Abstract
Objective
To investigate the harvest and application of hamstring grafts for canine cranial cruciate ligament (CrCL) reconstruction.
Study Design
Experimental study.
Animals
Four adult female hounds, weighing 26.3 ± 1.6 kg (mean ± SEM).
Methods
One stifle in each dog was randomly chosen for hamstring graft CrCL reconstruction after native CrCL transection. Arthroscopy was performed to evaluate graft integrity at 12 weeks. Gait analysis and stifle radiographs were performed preoperatively and up to 52 weeks after graft placement. Dogs were killed 12 (n = 2) or 52 weeks (n = 2) after CrCL reconstruction. Tissues were evaluated grossly and with light and confocal laser microscopy.
Results
Hamstring grafts were intact in all stifles at 12 weeks (n = 4) and 52 weeks (n = 2). Grossly, there was no osteoarthritis in stifles at 12 weeks and only chondrophytes along the trochlear ridges at 52 weeks. Minimal radiographic evidence of osteoarthritis developed in stifles with grafts during the study. Lameness in limbs with grafts resolved by 52 weeks. Graft tissue was highly vascular, ligamentized, and undergoing active remodeling at 12 weeks. Fifty-two weeks after graft placement, intraarticular graft tissue was well vascularized, mature, and encapsulated by synovium, and graft-bone interfaces were characterized by Sharpey’s fiber insertions. There was no evidence of graft necrosis using confocal laser microscopy at either time point.
Conclusions
The hamstring graft technique may be a viable method of canine CrCL reconstruction.
Clinical Relevance
Hamstring grafts may be an alternative technique for canine CrCL reconstruction. Further study is needed before clinical application.
Rupture of the cranial cruciate ligament (CrCL) and associated degenerative changes are the most common debilitating lesions in the canine stifle.1–3 Methods of CrCL deficient joint stabilization include intraarticular CrCL reconstruction, extraarticular procedures to stabilize the joint by altering periarticular structures, and tibial plateau leveling.4–14 In retrospective studies, little variation in clinical results has been reported between ligament replacement techniques and extracapsular stabilization techniques.12
An optimal graft for CrCL reconstruction would use a material of sufficient strength and minimum harvest morbidity, would have accurate, reliable placement and fixation, would allow immediate weight bearing and full range of motion, and would be performed with limited morbidity.15 The most common autograft materials for canine CrCL reconstruction are the patellar tendon and fascia lata.1 Hamstring grafts consisting of the tendons of the semitendinosus and gracilis are gaining popularity in human surgery, with reported benefits over standard patellar tendon grafts, including superior initial strength, faster biologic incorporation, better joint stability, and minimal pain and donor site morbidity.16 Based on the reported benefits of the hamstring graft and using established surgical techniques for canine CrCL reconstruction, we sought to design a hamstring graft technique customized for the dog.
Our study was designed to investigate the harvest and application of hamstring grafts in canine CrCL deficient stifles. We hypothesized that hamstring grafts would undergo standard ligamentization, would develop strong bony insertions, and would be viable 52 weeks after graft placement.
MATERIALS AND METHODS
Experimental Design
Four skeletally mature female crossbreed hounds, weighing 23.5 to 29 kg (mean ± SEM weight, 26.3 ± 1.6 kg) were used. Before inclusion, all dogs were evaluated for hind-limb lameness and radiographic evidence of OA with force plate gait analysis and stifle radiographs. One stifle in each dog was randomly chosen for hamstring graft CrCL reconstruction after native CrCL transection. Twelve weeks after graft placement, stifle arthroscopy was used to assess hamstring graft integrity.17 Two dogs were killed at the end of the arthroscopic procedure. A modified retinacular imbrication technique (MRIT) was performed after arthroscopic surgery in the other 2 dogs to reduce intraarticular stresses on the graft, given that fat pad and synovium were removed to evaluate graft insertion sites.13 The sutures were cut percutaneously 12 weeks later. Two dogs were killed 52 weeks after graft placement. Assessment variables included manual stifle examination; radiographs; subjective and objective (force plate) gait analysis; and, after death, gross, light, and confocal laser microscopic graft evaluation.
Surgical Procedure
Dogs were premedicated with acepromazine (0.10 mg/kg) and butorphanol tartrate (0.2 mg/kg), both administered subcutaneously. After 20 minutes, 5 mg/kg thiopental was administered intravenously (IV) for anesthetic induction. Dogs were intubated and maintained on halothane in oxygen administered with a semiclosed circle system. Before surgery, each dog was administered cephazolin (20 mg/kg, IV) prophylactically. All stifles were examined for drawer, range of motion, swelling, temperature, crepitus, patellar tracking, and varus-valgus deformity.
One randomly selected stifle from each dog was shaved, aseptically prepared for surgery, and draped, and a betadine-impregnated adhesive drape (Ioban 2; 3M Health Care, St. Paul, MN) was applied. A skin incision was made on the medial aspect of the limb extending from 5 mm medial to the distal aspect of the patella to 3 cm proximal to the talocrural joint (approximately two thirds the length of the tibia). The combined tendinous insertions of the gracilis and semitendinosus muscles were identified (Fig 1). While maintaining the combined bony insertion of both muscles and the surrounding dense connective tissue, the tendons were dissected free of their muscular origins. The insertion was then elevated, and the attached dense connective tissue and muscle fascia of the medial aspect of the cranial tibialis muscle were dissected free of their bony and muscular attachments to a level approximately 3 cm proximal to the talocrural joint, where they were sharply transected.
Fig 1.
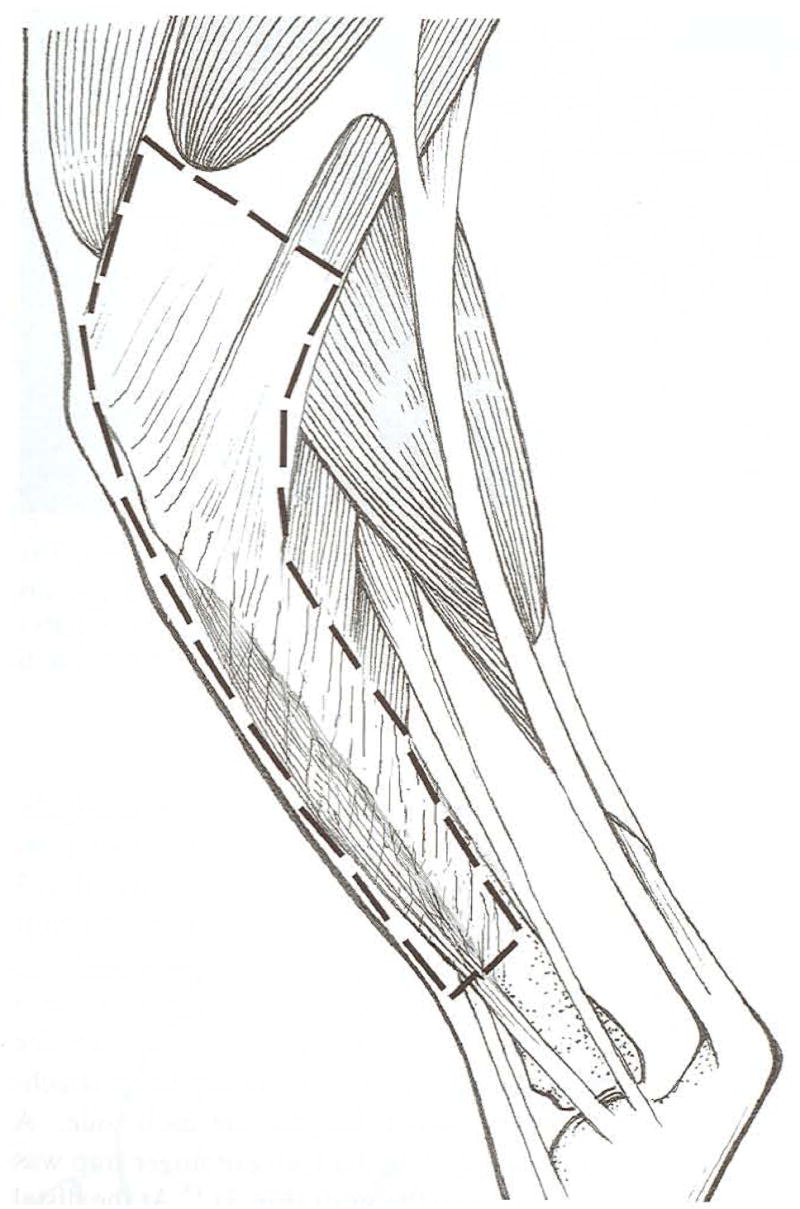
The canine hamstring graft is composed of the combined tendinous insertion of the semitendinosus and gracilis muscles, attached dense connective tissue, and fascia from the medial aspect of the cranial tibialis muscle. The bony insertion of both tendons is maintained while they are dissected free of their muscular origins. The attached dense connective tissue and muscle fascia of the medial aspect of the cranial tibialis muscle are dissected free of their bony and muscular attachments to a level approximately two thirds the length of the tibia.
