Abstract
α-Galactosylceramide (α-GalCer), a glycolipid that stimulates natural killer T (NKT) cells to produce both T helper (Th)1 and Th2 cytokines, has shown antitumor effects in mice but failed in clinical trials. We evaluated 16 analogs of α-GalCer for their CD1-mediated T cell receptor (TCR) activation of naïve human NKT cells and their anticancer efficacy. In vitro, glycolipids containing an aromatic ring in their acyl tail or sphingosine tail were more effective than α-GalCer in inducing Th1 cytokines/chemokines, TCR activation, and human NKT cell expansion. None of these glycolipids could directly stimulate human dendritic cell maturation, except for a glycolipid with an aromatic ring on the sphingosine tail. Here, we show that glycolipids activated the TCR of NKT cells with phosphorylation of CD3ε, ERK1/2, or CREB, which correlated with their induction of Th1 cytokines. Notably, the extent of NKT cell activation when glycolipid was presented by antigen-presenting cells was greater than when glycolipid was presented by non-antigen-presenting cells. In vivo, in mice bearing breast or lung cancers, the glycolipids that induced more Th1-biased cytokines and CD8/CD4 T cells displayed significantly greater anticancer potency than α-GalCer. These findings indicate that α-GalCer analogs can be designed to favor Th1-biased immunity, with greater anticancer efficacy and other immune-enhancing activities than α-GalCer itself.
Keywords: antigen presentation, CD1, α-galactosylceramide
Natural killer T (NKT) cells represent a subset of T lymphocytes that share receptors with both conventional T cells and NK cells (1, 2). A major fraction of the NKT cells that expresses a semiinvariant T cell receptor (TCR) (Vα24-Jα18 in humans and Vα14-Jα18 in mice) together with NK cell markers, NK1.1 (NKR-P1 or CD161c), is designated as the invariant NKT (iNKT) cell fraction. Several studies (2–4) have demonstrated that NKT cells specifically recognize the glycolipid α-galactosylceramide (α-GalCer) and that recognition requires that the glycolipid be presented by CD1d (2, 3). In vitro stimulation of mouse spleen cells by α-GalCer led to proliferation of NKT cells and production of both IFN-γ and IL-4. Murine studies have shown that cells can be rapidly activated by immature dendritic cells (iDCs) bearing α-GalCer and that the activated iNKT cells can in turn induce full maturation of DCs (5, 6). Although these observations indicate that cooperative interaction between iNKT cells and DCs is associated with DC maturation, to our knowledge no study has yet explored the direct effect of glycolipids on iDCs.
The production of Th1 cytokines is thought to correlate with antitumor, antiviral/antibacterial, and adjuvant activities, whereas Th2 cytokine production is thought to subdue autoimmune diseases (7–9). Treatment of mice with α-GalCer has been shown to suppress tumor metastasis to liver, lung, and lymph nodes (10–13). In two phase I clinical trials in patients with advanced cancers who were injected with α-GalCer (14) or α-GalCer-loaded iDCs (15), a distinct activation of the immune system was observed in those patients who had a detectable Vα24+Vβ11+ NKT cell number prior to treatment (14). Although there was no durable tumor regression, stable disease was noted in several patients, without any toxicity (14–17), and some patients even showed a transient reduction of serum tumor markers or tumor size (15). The lack of significant anticancer activity of α-GalCer in several clinical trials may be due to the effect of IFN-γ counteracted by IL-4, resulting in no net benefit (14–17). Therefore, it may be desirable to identify compounds that preferentially induce Th1 cytokine responses (18, 19).
Several natural and synthetic analogs of α-GalCer have been investigated for their effects on NKT cell activation (20–25), and the results suggest that modification of the lipid chain in the α-GalCer structure might favor Th1 response. Recently, a series of structure-based synthetic α-GalCer analogs were developed by our groups and shown to induce human NKT cells to selectively release IL-4 or IFN-γ in vitro (26–30). In the present study, we examined in detail the capacity of glycolipid analogs to induce the activation and expansion of NKT cells and the maturation of DCs in vitro. We also studied the anticancer activities of these analogs in vivo, with special focus on Th1/Th2 cytokine induction and changes in the immune effector cell subpopulation.
Results
Cytokine Secretion Profile of NKT Cells Upon Stimulation with Glycolipids.
