Abstract
Growth factor signaling is usually analyzed in isolation without considering the effect of ligand occupancy of transmembrane proteins other than the growth factor receptors themselves. In smooth muscle cells, the transmembrane protein Src homology 2 domain containing protein tyrosine phosphatase substrate-1 (SHPS-1) has been shown to be an important regulator of insulin-like growth factor-I (IGF-I) signaling. SHPS-1 is phosphorylated in response to IGF-I, leading to recruitment of Src homology 2 domain tyrosine phosphatase (SHP-2). Subsequently, SHP-2 is transferred to IGF-I receptor and regulates the duration of IGF-I receptor phosphorylation. Whether ligand occupancy of SHPS-1 influences SHPS-1 phosphorylation or SHP-2 recruitment, thereby altering growth factor signaling, is unknown. Previous studies have shown that integrin associated protein (IAP) associates with SHPS-1. We undertook these studies to determine whether this interaction controlled SHPS-1 phosphorylation and/or SHP-2 recruitment and thereby regulated IGF-I signaling. Disruption of IAP-SHPS-1 binding, by using an IAP monoclonal antibody or cells expressing mutant forms of IAP that did not bind to SHPS-1, inhibited IGF-I–stimulated SHPS-1 phosphorylation and SHP-2 recruitment. This was associated with a lack of SHP-2 transfer to IGF-I receptor and sustained receptor phosphorylation. This resulted in an inability of IGF-I to stimulate sustained mitogen-activated protein kinase activation, cell proliferation, and cell migration. The effect was specific for IGF-I because disruption of the IAP–SHPS-1 interaction had no effect on platelet-derived growth factor-stimulated SHPS-1 phosphorylation or cell migration. In summary, our results show that 1) ligand occupancy of SHPS-1 is a key determinant of its ability to be phosphorylated after IGF-I stimulation, and 2) the interaction between IAP and SHPS-1 is an important regulator of IGF-I signaling because disruption of the results in impaired SHP-2 recruitment and subsequent inhibition of IGF-I–stimulated cell proliferation and migration.
INTRODUCTION
Insulin-like growth factor-I (IGF-I) is a potent stimulator of smooth muscle cell (SMC) migration and proliferation (Jones et al., 1996). There is increasing evidence to show that the ability of IGF-I to initiate intracellular signaling is regulated not only by its association with its own transmembrane receptor but also by other transmembrane proteins such as the αVβ3 integrin (Zheng and Clemmons, 1998; Maile and Clemmons, 2002a), integrin associated protein (IAP) (Maile et al., 2002), and Src homology 2 domain containing protein tyrosine phosphatase substrate-1 (SHPS-1) (Maile and Clemmons, 2002b).
SHPS-1 was identified as a tyrosine-phosphorylated protein that binds to SHP-2 in v-SRC–transformed fibroblasts (Noguchi et al., 1996) and in insulin-stimulated Chinese hamster ovary cells (Fujioka et al., 1996). The cytoplasmic region of SHPS-1 contains two immunoreceptor tyrosine-based inhibitory motifs (Kharitonenkov et al., 1997) that are phosphorylated in response to various mitogenic stimuli (Noguchi et al., 1996; Kharitonenkov et al., 1997; Stofega et al., 1998; Maile and Clemmons, 2002b) and integrin-mediated cell attachment (Fujioka et al., 1996; Noguchi et al., 1996; Takada et al., 1998). This phosphorylation generates binding sites for the recruitment and activation of Src homology 2 domain tyrosine phosphatase (SHP-2) that in turn dephosphorylates SHPS-1 (Fujioka et al., 1996; Noguchi et al., 1996; Kharitonenkov et al., 1997; Takada et al., 1998).
In stably attached smooth muscle cells (SMCs), SHP-2 is localized to a site close to the cell membrane from where it is transferred to the SHPS-1 after IGF-I–stimulated SHPS-1 phosphorylation (Maile and Clemmons, 2002b). This recruitment of SHP-2 is followed by the dephosphorylation of SHPS-1 and the transfer of SHP-2 to the IGF-I receptor (IGF-IR) where it subsequently dephosphorylates this substrate (Maile and Clemmons, 2002b). The importance of SHPS-1 phosphorylation in regulating IGF-IR phosphorylation has been demonstrated in cells expressing a truncated form of SHPS-1 in which the SHP-2 binding sites have been deleted. In these cells, transfer of SHP-2 to both SHPS-1 and the IGF-IR is blocked and sustained phosphorylation of both molecules is evident (Maile and Clemmons, 2002b).
IAP was first identified by its ability to associate with αVβ3 (Brown et al., 1990) and to increase the affinity of the integrin for its ligands (Brown et al., 1990). IAP consists of a N-terminal (extracellular) Ig variable type domain followed by five membrane spanning hydrophobic helices and a cytoplasmic tail (Rosales et al., 1992).
IAP has been shown to bind to SHPS-1 (Jiang et al., 1999; Seiffert et al., 1999; (Babic et al., 2000; Oldenborg et al., 2000; Vernon-Wilson et al., 2000; Yoshida et al., 2002). The amino terminal Ig domain of IAP and the extracellular Ig variable domain of SHPS-1 are sufficient for their physical interaction (Seiffert et al., 1999; Vernon-Wilson et al., 2000). Although disruption of IAP–SHPS-1 interaction has been shown to result in changes in cellular function (Babic et al., 2000; Oldenborg et al., 2000; Vernon-Wilson et al., 2000) the effect of IAP binding to SHPS-1 on growth factor-stimulated SHPS-1 phosphorylation and SHP-2 recruitment has not been reported. The first aim of these studies was to determine whether an association between IAP and SHPS-1 could be detected in SMCs. Second, we wished to determine the effect of IAP association with SHPS-1 on IGF-I–stimulated SHPS-1 phosphorylation and subsequent SHP-2 recruitment and then to examine the effect of disrupting the interaction between IAP and SHPS-1 on IGF-IR–dependent biological actions in SMCs.
