs are a highly conserved family of serine/threonine protein kinases (Bokoch, 2003). Nearly all eukaryotes contain one or more PAK genes (Hofmann et al., 2004). PAK family members regulate cellular proliferation, differentiation, transformation, and survival. They also play important roles in cytoskeleton rearrangement during cell migration. Expression of constitutively active PAK stimulates ruffle formation and inhibits stress fibers (Manser et al., 1997; Sells et al., 1997). Increases in PAK expression and activity have been correlated with progression of colorectal carcinomas to metastasis (Carter et al., 2004) and enhanced motility and invasiveness of human breast cancer cells (Vadlamudi et al., 2000). In mammals, PAKs can be grouped into two subfamilies: group A (PAK1, 2 and 3) can be activated by small GTPases such as Rac-GTP or Cdc42-GTP binding (Bokoch, 2003). Group B (PAK 4, 5 and 6) can interact with Cdc42-GTP but are not activated by this binding. From the crystal structure of PAK1 (Lei et al., 2000), it appears that the inactive state is maintained by the N-terminal autoregulatory region (residues 83–149) binding to and inhibiting the C-terminal catalytic kinase domain. This autoregulatory region inhibits PAK1 even when supplied as an independent fragment. Binding of GTP-bound Rac (or active Cdc42) releases this inhibition. PAK1 then autophosphorylates Thr423 in its activation loop to stabilize the active state (Lei et al., 2000). In addition, PAKs 1–3 can be activated by Rac/Cdc42-independent mechanisms such as by caspase-mediated cleavage, by membrane recruitment via adapter proteins, and by sphingolipids (Bokoch, 2003).
Like cAMP and Ca2+, cGMP is a ubiquitous second messenger mediating cellular responses to various exogenous and endogenous signaling molecules. cGMP controls diverse physiological functions such as relaxation of vascular smooth muscles, phototransduction, epithelial electrolyte transport, bone growth, leukocyte migration, axonal guidance, sperm motility, platelet spreading, and vascular permeability (Lucas et al., 2000). cGMP regulates physiological processes by activating protein kinases, gating specific ion channels, and modulating cellular cyclic nucleotide concentrations through phosphodiesterases (Lucas et al., 2000). The conversion of GTP to cGMP is catalyzed by guanylyl cyclases (GCs). There are two types of GCs in mammals that are expressed in nearly all cell types: the soluble and the membrane-bound GCs (Lucas et al., 2000). The soluble GCs are generally activated when NO (nitric oxide) binds to the attached prosthetic heme group. Seven membrane-bound GCs (also named transmembrane or particulated GCs) have been identified in the human genome (Lucas et al., 2000). GC-A and GC-B are natriuretic peptide receptors. GC-C can be activated by bacterial heat-stable enterotoxins, guanylin and uroguanylin. The extracellular ligands for GC-D, GC-E, GC-F and GC-G are not known. The activity of transmembrane GCs can also be modulated by other receptor signals through intracellular signaling pathways. GC-E and GC-F, found in the retina, can be modulated by a group of retinal-specific cellular proteins named GCAPs (guanylyl cyclase activating proteins) in a calcium-dependent manner (Palczewski et al., 1994). Moreover, in genetic studies of olfaction in C. elegans, mutants defective in olfaction sensory response have been obtained including daf-11 and odr-1, two transmembrane GCs (Birnby et al., 2000; L'Etoile and Bargmann, 2000). Reintroduction of the intracellular domain of odr-1 rescued the defective phenotype (L'Etoile and Bargmann, 2000). Transmembrane GCs function in regulating cell migration and actin cytoskeletal reorganization. The sea urchin sperm membrane-bound GC is critical for sperm chemotaxis to the eggs (Bentley et al., 1986). In addition, Dictyostelium mutants lacking GCs are defective in cell chemotaxis (Bosgraaf et al., 2002). Furthermore, the Drosophila transmembrane GC Gyc76C has been genetically demonstrated to be essential for axonal guidance (Ayoob et al., 2004).
Although various types of membrane signaling receptors such as growth factor receptor tyrosine kinases can increase cellular cGMP levels (Coffey et al., 1988; Scheving et al., 1985), the molecular mechanism by which these receptors increase cGMP is not known. Here we have discovered a new signaling pathway linking the small GTPase Rac to the increase of cellular cGMP. Our data show that transmembrane GCs can be directly stimulated by intracellular PAK kinases. Previous studies have shown that second messengers such as cGMP and Rho-family small GTPases such as Rac regulate cell migration and actin cytoskeletal reorganization. However, the link between cGMP and Rac has, until now, been missing. Our finding bridges these two important regulators of cellular physiological functions.
RESULTS
Rac Increases Cellular cGMP Levels
In our search for signal transducing molecules that could increase the activity of GCs, we found that Rac1 could increase the GC activity in cells (Fig. 1A). Untransfected CHO cells have low levels of cellular cGMP that are below the detection limit of our method. Therefore, to reliably measure changes of cellular cGMP, we established CHO cell lines that stably express GC-E (CHO-GC-E cells) (here we use GC-E as an example) (Supplementary Fig. 1A). Transient expression of a constitutively activated mutant of Rac1 (Rac1G12V) in these CHO-GC-E cells led to an increase (~ 3-fold) of cellular cGMP levels (Fig. 1A). This stimulatory effect is specific to Rac1 since the other two members of the Rho-family small GTPases, RhoA and Cdc42, did not increase the cellular cGMP levels when their constitutively activated mutant forms [RhoA(G14V) and Cdc42(G12V)] were transiently expressed in CHO-GC-E cells (Fig. 1A). The lack of stimulation by RhoA and Cdc42 was not due to poor expression of these proteins in CHO cells (Fig. 1A, bottom Western blots). Furthermore, expression of RhoA(G14V) and Cdc42(G12V) in fibroblast cells led to the formation of actin stress fibers and filopodia, respectively (Supplementary Fig. 1B), indicating that these expressed proteins were functional.
Figure 1.