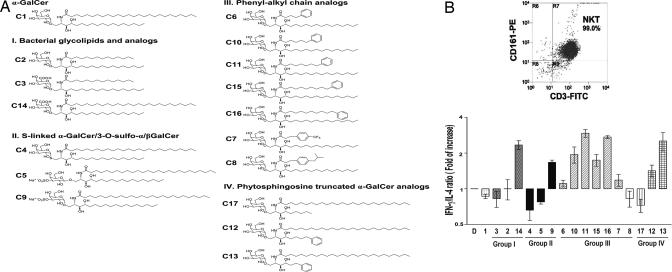
To identify glycolipids that elicit preferential Th1-biased responses among 16 analogs of α-GalCer (C1), we first examined the capacity of these analogs to induce 17 cytokines and five chemokines in human CD161+CD3+ NKT cells when presented by DCs. These glycolipid analogs were categorized, on the basis of their structure, into four groups (Fig. 1A). Aromatic compounds from groups III and IV, especially C11, C16, and C13, induced a significantly greater secretion of IFN-γ than did the parent compound, C1, whereas all analogs elicited slightly less IL-4 than did C1 [see supporting information (SI) Fig. 6A]. When expressed as the IFN/IL-4 ratio (Fig. 1B), compounds C9, C12, C13, C14, and all group III compounds were more Th1-biased, whereas C1, C3, C4, C5, C8, and C17 were Th2-biased (Fig. 1B). A similar trend was noted when other Th1 (IL-2, IL-12) and Th2 (IL-6, IL-10) cytokines were analyzed (SI Fig. 6B). The induction of 17 cytokines and five chemokines is shown in SI Table 1. Some glycolipids showed a greater induction in chemokines than did C1; for example, C13 elicited a striking increase in chemokines such as MIP-1α, MCP-1, and IL-8. Aromatic compounds C10, C11, and C16 displayed a greater induction of IL-3, granulocyte/macrophage colony-stimulating factor (GM-CSF), and IL-15. To our knowledge, the induction of chemokines (RANTES, IL-8, MCP-1) and several cytokines (IL-3, IL-15) in human NKT cells by glycolipids has not been previously reported.
Fig. 1.
Structures of α-GalCer analogs and their effect on Th1/Th2 cytokine production by human NKT cells. (A) Structure of α-GalCer and 16 synthetic glycolipid analogs, which were divided into four groups: bacterial origin (I), modification with sulfonation (II), phenol-alkyl chain analogs (III), and phytosphingosine truncated analogs (IV). To assess Th1/Th2 cytokine production by human NKT cells, human CD161+/CD3+ NKT cells (1 × 105) were cultured with 5 × 104 autologous immature CD14+ DCs pulsed with the indicated glycolipid antigens at 10 μg/ml for 18 h. Cytokines in the supernatants were measured with the Luminex 100 system in accordance with the assay protocol. (B Left) Purity of human CD161+/CD3+ NKT cells. (Right) Ratio of IFN-γ over IL-4, normalized DMSO control. Assays were performed in triplicate, and data are given as mean ± SD.
Aromatic Glycolipids Are More Effective in Expanding Human NKT Cells in Vitro.
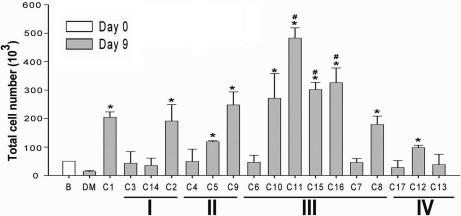
To examine the effects of glycolipids on NK or iNKT cell expansion, we first isolated CD56+ cells from peripheral blood mononuclear cells as a mixture of naive NK (CD56+/CD3−) and NKT (CD56+/CD3+) cells. On day 9 after exposure to glycolipids presented by DCs, the expansion/survival of NK and NKT cells and of a subpopulation of NKT cells, iNKT cells (CD161+/Vα24+/CD56+/CD3+), was determined by flow cytometry. As shown in Fig. 2 and SI Fig. 7, a significant increase in iNKT cells over control was noted upon stimulation with C1, C2, C8, C9, C10, C11, C12, C15, and C16. Among these analogs, several aromatic compounds from group III, especially C11, C15, and C16, were more effective than C1. The total number of NKT cells followed a similar pattern of increase as iNKT cells (data not shown); however, glycolipids did not display significant impact on the NK cell number (data not shown).
Fig. 2.
The studied glycolipids promote the in vitro expansion of human iNKT cells. Human CD56+ cells (2 × 106; NK/NKT mixtures) were cultured with 4 × 105 autologous immature DCs pulsed with the indicated glycolipid at 3 μg/ml on day 2 for 18 h. The percentage of CD161+/Va24TCR+ cells in the NK/NKT mixtures was gated by flow cytometry on day 9, and the total number of Va24i NKT cells is shown. Assays were performed in triplicate, and data are presented as mean ± SD. Similar results were obtained in three independent experiments. ∗, P < 0.05, compared with DMSO; #, P < 0.05, compared with C1.
Promotion of Maturation of Monocyte-Derived DCs in Vitro by Glycolipids.
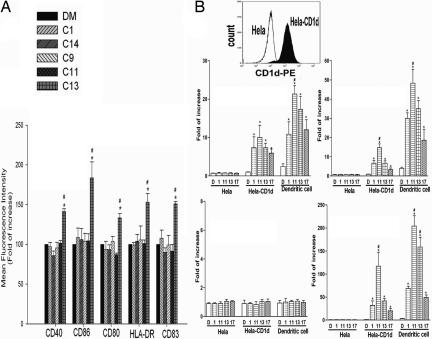
It is unclear whether the maturation of DCs in vivo occurs directly, from the effect of α-GalCer on iDCs, or indirectly as a result of the interaction of NKT cells with iDCs. Thus, we evaluated the ability of these glycolipid analogs to promote the maturation of human DCs from iDCs in vitro by measuring the expression of DC maturation markers CD40, CD54, CD80, CD83, CD86, CD209, and HLA-DR (MHC II molecule) using flow cytometry. C13 is the only glycolipid shown to significantly increase the expression levels of CD40, CD80, CD83, CD86, and HLA-DR; no significant changes were seen in other markers (Fig. 3A and SI Fig. 8A). Interestingly, incubation with C13 also led to elongation of DCs, along with dendrite extension (SI Fig. 8B).