MATERIALS AND METHODS
Human IGF-I was a gift from Genentech (South San Francisco, CA) and polyvinyldifluoride membrane (Immobilon P) was purchased from Millipore (Bedford, MA). Autoradiographic film was obtained from Eastman Kodak (Rochester, NY). Fetal bovine serum, Dulbecco's modified medium, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). The IGF-IR β chain antibody and the monoclonal phosphotyrosine antibody (PY99) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal SHP-2 and SHPS-1 antibodies were purchased from Transduction Laboratories (Lexington, KY). The monoclonal antibody (mAb) against IAP, B6H12, was purified using protein G-Sepharose from Pierce Chemical (Rockford, IL) according to the manufacturer's protocol from B cell hybridoma-conditioned media that had been obtained from cells purchased from the American Type Culture Collection (Manassas, VA). The anti FLAG mAb was purchased from Sigma-Aldrich (St. Louis, MO). The antibody against the dual phosphorylated (active) form of p42/p44 mitogen-activated protein kinase (MAPK) and the antibody against total p42/p44 MAPK protein were purchased from Cell Signaling Technology (Beverly, MA). A mouse isotype-specific control (IgGi) was purchased from Sigma-Aldrich, and polyclonal antibody control sera was obtained in house after a central ear artery bleed of a nonimmunized rabbit. All other reagents were purchased from Sigma-Aldrich unless otherwise stated.
Porcine aortic SMCs (pSMCs) were isolated as described previously (Gockerman et al., 1995) and maintained in Dulbecco's modified medium supplemented with glucose (4.5 gm/l), penicillin (100 U/ml), streptomycin (100 μg/ml) (DMEM-H), and 10% fetal bovine serum (FBS) in 10-cm tissue culture plates (Falcon Laboratory, Franklin Lakes NJ). The cells were used between passage 5 and 16.
Generation of Expression Vectors
Full-Length Porcine IAP with a C-Terminal FLAG Epitope (IAPfl). Full-length porcine IAP was cloned by reverse transcription-polymerase chain reaction (PCR) from a cDNA library that had been derived from pSMCs that had been isolated as described previously (Gockerman et al., 1995). The 5′ primer sequence 5′-ATGTGGCCCTGGTGGTC corresponded to nucleotides 121–139 of the porcine sequence. The 3′-primer sequence was complementary to nucleotides 1005–1030 with the addition of bases encoding the FLAG sequence (underlined) and a stop codon. The sequence was 5′-TCATTTGTCGTCGTCGTCTTTGTAGTCGGTTGTATAGTCT 3′. After sequencing, the cDNA was cloned into the pcDNA V5 his 3.1 vector (Invitrogen).
IAP with Truncation of Extracellular Domain at Residue 135 and Containing a C-terminal FLAG Epitope (IAPcyto). The pcDNA V5 his 3.1 vector containing the IAPfl cDNA sequence was linearized and the mutant form of IAP was generated using PCR with a 5′ oligonucleotide encoding bases 527–556 (5′ TCTCCAAATGAAAAATCCTCATTGTTATT 3′) and the same 3′ oligonucleotide that was used to generate the IAPfl. The PCR product was cloned in to pcDNA V5 his 3.1.
IAP in which Cysteine 33 and 261 Are Substituted with Serine Residues Containing a C-Terminal FLAG Epitope (IAPc-s). The IAPfl cDNA was subcloned in a pRcRSV expression vector and it was used as a template to perform single-stranded mutagenesis to incorporate the two substitutions. The pRcRSV vector contains a neomycin derivative (G418) resistance gene and a bacteriophage origin of replication (F1) gene that permits direct single-stranded mutagenesis of the cDNA. Two oligonucleotides encoding the base substitutions were used. They were C33S, complementary to nucleotides 204–225, except for a base substitution to encode a serine (underlined) 5′ GTAACAGTTGTATTGGAAACGGTGAATTCTA 3′; and C261S, complementary to nucleotides 888–918, except for the base substitution to encode the serine residue (underlined) 5′ CCATGCACTGGGGTAGACTCTGAGACGCAG. After sequencing, the DNA constructs were subcloned into pMEP4 expression vector (Invitrogen).
Transfection of pSMCs
Cells that had been grown to 70% confluence were transfected with one of three IAP cDNA constructs as described previously (Imai et al., 1997). Hygromycin-resistant pSMCs were selected and maintained in DMEM-H containing 15% FBS and 100 μg/ml hygromycin as described previously (Imai et al., 1997). Expression of protein levels was assessed by preparing whole cell lysates and visualizing FLAG protein expression by immunoblotting as described below. Transfected pSMCs that were obtained from two transfections performed independently were used in subsequent experiments and the results that were obtained were consistent between the two groups of cells.
IGF-I Stimulation of Cells
Quiescent SMCs were pretreated with either B6H12 or IgGi (both at a concentration of 4 μg/ml) in serum free medium (SFM). Cells were then exposed to IGF-I (100 ng/ml) or to platelet-derived growth factor (PDGF) (10 ng/ml) for appropriate lengths of time.
Immunoprecipitation and Immunoblotting
Tyrosine phosphorylation and association between proteins was determined by immunoprecipitation and immunoblotting of cell lysates as we have described previously (Maile and Clemmons, 2002a). The lysis buffer contained: 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EGTA plus 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 1 μg/ml aprotinin. For the immunoprecipitation cell lysates were incubated overnight at 4°C with the appropriate antibody; IGF-IR, SHPS-1 nonimmune rabbit serum as a control or B6H12 or IgGi by using a 1:500 dilution. For immunoblotting, membranes were incubated with one of six primary antibodies (IGF-IR, SHP-2, SHPS-1, PY99, B6H12 or FLAG, all using a 1:500 dilution) overnight at 4°C. Binding of the peroxidase labeled secondary antibody was visualized using enhanced chemiluminescence following the manufacturer's instructions (Pierce Chemical), and the immune complexes were detected by exposure to autoradiographic film or by using the GeneGnome charge-coupled device imaging system (Syngene, Cambridge, UK).
Chemiluminescent images obtained were scanned using a DuoScan T1200 (AGFA Brussels, Belgium), and band intensities of the scanned images were analyzed using NIH Image, version 1.61. The Student's t test was used to compare differences between treatments. The results that are shown are representative of at least three separate experiments
Assessment of p42/p44 MAPK Activation
The effect of B6H12 on IGF-I activation of p42/44 MAPK was determined, as we have described previously (Imai and Clemmons, 1999), by immunoblotting of cell lysates with an antibody that is specific for the dual phosphorylated (threonine202 and tyrosine204) protein (at a dilution of 1:1000).
Cell Wounding and Migration Assay
Wounding of cell monolayers that had been grown to confluence over 7 days was performed as described previously (Jones et al., 1996). The wounded monolayers were incubated with SFM (plus 0.2% FBS) with or without 100 ng/ml IGF-I or PDGF (10 ng/ml). The cells were then fixed and stained (Diff Quick; Dade Behring, Newark, DE), and the number of cells migrating into the wound area was counted. At least five of the previously selected 1 mm areas at the edge of the wound were counted for each data point.