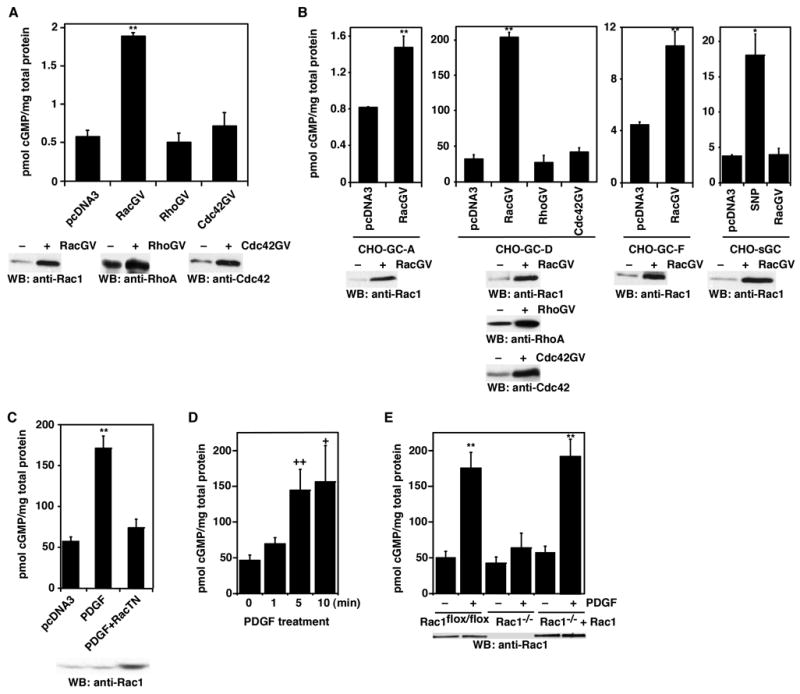
Rac1 stimulates GC activity in cells. A, Increase of GC-E activity by Rac1 in CHO-GC-E cells. Rac1(G12V), RhoA(G14V), CDC42(G12V), and the control vector pcDNA3 plasmid DNAs were transiently transfected into CHO cells that stably express GC-E. Cellular cGMP levels were determined. Bottom panels: Western blots show the expression of transfected Rac1(G12V), RhoA(G14V), and Cdc42(G12V) proteins, comparing to pcDNA3 vector-transfected cells. B, Effect of Rac1 on the activity of GC-A, GC-D, GC-F and soluble GC. Rac1(G12V) and the control vector pcDNA3 plasmid DNAs were transiently transfected into CHO cells stably expressing GC-A, GC-D, GC-F, or sGC (α1β1 isoform). Cellular cGMP levels were determined. Bottom panels: Western blots show the expression of transfected Rac1(G12V) proteins, comparing to pcDNA3 vector-transfected cells. C, Dominant-negative Rac1(T17N) inhibited the stimulation of GC-D by PDGF. Plasmid DNAs for Rac1(T17N) or pcDNA3 vector were transiently transfected into CHO-GC-D cells. Cellular cGMP levels were measured with or without PDGF stimulation (20 ng/ml for 5 min). D, Time course of cGMP accumulation after PDGF treatment. Data are shown as mean ± s.d. of three to five experiments. E, Rac1 deficiency blocked the stimulation of GC-D by PDGF. Rac1flox/flox cells, before or after infection of Cre-retrovirus, stably transfected with GC-D were treated with or without PDGF (20 ng/ml for 5 min). Rac1−/− +Rac1 cells were Rac1−/− cells infected with retroviruses containing wild-type Rac1. Cellular cGMP levels were measured. Error bars show mean ± s.d., **, P < 0.001; *, P < 0.005; ++, P < 0.01; +, P < 0.05 (Student’s t-test).
To examine whether Rac1 regulation of GC-E is extensive to other GCs, we tested the effect of Rac1 on the activity of GC-A, GC-D, GC-F and the α1β1 soluble GC. Rac1(G12V) was transiently expressed in cells stably expressing GC-A, GC-D, GC-F, or soluble GC. As shown in Fig. 1B, Rac1(G12V) increased the activity of GC-A, GC-D, and GC-F. Rac1 had no effect on the activity of soluble GC even though the cells expressing soluble GC responded to the soluble GC activator sodium nitroprusside (Fig. 1B). Furthermore, neither RhoA(G14V) nor Cdc42(G12V) had any effect on GC-D activity (Fig. 1B). These data indicate that Rac1 regulation of transmembrane GCs is a general phenomenon.
To verify that membrane signaling receptors could use this Rac-mediated pathway to modulate cellular cGMP levels, we tested PDGFR as a representative of the growth factor receptor tyrosine kinases. In serum-starved CHO-GC-D cells, PDGF treatment led to higher cellular cGMP levels (Fig. 1C); this increase is comparable to that induced by Rac1(G12V) in CHO-GC-D cells (Fig. 1B). Furthermore, expression of a dominant-negative Rac1 mutant [Rac1(T17N)] blocked PDGF-induced increase of cellular cGMP in CHO-GC-D cells (Fig. 1C and Supplementary Fig. 1C). In addition, cellular cGMP accumulation was observed beginning at 1 min after PDGF treatment (Fig. 1D), similar to Rac activation by PDGF (Itoh et al., 2002). Moreover, in Rac1-deficient mouse embryonic fibroblast (MEF) cells, PDGF-induced increase of cellular cGMP was blocked (Fig. 1E). Re-expression of Rac1 in these Rac1-deficient cells restored the cGMP response (Fig. 1E). These data demonstrate that Rac relays the PDGF signal to increase GC activity.
PAK Mediates Rac and PDGF Effect on Cellular cGMP Levels
To understand the molecular mechanism by which Rac1 increases the activity of GCs, we studied the participation of several downstream targets of Rac1 in this regulatory process. We found that PAK is required for this Rac1 function (Fig. 2). Transient expression of the PAK1 autoinhibitory domain PAK1-(83–149), which inhibits PAK function (Frost et al., 1998), blocked the stimulation of GC-E by Rac1(G12V) (Fig. 2A and Supplementary Fig. 1D). On the other hand, expression of PAK1-(83–149)L107F, which lacks the inhibitory effect on PAK1, did not block Rac1(G12V)-induced cellular cGMP accumulation (Fig. 2A). Furthermore, consistent with a positive role for PAK in this pathway, we observed that expression of a constitutively active mutant of PAK1 [PAK1-(165–544)] in CHO-GC-E cells increased the cellular cGMP levels (Fig. 2B). Moreover, the participation of PAK in this modulatory process was verified in the case of PDGF treatment in CHO-GC-D cells (Fig. 2C). Expression of the inhibitory PAK1 mutant PAK1-(83–149) was shown to block the stimulation of GC-D activity by PDGF, while the non-inhibitory mutant PAK1-(83–149)L107F did not (Fig. 2C). These findings demonstrate that active PAK can substitute for active Rac1 in stimulating GC, and that PAK is needed to relay the Rac1 and PDGF signals to GC.
Figure 2.