The graft was then trimmed to a width of 1.5 cm (Fig 2). A Backhaus towel clamp was used to twist the graft from the distal end until there was a 360° tissue turn every 4 to 5 mm, giving a final graft diameter of about 4.5 mm. The graft was twisted to increase the diameter and to mimic the multifascicular nature of the native CrCL Suture (size 3 polyglactin 910) was passed through the center of the proximal aspect of the graft just distal to the bony attachment until there were equal lengths on each side. A transfixation suture was tied, and a Chinese finger trap was placed around the length of the graft (Fig 3).18 At the distal end of the graft, another transfixation suture was tied, and the suture ends were left long after removal of the needle. The graft was then wrapped in saline-soaked gauze sponges.
Fig 2.
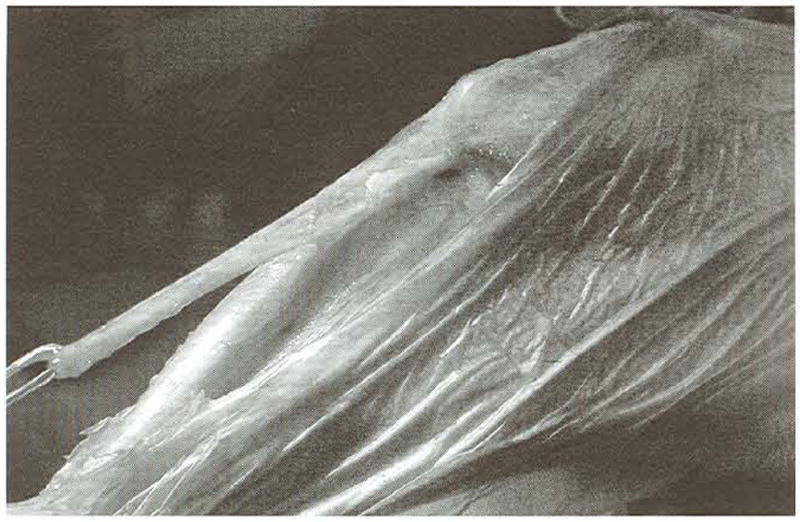
The hamstring graft immediately after harvest. The combined bony insertion of the semitendinosus and gracilis muscles is maintained. The photograph shows the relative length of the graft but not the width because the graft tissue is curled slightly.
Fig 3.
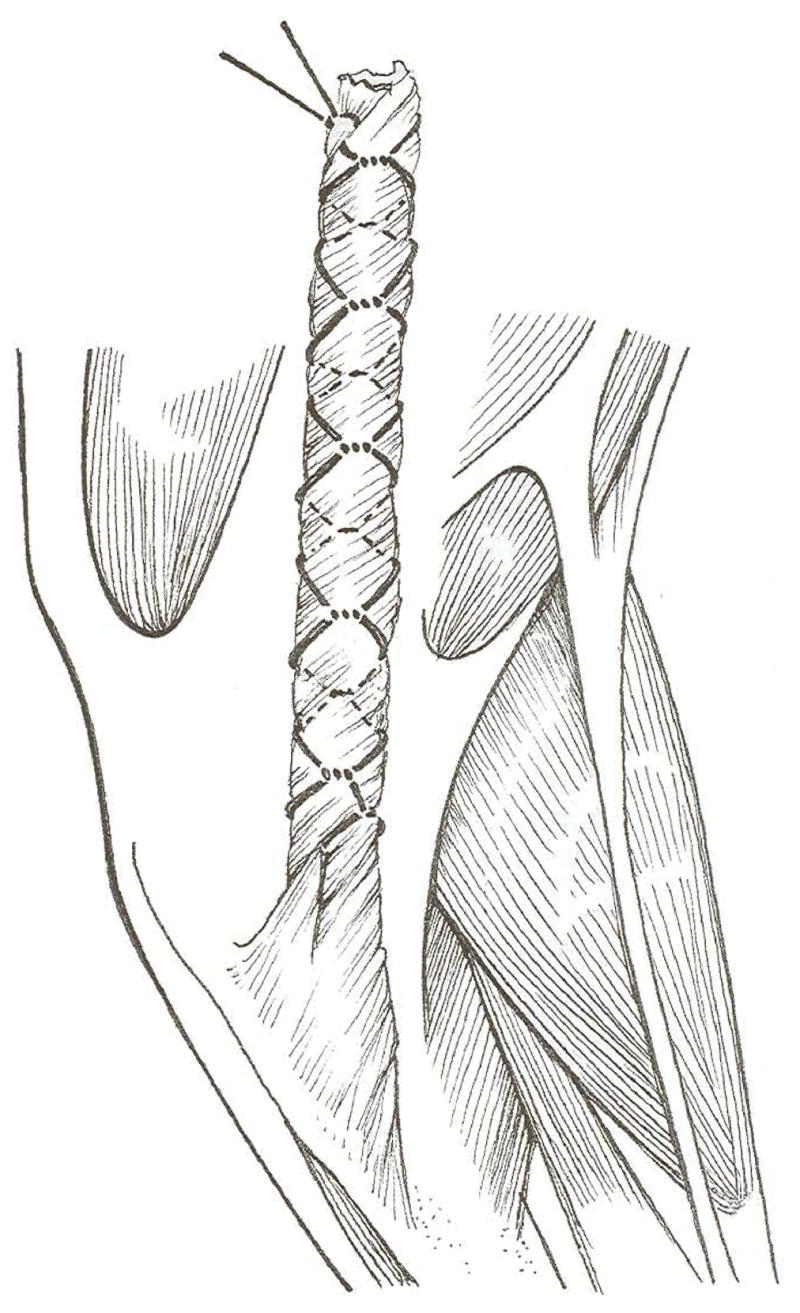
The hamstring graft is trimmed to a width of about 1.5 cm and then twisted to give a 360° tissue turn every 4 mm. A transfixation suture is tied just distal to the combined bony insertion of the gracilis and semitendinosus, and the graft is wrapped in a Chinese finger trap composed of size 3 polyglactin 910.
A 3-cm arthrotomy was made into the medial aspect of the femorotibial joint through the proximal aspect of the medial skin incision. The intact CrCL was transected with a No. 12 scalpel blade. A positive drawer sign confirmed complete transection. An aiming device (Adapteur Drill Guide C-Ring and Tibial Tunnel Marking Hook; Arthrex, Naples, FL) was used to guide a 4.5-mm drill bit (Synthes, Paoli, PA) powered with a Stryker drill (Stryker, Kalamazoo, MI) through the tibia to the site of the anatomic CrCL insertion. Saline solution (0.9% NaCl) lavage was applied during drilling. The suture ends on the graft were grasped with a curved hemostatic forcep, and the graft was pulled through the tibial tunnel (Fig 4).
Fig 4.
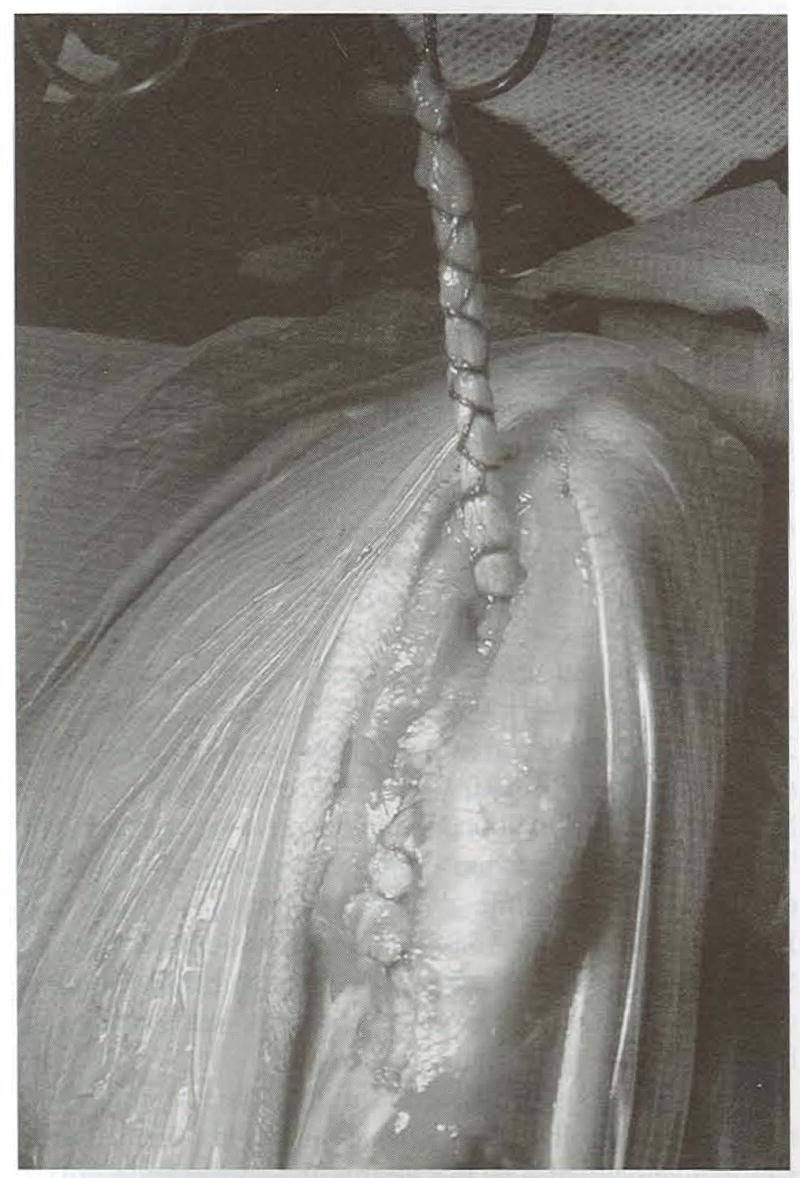
Hamstring graft within a Chinese finger trap that has been pulled through the tibial tunnel. The graft enters the tibia tunnel just proximal to the combined bony insertion of the semitendinosus and gracilis tendons and exits at the anatomic insertion of the CrCL.