Fig. 3.
The studied glycolipids promote maturation of human monocyte-derived DCs and CD1d-dependent TCR activation of human NKT cells. Immature human DCs (2 × 106) were incubated with 3 μg/ml of indicated glycolipids or vehicle for 48 h. (A) Expression levels (mean fluorescence intensity) of surface CD40, CD80, CD86, CD83, and HLA-DR on DCs. (B) Activation of TCR on NKT cells. HeLa, HeLa-CD1d (Top), or autologous iDCs were loaded with compounds C1, C11, C13, and C17 at 10 μg/ml, or DMSO, for 2 h and then added to 3 × 105 naïve CD161+/CD3+ NKT cells. After 5–10 min stimulation, the cells were washed with PBS and lysed with Beadlyte cell signaling universal assay buffer. The intracellular levels of phospho-CD3ε (phosphotyrosine) (Middle Left), phospho-ERK1/2 (Thr-185/Tyr-187) (Middle Right), phospho-Syk (phosphotyrosine) (Bottom Left), and phospho-CREB (Ser-133) (Bottom Right), and phospho-Syk (phospho-tyrosine) were measured with the Luminex 100 system and expressed as median fluorescence intensity, which was normalized to the amount of total input protein. ∗, P < 0.05, compared with DMSO; #, P < 0.05, compared with C1.
Aromatic Compounds Induce CD1d-Dependent TCR Activation of Naïve Human NKT Cells.
To demonstrate the activation of TCR on naïve NKT (CD161+CD3+) cells by glycolipids, we examined the induction of phospho-CD3ε (phosphotyrosine), ERK/MAP kinase 1/2 (Thr-185/Tyr-187), and CREB (Ser-133). To discern whether TCR activation is CD1d-dependent, we compared the effects of glycolipids presented by HeLa-CD1d, overexpressing human CD1d, and control HeLa cells. We also compared the capacity of HeLa-CD1d [nonprofessional antigen-presenting cells (APCs)] and iDCs (professional APCs) in presenting these glycolipids to NKT cells. As shown in Fig. 3B, compounds C1, C11, C13, and C17 increased CD3ε phosphorylation by 7.3-, 10-, 7.3-, and 5.9-fold over control (1% DMSO), respectively, when presented by HeLa-CD1d cells and 10.8-, 21.3-, 17.3-, and 12-fold, respectively, when presented by DCs (Fig. 3B). For ERK1/2 phosphorylation, compounds C1, C11, C13, and C17 induced 6.6-, 14.6-, 6.6-, and 3.3-fold increase, respectively, with HeLa-CD1d cells and 30-, 48.3-, 35-, and 18.6-fold increase, respectively, with DCs. Induction of CREB phosphorylation was even more striking. Compounds C1, C11, C13, and C17 induced 32-, 117-, 41-, and 20-fold expression, respectively, when presented by HeLa-CD1d cells and 68-, 204-, 158-, and 49-fold increase, respectively, when presented by DCs. This study demonstrated TCR activation by α-GalCer and its analogs and compared the extent of NKT cell activation upon presentation of glycolipid by APCs and non-APCs. Overall, compounds C11 and C13 appeared to be stronger in TCR activation than compounds C1 and C17, which was consistent with their greater induction of Th1 cytokines. For these four glycolipids, TCR was activated more potently when they were presented by DCs than by HeLa-CD1d cells, especially with C13. Conversely, none of these compounds showed any effect on phosphorylation of CD3ε, ERK1/2, or CREB in NKT cells when cocultured with control HeLa cells. In addition, none of these compounds have any effect on the phosphorylation of Syk, a protein kinase known to play a role in B cell receptor signaling but not in the TCR pathway (Fig. 3B). These findings suggest that aromatic compounds induce a strong TCR activation in a CD1d-dependent manner and that the extent of activation is greatly enhanced when presented by professional APCs as compared with nonprofessional APCs.
Cytokine/Chemokine Production in Response to Glycolipids Administered Intravenously in Vivo.