Assessment of Cell Proliferation
The effect of B6H12 or an IgGi (4 μg/ml) on IGF-I–stimulated cell proliferation was carried out essentially as we have described previously (Nam et al., 2002). A 2-h preincubation in the presence or absence of B6H12 or an IgGi (4 μg/ml) was followed by the addition of IGF-I (100 ng/ml). Cells were incubated for 48 h, and final cell number in each well determined. Each treatment was performed in triplicate The Student's t test was used to compare differences between treatments. The results that are shown represent the mean (± SEM) from three separate experiments.
RESULTS
IAP Associates with SHPS-1 in Stably Attached pSMCs via Its Extracellular Domain
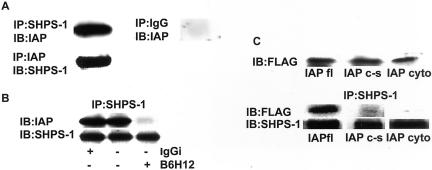
Figure 1A shows that in stably attached quiescent SMCs, there is detectable association between IAP and SHPS-1 as determined by coimmunoprecipitation experiments with both anti-IAP and anti SHPS-1 antibodies for immunoprecipitation.
Figure 1.
Coprecipitation of IAP with SHPS-1 and disruption with anti IAP antibody. (A) Cell lysates were either immunoprecipitated with an anti-IAP antibody and coprecipitation of SHPS-1 determined by immunoblotting with anti-SHPS-1 antiserum or they were immunoprecipitated with anti SHPS-1 antibody and coprecipitation of IAP determined by immunoblotting with an anti-IAP antibody. As a control cell lysates were also immunoprecipitated with nonimmune rabbit serum (IgG) and immunoblotted with an anti-IAP antibody. (B) Quiescent pSMCs were incubated for 2 h ± the addition of the anti-IAP mAb, B6H12 or IgGi (both at 4 μg/ml). Coprecipitation of IAP with SHPS-1 was then determined by immunoprecipitating with an SHPS-1 antibody and immunoblotting with an anti-IAP antibody. The amount of SHPS-1 protein in each lane is shown in the lower row. (C) Expression of FLAG-labeled IAP and association with SHPS-1. Top, expression of FLAG-labeled IAP was determined by immunoblotting whole-cell lysates from cells transfected with each of the IAP cDNA constructs by using an anti-FLAG antibody. The results as scanning units are lane 1, 38,018; lane 2, 39,274; and lane 3, 46,779. Bottom, cell lysates were immunoprecipitated with an anti-SHPS-1 antibody and then coprecipitation of FLAG-labeled IAP was determined by immunoblotting with an anti-FLAG antibody. The amount of SHPS-1 that was immunoprecipitated in each lane is shown in the lower row. The results shown in this figure are representative of at least three independent experiments with similar results.
To investigate the role of IAP association with SHPS-1 in IGF-IR signaling, we developed two experimental models in which we disrupted the association between IAP and SHPS-1. The first approach was to use an anti-IAP monoclonal, B6H12, to interfere with the binding of the two proteins. This antibody has been shown to be specific for IAP (Brown et al., 1990). Figure 1B shows that after incubation of quiescent pSMCs with the anti-IAP mAb (B6H12), the interaction between IAP and SHPS-1 is significantly reduced (a 75 ± 7.5% reduction [mean ± SEM, n = 3, p < 0.05]). Preincubation with the IgGi control had no effect on the association between the two proteins.
As we have reported previously, the B6H12 antibody can also disrupt IAP association with αVβ3 and thereby alter IGF-I signaling (Maile et al. 2002). The binding between IAP and SHPS-1 specifically requires an intact disulfide bond in IAP between cysteine 33 in the extracellular domain and cysteine 261 within the putative transmembrane domain (Rebres et al., 2001). If this bond is disrupted by mutagenesis, the interaction of IAP with αVβ3 is preserved but binding to SHPS-1 is eliminated. We therefore generated and expressed a mutant form of IAP in which the association between IAP and SHPS-1 would be predicted to be specifically disrupted, whereas the association with αVβ3 would be preserved. This allowed us to distinguish between the effects of disrupting IAP-SHPS-1 binding from disrupting IAP-αVβ3. Additonally, we expressed a second mutant form of IAP in which the entire extracellular domain was truncated. Figure 1C (top) shows the level of expression of three forms of IAP that were used in subsequent experiments. The results show that the cells are expressing equal amounts of 1) FLAG-tagged full-length IAP (IAPfl); 2) the FLAG-tagged mutant form of IAP in which the two cysteine residues 33 and 261 had been substituted with serines (IAPc-s); and 3) the FLAG-tagged mutant form of IAP in which the complete extracellular domain has been deleted at amino acid residue 135 (IAPcyto).
To determine whether mutagenesis disrupted IAP association with SHPS-1, we immunoprecipitated SHPS-1 from each of these cell populations and immunoblotted with an anti-FLAG antibody. A representative experiment shown in Figure 1C shows that disruption of the extracellular domain of IAP alters its ability to associate with SHPS-1. When the results from three experiments were analyzed, it was determined that expression of IAPcyto resulted in a significant 88 ± 6.4% (mean ± SEM, n = 3, p < 0.05) reduction in IAP association with SHPS-1 compared with cells expressing IAPfl. Because truncation of the extracellular domain of IAP also disrupts its association with αVβ3, we analyzed the SHPS-1–IAP interaction in cells expressing the IAPc-s mutation. In cells expressing IAPc-s, there was a significant 81 ± 4.5% (mean ± SEM, n = 3 p < 0.05) reduction in IAP association with SHPS-1 compared with cells expressing IAPfl. The control immunoblots show that similar levels of SHPS-1 were immunoprecipitated.
