Abstract
Converging information on medical issues, motor ability, and cognitive outcomes is essential when addressing long-term clinical management in children with holoprosencephaly. This study considered whether adding more informative structural indices to classic holoprosencephaly categories would increase prediction of cognitive outcomes. Forty-two children with holoprosencephaly were examined to determine the association of deep gray nuclei abnormalities with cognitive abilities and the effect of motor skill deficits on cognitive performance. Additionally, a cognitive profile was described using the Carter Neurocognitive Assessment, an experimental diagnostic instrument designed specifically for young children with severe neurodevelopmental dysfunction. Findings indicated that nonseparation of the deep gray nuclei was significantly associated with the cognitive construct of vocal communication, but not with the cognitive constructs of social awareness, visual attention, or auditory comprehension. Importantly, motor skill deficits did not significantly affect performance on the Carter Neurocognitive Assessment. This study is the first investigation to provide a descriptive overview of specific cognitive skills in this group of children. The results also strongly suggest that this feature of the brain’s structure does not predict all aspects of neurodevelopmental function. These findings contribute a critical component to the growing body of knowledge regarding the medical and clinical outcomes of children with holoprosencephaly.
Introduction
Advances in technology, particularly magnetic resonance imaging, have allowed identification of previously undiagnosed children with less severe forms of holoprosencephaly [1]. Moreover, many children with mild to moderate forms of holoprosencephaly are surviving into childhood and beyond [1]. As a consequence, more sensitive diagnostic indices have been sought that might provide additional guidance as to longer-term clinical management. Recent studies describe such an indicator, namely the degree of nonseparation of the deep gray nuclei, that may well provide convergent information when added to traditional anatomic measures [1–3]. Specifically, abnormal separation of the deep gray nuclei has been found to be correlated with neurodevelopmental dysfunction, particularly in the areas of gross motor ability, upper extremity function, and language development [3]. One barrier to optimal multidisciplinary management is that the cognitive skills of children with holoprosencephaly have never been adequately described owing to the difficulty in assessing such skills when severe motor and expressive language deficits coexist. The present study attempts to address such issues.
Holoprosencephaly is a brain malformation that results from a primary defect in induction and patterning of the rostral neurotube (basal forebrain) due to underexpression of the ventralizing genes during the first 4 weeks of embryogenesis [4,5]. This defect results in incomplete cleavage of the cerebral hemispheres and, when the rostrocaudal gradient of genetic expression extends to the midbrain, is frequently associated with midline craniofacial malformations [5–7]. In addition, deeper midline structures, including the caudate nuclei, thalamic nuclei, lentiform nuclei, and hypothalamic nuclei exhibit varying degrees of nonseparation [2]. Most children with holoprosencephaly have severe motor impairment with varying degrees of hypertonicity or hypotonicity. Only a limited number develop functional expressive speech and language skills. Medical complications may include seizures, need for a gastroesophageal tube for feeding, and diabetes insipidus. Both environmental and genetic causes of holoprosencephaly have been identified. Possible etiologies include maternal diabetes, infections or drug use during pregnancy, deficits in cholesterol biosynthesis, chromosomal abnormalities, and genetic defects [8–11]. Six human genes have been found to be associated with holoprosencephaly (SHH, SIX3, ZIC2, TGIF, PTCH, and DKK), and seven other defective chromosome loci have been linked to this diagnosis. Nonetheless, these genetic anomalies together account for less than 20% of known cases [5,12].
Based on the degree of hemispheric nonseparation, holoprosencephaly traditionally has been classified into three types: alobar, semilobar, and lobar [13]. Alobar holoprosencephaly is the most severe type. These children are often observed to have more significant midline craniofacial defects and a shortened lifespan [14]. Semilobar holoprosencephaly, the most common type, is characterized by nonseparation of the anterior brain structures including the cortex, basal ganglia, and thalamus with separation of the hemispheres observed in the posterior region of the brain. Lobar holoprosencephaly is the least severe of the major types of holoprosencephaly. In addition, there is a fourth type known as middle interhemispheric variant [15]. This classification is characterized by an abnormal midline connection of the cerebral hemispheres in the posterior frontal and parietal regions [16]. This group as a whole tends to be higher functioning.
The extent of hemispheric nonseparation falls along a spectrum, and it is not always straightforward to categorize an individual case into the traditional classifications [1]. Further, traditional categories such as semilobar holoprosencephaly are not highly predictive of cognitive and motor outcome, mainly because a number of important features, such as divergent cortical malformations and variations in separation of deep gray nuclei, cannot be incorporated into this rather inflexible classification scheme [1]. Thus, abnormal separation of the deep gray nuclei in holoprosencephaly may be just as important in predicting outcome and function [2,3]. Simon’s group [16] found that some degree of hypothalamic noncleavage was present in 100% of the holoprosencephaly cases studied. Some degree of caudate noncleavage was observed in 96% of the cases, and this was more common than noncleavage of the lentiform nuclei (85%). Noncleavage of the thalamus was least common but still occurred in over half (67%) of the cases studied.