To evaluate the induction of cytokine/chemokine in vivo, α-GalCer and seven of its analogs were injected i.v. into BALB/c mice (see SI Materials and Methods). SI Fig. 9A shows the serum level of IFN-γ at 2 and 18 h after injection. C1 induced the highest level of IFN-γ secretion at 18 h, followed by C9 and C16; the remaining glycolipids were much less potent. SI Fig. 9B shows the level of IL-4 after injections of glycolipids. α-GalCer was again superior to other compounds in stimulating the secretion of IL-4, which peaked at 2 h and returned to baseline by 18 h. The level of IL-4 was followed by compounds C13, C11, and C16. When the data were expressed as the IFN-γ/IL-4 ratio, all of the tested glycolipids elicited less Th2 response than α-GalCer at 2 h, and all compounds induced Th1-bias responses at 18 h (SI Fig. 9C). Along with IFN-γ and IL-4, other cytokines and chemokines also increased significantly in sera in response to these studied glycolipids. These cytokines/chemokines included IL-2, IL-6, KC, IL-10, IL-12, IL-13, GM-CSF, TNFα, RANTES, MCP-1, and MIP-1, which are summarized in SI Table 2. Several glycolipids elicited more Th1-biased cytokines and chemokines than C1. For example, aromatic compounds C11, C13, and C16 induced striking rises in IL-2, IL-12, MIP-1β, and MCP-1, and C14 displayed the highest induction of IL-3, IL-12p40, and GM-CSF among all the compounds we studied.
In Vivo Effects of Glycolipids on Splenic Immune Effector Cells.
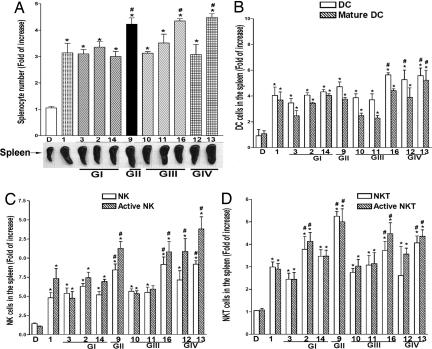
Next, we analyzed the populations of immune cells in the spleens of BALB/c mice 72 h after injection with 1 of 10 glycolipids representative of groups I–IV. As shown in Fig. 4A, after i.v. administration all compounds induced significant expansion in splenocytes, with C9, C13, and C16 showing greater potency than C1.
Fig. 4.
Expansion and activation of murine splenocytes after injection of glycolipids. Spleens from BALB/c mice (n = 4 per group) were harvested 72 h after i.v. injection of glycolipids. The spleens of mice injected with 100 μl of 1% DMSO appear grossly normal. There is no significant difference in subpopulation of splenocytes between normal untreated and DMSO-treated mice. (A) Total number of nucleated cells and spleen size. (B–D) Population of innate immune cells including mature DCs (CD11C+/CD80+/CD86+) (B), activated NK cells (U5A2-13Ag+/CD3−/CD69+) (C), and activated NKT cells (U5A2-13Ag+/CD3+/CD69+) (D). Assays were performed in duplicate, and data are shown as mean ± SD. ∗, P < 0.05, compared with DMSO; #, P < 0.05, compared with C1.
The cell-expanding/activation capacity of each compound for DC, NK, NKT, B, CD4 T, and CD8 T cells is shown in Fig. 4 B–D and SI Fig. 10. All 10 glycolipids elicited increases in DC, NK, NKT, and B cell numbers and activated CD8/CD4 ratios. Aromatic compounds C12, C13, and C16 induced significantly greater rises in total and mature DCs than did α-GalCer (Fig. 4B). C9, C12, C13, and C16 displayed the best capacity for expansion/activation of NK and NKT cells (Fig. 4 C and D). C2, C9, C10, C11, and C16 displayed a greater capacity for expansion/activation of B cells than did α-GalCer (SI Fig. 10A). Among the T cell subpopulations, all analogs tested induced a rise in CD8/CD4 ratio, with C11, C13, C14, and C16 being more potent than α-GalCer (SI Fig. 10B).
Assessment of Anticancer Efficacy of Glycolipids.
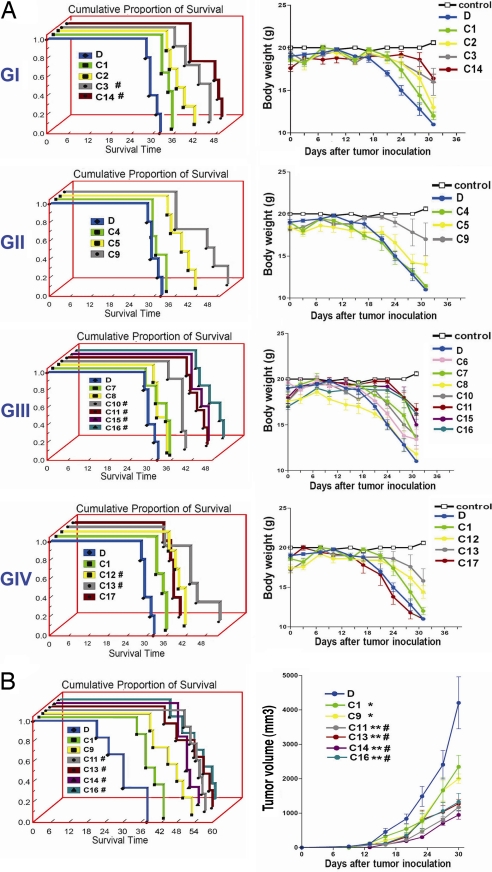
To determine the anticancer efficacy of these glycolipids, we studied mouse models of metastatic lung cancer by using the TC1 cell line and a s.c. tumor model of breast cancer by using the 4T1 cell line in syngeneic immunocompetent mice (C57BL/6 and BALB/c, respectively) (see SI Materials and Methods). SI Fig. 11A shows the results of a representative experiment with reduced number of tumor nodules on the lung surface of mice treated with C11, which exhibited the best Th1-biased cytokine secretion profile in vitro. The effects of i.v. administration of 16 glycolipids from groups I–IV and α-GalCer on the survival of TC1 tumor-bearing mice are shown in Fig. 5A. Significant prolongation of survival and reduced weight loss were observed with all compounds except C4, C6, C7, C8, and C17 (Fig. 5A). Moreover, eight glycolipids (C3, C10, C11, C12, C13, C14, C15, and C16) showed significantly greater anticancer efficacy than C1. Next, we tested the antitumor efficacy of these eight glycolipids and α-GalCer administered i.v. in mice bearing 4T1 breast cancer. The reduced tumor size in mice 16 days after treatment with C11 is shown in SI Fig. 11B, as an example. All eight compounds were able to suppress tumor growth and prolong survival as compared with control, and C11, C13, C14, and C16 were significantly more effective than α-GalCer (Fig. 5B and data not shown).