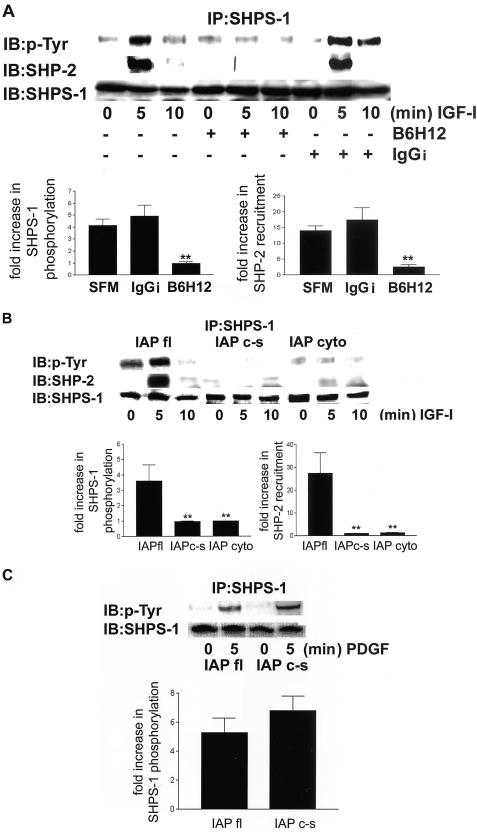
Blocking IAP–SHPS-1 Association Inhibits IGF-I–Stimulated SHPS-1 Phosphorylation and SHP-2 Recruitment
To determine the functional consequences of the loss of this physical association between IAP and SHPS-1, we examined SHPS-1 phosphorylation in response to IGF-I in wild-type cells pretreated with the anti-IAP mAb, B6H12. A representative experiment is shown in Figure 2A. When cells were exposed to SFM or control IgGi, IGF-I increased SHPS-1 phosphorylation by 4.1 ± 0.9 (mean ± SEM n = 3)-fold and 4.9 ± 1.45 (mean ± SEM, n = 3), respectively. In contrast, in cells exposed to B6H12 IGF-I stimulated phosphorylation 0.93 ± 0.12 (mean ± SEM, n = 3)-fold and this was significantly reduced (p < 0.05). This reduction in SHPS-1 phosphorylation in the presence of B6H12 is associated with a significant decrease (p < 0.05) in IGF-I–stimulated recruitment of SHP-2 to SHPS-1. Whereas SHP-2 association was increased 14 ± 1.5-fold increase in control cells and 17.3 ± 3.85-fold in cells preincubated with the IgGi (mean ± SEM, n = 3) after B6H12 exposure the increase was only 1.8 ± 1.1 (mean ± SEM, n = 3)-fold. When the results of the cells exposed to B6H12 were compared with the SFM or IgGi control cultures, the differences were significant (p < 0.05).
Figure 2.
(A) SHPS-1 phosphorylation and SHP-2 recruitment to SHPS-1 in response to IGF-I after disruption of the association between IAP and SHPS-1 by the anti-IAP antibody B6H12. Quiescent cells were incubated for2h ± B6H12 antibody or IgGi (both at 4 μg/ml) and then exposed to IGF-I (100 ng/ml) as indicated. Cell lysates were immunoprecipitated with an anti-SHPS-1 antibody and then SHPS-1 phosphorylation was determined by immunoblotting with a pTyr. The association of SHP-2 with SHPS-1 was visualized by immunoblotting by using an anti SHP-2 antibody. The amount of SHPS-1 protein in each lane is shown in the lower row. The fold increase in SHPS-1 phosphorylation and SHP-2 recruitment after IGF-I stimulation as determined by scanning densitometric analysis of Western immunoblots from three separate experiments is shown. **p < 0.05 when the response to IGF-I of cells preincubated with B6H12 is compared with the IGF-I response in cells preincubated in SFM or control IgGi. (B) SHPS-1 phosphorylation and SHP-2 recruitment in response to IGF-I after disruption of the association between IAP and SHPS-1 in cells expressing mutated forms of IAP. Cells were exposed to IGF-I (100 ng/ml) for various periods. Cell lysates were immunoprecipitated with an anti-SHPS-1 antibody and SHPS-1 phosphorylation was determined by immunoblotting with an anti-phosphotyrosine antibody (pTyr). The association of SHP-2 was visualized by immunoblotting using an anti SHP-2 antibody. The amount of SHPS-1 protein in each lane is shown in the lower row. The increase in SHPS-1 phosphorylation and SHP-2 recruitment after IGF-I stimulation as determined by scanning densitometric analysis of Western immunoblots from three separate experiments is shown. **p < 0.05 when the response of cells expressing either of the mutant forms of IAP to IGF-I is compared with the IGF-I response in cells expressing IAPfl. (C) SHPS-1 phosphorylation in response to PDGF. Cells were exposed to PDGF (10 ng/ml) for 5 min. After cell lysis and immunoprecipitation with an anti-SHPS-1 antibody, SHPS-1 phosphorylation was determined by immunoblotting with a pTyr.
The Extracellular Domain of IAP Is Required for IGF-I–stimulated SHPS-1 Phosphorylation and SHP-2 Recruitment
To confirm that blocking IAP binding to SHPS-1 inhibited IGF-I–stimulated SHPS-1 phosphorylation, the ability of IGF-I to stimulate SHPS-1 phoshorylation in cells expressing the mutant forms of IAP was compared with cells expressing wild-type IAP. The results from a representative experiment are shown in Figure 2B. IGF-I stimulated a 3.6 ± 0.8 (mean ± SEM, n = 3)-fold increase in SHPS-1 phosphorylation in cells expressing IAPfl. In contrast, cells expressing the IAPcyto or the IAPc-s mutant had a 1.03 ± 0.08- and 1.03 ± 0.13 (mean ± SEM, n = 3)-fold increases in SHPS-1 phosphorylation in response to IGF-I. These responses were significantly less than in cells expressing IAPfl (p < 0.05).
Consistent with the results obtained using B6H12 the lack of SHPS-1 phosphorylation observed in the cells expressing the mutant forms of IAP is associated with an inhibition in SHP-2 recruitment to SHPS-1 in response to IGF-I (Figure 2B) (p < 0.05 when the results obtained will cells expressing either mutant was compared with cells expressing IAPfl).
Because SHPS-1 has been shown to be phosphorylated in response to several growth factors, we wished to investigate the specificity of the requirement of IAP binding to SHPS-1. Figure 2C shows that PDGF induces a 5. 3 ± 1 (mean ± SEM, n = 2)-fold increase in SHPS-1 phosphorylation after 5-min exposure in cells expressing IAPfl. However, in contrast to IGF-I, PDGF stimulated a 6.8 ± 0.8 (mean ± SEM, n = 2)-fold increase in cells expressing IAPc-s and this was not significantly different compared with the response of the IAPfl cells.