A 3-crn incision through skin and subcutaneous tissue was centered over the lateral fabella at a 45° angle to the long axis of the femur. With the joint in extreme flexion, the forcep was passed caudal to the lateral fabella and into the intercondylar notch through the lateral skin incision. The graft was then gently pulled through the joint and over the top of the lateral aspect of the femoral condyle. Gentle tension was applied to the graft until there was no elicitable drawer motion. With the joint in approximately 135° of flexion (standing angle), the graft was secured to the femur with a belt buckle technique just proximal and cranial to the lateral fabella with 3, 7 × 7-mm bone staples using a powered metaphyseal stapler (Stapilizer, 3M Health Care), and the long suture tags were removed (Fig 5).2 The graft was also secured to the tibia with 2 bone staples equally spaced between the bone tunnel and tendinous bony insertion to prevent motion along the edge of the bone tunnel (Fig 6).
Fig 5.
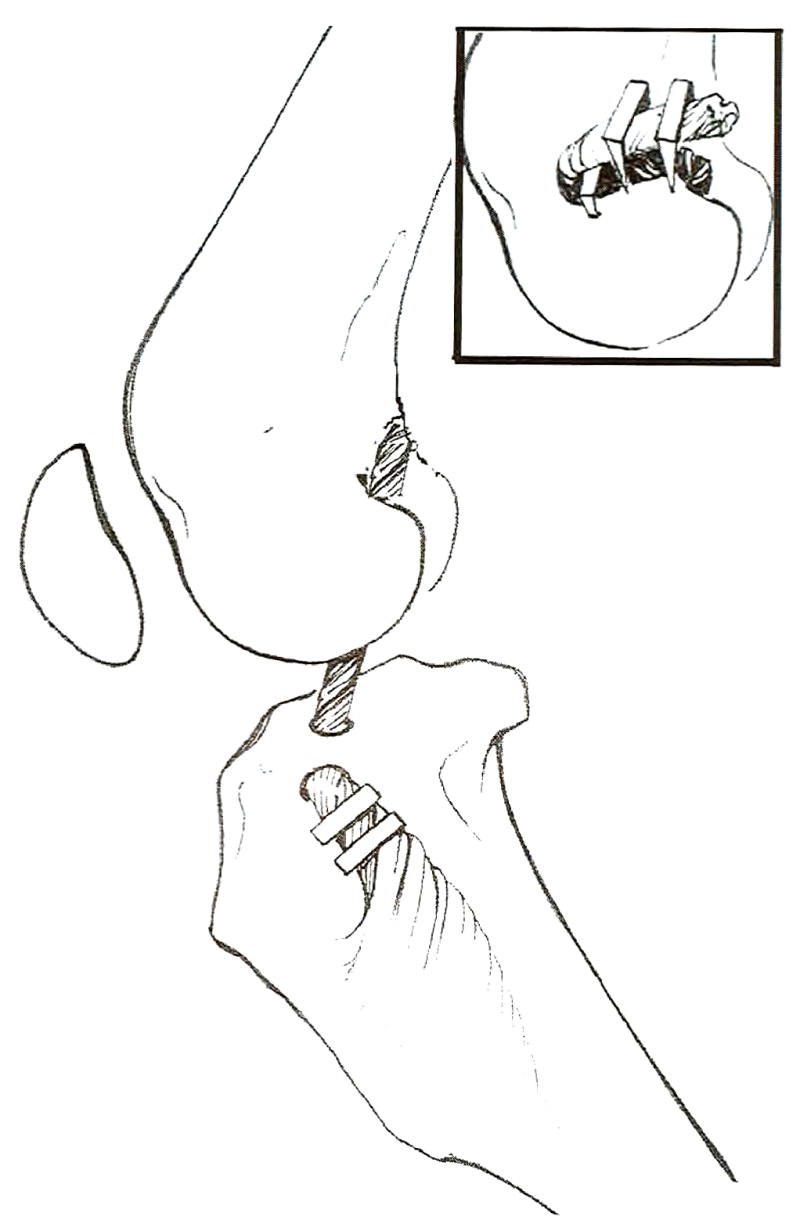
The graft is secured to the femur with a belt buckle technique just proximal and cranial to the lateral fabella with 3, 7 × 7-min bone staples using a powered metaphyseal stapler (Stapilizer; 3M, St. Paul, MN).
Fig 6.
Photograph of 2 bone staples used to secure the graft to the tibia just proximal to the bony insertion to prevent motion along the edge of the bone tunnel.
The lateral femoral incision was closed routinely. The medial incision was closed as follows: Synovial joint capsule was approximated with size 3-0 polyglecaprone 25 sutures, subcutaneous tissues were approximated with size 3-0 polyglyconate sutures, the subcuticular layer was apposed with size 2-0 polyglecaprone 25 sutures, and skin apposition was achieved with skin staples. Graft tissue harvest was superficial and caused minimal tissue disruption. Care was taken during subcutaneous tissue apposition to obliterate any potential tissue space created by the harvest.
Butorphanol tartrate (0.2 mg/kg, IV) was administered once during anesthetic recovery and then intramuscularly every 4 hours for 24 hours as required for postoperative analgesia. Each dog was administered etodolac (10 mg/kg, every 24 hours; EtoGesic, Fort Dodge, IA) and enrofloxacin (15 mg/kg, every 24 hours; Baytril; Bayer Animal Health, Shawnee Mission, KS) beginning 24 hours after and continuing for 10 days after surgery. All dogs were confined to 4 × 6 feet2 runs during the study.
Arthroscopy
Arthroscopy was performed on the stifles with hamstring grafts 12 weeks after graft placement to evaluate graft integrity.17 Dogs were anesthetized and prepared for surgery as described above. The stifle was distended with 7 mL of sterile saline solution. A 0.5-cm incision was made through skin and joint capsule on the lateral aspect of the stifle 0.5 cm proximal to the tibia and just cranial to the lateral digital extensor tendon. A 2.7-mm blunt trocar in a 3-mm cannula was used to enter the joint through the incision. The trocar was replaced by a 2.7-mm, 30° arthroscope (Storz, Goleta, CA). Joint distension was maintained with sterile saline solution using gravity flow.
A 4-mm synovial shaver (APEX; Linvatec, Largo, FL) was inserted into the joint through a 0.5-cm incision in the medial aspect of the joint, just proximal to the tibial plateau and medial to the patellar tendon. Synovium and infrapatellar fat pad were removed until the entire graft, including both bony insertions, could be seen clearly. Each graft was evaluated to determine the presence of tissue resembling a ligament and to determine that it was positioned correctly within the joint.17 The grafts were gently manipulated with a metal probe to assess the security of the proximal and distal attachments and the relative tautness of the graft with the joint in approximately 135° of flexion. The joint was lavaged for 1 minute with sterile saline solution, and incisions were closed routinely.
A modified retinacular imbrication technique was used to protect the graft after arthroscopy in the 2 surviving dogs because of uncertainty of the effect of fat pad and synovium removal on graft viability. Briefly, a skin incision was made on the lateral aspect of the stifle extending from the lateral fabella to the tibial tuberosity. The lateral fabella was exposed, and 2, size 80 nylon sutures (Sufix; Sufix USA, Highpoint, NC) were passed around the caudal aspect of the fabella with a ½ curved cutting edge needle. They were then passed through a 2-mm hole drilled in the tibial tuberosity, 1 cm distal to the tibial plateau and 1 cm caudal to the most cranial aspect of the tibial tuberosity, from lateral to medial and then back just caudal to the patellar tendon and proximal to the tibial plateau. With the stifle in approximately 135° of flexion, the sutures were tied in surgeons’ knots at the level of the lateral fabella. Closure was routine. Postoperative care was identical to that described above.
Twelve weeks after suture placement, the dogs were anesthetized as described, and stifles containing grafts were shaved, aseptically prepared, and draped routinely. A percutaneous stab incision was made over the MRIT sutures just caudal to the point of the tibial tuberosity, and the sutures were transected with a No. 12 scalpel blade. A single simple 2-0 nylon skin suture was used to appose skin edges.