PAK1 is downstream of Rac1 in the GC stimulation pathway. A, Constitutively active Rac1(G12V) increased cellular cGMP levels. This Rac stimulatory effect was blocked by PAK autoinhibitory domain [PAK1-(83–149)]. A mutated PAK1-(83–149)L107F did not interfere with the Rac1(G12V) effect. Plasmid DNAs for Rac1(G12V), PAK1-(83–149), and/or PAK1-(83–149)L107F were transiently transfected into CHO-GC-E cells. Cellular cGMP levels were measured. B, Constitutively active PAK (caPAK) [PAK1-(165–544)] increased GC-E activity. Plasmid DNAs for the caPAK, Rac1(G12V), and/or Rac1(T17N) were transiently transfected into CHO-GC-E cells. Cellular cGMP levels were measured. Bottom panels: Western blots show the expression of transfected Rac1(G12V), Rac1(T17N), or myc-tagged PAK1-(165–544) proteins. C. The PAK autoinhibitory domain PAK1(83–149) blocked PDGF-induced cGMP increase in CHO-GC-D cells. The mutated PAK1-(83–149)L107F did not interfere with the PDGF effect. Data are shown as mean ± s.d. of three to five experiments. **, P < 0.001 (Student’s t-test).
Direct Stimulation of GC-E by Activated PAK
To learn how PAK regulates the activity of GC-E, we examined the possibility that GC-E activity is stimulated directly by PAK. We purified recombinant PAK1 and PAK2, the intracellular domain of GC-E (GC-E-intra) and Rac1 from either Sf9 cells or E. coli (Fig. 3A). Purified PAK2 (in the presence of Mg2+-ATP) by itself increased the activity of purified GC-E-intra by ~ two-fold (Fig. 3B). Addition of Rac1-GTPγS to activate PAK2 led to more than an 8-fold increase of GC-E-intra activity (Fig. 3B). The stimulatory effect was due to PAK2 since omission of PAK2 or boiled PAK2 sample had no effect on the activity of GC-E-intra even in the presence of Rac1-GTPγS (Fig. 3B). Furthermore, similar results were obtained with purified PAK1 (Fig. 3B). Moreover, the activation of GC-E by PAK2 (in the presence of Rac1-GTPγS) was dose-dependent with an EC50 value of ~42 nM (Fig. 3C). Therefore, purified PAK directly activates purified GC-E.
Figure 3.

Direct stimulation of purified GC by purified PAK. A, Coomassie blue staining of purified recombinant proteins. B, Direct stimulation of GC-E-intra by PAK. The activity of purified GST-GC-E-intra was assayed in vitro in the presence or absence of purified GST-PAK1, GST-PAK2, GST-PAK1(K298A), GST-PAK2(K278A), and/or Rac1-GTPγS. Data are shown as mean ± s.d. of three experiments. **, P < 0.001; *, P < 0.005; ++, P < 0.01 (Student’s t-test). C. Dose-response curve of the activation of purified GST-GC-E-intra by purified GST-PAK2 in the presence of Rac1-GTPγS.
Phosphorylation-independent Activation of GC by Activated PAK
To further study the biochemical mechanism by which PAK activates GC-E, we investigated whether PAK stimulates GC through protein phosphorylation. Using in vitro phosphorylation assays, we found that GC-E-intra was not phosphorylated by PAK2 (with or without Rac1-GTPγS) (Fig. 4A). The purified PAK2 was catalytically competent since it is capable of autophosphorylation, which was enhanced by Rac1-GTPγS (Fig. 4A). To rule out the possibility that purified GC-E-intra from E. coli was already phosphorylated, we pre-treated purified GC-E-intra with calf intestinal alkaline phosphatase. After removal of the phosphatase by chromatography, a phosphorylation assay was performed and no phosphorylation of GC-E-intra by PAK2 was observed (Fig. 4B). To demonstrate that GC-E-intra was phosphorylatable by a protein kinase, we showed that purified GC-E-intra could be phosphorylated by protein kinase A (Supplementary Fig. 2A). These data suggest that PAK does not work through direct phosphorylation of GC-E.
Figure 4.

Auto-phosphorylation of PAK is necessary for GC activation by PAK. A, PAK did not phosphorylate GC-E. Purified GST-PAK2, Rac1-GTPγS, and/or GC-E-intra-His6 were mixed as indicated. In each lane, γ-32P-ATP was included. The positions of GST-PAK2 and GC-E-intra-His6 are indicated on the right. B, PAK did not phosphorylate phosphatase-pretreated GC-E. C, Kinase-dead PAK1 could not stimulate GC-E and GC-D in cells. D, Phosphorylation state of purified PAK proteins. Purified GST-fusion proteins treated with or without calf intestinal alkaline phosphatase (CIP). These protein samples were then separated on SDS-PAGE and Western blotted with anti-phospho-Thr423-PAK1/phospho-Thr402-PAK2 antibody. E, Dephosphorylation of PAK abolished its stimulatory effect on GC. F. Purified GC-D-intra activation by purified PAK2 in the presence of purified Rac1-GTPγS or Cdc42-GTPγS. Purified PAK1(K298A/T423E) and phosphorylated-PAK1(K298A) could also activate GC-D-intra. Data are shown as mean ± s.d. of three experiments. **, P < 0.001; *, P < 0.005 (Student’s t-test).
We noticed that a PAK1 kinase-dead mutant [PAK1-(165-544)(K298A)] could not stimulate GC-E or GC-D in cells (Fig. 4C). Furthermore, purified PAK1(K298A) or PAK2(K278A) proteins did not activate purified GC-E-intra with or without Rac1-GTPγS (Fig. 3B). These data suggest that the kinase activity of PAK is needed for its stimulatory effect on GC. Since PAK does not directly phosphorylate GC, we examined the autophosphorylation of PAK. PAK1 can autophosphorylate itself on several serine and threonine residues including Thr423 (Thr402 in PAK2) at the activation loop of the catalytic domain. Phosphorylation of Thr423 is critical for the conformational change of the activation loop, assembly of the active configuration of the catalytic domain, and activation of PAK1 (Lei et al., 2000). Therefore, we analyzed the phosphorylation state of purified wild-type PAK2 and PAK1 as well as the kinase-dead PAK1(K298A) or PAK2(K278A) proteins. Western blots with anti-phospho-Thr423-PAK1/phospho-Thr402-PAK2 antibody showed that the wild-type PAK1 and PAK2 proteins were phosphorylated while PAK1(K298A) and PAK2(K278A) proteins were not (Fig. 4D). To investigate whether the phosphorylation of PAK is essential for its ability to activate GC, we treated the purified wild-type PAK2 protein with alkaline phosphatase. After removing the phosphatase through chromatography, we found that the de-phosphorylated PAK2 could no longer activate GC-E-intra (Fig. 4E). The de-phosphorylated PAK2 could still be activated by Rac1-GTPγS in the presence of ATP leading to Thr402 re-phosphorylation; this re-phosphorylated PAK2 regained its ability to stimulate GC-E-intra (Fig. 4E). In addition, we examined the effect of purified PAK2 on the activity of purified GC-D-intra. As shown in Fig. 4F, PAK2 (in the presence of Rac1-GTPγS) increased the activity of GC-D-intra. Although Cdc42 did not activate GC in cells, purified PAK2 activated by purified Cdc42-GTPγS increased GC-D-intra activity in vitro, as would be expected (Fig. 4F). It is not clear why Cdc42 does not activate GCs via PAK in cells. One possible explanation might be that the subcellular localization and/or duration of PAK activated by Rac and by Cdc42 are different. Furthermore, we mutated Thr423 of PAK1 to Glu to mimic the autophosphorylation after activation by Rac. As shown in Fig. 4F, purified PAK1(K298A/T423E) significantly increased the activity of GC-D-intra in the absence of Rac1-GTPγS. Moreover, we used immunoprecipitated myc-tagged PAK1 to phosphorylate GST-PAK1(K298A). This phosphorylated PAK1(K298A) increased the activity of GC-D-intra (Fig. 4F). Therefore, although PAK does not phosphorylate GC, its own phosphorylation is required for its ability to activate GC.