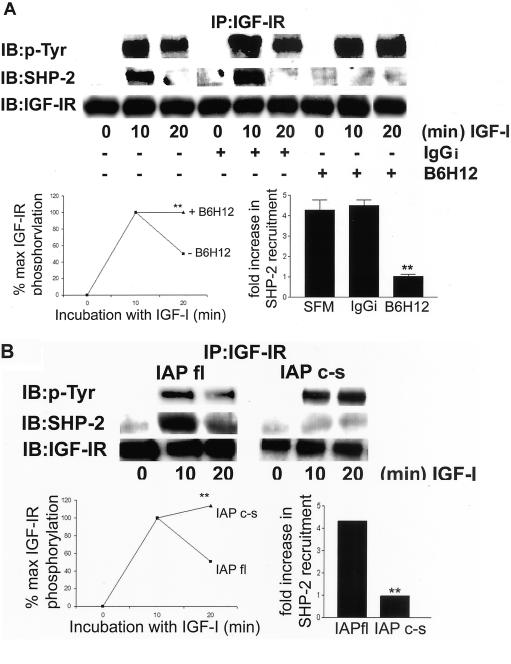
The Association between the Extracellular Domain of IAP and SHPS-1 Regulates the Duration of IGF-IR Phosphorylation via Its Modulation of SHP-2 Recruitment
Phosphorylation of SHPS-1 is required for SHP-2 transfer to the IGF-IR and thereby regulates the duration of IGF-IR phosphorylation (Maile and Clemmons, 2002b); therefore, we examined recruitment of SHP-2 to IGF-IR and the duration of IGF-IR phosphorylation in cells pretreated with B6H12 and in cells expressing the mutant forms of IAP. In control cells IGF-I stimulates a 4.3 ± 0.5 (mean ± SEM, n = 3)-fold increase in SHP-2 recruitment to the IGF-I receptor after a 10-min exposure to IGF-I (Figure 3A). However, in cells pretreated with B6H12, there is no significant increase seen in SHP-2 recruitment to IGF-IR (1.1 ± 0.05-fold; mean ± SEM, n = 3), and this response to IGF-I is significantly lower compared with control or IgGi-treated cells (p < 0.05). Consistent with our previous results (Maile and Clemmons, 2002b) the recruitment of SHP-2 to the IGF-IR precedes a 50 ± 0.06% reduction in IGF-I receptor phosphorylation that is observed after 20 min IGF-I stimulation. Following B6H12 pretreatment there is no significant difference in the level of IGF-IR phosphorylation after 10 min exposure to IGF-I. In contrast to control cells however, there is no apparent reduction in IGF-IR phosphorylation after 20 min. To confirm that the lack of SHP-2 recruitment to the IGF-IR in the cells pretreated with B6H12 was due to specifically to the disruption of the IAP–SHPS-1 interaction, we examined IGF-IR phosphorylation in cells expressing IAPc-s. Figure 3B shows that in these cells, there is no increase in the recruitment of SHP-2 to the IGF-IR in response to IGF-I and this is associated with is a significant reduction in the amount of IGF-IR dephosphorylation observed 20 min after IGF-I stimulation.
Figure 3.
IGF-IR phosphorylation time course and SHP-2 recruitment after disruption of the interaction between IAP and SHPS-1. (A) Quiescent cells were incubated ± B6H12 or IgGi (4 μg/ml) and then exposed to IGF-I (100 ng/ml) for various lengths of time. After lysis and immunoprecipitation with an anti-IGF-IR antibody phosphorylation of the receptor was determined by immunoblotting with a pTyr. The association of SHP-2 was determined by immunoblotting with an anti SHP-2 antibody. The amount of IGF-IR protein in each lane is shown in the lower row. The level of tyrosine phosphorylation of IGF-IR as a percentage of maximum phosphorylation detected as determined by scanning densitometric analysis of Western immunoblots from three separate experiments is shown. The increase in SHP-2 recruitment after IGF-I stimulation as determined by scanning densitometry analysis of Western immunoblots from three separate experiments is also shown. **p < 0.05 when the response of cells preincubated with B6H12 to IGF-I is compared with cells preincubated in SFM alone or containing control IgGi. (B) Cells were incubated with IGF-I (100 ng/ml) for various times. After lysis and immunoprecipitation with an anti-IGF-IR antibody, phosphorylation of the receptor was determined by immunoblotting with a pTyr. The association of SHP-2 was determined by immunoblotting with an anti-SHP-2 antibody. The amount of IGF-IR protein in each lane is shown in the lower row. The changes IGF-IR phosphorylation and SHP-2 recruitment after IGF-I stimulation as determined by scanning densitometric analysis of Western immunoblots from three separate experiments is shown. **p < 0.05 when the response of cells expressing IAPc-s that were stimulated with IGF-I is compared with the response of cells expressing IAPfl.
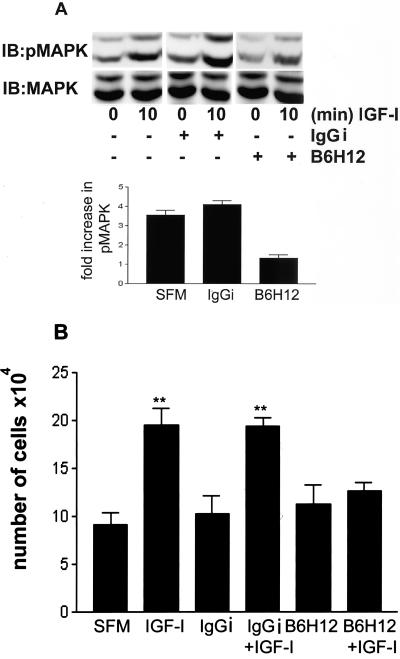
IGF-I–stimulated MAPK Activity and Cell Proliferation Are Inhibited after Disruption of SHP-2 Transfer
Previous studies have shown that expression of an inactive form of SHP-2 results in inhibition of IGF-I–stimulated MAPK, suggesting that transfer of SHP-2 to signaling molecules is necessary for IGF-I–stimulated MAPK activity (Manes et al., 1999). To examine the consequence of the lack of SHP-2 transfer after the disruption of IAP–SHPS-1 binding, we examined the activation of MAPK in response to IGF-I in the presence of B6H12.
Figure 4A shows after 10 min, IGF-I treatment stimulates a significant increase in the activation of MAPK as determined by the assessment of the dual phosphorylation of p42/p44 MAPK (3.6 ± 0.2-fold, mean ± SEM, n = 3), and this was not significantly different from cells preincubated with IgGi (4.4 ± 0.3, mean ± SEM, n = 3). However, when cells were preincubated with B6H12, IGF-I was unable to stimulate a significant increase in p42/p44 MAPK phosphorylation (1.3 ± 0.1-fold, mean ± SEM, n = 3) (p < 0.05 when cells are compared with control, IGF-I–stimulated cells).
Figure 4.
(A) Phosphorylation of MAPK and cell proliferation in response to IGF-I. Cells were plated and grown as described in MATERIALS AND METHODS before a 2-h incubation ± B6H12 or IgGi (both at 4 μg/ml) and then treated with IGF-I (100 ng/ml) for 10 min. The level of p42/44 MAPK phosphorylation was determined by immunoblotting with a phosphospecific MAPK antibody. The total amount of MAPK in each sample was determined by immunoblotting with a MAPK antibody. (B) Cells were plated and grown as described in MATERIALS AND METHODS before a 2-h incubation ± B6H12 or IgGi (both at a concentration of 4 μg/ml) and then treated with IGF-I (100 ng/ml) for 48 h. Cell number in each well was then determined. Each data points represent the mean of three independent experiments. **p < 0.05 when the response of cells incubated in the presence of B6H12 is compared with cells incubated with control IgGi or in the absence of antibody.