Gait Analysis
Force plate gait analysis was performed preoperatively and 12 weeks after graft placement for all dogs and 16, 24, 30, 36, and 52 weeks after graft placement for 2 dogs using a force plate (OR6-6-1000 Biomechanics Platform with SGA6-4 Signal Conditioner/Amplifier; Advanced Medical Technology, Inc., Newton, MA) connected to a commercially available satellite data acquisition system (VET-DATA v2.03, Acquire v5.0, Mininet v4.0 and Update v1.1: Sharon Software Inc. Dewitt, MI). A single handler trotted dogs for all gait trials, and gait evaluations were performed before any other procedures.
A trial was considered successful if a forepaw contacted the force plate followed by contact of the ipsilateral hind-paw at a velocity of 1.80 to 2.80 m/s and acceleration of 0.9 to −0.9 m/s/s. Three successful passes for each limb were recorded at each time point. Vertical impulse (VI), defined as total force applied over time, and peak vertical force (PVF) were recorded and normalized to body weight. Mean (±SEM) PVF and VI were calculated for control (unoperated) and hamstring graft limbs at each lime point.
Radiographic Evaluation
Caudocranial and lateromedial radiographs were taken preoperatively, and lateromedial radiographs were performed 4, 8, and 12 weeks after graft placement for all dogs and 16, 24, 30, 36. and 52 weeks after graft placement for 2 dogs. All radiographs were performed with the knees in approximately 135° of flexion. Radiographs were evaluated for signs of osteoarthritis progression, including the presence of osteophytes, enthesiophytes, and subchondral bone sclerosis at each time point. Dogs were anesthetized as described previously for all radiographs.
Confocal Laser Microscopy
Immediately after death and following gross inspection, samples were collected for confocal laser microscopy. Femur-graft-tibia specimens were dissected free of all soft tissue immediately after harvest. The graft was removed by sharp transection at its most proximal and distal intraarticular attachments. Three, 1-mm transverse sections from the center of the intraarticular expanse of each graft and contralateral CrCL were collected. Sections were analyzed for fibrocyte viability the same day. Sections were stained by incubation in a 1.0 mL phosphate buffered saline (PBS) containing 0.4 μL calcein (acetoxymethyl ester) per 13 μL ethidium homodimer (Molecular Probes, Eugene, OR) for 30 minutes at room temperature.
Viable and nonviable cells differ in their ability to exude fluorescent dyes.19 Specifically, viable cells with intact plasma membranes and active cytoplasm metabolize calcein and fluoresce green, whereas ethidium homodimer penetrates dead, damaged, or dying cells and stains their nuclei red. For analysis, sections were placed on a glass slide and moistened by several drops of PBS. Using the triple-labeling technique, a confocal laser microscope (MRC-1024: Bio-Rad, Hemel Hempsted/Cambridge. United Kingdom) equipped with a krypton/argon laser and necessary filter systems (fluorescein:522DF32 and rhodamine: 585EFLP) was used to examine the sections. Signals emitted from double-stained specimens can be distinguished because of their different absorption and emission spectra with this technique.19 The images were viewed on an RGB screen and stored digitally on a personal computer.
Histology
After removal of graft and CrCL sections for confocal microscopy, graft tissue from the 12-week dogs and all tissues from the 56-week dogs were fixed in 10% buffered formalin. Intraarticular graft tissue and CrCLs from all dogs were embedded in paraffin blocks and sectioned at 5 μm. Graft and CrCL tissue was stained with hematoxylin and eosin (H&E) and Masson’s trichrome. Femoral and tibial tissue from the 52-week dogs was decalcified and sectioned in paraffin blocks at 5 μm. Tibial sections were sectioned saggitally, and femoral sections were sectioned coronally and transversely. Bone sections were stained with H&E and safranin O. The intraarticular graft, interosseous (tibial) graft, graft-tibia, and graft-femur interfaces were examined with light and polarized light microscopy.
RESULTS
Surgical Procedure
There was moderate soft-tissue swelling along the graft harvest site in the immediate postoperative period after CrCL reconstruction in all dogs. The swelling resolved over 7 days. Each of the dogs had moderate stifle effusion immediately postoperatively. The effusion slowly resolved over 30 days. After surgical CrCL reconstruction, dogs were nonweight bearing for approximately 5 days, toe touching for the next 5 days, and lame for the next 2 weeks, for a total of about 4 weeks until lameness could no longer be subjectively appreciated. After stifle arthroscopy and MRIT suture placement, there was moderate stifle effusion and soft-tissue swelling over the sutures. The soft-tissue swelling and stifle effusion both resolved over 21 days. The resolution of lameness after stifle arthroscopy and placement of MRIT sutures was very similar. There were no complications associated with either surgical procedure, and the graft harvest site healed with minimal cicatrix formation in all dogs.
Grafts were present and intact 12 weeks and 52 weeks after surgery at necropsy (Fig 7). On close inspection, the graft was firmly attached at the normal tibial insertion site of the CrCL and on the femur from the normal CrCL origin to the fixation site. Grossly, there were no signs of OA in stifles evaluated 12 weeks after graft placement and only chondrophytes along the abaxial surfaces of the trochlear ridges in stifles evaluated 52 weeks after graft placement. Capsular thickening was not appreciated in the stifles with hamstring grafts during necropsy 12 weeks after surgery, and the lateral joint capsule was significantly thickened in association with the MRIT sutures in stifles containing grafts 52 weeks after CrCL reconstruction. There was slight fibrillation of the axial margin of the medial meniscus of 1 stifle evaluated 52 weeks after graft placement. There were no other grossly detectable changes in the menisci at either time point.
Fig 7.
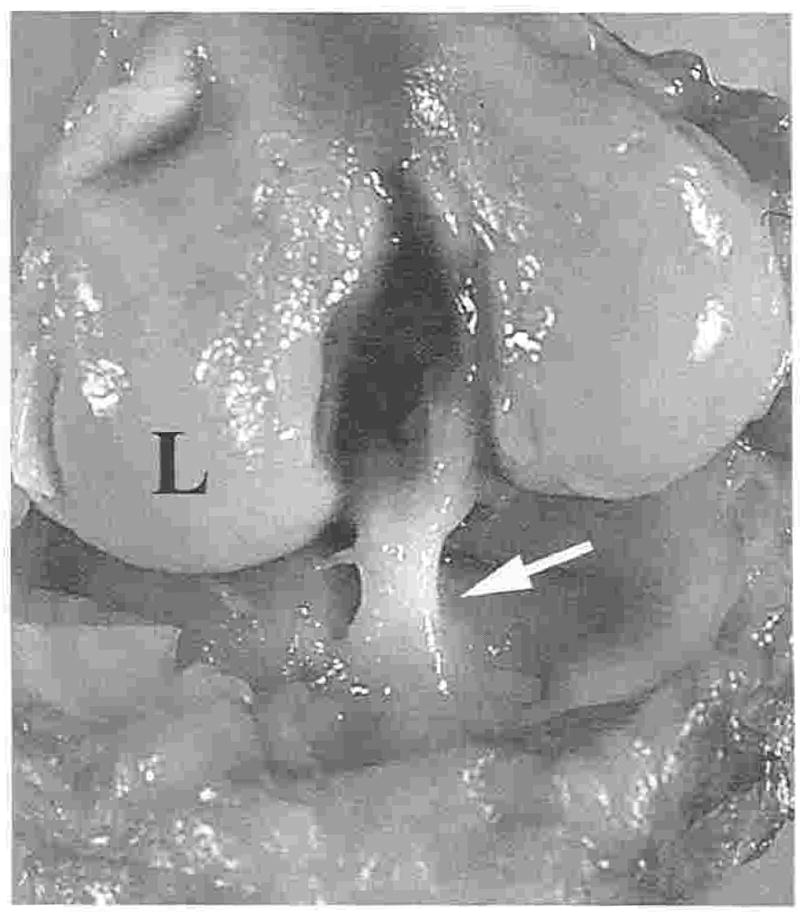
Hamstring graft (white arrow) at 52 weeks. L, lateral aspect of the femoral condyle.
Radiographic Evaluation
All radiographs performed preoperatively were within normal limits, and radiographs from control limbs remained normal throughout the study. Radiographs from stifles with hamstring grafts all had bony proliferation on the cranial surface of the tibial plateau at the exit point of the tibial tunnel 8 weeks after graft placement (Fig 8). The changes remained stable in 1 of 2 dogs up to 52 weeks after surgery. The other dog’s stifle had a very slight increase in bony proliferation, which extended from the exit point of the tibial tunnel to the dorsal aspect of the tibial tuberosity at study end (Fig 9). The only other radiographically evident change was the appearance of enthesiophytes on the lateral fabella of 1 dog between 30 and 36 weeks after surgery.
Fig 8.