The Kinase Domain of PAK1 Directly Interacts with the Cyclase Domain of GC-E
Since GC-E-intra is not a substrate of PAK, we then used five different approaches to investigate the interaction of PAK and GC-E-intra. First, we expressed GFP-tagged GC-E-intra and myc-tagged full-length PAK1 in HEK-293 cells (Fig. 5A). While an anti-GFP antibody co-immunoprecipitated myc-PAK1 in GFP-GC-E-intra and myc-PAK1 co-transfected cells, this antibody did not co-immunoprecipitate myc-PAK1 in control cells co-transfected with GFP-GC-E-intra and myc vector, or with GFP and myc-PAK1 (Fig. 5A). Second, we examined the interaction of PAK and GC-E-intra in Sf9 cells (Fig. 5B). We generated recombinant baculoviruses harboring His6-tagged GC-E-intra or GST-tagged full-length PAK2. These recombinant baculoviruses were then used to co-infect the Sf9 cells. Glutathione agarose beads were used to pull-down GST or GST-PAK2 from the lysate. Western blots with anti-His6 antibodies revealed that GC-E-intra only co-precipitated with GST-PAK2, but not GST (Fig. 5B). These results demonstrate that, in both HEK-293 and Sf9 cells growing in the presence of serum (thus PAK is active), GC-E-intra forms a complex with PAK. Third, we examined the interaction of endogenous PAK and endogenous GC by using a co-immunoprecipitation assay. Endogenous GC-A from MEF cells was immunoprecipitated by antibodies directed against the C-terminus of GC-A (Fig. 5C). [The specificity of this GC-A antibody was also verified in GC-A transfected HEK-293 cells (Supplementary Fig. 2B).] Immunoblot analysis with anti-PAK1 antibody showed that PAK1 was found in the immunoprecipitate obtained with anti-GC-A antibody, but not in the immunoprecipitate obtained with control antiserum (Fig. 5C). Conversely, anti-PAK1 antibody co-immunoprecipitated GC-A in MEF cells (Fig. 5D). These results show that PAK1 interacts with GC-A in vivo.
Figure 5.
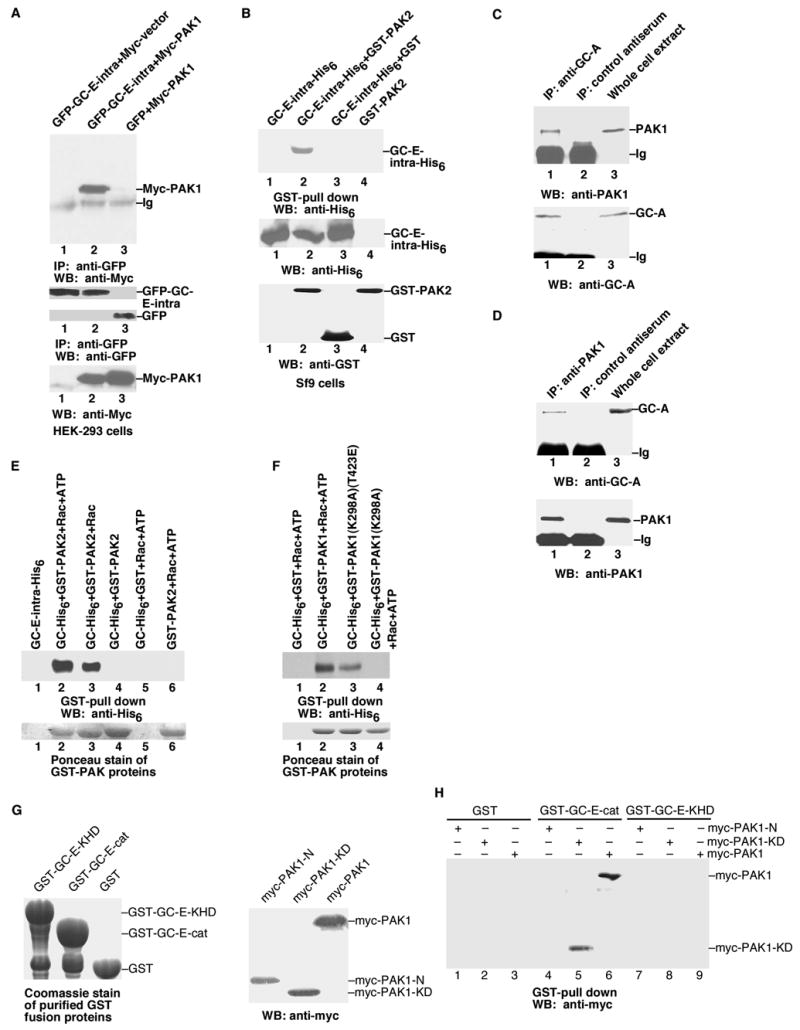
Direct interaction of PAK and GC-E. A, Co-immunoprecipitation of PAK1 and GC-E in HEK-293 cells. Bottom panels: anti-myc or anti-GFP antibodies were used to detect the protein expression of transfected genes in whole cell extracts. B, Co-immunoprecipitation of PAK2 and GC-E in Sf9 cells. C, PAK1 interacts with GC-A in vivo. Whole-cell lysates (MEF cells transfected with Rac1(G12V) to activate endogenous PAK) were subjected to immunoprecipitation with anti-GC-A antibody and immunoprecipitated proteins were analysed by immunoblotting with anti-PAK1 antibody. D, Anti-PAK1 antibody co-immunoprecipitated GC-A from MEF cells. Conditions were the same as in C. E and F, Interaction of purified PAK with purified GC-E in the presence of Rac-GTPγS. G Left panel: Coomassie staining of purified GST-GC-E-KHD (the kinase homology domain, residues 488 to 829), GST-GC-E-cat (the catalytic cyclase domain, residues 830 to 1059), or GST proteins used in the interaction assays. Right panel: Western blot with anti-myc antibody to show the expression of transfected myc-PAK1-N (residues 1 to 270), myc-PAK1-KD (residues 271 to 544), and myc-PAK1 in HEK-293 cells used for the interaction assays. H, Interaction of GC-E and PAK1. Purified GST, GST-GC-E-cat, or GST-GC-E-KHD proteins were mixed with lysates from HEK-293 cells transfected with myc-PAK1-N, myc-PAK1-KD, or myc-PAK1. Data are representative of three to four similar experiments.