Fig. 5.
Most of the studied glycolipids can prolong survival of mice bearing lung cancer or breast cancer, and several are more potent than α-GalCer. (A) C57BL/6 mice (n = 5 per group) were inoculated i.v. with mouse lung cancer cells, TC-1, and then treated i.v. with various glycolipids of choice (2 μg per mouse) or vehicle (100 μl of 1% DMSO) twice per week for 4 weeks. Shown are the Kaplan–Meier survival curves (Left) and changes in body weight (Right) of mice bearing lung cancer. The control is the mice without tumor inoculation. Results are shown according to glycolipid groups I–IV. Statistical analysis of survival ratio compared with control or C1 (in parentheses) yielded the following: C1: P = 0.027, C2: P = 0.019 (0.253), C3: P = 0.004 (0.025), and C14: P = 0.002 (0.002) from group I; C4: P = 0.804 (0.055), C5: P = 0.019 (0.140), and C9: P = 0.008 (0.106) from group II; C7: P = 0.118 (0.789), C8: P = 0.657 (0.057), C10: P = 0.009 (0.027), C11: P = 0.002 (0.002), C15: P = 0.002 (0.002), and C16: P = 0.002 (0.002) from group III; and C12: P = 0.018 (0.018), C13: P = 0.018 (0.012), and C17: P = 0.459 (0.070) from group IV. (B) BALB/c mice (n = 6) were inoculated s.c. with mouse breast cancer cells, 4T-1. Three days after tumor inoculation, the mice were treated i.v. with the indicated glycolipids or vehicle twice per week for 4 weeks. The tumor volume was recorded every 3 days for 1 month, and survival was monitored for up to 54 days. Shown are the survival ratio curves (Left) and tumor growth (Right) of mice after i.v. administration of the indicated glycolipids or vehicle. ∗, P < 0.05 and ∗∗, P < 0.001, compared with DMSO; #, P < 0.05, compared with C1.
Discussion
The ligands for CD1d, such as α-GalCer and its analog C-α-GalCer, have been shown to protect against tumors by activating NKT cells (10–13). The primary aim of the present study was to assess the immune-modulating capacity and anticancer efficacy of a series of α-GalCer analogs, some of which had been designed to better fit the CD1d groove, based on computer modeling. Previously, compounds C2, C3, and C9 were shown to induce IL-2 from mouse NKT cells and IL-4 and IFN-γ from human Vα24i NKT cells in vitro (27–30). A recent study (26) has demonstrated that group III compounds, including C10, C11, and C16, are more potent than α-GalCer in inducing IFN-γ and IL-4 in vitro. The present study not only confirmed the in vitro induction of IFN-γ and IL-4 but also examined the profiles of 20 additional Th1/Th2 cytokines and chemokines released from human NKT cells, as well as their production in mice after in vivo administration of glycolipids. In addition, five compounds (C4, C12, C13, C14, and C15) were evaluated for their in vitro and in vivo immune-modulating effects. All except C4 induced more Th1-biased immunity than did α-GalCer, especially C13 (derived from OCH) and C14 (from Sphingomonas bacterial glycolipid).