Given the cognitive role of the caudate nuclei [17] and the importance of the sensory pathways provided by the thalamic nuclei to the cerebral cortex [18,19], it is posited that there might be a clinically relevant association between abnormalities in deep gray structures and cognitive outcomes in children with holoprosencephaly. The caudate nuclei, in particular, receive all input to the basal ganglia and are intricately involved in the dorsolateral prefrontal circuit. This circuit has been implicated as a way-station for the processing of sensory and cognitive information during tasks such as organizing behavioral responses and verbal problem solving [18]; as a consequence, malformation of the caudate nuclei would affect performance on those cognitive tasks. Likewise, the lentiform nuclei are important for their motoric role and the thalamic nuclei for their involvement in the relay of sensory information to the cerebral cortex [18]; hence, these deep gray structures would also be likely to impact cognitive performance.
The primary aim of this study was to examine the potential impact of deep gray nuclei abnormalities on cognitive abilities, and to assess whether accounting for this impact added further predictive power within a holoprosencephaly category. The influence of motor skill deficits on cognitive performance was also explored. Moreover, a first attempt was made to describe the cognitive profile of children with holoprosencephaly using an experimental measure, which requires minimal motor sophistication to assess the cognitive constructs of social awareness, visual attention, auditory comprehension, and vocal communication. These findings contribute a critical, but rarely considered, component to the growing body of knowledge regarding the medical and clinical outcomes of children with holoprosencephaly. The focus to date in this area has been on medical issues, with consideration of cognitive outcomes rarely undertaken. However, ongoing investigation of the correlates of cognitive performance in children with congenital brain malformations will help physicians to make more informed medical judgments and will enable specialists and families to intervene appropriately in order to improve overall neurodevelopmental outcomes.
Methods
Participants
Forty-five children with holoprosencephaly were evaluated at one of the four Carter Centers for Brain Research in Holoprosencephaly and Related Brain Malformations, a national consortium funded by a nonprofit, private foundation. (For more information, see http://www.stanford.edu/group/hpe.) The Institutional Review Boards at each of the centers approved the study, and appropriate consents were obtained. Three children exhibiting moderate to severe visual or hearing impairments were excluded from the final analyses. Therefore, our final sample size was 42 children with holoprosencephaly.
Participants included 17 males and 25 females with a mean age of 3 years, 10 months (S.D. 3 years, 3 months) and an overall age range of 3 months to 15 years, 5 months. Three children (7%) in the study were classified as alobar type, 25 (60%) semilobar, 8 (19%) lobar, and 6 (14%) middle interhemispheric variant. Deep gray scores used to describe the degree of nonseparation of the deep gray nuclei were available for 30 of the children in the study. The majority of the children (60%) had composite deep gray scores from 0–3 (mild degree of nonseparation), 27% had scores of 4–7 (moderate degree of nonseparation) and 16% had scores of 8–11 (severe degree of nonseparation).
Two children in the sample were reported to have chromosomal abnormalities (13Q deletion; 47XX) and one child was known to have a mutation on one of the holoprosencephaly genes (TGIF). To date, a total of 16 of 94 (17%) patients from the Carter Centers database are known to have a mutation on one of the holoprosencephaly genes [20]. Thus, the group studied here was essentially a nonsyndromic subgroup of the holoprosencephaly population. Other clinical features found in this group and commonly observed in children with holoprosencephaly are summarized in Table 1. Note that information for each clinical feature was not mentioned in every medical record, thus data presented in the table are based on the total number of children for whom the information was available.
Table 1. Clinical features of holoprosencephaly sample.
| Clinical Features | Incidence* | % |
|---|---|---|
| Craniofacial defect | 13 of 27 | 48 |
| Microcephaly | 26 of 36 | 72 |
| Macrocephaly | 7 of 36 | 19 |
| Seizure medication | 12 of 34 | 35 |
| GE tube - feeding | 16 of 37 | 43 |
Abbreviation: GE = Gastroesophageal
Data were not reported in every medical record.
Assessments
Deep Gray Score
The deep gray score was obtained from the neuroimaging report for each child. According to this grading system [2], the deep gray nuclei (caudate, lentiform, and thalami) were graded on a scale from 0 to 3, with 0 = complete separation, 1 = less than 50% noncleavage or abnormal medial location, 2 = 50% to 99% noncleavage, and 3 = complete noncleavage. The degree of hypothalamic noncleavage was assessed using a scale of 0 to 2, with 0 = complete separation, 1 = partial (anterior) noncleavage, and 2 = complete noncleavage. The composite nonseparation score is called the Deep Gray Score (i.e., caudate grade + lentiform grade + thalamic grade + hypothalamic grade = Deep Gray Score).