Fourth, we studied the direct interaction with purified GST-PAK2 and purified His6-tagged GC-E-intra as well as purified His6-tagged GC-D-intra (Fig. 5E and Supplementary Fig. 2C). In the absence of Rac1-GTPγS, we did not observe any direct interaction of PAK2 and GC-E-intra (Fig. 5E, lane 4). On the other hand, when Rac1-GTPγS was included, PAK2 formed a complex with GC-E-intra, with or without ATP (Fig. 5E, lanes 2 and 3). Hence, a conformation of activated PAK, which can be achieved by the addition of Rac1-GTPγS, is needed for its interaction with GC-E-intra. Fifth, we further characterized the interaction by mapping the interacting domains on both PAK1 and GC-E (Fig. 5G and 5H). We purified GST-fusion proteins of the kinase-homology domain (residues 488 to 829) of GC-E (GST-GC-E-KHD) and the catalytic cyclase domain (residues 830 to 1059) of GC-E (GST-GC-E-cat). These GST-fusion proteins and the control GST were used to pull-down PAK1 from cell extracts prepared from HEK-293 cells transfected with myc-tagged full-length PAK1, myc-tagged N-terminal regulatory domain of PAK1 (residues 1 to 270), or myc-tagged PAK1 kinase domain (residues 271 to 544). We found that the kinase domain of PAK1 directly interacted with the catalytic cyclase domain of GC-E (Fig. 5H). Therefore, similar to the allosteric activation of transmembrane adenylyl cyclases by G proteins, PAK likely activates GC-E by direct contact with the cyclase domain to induce a conformational change.
GC is Required for PDGF-induced Fibroblast Cell Migration
We then studied the physiological significance of the Rac/cGMP signaling pathway. Since Rac1 and PAK are known to be critical players in cell migration although the detailed mechanism is not completely defined (Jaffe and Hall, 2005), we reasoned that Rac1/PAK could use multiple pathways or feedback loops to control cell migration, and that Rac1/PAK signaling through GC/cGMP is one of them. Indeed, cGMP, the product of GCs, is known to play a role in cell migration (Estensen et al., 1973). If that is the case, a decrease of the GC protein levels in cells by RNA interference should impair cell migration. We investigated this possibility in MEF cells. MEF cells mainly express endogenous GC-A (with low levels of GC-B and no GC-E) (Fig. 6A; data not shown). While a mouse GC-A specific siRNA reduced the protein level of GC-A, a control siRNA did not (Fig. 6A). [With a rhodamine-labeled control siRNA, the transfection efficiency was more than 90%.] Furthermore, the successful reduction of GC-A protein level by RNAi was confirmed by a functional assay: the cGMP production induced by the activation of GC-A with the extracellular ligand atrial natriuretic peptide (ANP) in GC-A siRNA-treated MEF cells was lower than that in control cells (Fig. 6B). Similarly, PDGF-induced cGMP increase was reduced by GC-A siRNA treatment and GC-A RNAi did not induce apoptosis or inhibit cell proliferation during the time course of cell migration assays (Fig. 6C, and data not shown).
Figure 6.

Role of GC-A in PDGF-induced cell migration. A, Western blot with anti-GC-A antibody showing the reduction of GC-A protein levels in GC-A siRNA cells compared to cells treated with a control siRNA. Bottom panels: Western blots with anti-tubulin, anti-Rac1, or anti-PAK1 antibodies show no changes in control and GC-A siRNA cells. B, ANP-induced accumulation of cellular cGMP was reduced in GC-A siRNA cells compared to cells transfected with a control siRNA. C, GC-A siRNA treatment reduced the cGMP levels induced by PDGF, compared to cells treated with a control siRNA. D, GC-A siRNA treatment, expression of dominant-negative Rac1(T17N) or PAK1-(83–149) reduced the cell migration induced by PDGF in a wound healing assay. E, GC-A siRNA treatment and expression of dominant-negative Rac1 or PAK1 mutant proteins reduced the cell migration induced by PDGF in a Boyden chamber assay. F, Re-expression of human GC-A in MEF cells treated with mouse GC-A siRNA rescued the cell migration induced by PDGF. G, GC-A siRNA treatment and expression of dominant-negative PAK1 mutant proteins reduced Rac1(G12V) induced cell migration. H, Expression of constitutively active Rac1(G12V) or PAK1-(165–544) increased the endogenous cGMP levels in MEF cells and HeLa cells. I, Time-lapse recordings of MEF cells treated with a control siRNA or GC-A siRNA. The position of cells at 0 min (before PDGF addition) was marked as red. The position of cells at ~3 hours was marked as green. Data are representative of three to four similar experiments. **, P < 0.001; *, P < 0.005; ++, P < 0.01 (Student’s t-test).