We found that presentation of glycolipids to human NKT cells in vitro led to an increase in Th1 cytokines (IFN-γ, IL-1β, IL-2, IL-3, IL-12, IL-15, and TNF-α) and Th2 (IL-4, IL-6, IL-10, and IL-13) and in chemokines (RANTES, MIP-1α, MCP-1,and IL-8) and GM-CSF. In general, aromatic compounds exhibited more potent effects on human NKT cells than did α-GalCer, likely because of their higher affinity to CD1d (26). In the murine system, i.v. administration of α-GalCer induced higher levels of IFN-γ and IL-4 in sera over other glycolipids. However, based on the ratio of IFN-γ/ IL-4, these studied compounds elicited more Th1-biased responses than did α-GalCer at 2 h and remained so up to 18 h. The types of cytokines/chemokines induced by glycolipids in mouse were consistent with those in human NKT cells. This suggested that NKT cell activation was probably the main effector contributing to the rapidly elevated cytokine/chemokine responses to these glycolipids in mouse. Chemokines control leukocyte trafficking during homeostasis and inflammation and provide the necessary link between innate and adaptive immunity. Although the expression of chemokine receptors on human Vα24-invariant NKT cells has been reported (31, 32), little information is available on the production of chemokines by NKT cells in response to glycolipid. In this study, we documented the induction of chemokines by glycolipids in both human and mouse systems, suggesting that certain α-GalCer analogs can influence a wide range of immune responses by inducing chemokines in addition to cytokines.
In this study, we also found that several glycolipids, especially group III compounds, were more potent than α-GalCer in expanding human iNKT cells. Because decreased numbers of iNKT cells in human peripheral blood mononuclear cells have been documented in patients with malignancies (33–35), expansion and activation of such patients' iNKT cells with group III compounds may be therapeutically beneficial (34, 35).
Previous studies (36) have demonstrated the ability of α-GalCer and α-C-GalCer to facilitate the maturation of both splenic and hepatic DCs in mice. Similarly, our data showed that all tested glycolipids induced mouse splenic DC maturation in vivo and that several of them were more potent than α-GalCer. It is noteworthy that, in vitro, C13 was able to directly augment the expression of costimulatory molecules such as CD40, CD86, CD80, HLA-DR, and CD83 on human DCs, along with dendrite elongation. Such direct effect is unique because α-GalCer, α-C-GalCer, and all the other glycolipid molecules examined in this study had no direct impact on DC maturation and likely acted indirectly through the feedback effect of NKT cells on DC (36, 37). As reported by Fujii et al. (5), α-GalCer could not stimulate DCs in the absence of NKT cells, which is consistent with our data (Fig. 3A, bar 2). Conversely, the mechanism of direct activation of DCs by C13 remains enigmatic. It is possible that in addition to CD1d, C13 may interact with other CD1 molecules (such as CD1 a, b, and c) or with other receptors (such as toll-like receptors or lectin on DCs), which in turn trigger the DC maturation process. Whether such interactions occur awaits further investigation.
We showed that glycolipids could activate TCR signaling pathways in human NKT cells with phosphorylation of CD3ε, ERK1/2, or CREB. Our finding of higher levels of phosphorylated CD3ε, ERK1/2, and CREB induced by C11 than by α-GalCer and OCH (C17) is consistent with the notion that stronger binding of glycolipid to CD1d induces a greater stimulation of TCR on NKT cells (26). It may also account for a more Th1-biased cytokine profile triggered by C11 as compared with α-GalCer, because ERK1/2 and CREB activations have been reported to play a role in the induction of many Th1 cytokines, such as IL-12 and IFN-γ (38–41). It is notable that C13 also triggers significant activation of TCR, presumably as a consequence of the unique ability of C13 to enhance expression of costimulatory molecules on DC.
Previous studies have shown that the secretion of Th1/Th2 cytokines/chemokines from NKT cells by glycolipid triggered activation of various types of immune cells. For example, α-GalCer induces IFN-γ and up-regulates CD40L on NKT cells (42), which stimulates the maturation of iDCs and the production of IL-12 from DCs (37). IFN-γ and IL-12 can also activate NK cells and T cells, whereas Th2 cytokines activate B cells (7–9). Thus, the observed activation/expansion of NK, DC, T, and B cells by several glycolipids, including α-GalCer, may be attributed to their capacity to activate NKT cells to produce Th1/Th2 cytokines.
It has been shown (1, 7–9) that the production of Th1 cytokines/chemokines, especially IFN-γ, IL-2, IL-12, MIP-α, MCP-1, and TNF-α, and their resulting activation of NK, NKT, and cytotoxic T cells, contributes to antitumor activity. Our findings of more potent antitumor activity for those glycolipid analogs displaying a better Th1-biased response than does α-GalCer with respect to these cytokine/chemokine inductions (SI Table 2) and immune cell activation (SI Fig 10B) are consistent with this notion. Although the exact mechanism by which these glycolipids induced more Th1-biased cytokines remains to be elucidated, it is noteworthy that a recent report (26) has shown that the aromatic compounds elicit more potent activation of human NKT cells than α-GalCer, likely because of their higher affinity to CD1d. This affinity may underlie our observation that these compounds could elicit stronger TCR activation in human NKT cells with phosphorylation of signalsomes sitting upstream of multiple cytokines /chemokines.