To examine the consequence of the disruption in IAP-SHPS-1 association on IGF-I action in SMCs we determined the effect of B6H12 on IGF-I stimulated cell proliferation. Figure 4B shows that in IGF-I stimulates a 2.2 ± 0.2-fold (mean ± SEM, n = 3) increase in cell proliferation after 48 h, and this was not significantly different from cells preincubated with control IgGi (2.0 ± 0.1, mean ± SEM, n = 3). However, when cells are incubated with B6H12, there is a significant reduction in IGF-I–stimulated cell proliferation (1.03 ± 0.01, mean ± SEM, n = 3, p < 0.05 compared with cells incubated in the absence of B6H12). This inhibition in cell proliferation is consistent with the inhibition of IGF-I stimulated MAPK activation.
Disruption of the IAP Interaction with SHPS-1 Inhibits IGF-I–stimulated Cell Migration
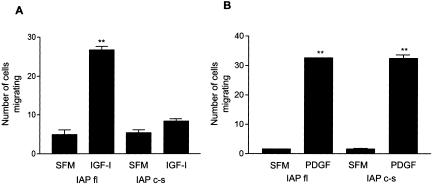
We have previously reported that the preincubation of pSMCs with B6H12 inhibits IGF-I–stimulated migration, in part, by altering the interaction between IAP and αVβ3 (Maile et al., 2002). To determine whether at least part of the effect B6H12 was also due to the inhibition of IAP binding to SHPS-1, we compared cell migration in response to IGF-I in cells expressing IAPfl and the IAPc-s mutant. In Figure 5A, it can be seen that IGF-I stimulated a significant increase in pSMC migration in cells expressing IAPfl. However, in cells expressing the IAPc-s mutant, IGF-I–stimulated migration is significantly reduced. In contrast, PDGF-stimulated cell migration of the IAPc-s cells is not significantly different compared with cells expressing full-length IAP (Figure 5B).
Figure 5.
IGF-I- and PDGF-stimulated cell migration in cells expressing full-length IAP and IAPc-s. Confluent cells were wounded then incubated ± IGF-I (100 ng/ml) (A) or PDGF (10 ng/ml) (B) for 48 h. The number of cells migrating across the wound edge in at least five preselected regions was counted. Each data point represents the mean ± SEM of three independent experiments. **p < 0.05 when migration of cells expressing IAPfl in the presence of IGF-I or PDGF is compared with incubation in SFM alone or when cells expressing IAPc-s stimulated with PDGF are compared with SFM alone.
DISCUSSION
The role of SHPS-1 in intracellular signaling has largely been attributed to the recruitment of SHP-2 to the phosphorylated tyrosines contained within ITIM motifs in the cytoplasmic tail of SHPS-1 and the subsequent activation of its phosphatase activity (Fujioka et al., 1996; Takada et al., 1998). This SHP-2 recruitment has been shown to be important for its transfer to growth factor receptors and downstream signaling molecules. The requirement of transfer of activated SHP-2 to downstream signaling molecules for growth factors such as IGF-I to stimulate their physiological actions has been strongly suggested by studies showing that expression of dominant negative forms of SHP-2 result in failure to properly activate growth factor-stimulated increases in MAPK (Milarski and Saltiel, 1994; Noguchi et al., 1994; (Pronk et al., 1994; Xiao et al., 1994; Yamauchi et al., 1995) and phosphatidylinositol 3-kinase (Ugi et al., 1996; Wu et al., 2001; Zhang et al., 2002). For IGF-I, it was specifically shown that expression of a dominant negative SHP-2 mutant resulted in a failure to activate MAPK or cell migration in response to IGF-I (Manes et al., 1999). The results from this study allow us to propose that 1) ligand occupancy of SHPS-1 by IAP is necessary for IGF-I–stimulated SHPS-1 phosphorylation, and 2) the interaction between the IAP and SHPS-1 is a key regulator of IGF-I signaling in SMCs because disrupting the interaction leads to failure to recruit and transfer SHP-2 and to attenuation of several IGF-I–stimulated effects. Specifically, disruption of the interaction between the two proteins using two independent approaches resulted in a loss of SHP-2 recruitment to SHPS-1 and subsequent transfer to the IGF-IR, which was reflected in prolonged IGF-IR phosphorylation. The consequence of lack of SHP-2 recruitment and transfer was evident in the inability of IGF-I to stimulate MAPK activation and subsequently cell proliferation or cell migration. Although not specifically examined in this study, we cannot rule the possibility that the disruption in SHP-2 recruitment may also result in an inhibition of the PI 3 kinase pathway and that this contributes to the inhibition of cell migration in response to IGF-I.
The consequence of disrupting the interaction between SHPS-1 and IAP has been studied in other systems that have not been shown to be dependent upon growth factor receptor activation. These studies have shown that anti-IAP monoclonal antibodies block the attachment of cerebellar neurons, erythrocytes, and thymocytes to a substratum containing P84 (a brain homolog of SHPS-1) (Jiang et al., 1999; Seiffert et al., 1999). That this interaction might play a role in cell-to-cell attachment was substantiated in experiments that demonstrated that the expression of the extracellular domain of SIRPα in SIRP negative cells supported adhesion of primary hematopoietic cells and this interaction was inhibited by anti-IAP monoclonal antibodies (Vernon-Wilson et al., 2000). In general, these studies have analyzed the role of this interaction in mediating intercellular attachment. However, cell adhesion molecules that are expressed on the cell surface such as integrins are important not only for cell attachment but also for the regulation of cell proliferation, survival, and differentiation (Mauro et al., 2003). The regulation of growth factor signaling by integrin receptors has been well documented. We have previously reported that ligand occupancy of αVβ3 is necessary for IGF-I–stimulated receptor signaling and a similar cooperative relationship between integrin receptors and the PDGF, epidermal growth factor, and fibroblast growth factor growth factor receptors has also been described (Miyamoto et al., 1996). Previous studies (Kuzmenko et al., 1998; Andre et al., 1999; Mauro et al., 2001; Maile et al., 2002) have explored the role of growth factor receptors in activating cell adhesion molecule affinity, but there is minimal published data regarding the ability of cell adhesion molecules to regulate growth factor action. The results from this study demonstrate that the interaction of the cell-to-cell adhesion molecules (e.g., IAP and SHPS-1) plays an important regulatory role in IGF-I signaling. Given the importance of cell-to-cell adhesion molecules in regulating cell function, it is tempting to speculate that the regulation of IGF-I signaling by cell-to-cell adhesion molecules is a general mechanism for regulating IGF-I actions. Although PDGF signaling was not affected by disruption of the IAP–SHPS-1 interaction, this finding does not exclude the possibility that other cell-to-cell adhesion molecules could play a similar role in regulating signaling by PDGF and other growth factors.