Composite Motor Score
The Composite Motor Score was derived from components of the motor examination that included spasticity, dystonia, choreoathetosis, and hypotonia. These abnormalities were graded using a standardized scoring system described in a previous study [3]. The subscores were based on degree of severity: 0 = none, 1 = mild, 2 = severe, with the exception of spasticity where 2 = moderate and 3 = severe. The Composite Motor Score was the sum of the subscores (i.e., spasticity grade + dystonia grade + choreoathetosis grade + hypotonia grade = Composite Motor Score).
Carter Neurocognitive Assessment Severity Score
The Carter Neurocognitive Assessment, an experimental test designed specifically for children with severe motor and expressive language deficits, was administered to all participants [21]. For purposes of this study, a severity score was derived for each subscale of the Carter Neurocognitive Assessment using items that were considered to represent significant milestones in development. The subscales included the following cognitive constructs: Social Awareness, Visual Attention, Auditory Comprehension, and Vocal Communication. Each milestone and its corresponding grade are delineated in Table 2, with scores ranging from 0 to 5. A score of 0 indicates attainment of all five milestones (i.e., best performance), and a score of 5 indicates that none of the milestones were attained (i.e., most severe level of deficit).
Table 2. Carter Neurocognitive Assessment Severity Scale.
| Performance Rating | Severity Score | Social Awareness | Visual Attention | Auditory Comprehension | Vocal Communication |
|---|---|---|---|---|---|
| Best performance (less severe) | 0 | Indicates yes/no | Looks for object after visual displacement | Looks at 3/3 objects | Produces 2–3 word utterances |
| 1 | Joint attention following point | Visually anticipates path when object disappears | Looks at 3 pictures in 2-way discrimination task | Labels 1 object | |
| 2 | Anticipates an event | Looks for object hidden under single cup | Follows direction to look at object | Produces 4 different consonant- vowel combinations | |
| 3 | Responds to mirror image | Alternates glance between two objects | Recognizes 2 words | Vocalizes one consonant | |
| 4 | Smiles or vocalizes in response to adult | Tracks moving object | Looks to source of sound via eye gaze | Produces vowel sounds | |
| Poorest performance (most severe) | 5 | None of the above | None of the above | None of the above | None of the above |
Statistical Analyses
The primary outcome variable in the analyses was cognitive performance as measured by the severity score derived from the Carter Neurocognitive Assessment. The independent variables were the deep gray scores and the composite motor scores. The mediating effects of sex and age were also examined.
The Carter Neurocognitive Assessment is an assessment designed to measure specific skills up to the 2-year level of cognitive development. All children tested were thought to be functioning below this level based on the clinical assessments completed before referral to the study. Participation was restricted to children diagnosed with holoprosencephaly who are known to have significant developmental delays. Given the wide age range, we examined the potential mediating effects of chronological age. Subjects were divided into two groups. The first consisted of children aged 0 to 3 years, 11 months of age and thus within 2 years of the normal cognitive developmental range targeted by the Carter Neurocognitive Assessment. The second group included children outside of that range who were 4 years of age and older. A Cochran-Mantel-Haenszel test was performed to determine if age was associated with the cognitive scores, the deep gray scores, or the motor scores. As cognitive performance did differ in three of the Carter Neurocognitive Assessment subscales based on age groupings (see below), all subsequent analyses took age into account. Because of the modest sample size, exact tests of homogeneity were performed to test the hypothesis of independence.
Spearman rank correlations were used to measure the strength of the associations among the Carter Neurocognitive Assessment severity scores, Deep Gray Scores, and Composite Motor Scores. However, because of the small sample size, the results of this test were interpreted with reliance upon the exact tests for assessment of significance of independence between the outcome and independent variables.
In addition, 12 of 34 (35%) of the children for whom information was available were reported to have seizure activity and were taking seizure medication at the time of the study. This subgroup of children were matched by deep gray scores and chronological age with eight children who did not have a history of seizures and were not taking seizure medication. Paired, two-tailed t tests were performed to investigate whether the children on seizure medication performed differently from children with holoprosencephaly who did not have seizures.
Results
Age and Sex Effects
Sex did not significantly affect performance scores on the Carter Neurocognitive Assessment, Deep Gray Scores, or Composite Motor Scores. There were no significant age group differences for the Deep Gray Scores, Composite Motor Scores, or the Vocal Communication subscale score of the Carter Neurocognitive Assessment. However, there was a significant statistical difference between age groups for the other three subscales of the Carter Neurocognitive Assessment: Social Awareness, Visual Attention, and Auditory Comprehension (exact Cochran-Mantel-Haenszel test, X2 = 6.3, P = 0.01; X2 = 10.7, P < 0.001; X2 = 9.7, P < 0.001, respectively). The children in the older group performed better than the younger group on these subscales. Therefore age was considered a mediating variable and was accounted for in all further analyses.