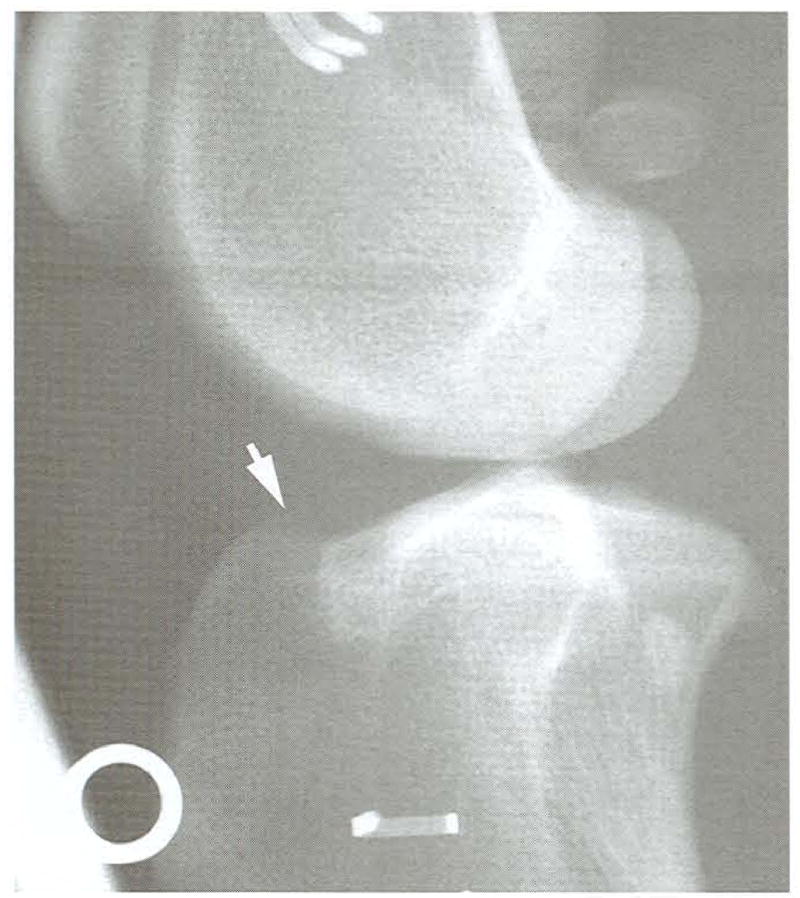
Lateromedial radiograph of a stifle at 8 weeks. Slight bony proliferation is evident at the proximal exit point of the tibial tunnel (white arrow). Radiodense artifacts are from a positioning device.
Fig 9.
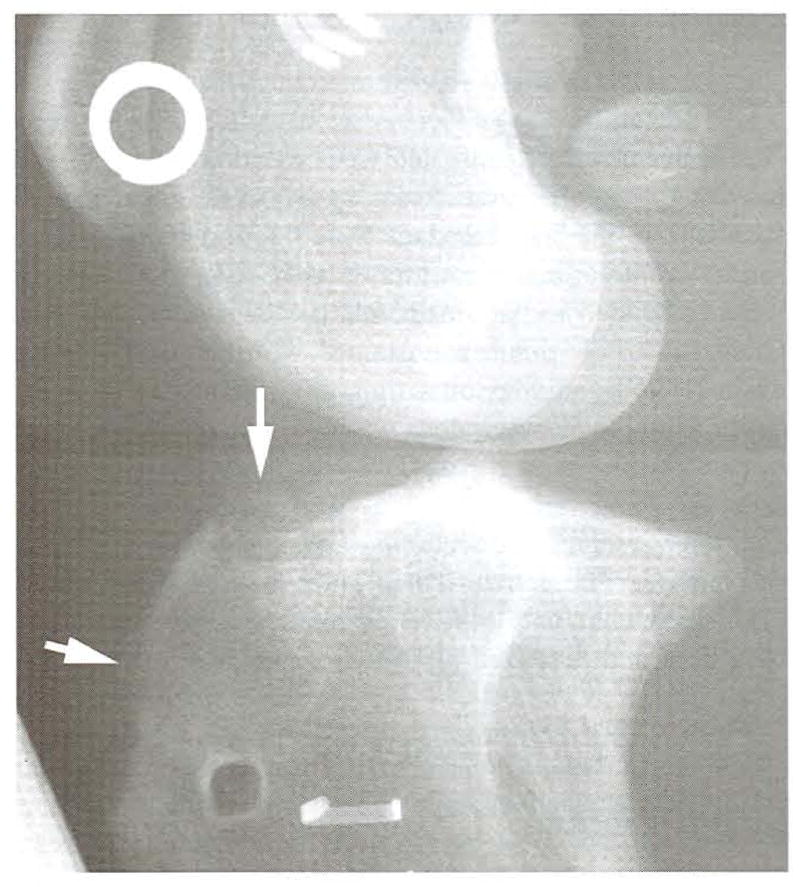
Lateromedial radiograph of the stifle in Fig 8 at 52 weeks. There is limited bony proliferation evident from the proximal exit point of the tibial tunnel to the proximal aspect of the tibial crest (white arrows). Radiodense artifacts are from a positioning device.
Gait Analysis
There was no apparent lameness evident in PVF or VI values on control limbs or those to receive grafts before surgery (Fig 10). Twelve weeks after hamstring CrCL reconstruction, mean PVF on limbs with grafts was 55% of the preoperative value and 57% of the control limb value. Mean VI was 50% of that on the control limb and 55% of the preoperative value. Despite second look arthroscopy. MRIT procedure, and percutaneous MRIT suture release, lameness on the limbs with hamstring grafts appeared to resolve over the study period. Fifty-two weeks after graft placement, mean PVF on limbs with grafts was 98% of that on control limbs and 79% of the preoperative value, whereas the mean on control limbs was 93% of the preoperative value. Similarly, mean VI was 90% of that on the control limb and 101% of the preoperative value, whereas the mean value on control limbs was 86% of the preoperative value. The gait information is presented only as an objective description of the effects of graft placement and subsequent procedures on gait over the study period. Given the very limited number of dogs in this study, statistical analysis was not performed.
Fig 10.
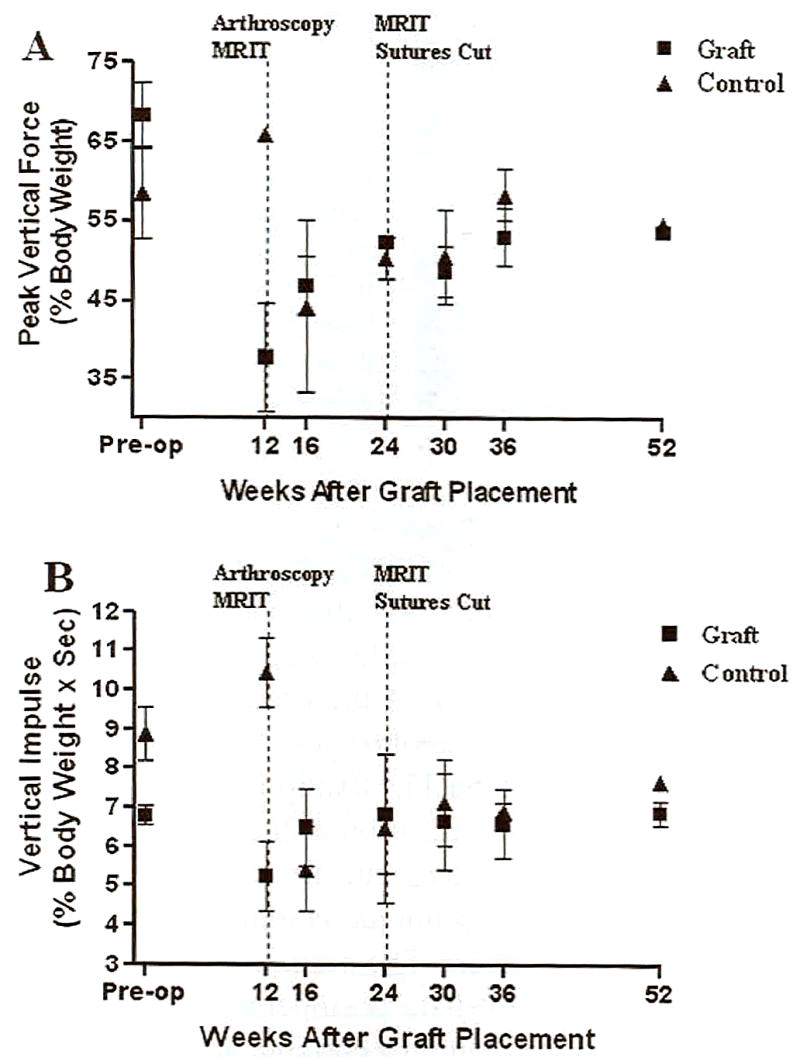
Peak vertical force (A) and vertical impulse (B) on limbs with hamstring grafts and contralateral control limbs over the course of the study.
Confocal Laser Microscopy
Sections collected from the central aspect of each graft were comparably sized with sections collected from the contralateral CrCL (Fig 11). All of the central sections from each graft specimen contained viable cells as indicated by a generalized green fluorescence over the entire graft surface, comparable with the native CrCL.