SHPS-1 phosphorylation in macrophages was increased after contact with red blood cells expressing IAP when they were compared with red blood cells not expressing IAP (Oldenborg et al., 2000). That result suggested that IAP binding to SHPS-1 was stimulating phosphorylation. In this study, we have demonstrated that ligand occupancy of SHPS-1 by IAP is required for IGF-I–stimulated SHPS-1 phosphorylation. SHPS-1 has been shown to be phosphorylated directly by the insulin receptor kinase (Fujioka et al., 1996). Given the homology between the tyrosine kinase domains in the insulin and IGF-IR (e.g., 84%), it is possible that SHPS-1 is also a direct substrate for the IGF-IR kinase. If the IGF-IR kinase is directly phosphorylating SHPS-1, then IAP binding to SHPS-1 could modulate this process by either translocating SHPS-1 into proximity with the IGF-IR kinase or by altering the conformation of the SHPS-1 cytoplasmic domain, thus making its tyrosines residues more accessible to phosphorylation by the IGF-IR kinase. Using either model disruption of the SHPS-1–IAP interaction would be predicted to have the opposite effect. Because PDGF could still stimulate SHPS-1 phosphorylation in the absence of IAP binding to SHPS-1, this suggests that PDGF and IGF-I may stimulate SHPS-1 phosphorylation via two different kinases. SHPS-1 phosphorylation in response to integrin engagement has been shown to be mediated via a Src family kinase (Fujioka et al., 1996). Although the kinase responsible for PDGF-stimulated phosphorylation has not been described, our results suggest that the phosphorylation of SHPS-1 by IGF-I and PDGF is differentially regulated by SHPS-1 ligand occupancy.
By virtue of its ability to stimulate SMC migration and proliferation, IGF-I has been proposed to be an important contributor to the development of atherosclerosis (Jones et al., 1996). In mice in which IGF-I was overexpressed in SMCs, there was an increase in the rate of neointimal formation after carotid injury that seemed to have resulted from increased SMC proliferation and migration. The effect was apparent despite equivalent levels of serum IGF-I in plasma compared with control animals, suggesting a paracrine effect of locally produced IGF-I (Zhu et al., 2001). Given the apparent role of IGF-I in the development of atherosclerosis and the effect of this interaction on IGF-I signaling, it is likely that this system may play a role in the development of atherosclerosis and disruption of the interaction of IAP–SHPS-1 may represent a novel therapeutic strategy to specifically inhibit IGF-I action. Current approaches to target IGF-I signaling have focused on blocking the activity of the receptor itself by using antibodies or peptides. Disrupting cell-to-cell adhesion molecule interactions that specifically inhibit growth factor signaling offers a novel therapeutic strategy. It is also possible that this approach, which uses a distinct molecular mechanism, might work in synergy with other strategies.
In summary, our results have shown that the interaction of two specific cell surface proteins that have been shown to mediate cell adhesion is required for IGF-I–stimulated SHPS-1 phosphorylation and SHP-2 recruitment in SMCs. Disruption of this interaction results in impairment of IGF-I signaling and biological actions. Further studies will be necessary to understand the effect of specific ligands for both of these transmembrane proteins on their interaction and their effect on IGF-IR signaling. Because IAP also binds to αVβ3 it is also possible that these three proteins function coordinately as a unit. Analyzing how ligand occupancy of each component alters signaling by the entire complex is therefore an important goal of future studies.
Acknowledgments
We thank Laura Lindsey for help in preparing the manuscript. This work was supported by grant HL56850 from the National Institutes of Health.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0239. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0239.
References
- Andre, F., Rigot, V., Thimonier, J., Montixi, C., Parat, F., Pommier, G., Marvaldi, J., and Luis, J. (1999). Integrins and E-cadherin cooperate with IGF-I to induce migration of epithelial colonic cells. Int. J. Cancer 83, 497–505. [DOI] [PubMed] [Google Scholar]
- Babic, I., Schallhorn, A., Lindberg, F.P., and Jirik, F.R. (2000). SHPS-1 induces aggregation of Ba/F3 pro-B cells via an interaction with CD47. J. Immunol. 164, 3652–3658. [DOI] [PubMed] [Google Scholar]
- Brown, E., Hooper, L., Ho, T., and Gresham, H. (1990). Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 111, 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, Y., Matozaki, T., Noguchi, T., Iwamatsu, A., Yamao, T., Takahashi, N., Tsuda, M., Takada, T., and Kasuga, M. (1996). A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16, 6887–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gockerman, A., Prevette, T., Jones, J.I., and Clemmons, D.R. (1995). Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology 136, 4168–4173. [DOI] [PubMed] [Google Scholar]
- Imai, Y., Busby, W.H., Jr., Smith, C.E., Clarke, J.B., Garmong, A.J., Horwitz, G.D., Rees, C., and Clemmons, D.R. (1997). Protease-resistant form of insulin-like growth factor-binding protein 5 is an inhibitor of insulin-like growth factor-I actions on porcine smooth muscle cells in culture. J. Clin. Investig. 100, 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y., and Clemmons, D.R. (1999). Roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in stimulation of vascular smooth muscle cell migration and deoxyribonucleic acid synthesis by insulin-like growth factor-I. Endocrinology 140, 4228–42235. [DOI] [PubMed] [Google Scholar]
- Jiang, P., Lagenaur, C.F., and Narayanan, V. (1999). Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 274, 559–562. [DOI] [PubMed] [Google Scholar]
- Jones, J.I., Prevette, T., Gockerman, A., and Clemmons, D.R. (1996). Ligand occupancy of the alpha-V-beta3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc. Natl. Acad. Sci. USA 93, 2482–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov, A., Chen, Z., Sures, I., Wang, H., Schilling, J., and Ullrich, A. (1997). A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186. [DOI] [PubMed] [Google Scholar]