Effect of Deep Gray Nuclei Abnormalities and Motor Skill Deficits
Examination of children’s performance on the Carter Neurocognitive Assessment indicated that only the Vocal Communication subscale was associated with nonseparation of the deep gray structures. The pattern of associations was examined using the Spearman rank correlation. The results, summarized in Table 3, demonstrate that the strongest associations were between vocal communication and the lentiform nuclei (r = 0.41, P = 0.02), thalamic nuclei (r = 0.42, P = 0.03), and the total Deep Gray Score (r = 0.40, P = 0.02). These results were confirmed by exact tests of independence (X2 = 4.9, P = 0.03, X2 = 4.3, P = 0.04, X2 = 5.7, P = 0.01, respectively). Children with a higher Deep Gray Score (i.e., more severe separation abnormalities) had a higher severity score on the Vocal Communication subscale (i.e., more severe deficits in vocal communication).
Table 3. Spearman rank correlations between Carter Neurocognitive Assessment, Deep Gray Scores, and Composite Motor Scores.
| Carter Neurocognitive Assessment Subscales
|
||||
|---|---|---|---|---|
| Deep Gray Structures | Social Awareness | Visual Attention | Auditory Comprehension | Vocal Communication |
| Caudate nuclei | .14 | .33 | .24 | .32 |
| Lentiform nuclei | .13 | .29 | .19 | .41* |
| Thalamic nuclei | .24 | .32 | .27 | .42* |
| Hypothalamus | .07 | .09 | .03 | .29 |
| Total Deep Gray Score | .19 | .32 | .21 | .40* |
| Composite Motor Score | −.05 | −.01 | .08 | .18 |
P < 0.05.
Note: Boldface indicates significant values.
Analysis of the Composite Motor Score and Carter Neurocognitive Assessment severity scores revealed no significant associations between gross motor skill deficits and performance on the Carter Neurocognitive Assessment subscales.
Effect of Seizures
Children who had seizures and were taking medication for seizures performed at significantly lower levels than the children who did not have a history of seizures on all subscales of the Carter Neurocognitive Assessment, except the Vocal Communication subscale: Visual Attention, t(17) = 2.313, P < 0.05; Auditory Comprehension, t(17) = 2.397, P < 0.05; Social Awareness, t(17) = 2.548, P < 0.05.
Cognitive Profile
The cognitive profile of the group was evaluated by looking at the mean scores for the cognitive constructs assessed on the Carter Neurocognitive Assessment: Social Awareness, Visual Attention, Auditory Comprehension, and Vocal Communication. Performance on the Carter Neurocognitive Assessment (n = 42) revealed the following mean severity scores for each subscale: Social Awareness 1.43 (range 0–5, S.D. 1.50), Visual Attention 1.81 (range 0–5, S.D. 1.74), Auditory Comprehension 2.10 (range 0–5, S.D. 1.68), and Vocal Communication 2.86 (range 0–5, S.D. 1.52) as indicated in Table 4. It should be emphasized that this is a severity scale, therefore the higher the score the more severe the deficit. Further, a Kruskal-Wallis test statistic indicated that there was a significant difference in performance among the Carter Neurocognitive Assessment subscales (x2 = 15.40, P = 0.001) after accounting for age. Specifically, Social Awareness and Visual Attention scores were significantly better (i.e. less severe) than Vocal Communication scores (Fig 1).
Table 4. Mean severity scores for Carter Neurocognitive Assessment subscales.
| Subscale | Mean Severity Score (Range 0–5) | S.D. |
|---|---|---|
| Social Awareness | 1.43 | 1.50 |
| Visual Attention | 1.81 | 1.74 |
| Auditory Comprehension | 2.10 | 1.68 |
| Vocal Communication | 2.86 | 1.52 |
Figure 1.
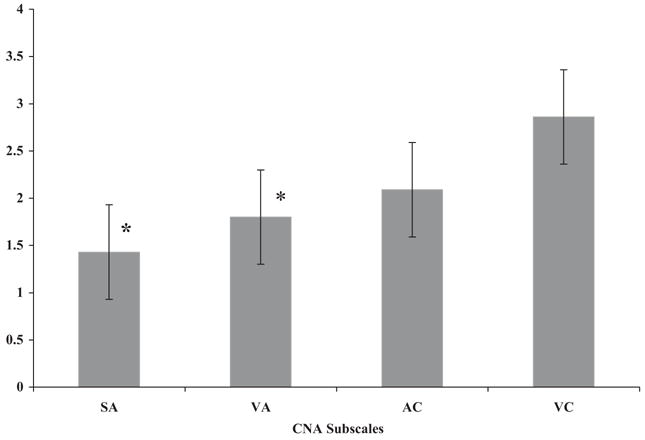
Mean Carter Neurocognitive Assessment (CNA) severity scores. SA = Social Awareness Subscale; VA = Visual Attention Subscale; AC = Auditory Comprehension Subscale; VC = Vocal Communication Subscale. *Performance on Social Awareness and Visual Attention subscales was significantly better than Vocal Communication subscale.