Having successfully reduced the GC-A protein level by RNAi, we used two different approaches to investigate whether the reduction of GC-A protein level would interfere with PDGF-induced cell migration. First, in an in vitro wound-healing assay, while PDGF induced MEF cell migration, the migration of GC-A siRNA-treated cells was reduced (Fig. 6D). These data demonstrated that GC-A is important for PDGF-induced cell migration. Second, we further confirmed the role of GC in PDGF-induced cell migration by Boyden chamber assay (Fig. 6E). Similar results were observed when a second different siRNA against GC-A was used (Supplementary Fig. 3A and 3B). Furthermore, PDGF-induced MEF cell migration depends on Rac and PAK since expression of dominant-negative Rac1(T17N) and PAK1-(83–149) proteins reduced the cell migration (Fig. 6D and 6E). Moreover, re-expression of human GC-A in mouse GC-A siRNA-treated MEF cells rescued the PDGF-induced cell migration (there are 6 nucleotide mismatches between mouse and human GC-As in the sequence against which the siRNA was designed) (Fig. 6F). In addition, GC-A siRNA inhibited Rac1(G12V)-induced cell migration (Fig. 6G). Also, expression of dominant-negative PAK1-(83–149) proteins reduced Rac1(G12V)-induced cell migration (Fig. 6G). Moreover, expression of constitutively active Rac1(G12V) or PAK1-(165–544) increased cellular cGMP levels in MEF cells and in HeLa cells (Fig. 6H). Additionally, from time-lapse recordings, control siRNA-treated MEF cells migrated in response to PDGF (Fig. 6I and Supplementary Movie 1). On the other hand, most GC-A siRNA-treated cells did not move much (Fig. 6I and Supplementary Movie 2 and 3). ANP treatment alone or expression of constitutively active PAK alone was not sufficient to induce MEF cell migration, and dominant-negative mutants of Rac1 and PAK1 did not interfere with the direct activation of GC-A by ANP (data not shown). Thus, an increased cGMP level (or a gradient) is required, but by itself not sufficient, for cell migration. Together, these data show that GCs, like Rac and PAK, play a critical role in cell migration.
Role of GC in PDGF-induced Lamellipodium formation
To further explore the molecular mechanism by which GC is required for PDGF-induced fibroblast cell migration, we studied the potential role for the Rac/cGMP pathway in lamellipodium formation. Lamellipodia are protruding membrane structures at the leading edge of migrating cells. Rac1 and PAK have been shown to mediate PDGF-induced lamellipodium formation (Ridley et al., 1992; Sells et al., 1999). We thus investigated whether the Rac/cGMP pathway is involved in lamellipodium formation. As shown in Fig. 7A and 7B, migrating MEF cells (treated with a control siRNA) at the edge of a wound in the presence of PDGF displayed lamellipodia (~85% of the cells). Significantly, GC-A siRNA treatment led to the reduction of lamellipodia in the presence of PDGF (~25% of cells with lamellipodia). Furthermore, expression of constitutively active HA-tagged Rac1(G12V) induced the formation of lamellipodia or membrane ruffles (in ~100% of infected cells) (Fig. 7C and 7D). The infected cells were identified by immunostaining with the anti-HA antibody (shown in the inserts of the figures). This effect was inhibited by GC-A RNAi treatment (only ~10% of Rac1(G12V) infected cells displayed lamellipodia) (Fig. 7C and 7D). Moreover, although ANP alone was not sufficient to induce MEF cell migration, it induced transient formation of lamellipodia (Fig. 7E). These data suggest that GC is involved in PDGF and Rac1 induced lamellipodium formation.
Figure 7.

GC-A is required for PDGF-induced lamellipodium formation. A, PDGF (20 ng/ml for 30 min) induced the formation of lamellipodia (indicated by arrowheads) in control siRNA-treated cells at the edge of the wound. GC-A siRNA treatment blocked PDGF-induced lamellipodium formation. Data are representative of three to four similar experiments. B, Quantification of the data from experiments in A. C, GC-A RNAi treatment inhibited the formation of lamellipodia induced by HA-tagged constitutively active Rac1 [Rac1(G12V)]. Infected cells were identified with anti-HA antibody immuno-staining (inserts). Data are representative of three to four similar experiments. D, Quantification of the data from experiments in C. E, While PDGF induced persistent lamellipodia (indicated by arrowheads), ANP (10 μM) induced transient formation of lamellipodia (at 10 min). F, Cells were fixed after treated with or without PDGF (20 ng/ml for 2 hours) after wounding and stained with anti-tubulin antibodies (Red), anti-pericentrin antibodies (to visualize MTOC)(Green), and Hoechst (to visualize the nucleus)(Blue). Left panels: low magnification. Right panels: high magnification. The large arrow indicates the direction of the wound. G, The percentage of cells at the wound edge with their MTOC facing the wound was measured. A minimum of 100 cells were scored. H, Schematic diagram of the activation of membrane-bound GCs by intracellular signals. Error bars show mean ± s.d., **, P < 0.001 (Student’s t-test).
We also investigated the potential effect of GC on cell polarity. In the presence of PDGF, cells at the wound edge become polarized and extend a long protrusion into the wound. The microtubule organizing center (MTOC) (stained with anti-pericentrin antibodies) and the microtubule cytoskeleton (stained with anti-tubulin antibodies) reorganize to face the wound (Etienne-Manneville and Hall, 2001)(Fig. 7F). GC-A RNAi did not affect the cell polarity. In both control siRNA-treated cells and GC-A siRNA-treated cells, PDGF induced 60~70% of cells in the front row to exhibit a polarized MTOC (Fig. 7G). In the absence of PDGF, random orientation of the MTOC facing the wound corresponds to ~30% of cells (Fig. 7G). Therefore, GC-A RNAi did not prevent the formation of protrusions and the polarization of the MTOC.
DISCUSSION
We have uncovered a new cellular signaling pathway that links the small GTPase Rac and the second messenger cGMP (Fig. 7H). Since Rac and PAK have been linked to various receptor signaling, this new Rac/PAK/GC/cGMP pathway could provide a general mechanism for various types of signaling receptors to increase the cellular concentrations of cGMP. We have shown here that PAK directly increases the activity of purified GC. Other purified protein kinases including PKC, PKA, Src, Abl, or Csk had no effect on the activity of GC-E or GC-D in vitro (unpublished data), suggesting that the effect on GCs is rather specific to PAK kinases. PAK does not phosphorylate GC. Rather, PAK contacts the cyclase domain of GC, likely causing a conformational change and activation of GC. This allosteric mechanism (direct contact and conformational change) is similar to the proposed mechanisms for the activation of membrane-bound GCs by their ligands such as ANP, of soluble GCs by NO, and adenylyl cyclases by heterotrimeric G proteins (Lucas et al., 2000; Tesmer et al., 1997).
The phosphorylation-independent function of PAK1 has been described before although the molecular mechanism was not known (Bokoch, 2003; Daniels et al., 1998; Frost et al., 1998; Sells et al., 1997). For example, PAK1 induces lamellipodium formation and membrane ruffling in fibroblasts and neurite outgrowth in PC12 cells, independent of its catalytic activity (Daniels et al., 1998; Frost et al., 1998). Furthermore, a recent crystal structure of the dimer of the kinase domain of EGFR could provide a potential structural basis for how the PAK kinase domain allosterically activates GCs (Zhang et al., 2006). In this asymmetric dimer of the EGFR kinase domain, one kinase domain (using its C-terminal lobe) allosterically activates the second kinase domain (by contacting the N-terminal lobe and changing the conformation of the active site of the second kinase domain into the active state) (Zhang et al., 2006). This activation does not involve the phosphorylation of one kinase domain by the other kinase domain. The first kinase domain acts as an allosteric activator.