- Kuzmenko, Y., Kern, F., Bochkov, V., Tkachuk, V., and Resink, T. (1998). Density- and proliferation status-dependent expression of T-cadherin, a novel lipoprotein-binding glycoprotein: a function in negative regulation of smooth muscle cell growth? FEBS Lett. 434, 183–187. [DOI] [PubMed] [Google Scholar]
- Maile, L.A., and Clemmons, D.R. (2002a). The alphaVbeta3 integrin regulates insulin-like growth factor I (IGF-I) receptor phosphorylation by altering the rate of recruitment of the Src-homology 2-containing phosphotyrosine phosphatase-2 to the activated IGF-I receptor. Endocr. J. 143, 4259–4264. [DOI] [PubMed] [Google Scholar]
- Maile, L.A., and Clemmons, D.R. (2002b). Regulation of insulin-like growth factor I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. J. Biol. Chem. 277, 8955–8960. [DOI] [PubMed] [Google Scholar]
- Maile, L.A., Imai, Y., Clarke, J.B., and Clemmons, D.R. (2002). Insulin-like growth factor I increases alphaVbeta 3 affinity by increasing the amount of integrin-associated protein that is associated with non-raft domains of the cellular membrane. J. Biol. Chem. 277, 1800–1805. [DOI] [PubMed] [Google Scholar]
- Manes, S., Mira, E., Gomez-Mouton, C., Zhao, Z.J., Lacalle, R.A., and Martinez, A.C. (1999). Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase regulation of cell motility. Mol. Cell. Biol. 4, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro, L., Bartucci, M., Morelli, C., Ando', S., and Surmacz, E. (2001). IGF-I receptor induced cell-cell adhesion of MCF-7 breast cancer cells requires the expression of ZO-1. J. Biol. Chem. 276, 39892–39897. [DOI] [PubMed] [Google Scholar]
- Mauro, L., Salerno, M., Morelli, C., Boterberg, T., Bracke, M., and Surmacz, E. (2003). Role of the IGF-I receptor in the regulation of cell-cell adhesion: implications in cancer development and progression. J. Cell Physiol. 194, 108–116. [DOI] [PubMed] [Google Scholar]
- Milarski, K.L., and Saltiel, A.R. (1994). Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J. Biol. Chem. 269, 21239–21243. [PubMed] [Google Scholar]
- Miyamoto, S., Teramoto, H., Gutkind, J.S., and Yamada, K.M. (1996). Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135, 1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, T., Busby, W.H., Jr., Rees, C., and Clemmons, D.R. (2000). Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology 141, 1100–1106. [DOI] [PubMed] [Google Scholar]
- Noguchi, T., Matozaki, T., Fujioka, Y., Yamao, T., Tsuda, M., Takada, T., and Kasuga, M. (1996). Characterization of a 115-kDa protein that binds to SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in Chinese hamster ovary cells. J. Biol. Chem. 271, 27652–27658. [DOI] [PubMed] [Google Scholar]
- Noguchi, T., Matozaki, T., Horita, K., Fujioka, Y., and Kasuga, M. (1994). Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 14, 6674–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg, P.A., Zheleznyak, A., Fang, Y.F., Lagenaur, C.F., Gresham, H.D., and Lindberg, F.P. (2000). Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054. [DOI] [PubMed] [Google Scholar]
- Pronk, G.J., de Vries-Smits, A.M., Buday, L., Downward, J., Maassen, J.A., Medema, R.H., and Bos, J.L. (1994). Involvement of Shc in insulinand epidermal growth factor-induced activation of p21ras. Mol. Cell. Biol. 14, 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebres, R.A., Vaz, L.E., Green, J.M., and Brown, E.J. (2001). Normal ligand binding and signaling by CD47 (integrin associated protein) requires a long range disulphide bond between the extracellular and membrane-spanning domains. J. Biol. Chem. 276, 34607–34616. [DOI] [PubMed] [Google Scholar]
- Rosales, C., Gresham, H.D., and Brown, E.J. (1992). Expression of the 50-kDa integrin-associated protein on myeloid cells and erythrocytes. J. Immunol. 149, 2759–2764. [PubMed] [Google Scholar]
- Seiffert, M., Cant, C., Chen, Z., Rappold, I., Brugger, W., Kanz, L., Brown, E.J., Ullrich, A., and Buhring, H.J. (1999). Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 94, 3633–3643. [PubMed] [Google Scholar]
- Stofega, M.R., Wang, H., Ullrich, A., and Carter-Su, C. (1998). Growth hormone regulation of SIRP and SHP-2 tyrosyl phosphorylation and association. J. Biol. Chem. 273, 7112–7117. [DOI] [PubMed] [Google Scholar]
- Takada, T., et al. (1998). Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J. Biol. Chem. 273, 9234–9242. [DOI] [PubMed] [Google Scholar]
- Ugi, S., Maegawa, H., Kashiwagi, A., Adachi, M., Olefsky, J.M., and Kikkawa, R. (1996). Expression of dominant negative mutant SHPTP2 attenuates phosphatidylinositol 3′-kinase activity via modulation of phosphorylation of insulin receptor substrate-1. J. Biol. Chem. 271, 12595–12602. [DOI] [PubMed] [Google Scholar]
- Vernon-Wilson, E.F., Kee, W.J., Willis, A.C., Barclay, A.N., Simmons, D.L., and Brown, M.H. (2000). CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur. J. Immunol. 30, 2130–2137. [DOI] [PubMed] [Google Scholar]
- Wu, C.J., O'Rourke, D.M., Feng, G.S., Johnson, G.R., Wang, Q., and Greene, M.I. (2001). The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene 20, 6018–6025. [DOI] [PubMed] [Google Scholar]
- Xiao, S., Rose, D.W., Sasaoka, T., Maegawa, H., Burke, T.R., Jr., Roller, P.P., Shoelson, S.E., and Olefsky, J.M. (1994). Syp (SH-PTP2) is a positive mediator of growth factor-stimulated mitogenic signal transduction. J. Biol. Chem. 269, 21244–21248. [PubMed] [Google Scholar]
- Yamauchi, K., Milarski, K.L., Saltiel, A.R., and Pessin, J.E. (1995). Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc. Natl. Acad. Sci USA 92, 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, et al. (2002). Interaction between Src homology 2 domain bearing protein tyrosine phosphatase substrate-1 and CD47 mediates the adhesion of human B lymphocytes to nonactivated endothelial cells. J. Immunol. 168, 3213–3220. [DOI] [PubMed] [Google Scholar]
- Zhang, S.Q., Tsiaras, W.G., Araki, T., Wen, G., Minichiello, L., Klein, R., and Neel, B.G. (2002). Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22, 4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, B., and Clemmons, D.R. (1998). Blocking ligand occupancy of the alphaVbeta3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA 95, 11217–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, B., Zhao, G., Witte, D.P., Hui, D.Y., and Fagin, J.A. (2001). Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology 142, 3598–3606. [DOI] [PubMed] [Google Scholar]