Discussion
It was not surprising that the degree of nonseparation of the deep gray nuclei was significantly associated with vocal communication skills, given the role these nuclei play in motor control [22] and thus motor-speech components. Vocal communication is the one construct assessed on the Carter Neurocognitive Assessment that requires specific fine motor skills and is also the most significantly delayed skill area in this subject sample. Vocal communication typically does not develop beyond a few vocalizations or word approximations, even among older children with holoprosencephaly, and this is observed in the study sample. The exception in the findings of this study was among the children in the middle interhemispheric variant group who have more complete separation of the hemispheres (i.e., lower Deep Gray Scores), and thus have more advanced expressive speech skills than the children with traditional holoprosencephaly. These children were producing utterances of 3 to 5 words in length. This finding is supported by the work of Plawner et al. [3] when the language component of their clinical severity scale is examined. Specifically, Plawner et al. focused on expressive speech skills, comparable to the items assessed on the Carter Neurocognitive Assessment severity index, finding that children with milder holoprosencephaly types achieved better expressive language function (i.e., production of speech sounds, words, and sentences) [3].
In contrast, the degree of nonseparation of the deep gray nuclei did not produce the expected impact on the cognitive constructs of Social Awareness, Visual Attention, and Auditory Comprehension. The lack of correlations between performance on these subscales and the deep gray scores might suggest that areas of the brain influencing social awareness, visual attention, and auditory comprehension are somewhat spared with respect to deep gray structural malformations or that the structures responsible for these skills lie in more lateral and dorsal regions than the nuclei that were studied. Further, two of these three areas are more heavily dependent on frontal and prefrontal cortex and executive function.
The pattern of correlations observed in the present study may also be accounted for by recent data on pathogenesis described by Sarnat and Flores-Sarnat. Their proposed classification system for human nervous system malformations integrates structural morphology and patterns of genetic expression on three axes: vertical (dorsoventral and ventrodorsal), longitudinal (rostrocaudal and caudo-rostral), and horizontal (mediolateral and lateromedial) [5]. The mediolateral gradient is observed in the most severely disorganized cerebral cortex in paramedian regions, less disordered cortex more laterally, and the cortex may be normally laminated with normal architecture in the most lateral regions. The extent to which this mediolateral gradient is expressed genetically could explain why only a limited number of children with holoprosencephaly develop functional speech and expressive language skills, as well as the variability in performance in the other cognitive areas and the relative strength observed in most of the children in the area of social awareness. The incorporation of traditional morphologic criteria with molecular genetics allows the pattern of expression to be recognized even if the exact gene is not yet identified [5]. Although multiple mechanisms of pathogenesis may converge to produce a similar anatomic end result [5], the clinical manifestations might be quite variable as was observed when looking at the cognitive performance of children with varying degrees of severity of holoprosencephaly based on the traditional classifications.
Of key importance in our findings is that no correlations emerged between the Composite Motor Scores and the Carter Neurocognitive Assessment subscales. The Composite Motor Score captures gross motor abnormalities (i.e., spasticity, choreoathetosis, hypotonia, dystonia); hence on most traditional measures of early cognition there is a decrease in performance when motor abnormalities are present, as many of the tasks require a motor response, such as reaching or pointing [21]. However, the Carter Neurocognitive Assessment requires minimal motor sophistication. It relies primarily on eye gaze and facial expressions to indicate a response; this suggests that the Carter Neurocognitive Assessment is a more sensitive diagnostic tool for this population, as their motor skill impairment is not detrimental to performance, with the exception of vocal communications skills. As reported in Plawner’s study, only the measure for dystonia within the Composite Motor Score correlated with the degree of nonseparation of the caudate and lentiform nuclei [3]. These are the same deep gray structures that correlated with performance on the Vocal Communication subscale in the present study. Hence, only a small portion of the Composite Motor Score is associated with nonseparation of the deep gray nuclei or expressive communication skills.