Our in vivo study demonstrated that the studied glycolipids were effective in inducing serum cytokines and splenocyte proliferation and in activating DC, NK, and NKT cells, as well as adaptive immune responses. Moreover, our in vivo studies of murine lung and breast cancer models demonstrated that those compounds that elicited higher Th1 cytokines in human NKT cells in vitro tended to exhibit more potent anticancer activity. It is noteworthy that, previously, melanoma was the mouse tumor model most commonly used for demonstrating the anticancer efficacy of glycolipids such as α-GalCer and C-α-GalCer (11, 12, 22, 36, 42), perhaps because of the known susceptibility of melanoma to immune modulation. In this study, we chose to use more rigorous tumor models such as lung and breast cancers, which are not as responsive to immune manipulation. Thus, it is quite remarkable that among 16 glycolipids evaluated, four compounds (C11, C13, C14, and C16) were shown to be more effective than α-GalCer in both cancer models.
Materials and Methods
Glycolipid Analogs of α-GalCer and Cell Lines.
α-GalCer (C1) and its 16 analogs were synthesized and purified by column chromatography, as we have described recently (26–30). These glycolipids were separated into four groups on the basis of their chemical structures, as shown in Fig. 1A. Group I: C2, C3, and C14 are of bacterial origin. Group II: C4, C5, and C9 contain sulfur modification of O-linkage to ceramide (C4) or a sulfate group at 3″-OH of the galactose moiety (C5 and C9). Group III: C6, C7, C8, C10, C11, C15, and C16 are modified with an aromatic ring in their acyl tail. Group IV: C12, C13, and C17 contain truncated phytosphingosine.
All of the glycolipids were originally dissolved in 100% DMSO at a concentration of 1–2 mg/ml. For in vivo experiments, all compounds were diluted to 20 μg/ml in saline just before injection of 100 μl of diluted glycolipid or 100 μl of 1% DMSO into mice. HeLa and its CD1d transfect, HeLa-CD1d (43), were kindly provided by Mitchell Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA).
Isolation and Generation of Human NK Cell Lines and Immature Monocyte-Derived DCs.
The generation of α-GalCer-pulsed human NKT cell lines was done according to the methods of Fujio et al. (26), and these cells were used to assess cytokine response to the studied glycolipids (see Fig. 1). iDCs were derived from CD14+ cells in leukopaks after a 2-day incubation with 300 units/ml GM-CSF and 100 units/ml IL-4 (R & D Systems, Minneapolis, MN). After irradiation (3,000 rad), the iDCs were cultured together with autologous CD161+ cells in the presence of 100 ng/ml α-GalCer and 10 units/ml IL-2 for 10 days. After repeating this stimulation, NK cell lines were generated and shown to express CD161+/CD3+/Vα24iTCR+ (99% purity). To generate immature human monocyte-derived DCs, CD14+ cells in leukopaks were cultured in the presence of 300 units/ml GM-CSF and 100 units/ml IL-4 (R & D Systems) for 6 days. These DCs had an immature phenotype (CD14-CD80+CD86+CD83weak HLA-DR+) and exhibited higher CD1d expression than mature DCs. The iDCs were pulsed with various glycolipids at 3 μg/ml, and their phenotype and morphology were examined 48 h later.
The naïve NKT cells (CD161+/CD3+) used for TCR activation experiments (see Fig. 3B) were isolated by using indirectly conjugated anti-CD161 multisort microbeads and were further separated by anti-CD3 microbeads (Miltenyi Biotec, Auburn, CA). The isolated cells were incubated in the presence of 100 units/ml IL-2 (R & D Systems) and replenished with fresh medium every 3 days.
In Vitro Expansion of iNKT Cells.
Human CD56+ cells (NK/NKT mixtures) used for iNKT cell expansion experiments (see Fig. 2) were isolated from human leukopaks by using anti-CD56 microbeads (Miltenyi Biotec). Human CD56+ cells (NK/NKT mixtures) were cultured with 4 × 105 autologous immature CD14+ DCs pulsed with the indicated glycolipids at 3 μg/ml or 0.3% DMSO on day 2 for 18 h. On day 3, the suspension cells were transferred to a new dish, cultured in the presence of 100 units/ml IL-2 (R & D Systems), and replenished with fresh medium every 3 days. The population of CD161+/Va24TCR+ cells in the NK/NKT mixtures was gated by flow cytometry on day 9, and the total number of Va24i NKT cells were counted.
In Vitro Human NKT Cell Cytokine Secretion Assay.
Vα24i human NKT cells (1 × 105) were cocultured with 5 × 104 irradiated immature CD14+ DCs in the presence of the glycolipid compounds at 10 μg/ml in a 96-well flat-bottom plate. Cytokines/chemokines in the supernatant collected at 18 h were quantified with the Beadlyte human 22-plex multicytokine detection system (catalog no. 48-011; Upstate/Millipore, Billerica, MA) and determined by a Luminex 100 system (Luminex, Austin, TX) (44).
Human NKT Cell TCR Activation.
HeLa, HeLa-CD1d, or autologous iDCs were incubated on 24-well plate with compounds C1, C11, C13, and C17 at 10 μg/ml or with DMSO for 2 h, and then 3 × 105 naïve CD161+/CD3+ NKT cells were added. After 5–10 min stimulation, cells in suspension were transferred to tubes, washed with PBS, and lysed with Beadlyte cell signaling universal lysis buffer (Upstate/Millipore) at 4°C. The concentrations of phospho-CD3ε (phosphotyrosine), phospho-ERK1/2 (Thr-185/Tyr-187), phospho-CREB (Ser-133), and phospho-Syk (phosphotyrosine) in lysates were assessed with the Beadlyte phosphoprotein detection system (catalog no. 48-690; Upstate/Millipore) in accordance with the assay protocol (45) and were determined by a Luminex 100 system (Luminex). The value was normalized with the amount of total input protein.