Fig 11.
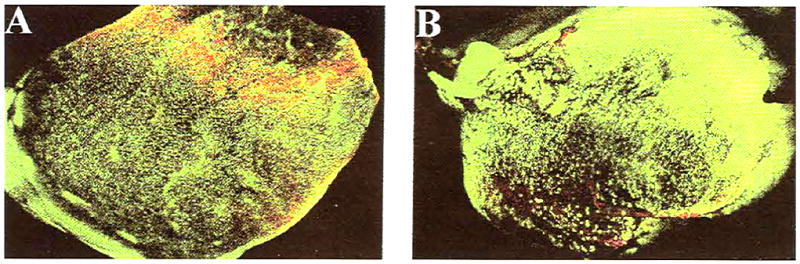
Confocal laser micrograph of midsubstance transverse sections of native CrCL (A) and hamstring graft (B) showing viable cells throughout the specimens (original magnification, ×10). In all confocal laser images, viable tissue fluoresces green, and nonviable tissue fluoresces red.
Histology
Histologic examination of transverse sections collected from intraarticular grafts 12 weeks after placement showed highly cellular, vascularized collagenous tissue with fibrocytes and collagen fibers arranged in numerous bundles throughout the sections (Fig 12). The graft was surrounded by a thin layer of loose connective tissue with occasional fibrocytes (Fig 13). Sections collected similarly from hamstring grafts 52 weeks after graft placement had highly organized, vascular collagenous tissue, with fibrocytes positioned between linearly arranged collagen fibers (Fig 14). A layer of synoviocytes approximately 4 cell layers thick surrounded the graft (Fig 15). Examination of the tibial tunnel revealed complete incorporation of the graft tissue. The collagenous tissue of the graft was highly organized, with occasional fibrocytes and vessels. Along the interface of the graft and tibia, Sharpey’s fibers extended from the graft into the cancellous bone (Fig 16). The same was true of the interface between the graft and femur both intra- and extraarticularly.
Fig 12.
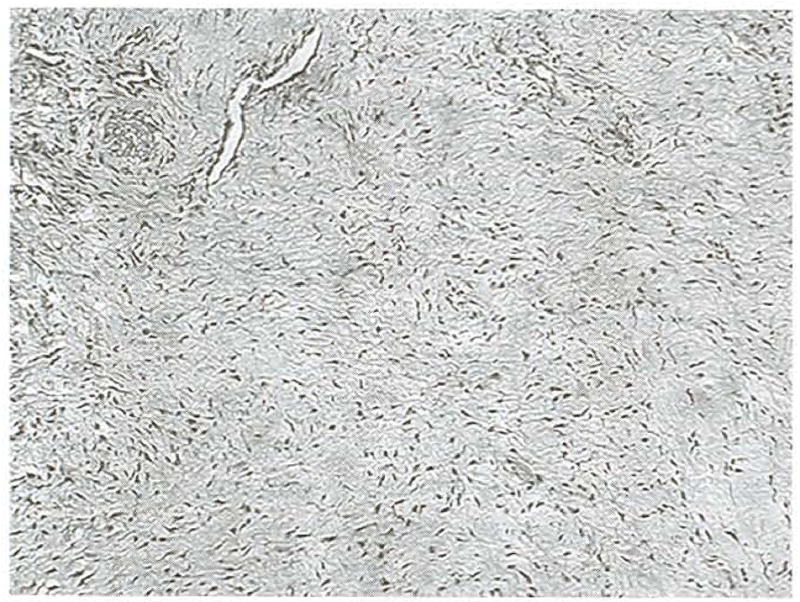
Transverse section of a hamstring graft collected from the central aspect of the intraarticular span at 12 weeks. The tissue is highly cellular and vascular., and fibrocytes and collagen fibers are arranged in bundles throughout the tissue section, (original magnification, ×20: Masson’s trichrome)
Fig 13.
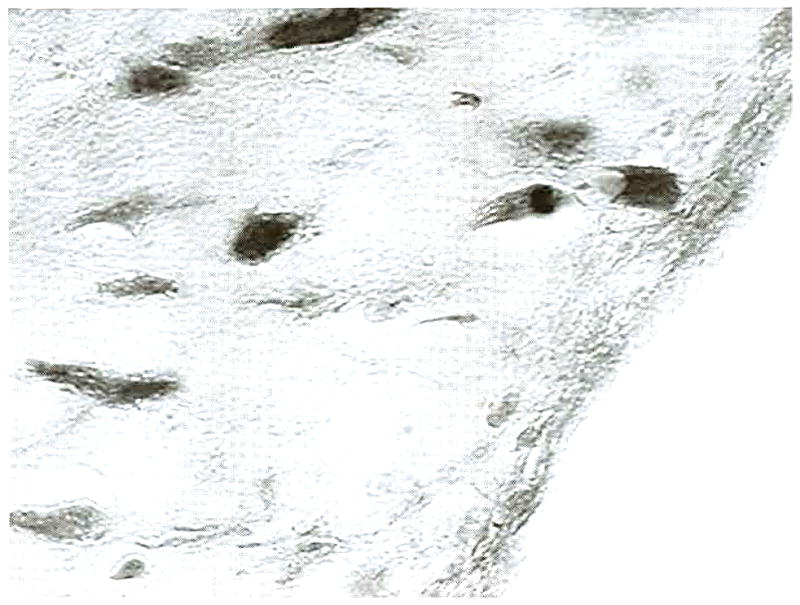
Graft edge from a transverse, 12-week graft section. The graft was contained within a thin layer of loosely organized connective tissue, which contained occasional fibrocytes. (original magnification, ×100; Masson’s trichrome)
Fig 14.
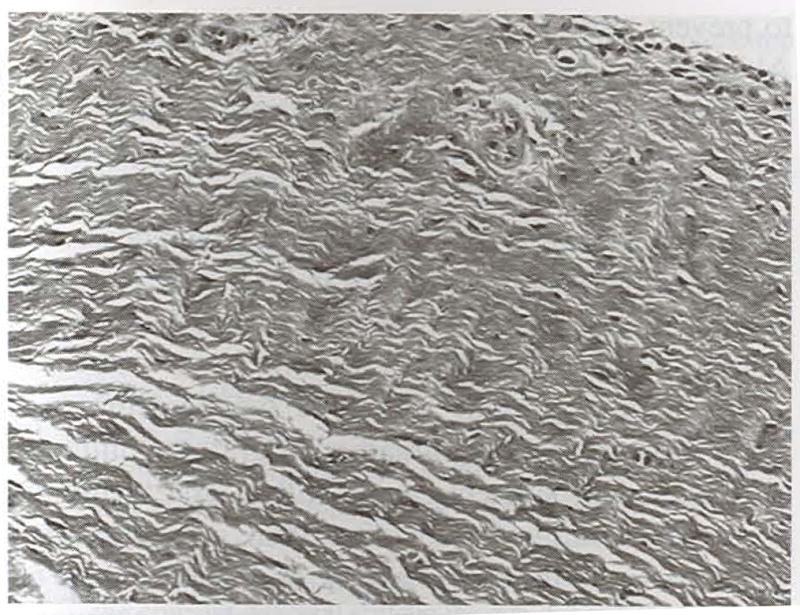
Transverse section of a hamstring graft collected from the central aspect of the intraarticular span at 52 weeks. The tissue is highly organized and vascular, and fibrocytes are positioned between linearly arranged collagen fibers. (original magnification, ×20; Masson’s trichrome)
Fig 15.
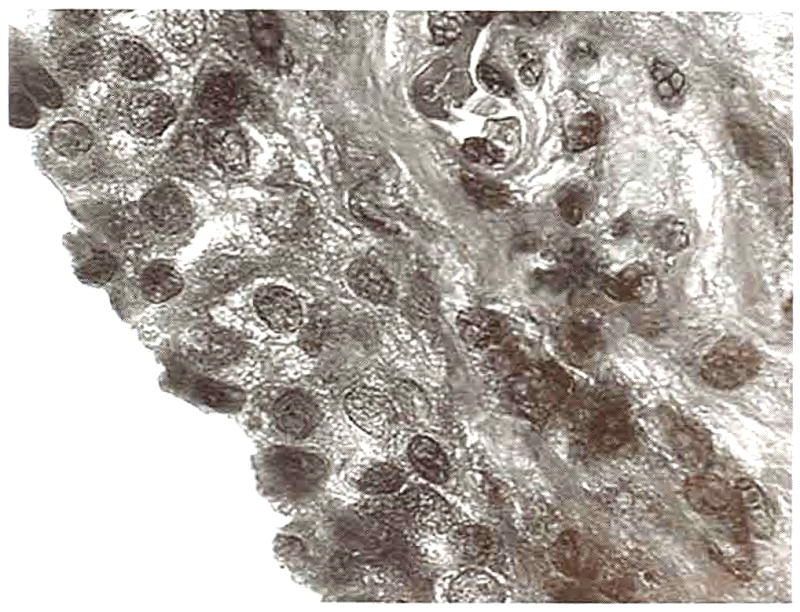
Graft edge from a transverse, 52-week graft section. A layer of synoviocytes approximately 4 cell layers thick surrounded the graft. (original magnification, ×100; Masson’s trichrome)
Fig 16.