There was a significant correlation between history of seizures and subsequent use of seizure medication and overall cognitive performance. This finding is not surprising as the physiologic damage to the brain that occurs as a result of repeated seizures is well documented in the literature [23]. The study participants who did not have seizures performed better than those who had a history of seizures and were under treatment. Interestingly, it did appear that matching the children with and without seizures by deep gray score and chronological age was a more sensitive measure than matching by holoprosencephaly classification and chronological age. The correlations were significant for all but the Vocal Communication subscale when the deep gray score was used, but not when the holoprosencephaly classification was used. These data suggest that the subgroup of children without seizures were spared the additional and possibly cumulative damage that can be caused by recurrent seizure activity, as well as the side effects of epileptic medications on attention and subsequent performance [24]. This premise could not be tested in our study group as medications prescribed varied widely by subject. It should also be noted that the responses required on the Vocal Communication Subscale are more motor-based than the range of responses accepted for the other subscales and, consequently, this scale may have been less impacted by seizure activity than the more cognitively based auditory and visual tasks. As mentioned earlier, the presence of seizures might be due to the degree of expression of the mediolateral gradient in the abnormal cortical architecture in holoprosencephaly in which the medial parts of the forebrain are more disorganized than the lateral parts [5,7,25]. Nonetheless, more than one third (35%) of the participants in the present study and almost one half (49%) of Plawner et al.’s [3] subjects had at least one seizure. Thus, this is an important clinical feature to bear in mind when looking at group performance of children with holoprosencephaly.
Use of the Carter Neurocognitive Assessment provides a more precise characterization of some specific cognitive constructs that can be observed in children with holoprosencephaly. In particular, social awareness, which included nonverbal communication skills (e.g., facial expressions) and visual attention were considered to be areas of relative strength for the children in this study. In fact, there was a significant difference in performance between the latter two subscales and the Vocal Communication subscale. This relative strength in social skills has been observed incidentally during clinical visits as most of the children are socially engaging and respond positively to faces and voices. It is also possible that social awareness skills, such as anticipatory reactions and social referencing, might index the degree of basic executive functioning.
Additional data from a broad laboratory-based battery that includes both behavioral measures and electrophysiology on the same sample are currently being analyzed and, to date, provide converging evidence that some of these early executive functions (including organizing and sequencing behaviors, sensitivity to contingent relations as well as memory retrieval strategies and speed of processing) seem to be surprisingly intact given the degree of frontal lobe pathology in this population [26,27].
As noted, very few children in our sample had been diagnosed with a genetic syndrome. In this context, it is important to emphasize that the prognosis in holoprosencephaly is much poorer for those with cytogenetic abnormalities, with only 2% surviving beyond 1 year, compared with 30–54% for those without cytogenetic anomalies [28]. This outcome might explain why our sample of children with holoprosencephaly is skewed toward nonsyndromic, more mildly affected cases than the literature has reported on in the past [9]. To provide further clarification of this issue, we performed our statistical analyses with and without the three children in the sample who were known to have genetic or chromosomal abnormalities. There were no significant differences in the results. Future studies that look at the cognitive performance of the children with specific mutations should be considered (that is, a more formal genotype/phenotype analysis), though this will be a challenge given the small numbers of children with documented genetic etiologies to date.
Limitations
An analysis of performance based on “traditional” classifications could not be conducted because of the disparity in the number of children in each group. However, in the largest group, children with a diagnosis of semilobar holoprosencephaly (n = 25, 60%), there was a great degree of variability in performance. Within this group, the Deep Gray Score provided a better descriptive account of individual performance than the traditional classification. As all these children were classified as semilobar, the considerable individual differences observed within this category could not be explained nor predicted. It appears likely that such converging assessments will provide better predictive power and enhanced support for ongoing clinical management decisions in children with severe neurodevelopmental dysfunction.
Although the lack of standardization of the Carter Neurocognitive Assessment could be considered a limitation, it should be evident that children with holoprosencephaly could not and should not be compared with a normative population given the severe physical and expressive language deficits typically encountered by these children. Their explorations of their surroundings and interactions with people and objects are quite different from children who have no physical or language handicaps. The inappropriate use of traditional assessments may be the reason for the low estimates of cognitive ability reported in such patients. The use of specific developmental milestones to create a severity scale for the Carter Neurocognitive Assessment has been demonstrated to be a useful strategy in determining a profile of relative strengths and weaknesses for specific cognitive constructs in this group of children with holoprosencephaly. The profile of skills generated by the Carter Neurocognitive Assessment is intended to provide caregivers and specialists with information to use as a guide when choosing intervention goals and strategies, not as a strict comparison of performance among constructs, given the experimental nature of the assessment tool.
Clearly, many variables interact with cognitive development. Given the developmental nature of the skills assessed on the Carter Neurocognitive Assessment and the use of specific milestones when deriving the severity scores, it is not surprising that there was an age effect on performance. Although the statistical analyses used here do a fairly good job of accounting for age effects, the authors are aware that a small subject sample was used in this study and that it was not designed as a longitudinal study. Therefore it is not clear what the age effect would be for an individual child over time. In addition, one might question the broad age range of children sampled in this study. However, it is important to keep in mind that holoprosencephaly is a rare disorder and most of these children exhibit severe motor and expressive language deficits. In fact, the older children were referred to the study because their cognitive skills could not be fairly assessed on traditional cognitive measures and their overall developmental level was judged to be below the 2-year level of development. Nonetheless, a similar profile of cognitive strengths and weaknesses was observed for both younger and older children in the sample. The Carter Neurocognitive Assessment provides a much needed clinical tool for multiply handicapped children as it assesses specific cognitive constructs using minimal motor sophistication, thus allowing a realistic inventory of skills not possible using adaptations of traditional measures. When such an assessment is used in combination with the anatomic indices described here, a more realistic picture of each child’s strengths and weaknesses can be assembled.