Supplementary Material
Acknowledgments
We thank the faculties of the vivarium at the National Defense Medical Center and of the Institute of Cellular and Organismic Biology, Academia Sinica, for technical assistance.
Abbreviations
- α-GalCer
α-galactosylceramide
- APC
antigen-presenting cell
- DC
dendritic cell
- iDC
immature DC
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- iNKT
invariant NKT
- TCR
T cell receptor
- Th
T helper.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703824104/DC1.
References
- 1.Bendelac A, Rivera MN, Park SH, Roark JH. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, Scherer DC, Singh N, Mendiratta SK, Serizawa I, Koezuka Y, Van KL. Immunol Rev. 1999;169:31–44. doi: 10.1111/j.1600-065x.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 5.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van KL. Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuji N, Ueda Y, Fujiwara H, Toh T, Yoshimura T, Yamagishi H. Clin Cancer Res. 2000;6:3380–3387. [PubMed] [Google Scholar]
- 11.Nakagawa R, Serizawa I, Motoki K, Sato M, Ueno H, Iijima R, Nakamura H, Shimosaka A, Koezuka Y. Oncol Res. 2000;12:51–58. doi: 10.3727/096504001108747521. [DOI] [PubMed] [Google Scholar]
- 12.Nakui M, Ohta A, Sekimoto M, Sato M, Iwakabe K, Yahata T, Kitamura H, Koda T, Kawano T, Makuuchi H, et al. Clin Exp Metastasis. 2000;18:147–153. doi: 10.1023/a:1006715221088. [DOI] [PubMed] [Google Scholar]
- 13.Nakui M, Iwakabe K, Ohta A, Sekimoto M, Sato M, Makuuchi H, Kawano T, Taniguchi M, Nishimura T. Jpn J Cancer Res (GANN) 1999;90:801–804. doi: 10.1111/j.1349-7006.1999.tb00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M., von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, et al. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 15.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 17.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, et al. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkers CR, Ovaa H. Trends Pharmacol Sci. 2005;26:252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 21.Schmieg J, Yang G, Franck RW, Van Rooijen N, Tsuji M. Proc Natl Acad Sci USA. 2005;102:1127–1132. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmieg J, Yang G, Franck RW, Tsuji M. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, Schmieg J, Tsuji M, Franck RW. Angew Chem Int Ed Engl. 2004;43:3818–3822. doi: 10.1002/anie.200454215. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Schmieg J, Tsuji M, Franck RW. Org Lett. 2004;6:4077–4080. doi: 10.1021/ol0482137. [DOI] [PubMed] [Google Scholar]
- 25.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, III, Teyton L, Bendelac A, Savage PB. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 26.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 27.Xing GW, Wu D, Poles MA, Horowitz A, Tsuji M, Ho DD, Wong CH. Bioorg Med Chem. 2005;13:2907–2916. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, Wilson IA, Wong CH. Proc Natl Acad Sci USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, et al. Proc Natl Acad Sci USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, Strominger JL, Brenner MB, Gumperz JE, Wilson SB, Luster AD. J Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Butcher EC, Johnston B. Trends Immunol. 2002;23:516–519. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 33.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 34.Molling JW, Kolgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, et al. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 35.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii SI, et al. Clin Cancer Res. 2006;12:5921–5923. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Proc Natl Acad Sci USA. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. J Immunol. 2001;166:6012–6018. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 38.Jones B, Chen J. EMBO J. 2006;25:2443–2452. doi: 10.1038/sj.emboj.7601148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renukaradhya GJ, Webb TJ, Khan MA, Lin YL, Du W, Gervay-Hague J, Brutkiewicz RR. J Immunol. 2005;175:4301–4308. doi: 10.4049/jimmunol.175.7.4301. [DOI] [PubMed] [Google Scholar]
- 40.Yano S, Ghosh P, Kusaba H, Buchholz M, Longo DL. J Immunol. 2003;171:2510–2516. doi: 10.4049/jimmunol.171.5.2510. [DOI] [PubMed] [Google Scholar]
- 41.Ortaldo JR, Winkler-Pickett R, Wigginton J, Horner M, Bere EW, Mason AT, Bhat N, Cherry J, Sanford M, Hodge DL, Young HA. Blood. 2006;107:1468–1475. doi: 10.1182/blood-2005-04-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- 43.Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. J Immunol. 1998;161:5124–5128. [PubMed] [Google Scholar]
- 44.Towne JE, Garka KE, Renshaw BR, Virca GD, Sim JE. J Biol Chem. 2004;279:13677–13688. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 45.Rhyne PW, Scull JD, Stiles LM, Eisinger DP. BioTechinques. 2003;35:624–629. doi: 10.2144/03353pf01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.