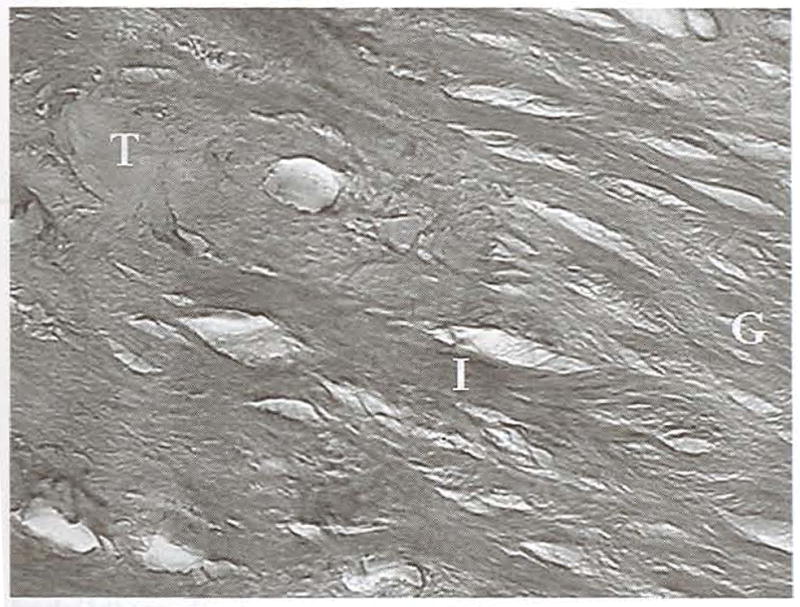
Saggital section of the interface (I) between the hamstring graft (G) and tibia (T) at 52 weeks. The graft tissue is highly organized, and Sharpey’s fibers extend from the graft into the cancellous bone. (original magnification, ×100; H&E)
DISCUSSION
A variety of graft sources have been used for intraarticular CrCL reconstructions to improve function and kinematics in CrCL deficient canine stifles, although the fascia lata and patellar tendon are presently most popular.1–7,13 Autogenous hamstring grafts composed of tendons of the semitendinosus and/or gracilis muscles have gained popularity in reconstruction of human anterior cruciate ligaments, with a number of reported benefits over standard patellar tendon reconstruction.16,20 A hamstring autograft, consisting of the combined tendinous insertion of the gracilis and semitendinosus muscles and cranial tibialis fascia, appears to be an appropriate material to reconstruct the ruptured CrCL in dogs. The graft is easy to harvest and create, provides adequate amounts of tissue for implantation, and has minimal harvest site morbidity. In our study, graft material was intact, viable, and well integrated into the parent tissue 1 year after CrCL reconstruction.
The harvest and composition of the canine hamstring graft is not entirely analogous to that in humans because of species differences in anatomy. Depending on technique, the human hamstring graft is composed of the combined tendinous insertion of the semitendinosus and gracilis.21 The attached fascia is dissected from the underlying musculature in strips, several of which may be grouped into a single graft. The canine hamstring graft includes the combined tendinous insertion of the semitendinosus and gracilis muscles with attached dense connective tissue and cranial tibialis fascia. This difference was necessary because the tissue collected using only tendon and fascia from the gracilis and semitendinosus was not consistently of sufficient length without significant dissection and tissue disruption. The harvest site along the medial tibia for approximately two thirds its length consistently produced a substantial graft of adequate length and width with minimal soft-tissue dissection. The hamstring graft technique described here also varies from other animal models, primarily rabbit and sheep, for reconstruction of the CrCL with hamstring tendon components. The techniques vary between studies and models, but the biggest differences between this canine application and others is the graft harvest site and the maintenance of the tendinous insertions of the graft we described.22,23
The combined tendinous insertion of the semitendinosus and gracilis augmented by 2 bone staples was used for distal hamstring graft fixation in the technique we described. The strength of the tendinous insertion was adequate, and bone staples were applied only to prevent potential abrasion of the graft along the edge of the bone tunnel. Femoral graft attachment was achieved with a belt buckle fixation, which allowed rapid surgical graft stabilization at the desired tension and remained intact throushout the study. The use of the belt buckle staple technique is a novel application for canine graft fixation. The weakest point in graft fixation is in the immediate postoperative period, and the belt buckle graft fixation technique has been shown to be biomechanically equivalent or superior to other methods, including the interference screw.20,24 Secure fixation allowing physiologic function enhances biologic fixation.25 Based on this information, we chose the belt buckle technique in an attempt to preclude graft fixation as a determinant of graft success. Although the technique worked well for this study, it may not be possible or appropriate for all hamstring graft applications. Standard graft stabilization techniques may be acceptable, although other techniques were not explored.
The ideal CrCL graft should have its points of fixation at the anatomic insertion site of the native anterior cruciate ligament on both the femur and tibia for normal ligament function and stifle joint motion. The method of intraarticular hamstring graft placement described here combines and augments attributes of established autogenous graft techniques for anatomic CrCL reconstruction in the dog. The over-the-top technique for intraarticular graft placement permits consistent anatomic CrCL reconstruction and avoids surgical time, trauma, and potential misdirection of a femoral tunnel.6,26 Grossly, the femoral graft attachment we used began at the intraarticular CrCL origin and continued around the lateral aspect of the condyle to the extraarticular surgical fixation site. The aiming device used to create the tibial tunnel permitted consistent placement of the distal graft attachment at the anatomic CrCL insertion, made easier by the fact that the proximal and distal aspects of the tunnel could be clearly and simultaneously viewed during tunnel creation. The hamstring reconstruction technique for the canine CrCL appears to be anatomically satisfactory.
Ligamentization and biologic incorporation of a soft-tissue graft to bone is ultimately necessary for successful ligament surgery when soft-tissue grafts are used for CrCL reconstruction.27 Histologically, midsubstance graft sections were consistent with the remodeling phase of ligamentization, 12 and 52 weeks after graft placement, although later sections showed a greater degree of maturity based on fiber organization, cell and vessel populations, and synovial encapsulation. 25,28,29 Graft tissue within the tibial tunnel 52 weeks after placement was histologically more mature than the intraarticular graft. This likely reflects continuous remodeling in the midsubstance in response to intraarticular stresses, whereas the bone segment section experiences little to no stresses after establishment of a strong bone graft interface. Tibial and femoral bone graft interfaces had direct tendon to bone Sharpey’s fiber association of a well-incorporated graft 52 weeks after placement.30 The relative size of the tibial tunnel and graft in this study simultaneously allowed graft passage through the tibia and a close fit between graft and bone, which is important in the development of the interface between bone and graft.27 Similarly, the mature Sharpey’s fiber association between graft and femur at both points examined supports a strong, stable initial fixation.
Despite the method of joint stabilization, canine stifle OA seems to progress in dogs with established joint disease.31,32 Conversely, in stifles with intraarticular grafts placed at the time of CrCL transection, similar to our study, there is no subsequent OA.6,33 Canine stifle instability is known to be an important contributing factor to OA progression and is the basis for established OA models.34 In our study, the tibial osteophytes that developed on the tibial tuberosity may have been contributed to by the MRIT sutures, and those on the tibial plateau were likely associated with the bone tunnel. The edges of the proximal aspect of the bone tunnel were not smoothed in surgery because of the soft-tissue disruption that would have been necessary to accomplish this. Taking into account the limited number of dogs in our study and the concurrent CrCL transection and graft placement, joint stability conferred by the hamstring graft appears to have been conducive to limited OA progression. Further studies are necessary for objective comparison of joint stability and OA progression between methods of CrCL reconstruction.
Arthroscopic second-look evaluations to assess graft stability were performed 12 weeks after graft placement based on the fact that 12 weeks has been shown to be adequate for graft ligamentization and integration both histologically and biomechanically.29,35 MRIT sutures were placed to protect the graft after arthroscopy because fat pad and synovium surrounding the graft were removed to clearly see the graft origin and insertion. With the uncertainty of the specific effect the removal of the tissue might have on the graft, we sought to reduce graft stresses postoperatively.28 This procedure would not be necessary in a clinical application of this technique, and we have since deemed it unnecessary after second-look arthroscopy. The sutures were cut 12 weeks after placement to prevent graft compromise by prolonged protection. Although the dogs in this study were subjectively lame for about 4 weeks after the initial graft implantation, similar to other described surgical stifle-stabilizing procedures, the subsequent procedures may have inhibited the recovery and resolution of lameness. Despite this, the lameness evident in PVF and VI values resolved to a level consistent with preoperative levels and to that of the contralateral limb.