Conclusions/Future Directions
The primary aim of this study was to consider how the structural abnormalities observed in children with holoprosencephaly might relate to a range of cognitive abilities, and further, what additional descriptive power deep gray nuclei scores might add to the traditional holoprosencephaly classifications. In addition, the influence of motor skill deficits on cognitive performance was evaluated. These goals reflect the need for more precise diagnosis of cognitive delays in children with holoprosencephaly than had been possible using the traditional classification system. The deep gray structures assessed were found to be significantly associated with vocal communication skills, a motor-based skill, but not with the other cognitive constructs observed. In addition, deficits in gross motor skill did not affect performance on the Carter Neurocognitive Assessment. This study describes the cognitive profile of children with holoprosencephaly in the domains of social awareness, visual attention, auditory comprehension, and vocal communication as assessed with an experimental measure, the Carter Neurocognitive Assessment, which requires minimal motor sophistication. These findings contribute a needed component to the growing body of knowledge regarding the medical and clinical outcomes of children with holoprosencephaly, as they suggest that neither the traditional holoprosencephaly categories nor the addition of deep gray nuclei abnormalities necessarily predict all aspects of neurodevelopmental function, an important consideration when making clinical management decisions and when counseling families. The data reported here will be of use to both pediatric neurologists and pediatricians who oversee the care of children with such disorders.
Given the multiple medical conditions that affect children with holoprosencephaly, neurodevelopmental outcomes are often overlooked. Further research of cognitive skill development in children with holoprosencephaly and similar disorders is essential, as the child’s performance levels influence decisions regarding multidisciplinary clinical management, intervention strategies, and importantly allow more optimal family counseling. The Carter Neurocognitive Assessment, a fairly new instrument [21], supports a more accurate and balanced view of overall abilities in children with holoprosencephaly, as well as other groups of children with multiple handicaps. As highlighted in this study, the Carter Neurocognitive Assessment has identified relative strengths in the areas of social awareness and visual attention skills for children with holoprosencephaly; thus intervention strategies that build on these strengths, such as the use of eye tracker technology, should be considered. Ongoing investigation of the relations between varying degrees of anatomic and structural abnormalities, emerging cognitive abilities, and the impact of appropriately focused interventions for children with congenital brain malformations is an important goal. Hopefully the information obtained will help pediatricians, pediatric neurologists, clinicians, and families to more accurately assess the developmental potential of affected children and lead to improved clinical management, better focused interventions, and an environment that fosters optimal neurodevelopmental outcomes.
Acknowledgments
This research was supported by grants from the Don and Linda Carter Foundation for Research into Holoprosencephaly and Related Brain Malformations, the Crowley-Carter Foundation, and the Communities Foundation of Texas. The Elizabeth H. Solomon Center for Neurodevelopmental Research, a grant to A.A.B. from NICHD (RO1-HD29419), and a Rutgers University Board of Trustees Excellence in Research Award to A.A.B. provided additional support.
We wish to thank the children who participated in this study and their caregivers. A special thank you goes out to Teresa Realpe Bonilla and Silvia Ortiz-Mantilla, M.D. for their thoughtful consideration and constructive criticism regarding this research. We also thank Wei Fang, Bianca Gray, Heather Kammann, Dr. Darla Swann, and Leeann Hammerschmitt, for their involvement with this project. Finally, we would like to thank Dr. James Barkovich for reviewing all of the neuroimaging reports and providing deep gray scores.