Transmembrane GCs could serve dual roles to increase cellular cGMP levels to different degrees, thus leading to possible different, sometimes opposing, physiological effects. Extracellular ligands such as ANP bind to transmembrane GCs and activate their cyclase activity leading to ~100–1000-fold increase of cellular cGMP levels. On the other hand, growth factor receptors and other membrane receptors could use the Rac/cGMP pathway or the GCAP proteins in retinal cells to stimulate the cyclase activity of transmembrane GCs from the interior of the cell leading to ~3–10-fold increase of cellular cGMP levels. As the effect of cGMP on physiological processes is dependent on cGMP concentrations (Elferink and VanUffelen, 1996), it is conceivable that activation of transmembrane GCs by growth factors and by ligands such as ANP could lead to different responses, depending on the cell type and the spatio-temporal dynamics of cGMP in living cells.
PAKs regulate a variety of physiological processes such as cell migration, morphological changes, gene expression, cell proliferation, and differentiation. Here we have shown that the PAK/GC/cGMP pathway is part of the regulatory mechanism by which PDGF induces fibroblast cell migration. The molecular mechanism of action of cGMP in cell migration is still poorly understood. One of the major targets of cGMP is cGMP-dependent protein kinase (PKG). A major substrate of PKG is VASP (vasodilator-stimulated phosphoprotein). Cells overexpressing VASP have lamellipodia that protrude faster than control cells, but these protrusions are rapidly withdrawn (Krause et al., 2003). Thus, VASP increases lamellipodium protrusion velocity but has an overall negative effect on whole-cell motility (Krause et al., 2003). PKG phosphorylation of VASP is proposed to relieve this negative effect on cell migration (Krause et al., 2003). There are other potential targets for cGMP in regulating cell migration. cGMP could regulate Ca2+ influx, intracellular Ca2+ concentration, myosin phosphatase activation and myosin phosphorylation (Sauzeau et al., 2000; Schlossmann et al., 2000; Surks et al., 1999). Furthermore, cGMP could positively regulate the activity of Rac and PAK, forming a positive feedback loop. 8-Br-cGMP, through PKG, could activate Rac and PAK in HEK293 cells (Hou et al., 2004). Although PKG did not directly phosphorylate Rac, they colocalize on membrane ruffles (Hou et al., 2004). This feedback regulation might be one of the many feedback loops that are essential for the repetitive behavior of cell migration.
The new Rac/cGMP pathway might have implications for studies on axonal guidance. The control of actin cytoskeletal reorganization lies at the core of axon guidance. Rho family GTPases play a key role in transmitting extracellular signals to changes in actin cytoskeletal structure and motility in growth cones. In Drosophila photoreceptor axonal guidance, both Rac and PAK have been genetically demonstrated to play critical roles (Hing et al., 1999; Newsome et al., 2000). However, the downstream signaling molecules have not yet been defined. On the other hand, in Xenopus spinal neurons and rat sensory neurons, the ratio of cAMP/cGMP is important in axonal guidance, yet the upstream signaling components modulating the cyclic nucleotide levels within neurons are not clear (Nishiyama et al., 2003; Song et al., 1998). Furthermore, the Drosophila transmembrane GC Gyc76C is essential for motor axon guidance downstream of Semaphorin-1a-Plexin A signaling (Ayoob et al., 2004). Moreover, mouse genetic studies have shown that cGMP-mediated signaling through PKG is required for spinal cord sensory neuron guidance and connectivity (Schmidt et al., 2002). Our finding that Rac/PAK regulates GC/cGMP provides new insights that could potentially unify these seemingly different axonal guidance pathways. Moreover, our discovery of the Rac/PAK/GC/cGMP pathway opens the way for further studies on the role of cGMP in Rac/PAK signaling, and of Rac/PAK in cGMP signaling in various biological processes.
EXPERIMENTAL PROCEDURES
Cell Culture and Co-Immunoprecipitation
CHO-GC cells were generated by stably transfecting CHO-K1 cells with plasmids encoding GCs. CHO-K1 cells were cultured in F12K nutrient mixture (Kaighn’s modification) containing 10% fetal bovine serum (Invitrogen). Transfections of CHO-K1 cells were performed with Transfast (Promega). HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. Transfections of HEK293 cells were performed using either calcium phosphate or Lipofectamine2000 (Invitrogen). MEF cells were routinely cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum.
Generation of Rac1-deficient cells from Rac1flox/flox mice has been described before (Gu et al., 2003; Guo et al., 2006). MEF cells isolated from E13.5 Rac1flox/flox mouse embryos were transduced with MIEG3-Cre retroviruses (with IRES-EGFP). EGFP+ cells were isolated using a FACS sorter. Deficiency of Rac1 was verified by western blot analysis with anti-Rac1 monoclonal antibody (Upstate Biotechnology). GC-D was transfected into these cells. For the re-expression of Rac1, retroviruses containing Rac1 were used to infect Rac1−/− MEF cells.
Co-immunoprecipitations and western-blots were performed as previously described (Ma et al., 2000; Wan et al., 1996). For the co-immunoprecipitation experiments of PAK1 and GC-A, anti-GC-A antibody was from FabGennix Inc. International (Shreveprot, LA) (Cat. # PGCA-100). Anti-PAK1 antibody was from Cell Signaling Technology (MA) (Cat. # 2602). The anti-pPAK1(T423)/pPAK2(T402) antibody was from Cell Signaling Technology (MA) (Cat. # 2601S).
Protein Purification
GST-tagged intracellular domain of GC-E (GST-GC-E-intra) was purified from E. coli. Histidine-tagged GC-E-intra protein was purified from High Five insect cells (Invitrogen). Baculoviruses containing GST-PAK1, GST-PAK2, GST-PAK1(K298A), GST-PAK2(K278A), GST-PAK1-KD(K298A), and GST-PAK1(K298A/T423E) were amplified in Sf9 cells and then used to infect 2 L of High-Five cells. The GST-tagged protein was purified using glutathione beads, followed by a Sephadex G-200 column (Pharmacia). His-tagged Rac1 was purified from E. coli using Ni-NTA beads (Qiagen). Rac1 was activated by incubating with 100 μM of GTPγS in the loading buffer (20 mM Tris, pH 8, 100 mM NaCl, 0.2 mM DTT, 1 mM EDTA) for 10 min at 30°C.