The hamstring graft technique we describe is a novel application of an intraarticular graft technique to stabilize CrCL-deficient canine stifles. The technique is no more surgically invasive than other reported techniques, and the recovery time and result, based on the limited population in this pilot study, is comparable.3,5,6 Graft collection is fairly simple, and the relative length of the tibial harvest site corresponds nicely to the length of graft necessary for each individual. This technique may not only prove useful for standard clinical application but as a new model for research surrounding autogenous CrCL grafts. The small number of subjects and the short duration of one of the groups limited this study. Further studies with larger subject numbers and a longer time frame are necessary to compare this technique with established techniques before clinical application.
Acknowledgments
The authors thank John Bogdanske, Jennifer Devitt, Daria Schwartz, and Kei Hayashi for their assistance in the completion of this project.
Supported in part by Smith and Nephew, Inc., Andover, MA, and by a grant from the National Institutes of Health-NIAMS.
References
- 1.Patterson RH, Smith GK, Gregor TP, et al. Biomechanical stability of four cranial cruciate ligament repair techniques in the dog. Vet Surg. 1991;20:85–90. doi: 10.1111/j.1532-950x.1991.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 2.Moore KW, Read RA. Rupture of the cranial cruciate ligament in dogs-part I. Compend Contin Educ Pract Vet. 1996;18:223–233. [Google Scholar]
- 3.Jevens DJ, DeCamp CE, Hauprman J, et al. Use of force-plate analysis of gait to compare two surgical techniques for treatment of cranial cruciate ligament rupture in dogs. Am J Vet Res. 1996;57:389–393. [PubMed] [Google Scholar]
- 4.Vaughan LC, Bowden JLR. The use of skin for the replacement of the anterior cruciate ligament in the dog. A review of 30 cases. J Small Anim Pract. 1964;5:167–171. [Google Scholar]
- 5.Dickenson CB, Nunamaker DM. Repair of ruptured anterior cruciate ligament in the dog: Experience of 101 cases using a modified fascial strip technique. J Am Vet Med Assoc. 1977;170:827–830. [PubMed] [Google Scholar]
- 6.Arnoczky SP, Tarvin GB, Marshall JL. The over-the-top procedure: A technique for anterior cruciate ligament substitution in the dog. J Am Anim Hosp Assoc. 1979;15:283–290. [Google Scholar]
- 7.Denny HR, Goodship AE. Replacement of the anterior cruciate ligament with carbon fiber in the dog. J Small Anim Pract. 1980;21:279–286. doi: 10.1111/j.1748-5827.1980.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 8.Gambardella PC, Wallace LJ, Cassidy F. Lateral suture technique for management of anterior cruciate ligament rupture in dogs: A retrospective study. J Am Anim Hosp Assoc. 1981;17:33–38. [Google Scholar]
- 9.Slocum B, Devine T. Cranial tibial wedge osteotomy: A technique for elimination of cranial tibial thrust in cranial cruciate ligament repair. J Am Vet Med Assoc. 1984;184:564–569. [PubMed] [Google Scholar]
- 10.Smith GK, Torg JS. Fibular head transposition for repair of the cruciate-deficient stifle in the dog. J Am Vet Med Assoc. 1985;187:375–383. [PubMed] [Google Scholar]
- 11.Knecht CD. Evolution of surgical techniques for cruciate ligament rupture in animals. J Am Anim Hosp Assoc. 1976;12:717–726. [Google Scholar]
- 12.Shires P. Intracapsular repairs for cranial cruciate ligament ruptures. Vet Clin North Am Small Anim Pract. 1993;23:761–776. doi: 10.1016/s0195-5616(93)50081-5. [DOI] [PubMed] [Google Scholar]
- 13.Korvic DL, Johnson AL, Schaeffer DJ. Surgeons’ preferences in treating cranial cruciate ligament ruptures in dogs. J Am Vet Med Assoc. 1994;205:1318–1323. [PubMed] [Google Scholar]
- 14.Reif U, Hulse DA, Hauptman JG. Effect of tibial plateau leveling on stability of the canine cranial cruciate-deficient stifle joint: An in vitro study. Vet Surg. 2002;31:147–154. doi: 10.1053/jvet.2002.31041. [DOI] [PubMed] [Google Scholar]
- 15.Clark R, Olsen RE, Larson BJ, et al. Cross-pin femoral fixation: A new technique for hamstring anterior cruciate ligament reconstruction of the knee. Arthroscopy. 1998;14:258–267. doi: 10.1016/s0749-8063(98)70141-0. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg TD, Deffner KT. ACL reconstruction: Semitendinosus tendon is the graft of choice. Orthopedics. 1997;20:396–398. doi: 10.3928/0147-7447-19970501-06. [DOI] [PubMed] [Google Scholar]
- 17.Passler JM, Babinski K, Schippinger G. Failure of clinical methods in assessing graft integrity after anterior cruciate ligament reconstruction: An athroscopic evaluation. Arthroscopy. 1999;15:27–34. doi: 10.1053/ar.1999.v15.0150021. [DOI] [PubMed] [Google Scholar]
- 18.Smeak DD. The Chinese finger trap suture technique for fastening tubes and catheters. J Am Anim Hosp. 1990;26:215–218. [Google Scholar]
- 19.Ohlendor C, Tomford WW, Mankin HJ. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res. 1996;14:413–416. doi: 10.1002/jor.1100140311. [DOI] [PubMed] [Google Scholar]
- 20.Beynnon BD, Uh BS, Pyne J, et al. Semitendinosus and gracilis tendon graft fixation for ACL reconstructions. Iowa Orthop J. 1996;16:118–121. [PMC free article] [PubMed] [Google Scholar]
- 21.Brown CH, Jr, Steiner ME, Carson EW. The use of hamstring tendons for anterior cruciate ligament reconstruction. Clin Sports Med. 1993;12:723–755. [PubMed] [Google Scholar]
- 22.Anderson K, Seneviratne AM, Izawa K, et al. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689–698. doi: 10.1177/03635465010290060301. [DOI] [PubMed] [Google Scholar]
- 23.Goradia VK, Rochat MC, Kida M, et al. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28:40–46. doi: 10.1177/03635465000280011901. [DOI] [PubMed] [Google Scholar]
- 24.Brand J, Jr, Weiler A, Caborn DNM, et al. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28:761–774. doi: 10.1177/03635465000280052501. [DOI] [PubMed] [Google Scholar]
- 25.Scranton PE, Jr, Lanzer WL, Ferguson MS, et al. Mechanisms of anterior cruciate ligament neovascularization and ligamentization. Arthroscopy. 1998;14:702–716. doi: 10.1016/s0749-8063(98)70097-0. [DOI] [PubMed] [Google Scholar]
- 26.Mitton GR, Ireland WP, Runyon CL. Evaluation of the instantaneous center of rotation of the stifle before and after repair of torn cruciate ligament by use of the over-the-top technique in dogs. Am J Vet Res. 1991;10:173–1731. [PubMed] [Google Scholar]
- 27.Greis PE, Burks RT, Bachus K, et al. The influence of tendon length and fit on the strength of a tendon-bone tunnel complex: A biomechanical and histologic study in the dog. Am J Sports Med. 2001;29:493–497. doi: 10.1177/03635465010290041901. [DOI] [PubMed] [Google Scholar]
- 28.McFarland EG. The biology of anterior cruciate ligament reconstructions. Orthopedics. 1993;16:403–410. doi: 10.3928/0147-7447-19930401-04. [DOI] [PubMed] [Google Scholar]
- 29.Ballock RT, Woo SLY, Lyon RB, et al. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: A long-term histologic and biomechanical study. J Orthop Res. 1989;7:474–485. doi: 10.1002/jor.1100070404. [DOI] [PubMed] [Google Scholar]
- 30.Tomita F, Yasuda K, Mikami S, et al. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461–476. doi: 10.1053/jars.2001.24059. [DOI] [PubMed] [Google Scholar]
- 31.Vasseur PB, Berry CR. Progression of stifle osteoarthrosis following reconstruction of the cranial cruciate ligament in 21 dogs. J Am Anim Hosp Assoc. 1992;28:129–136. [Google Scholar]
- 32.Elkins AD, Pechman R, Kearney MT, et al. A retrospective study evaluating the degree of degenerative joint disease in the stifle joint of dogs following surgical repair of anterior cruciate ligament rupture. J Am Anim Hosp Assoc. 1991;27:533–540. [Google Scholar]
- 33.Kirby BM. Decision-making in cranial cruciate ligament ruptures. Vet Clin North Am Small Anim Pract. 1993;23:797–819. doi: 10.1016/s0195-5616(93)50083-9. [DOI] [PubMed] [Google Scholar]
- 34.Chu Q, Lopez M, Hayashi K, et al. Elevation of a collagenase generated type II collagen neoepitope and proteoglycan epitopes in synovial fluid following induction of joint instability in the dog. Osteoarthritis Cartilage. 2002;10:662–669. doi: 10.1053/joca.2002.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson SG, Hulse DA, Hosan HA, et al. System behavior of commonly used cranial cruciate ligament reconstruction autografts. Vet Surg. 1989;18:459–456. doi: 10.1111/j.1532-950x.1990.tb01126.x. [DOI] [PubMed] [Google Scholar]