References
- 1.Hahn JS, Plawner LL. Evaluation and management of children with holoprosencephaly. Pediatr Neurol. 2004;31:79–88. doi: 10.1016/j.pediatrneurol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Simon EM, Hevner RF, Pinter JD, et al. Assessment of the deep gray nuclei in holoprosencephaly. Am J Neuroradiol. 2000;21:1955–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Plawner LL, Delgado MR, Miller VS, et al. Neuroanatomy of holoprosencephaly as predictor of function: Beyond the face predicting the brain. Neurology. 2002;59:1058–66. doi: 10.1212/wnl.59.7.1058. [DOI] [PubMed] [Google Scholar]
- 4.Golden JA. Towards a greater understanding of the pathogenesis of holoprosencephaly. Brain Dev. 1999;21:513–21. doi: 10.1016/s0387-7604(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 5.Sarnat HB, Flores-Sarnat L. Integrative classification of morphology and molecular genetics in central nervous system malformations. Am J Med Genet. 2004;126A:386–92. doi: 10.1002/ajmg.a.20663. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MM. Perspectives on holoprosencephaly: Part I. Epidemiology, genetics, and syndromology. Teratology. 1989;40:211–35. doi: 10.1002/tera.1420400304. [DOI] [PubMed] [Google Scholar]
- 7.Sarnat HB, Flores L. Neuropathologic research strategies in holoprosencephaly. J Child Neuro. 2001;16(12):918–32. doi: 10.1177/088307380101601211. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MM. Perspectives on holoprosencephaly: Part III. Spectra, distinctions, continuities, and discontinuities. Am J Med Genet. 1989;34:271–88. doi: 10.1002/ajmg.1320340232. [DOI] [PubMed] [Google Scholar]
- 9.Stashinko EE, Clegg NJ, Kammann HA, et al. A retrospective survey of perinatal risk factors of 104 living children with holoprosencephaly. Am J Med Genet. 2004;128A(2):114–9. doi: 10.1002/ajmg.a.30070. [DOI] [PubMed] [Google Scholar]
- 10.Ming JE, Muenke M. Holoprosencephaly: From Homer to Hedgehog. Clin Genet. 1998;53:155–63. doi: 10.1111/j.1399-0004.1998.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 11.Ming JE, Muenke M. Multiple hits during early embryonic development: Digenic diseases and holoprosencephaly. Am J Hum Genet. 2002;71:1017–32. doi: 10.1086/344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallis D, Muenke M. Mutations in holoprosencephaly. Hum Mutat. 2000;16:99–108. doi: 10.1002/1098-1004(200008)16:2<99::AID-HUMU2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.DeMyer W, Zeman W, Palmer CG. The face predicts the brain: Diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly) Pediatrics. 1964;34:256–63. [PubMed] [Google Scholar]
- 14.Barr M, Cohen MM. Holoprosencephaly survival and performance. Am J Med Genet. 1999;89:116–20. [PubMed] [Google Scholar]
- 15.Barkovich AJ, Quint DJ. Middle interhemispheric fusion: An unusual variant of holoprosencephaly. Am J Neuroradiol. 1993;14:431–40. [PMC free article] [PubMed] [Google Scholar]
- 16.Simon EM, Hevner RF, Pinter JD, et al. The middle interhemispheric variant of holoprosencephaly. Am J Neuroradiol. 2002;23:151–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia KP, Marsden CD. Brain. Pt 4. Vol. 117. 1994. The behavioral and motor consequences of focal lesions of the basal ganglia in man; pp. 859–76. [DOI] [PubMed] [Google Scholar]
- 18.Kandel ER, Schwartz JH, Jessell TM, editors. The functional organization of perception and movement: Principles of neuroscience. 4. New York: McGraw-Hill; 2000. pp. 338–48. [Google Scholar]
- 19.Best PJ, Weldon DA, Stokes KA. Lesions of mediodorsal thalamic nucleus cause deficits in attention to changes in environmental cues without causing sensory deficits. In: Diamond A, editor. The development and neural bases of higher cognitive function. New York: New York Academy of Sciences; 1990. pp. 705–14. [DOI] [PubMed] [Google Scholar]
- 20.Lacbawan F, Muenke M. Personal communication. 2005.
- 21.Leevers HJ, Roesler CP, Flax J, Benasich AA. The Carter Neurocognitive Assessment for children with severely compromised expressive language and motor skills. J Child Psychol Psychiatry. 2005;46(3):287–303. doi: 10.1111/j.1469-7610.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 22.Banich MT. Neuropsychology: The neural bases of mental function. Boston: Houghton Mifflin; 1997. Introduction to the nervous system; pp. 18–21. [Google Scholar]
- 23.Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5. doi: 10.1203/00006450-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: Morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 25.Dobyns WB, Reiner O, Carrozo R, Leadbetter DH. Lissencephaly: A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. J Am Med Assoc. 1993;270:2838–42. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 26.Roesler C, Flax J, Paterson S. Neurocognitive development in HPE: Cognitive profile of children with HPE. Invited presentation at: Third Annual NIH Conference on Holoprosencephaly: Midline and Laterality Development; Bethesda, Maryland. April 18–20, 2004. [Google Scholar]
- 27.Jing H, Flax J, Roesler CP, Choudhury N, Benasich AA. Auditory event-related responses in children with semilobar holoprosencephaly. Brain Dev. 2006;28:207–14. doi: 10.1016/j.braindev.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croen LA, Shaw GM, Lammer EJ. Holoprosencephaly: Epidemiologic and clinical characteristics of a California population. Am J Med Genet. 1996;64:465–72. doi: 10.1002/(SICI)1096-8628(19960823)64:3<465::AID-AJMG4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]