GST Pull-Down Assay
GST pull-down assays were done as previously described with purified proteins or with baculovirus-infected Sf9 cell lysates (Lowry et al., 2002; Ma et al., 2000).
Guanylyl Cyclase Activity Assay
In vivo cGMP concentrations were determined from cell lysates with the Direct cGMP Assay Kit from Assay Designs Inc. In general, cells were incubated for 18–24 hrs after transfection before the medium was changed to serum-free medium. Transfection efficiency of CHO cells was ~90% based on transfection with a GFP plasmid. After 24-hrs of starvation, cells were incubated with 0.5 mM IBMX for 30 min. Cells were washed once with ice-cold 1X PBS and lysed in 0.5% Triton X-100 containing 0.5 mM IBMX. We empirically determined the dilution factor for each cell lysate so that the experimental readings fall in the middle of the linear standard curve of the Direct cGMP Assay Kit.
For the in vitro guanylyl cyclase activity assay, purified proteins were mixed in 50 μl of reaction buffer (50 mM Hepes, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1 mM DTT, 1 mM GTP) and incubated for 20 min at 30°C. GTP was added last to start the reaction (there was no detergent in these reactions). The reaction was terminated by adding HCl to a final concentration of 0.1 M. The mixture was diluted 5–10 fold in 0.1 M HCl and the cGMP content was determined with the Direct cGMP Assay Kit from Assay Designs Inc.
RNA Interference
RNAi of GC-A was performed in MEF cells with Qiagen’s HiPerformance 2-for-Silencing siRNA duplexes. The target sequence for the first siRNA was CCGGACCACTACACCAAGCTA. There are 6 nucleotide differences between human and mouse GC-A in this region. The target sequence for the second siRNA was AACGCATTGAGTTGACACGAA. siRNA and Oligofectamine were diluted and mixed in OptiMEM I (Invitrogen) to allow the formation of siRNA-liposome complexes. After a 20-min incubation at room temperature, the mixture was overlayed onto MEF cells (50% confluent) cultured in medium without antibiotics. The transfected cells were analyzed 72–96 hr after transfection.
In Vitro Phosphorylation Assay
Phosphorylation assay was done as previously described (Wan et al., 1996).
Wound Healing Assays and Chamber Assay
In vitro wound-healing assays and Boyden chamber assays were performed as previously described (Shan et al., 2005; Yang and Huang, 2005).
Fluorescence microscopy
Fluorescence microscopy was done as previously described (Lowry et al., 2002).
MTOC Reorientation
MTOC reorientation (cell polarization) was analyzed as previously described (Etienne-Manneville and Hall, 2001). MTOCs were labelled with anti-pericentrin antibodies. Cells in which the MTOC was within the quadrant facing the wound were scored positive, and for each condition at least 100 cells were examined.
Supplementary Material
Acknowledgments
We thank A. Dizhoor for the GC-E and GC-F plasmids, M. H. Cobb for the PAK1 plasmids, D. Garbers for the cDNAs for GC-D, α1 and β1 subunits of soluble GC, and M. Lei for the pGEX-PAK1(K298A) plasmid. We are grateful to M. Lei, L. Levin, and members of our laboratory for critically reading the manuscript. This work was supported by grants (GM56904, AG14563 and AG23202) from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayoob JC, Yu HH, Terman JR, Kolodkin AL. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci. 2004;24:6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley JK, Shimomura H, Garbers DL. Retention of a functional resact receptor in isolated sperm plasma membranes. Cell. 1986;45:281–288. doi: 10.1016/0092-8674(86)90392-2. [DOI] [PubMed] [Google Scholar]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, Van Haastert PJ. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. Embo J. 2002;21:4560–4570. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, Konicek S, Hom J, Marshall M, Graff JR. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- Coffey RG, Davis JS, Djeu JY. Stimulation of guanylate cyclase activity and reduction of adenylate cyclase activity by granulocyte-macrophage colony-stimulating factor in human blood neutrophils. J Immunol. 1988;140:2695–2701. [PubMed] [Google Scholar]
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. Embo J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink JG, VanUffelen BE. The role of cyclic nucleotides in neutrophil migration. Gen Pharmacol. 1996;27:387–393. doi: 10.1016/0306-3623(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Estensen RD, Hill HR, Quie PG, Gogan N, Goldberg ND. Cyclic GMP and cell movement. Nature. 1973;245:458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J Biol Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Guo F, Debidda M, Yang L, Williams DA, Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J Biol Chem. 2006;281:18652–18659. doi: 10.1074/jbc.M603508200. [DOI] [PubMed] [Google Scholar]
- Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117:4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- Hou Y, Ye RD, Browning DD. Activation of the small GTPase Rac1 by cGMP-dependent protein kinase. Cell Signal. 2004;16:1061–1069. doi: 10.1016/j.cellsig.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- L'Etoile ND, Bargmann CI. Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron. 2000;25:575–586. doi: 10.1016/s0896-6273(00)81061-2. [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Huang J, Ma YC, Ali S, Wang D, Williams DM, Okada M, Cole PA, Huang XY. Csk, a critical link of g protein signals to actin cytoskeletal reorganization. Dev Cell. 2002;2:733–744. doi: 10.1016/s1534-5807(02)00175-2. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson BJ. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;424:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS, et al. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Scheving LA, Scheving LE, Tsai TH, Vesely DL. Epidermal growth factor enhances guanylate cyclase activity in vivo and in vitro. Endocrinology. 1985;116:332–336. doi: 10.1210/endo-116-1-332. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Werner M, Heppenstall PA, Henning M, More MI, Kuhbandner S, Lewin GR, Hofmann F, Feil R, Rathjen FG. cGMP-mediated signaling via cGKIalpha is required for the guidance and connectivity of sensory axons. J Cell Biol. 2002;159:489–498. doi: 10.1083/jcb.200207058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang XY. Synthetic analogues of migrastatin that inhibit mammary tumor metastasis in mice. Proc Natl Acad Sci U S A. 2005;102:3772–3776. doi: 10.1073/pnas.0500658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. see comments. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- Wan Y, Kurosaki T, Huang XY. Tyrosine kinases in activation of the MAP kinase cascade by G-protein- coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- Yang S, Huang XY. Ca2+ Influx through L-type Ca2+ Channels Controls the Trailing Tail Contraction in Growth Factor-induced Fibroblast Cell Migration. J Biol Chem. 2005;280:27130–27137. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


