Abstract
Overexpression of the Rho family member TC10α, disrupts adipocyte cortical actin structure and inhibits insulin-stimulated GLUT4 translocation when targeted to lipid raft microdomains. This appears to be independent of effecter domain function because overexpression of the wild-type (TC10/WT), constitutively GTP-bound (TC10/Q75L), and constitutively GDP bound (TC10/T31N) all inhibit adipocyte cortical actin structure and GLUT4 translocation. To examine the structural determinants responsible for these effects, we generated a series of chimera proteins between TC10 with that of H-Ras and K-Ras. Chimera containing the 79 (TC10–79/H-Ras), 41 (TC10–41/H-Ras), or 16 (TC10–16/H-Ras) amino acids of the TC10 amino terminal extension fused to H-Ras disrupted cortical actin and inhibited insulin-stimulated GLUT4 translocation. In contrast, the same amino terminal TC10 extensions fused to K-Ras had no significant effect on either GLUT4 translocation or cortical actin structure. Similarly, expression of TC10β was without effect, whereas fusion of the amino terminal 8 amino acid of TC10α onto TC10β resulted in an inhibition of insulin-stimulated GLUT4 translocation. Within the amino terminal extension point mutation analysis demonstrated that both a GAG and GPG sequences when lipid raft targeted was essential for these effects. Furthermore, expression of the amino terminal TC10 deletions ΔNT-TC10/WT or ΔNT-TC10/T31N had no detectable effect on cortical actin organization and did not perturb insulin-stimulated GLUT4 translocation. Surprisingly, however, expression of ΔNT-TC10/Q75L remained fully capable of inhibiting insulin-stimulated GLUT4 translocation without affecting cortical actin. These data demonstrate that inhibitory effect of TC10 overexpression on adipocyte cortical actin organization is due to the specific lipid raft targeting of the unusual TC10 amino terminal extension.
INTRODUCTION
The mechanisms and intracellular signaling pathways utilized by the insulin receptor to initiate biological responsiveness are quite complex. In the case of glucose uptake in adipose and striated muscle, the insulin-responsive glucose transporter protein (GLUT4) is predominantly localized to intracellular membrane compartments and undergoes a dramatic redistribution to the cell surface following insulin stimulation through a process termed translocation (Holman and Sandoval, 2001; Simpson et al., 2001; Bryant et al., 2002; Ducluzeau et al., 2002; Ploug and Ralston, 2002). Although this translocation process increases the amount of cell surface GLUT4, recent data has also suggested that the transport activity itself is also activated by insulin (Sweeney et al., 1999; Konrad et al., 2001; Somwar et al., 2001; Huang et al., 2002). In addition to understanding the translocation process and the mechanisms activating glucose uptake, the intracellular signaling pathways mediating these processes is also poorly understood.
Currently, it is well established that activation of the insulin receptor tyrosine kinase results in the tyrosine phosphorylation of IRS proteins (White, 1998; Giovannone et al., 2000). This results in the association and activation of the Type 1A phosphatidylinositol 3-kinase (PI 3-kinase) and the generation of phosphatidylinositol-3, 4,5-trisphosphate (PI3,4,5P3) at the plasma membrane (Alessi and Downes, 1998; Cantley, 2002; Saltiel and Pessin, 2002). PI3,4,5P3 serves to recruit the phosphoinositide-dependent protein kinase (PDK1) and protein kinase B (PKB) also know as Akt (Alessi et al., 1997; Anderson et al., 1998; Stephens et al., 1998). PDK1 also phosphorylates PKB as well as atypical protein kinase C isoforms zeta and lambda (PKCζ/λ) and evidence has been presented both for and against either PKB or PKCζ/λ as the critical target necessary for insulin-stimulated glucose uptake (Kohn et al., 1996; Alessi et al., 1997; Bandyopadhyay et al., 1997; Le Good et al., 1998; Imamura et al., 1999; Standaert et al., 1999; Wang et al., 1999; Kristiansen et al., 2001; Matsumoto et al., 2001; Yamada et al., 2002). Nevertheless, a substantially amount of data have established that PI 3-kinase activation and generation of PI3,4,5P3 are essential for insulin-stimulated GLUT4 translocation (Cheatham et al., 1994; Okada et al., 1994; Martin et al., 1996; Sharma et al., 1998; Vollenweider et al., 1999; Czech, 2000; Nakashima et al., 2000). Although PI 3-kinase function is necessary, numerous studies have also suggested that this pathway is not sufficient to mediate the full extent of insulin-stimulated GLUT4 translocation (Isakoff et al., 1995; Wiese et al., 1995; Krook et al., 1997; Guilherme and Czech, 1998; Egawa et al., 2002). More recently, a second insulin signaling pathway has been proposed that utilizes the APS/CAP/Cbl complex to recruit and activate an unusual member of the Rho family of small GTP binding proteins, TC10 in a PI 3-kinase–independent manner (Ribon and Saltiel, 1997; Ribon et al., 1998; Baumann et al., 2000; Chiang et al., 2001).
One surprising and unexplained aspect of these data was that overexpression of the wild-type (TC10/WT), a constitutively GTP-bound active mutant (TC10/Q75L) and a constitutively GDP-inactive mutant (TC10/T31N), all inhibited insulin-stimulated GLUT4 translocation (Chiang et al., 2001). Moreover, unlike other Rho family GTP binding proteins, TC10 contains a carboxyl terminal CAAX domain that targets TC10 to plasma membrane lipid raft microdomains (Murphy et al., 2001; Watson et al., 2001). The inhibitory effects of overexpressed TC10 were dependent on lipid raft microdomain compartmentalization, whereas targeting of TC10 to nonlipid raft domains of the plasma membrane had no effect (Watson et al., 2001, 2003). Because the inhibitory actions of TC10 are independent of effecter domain activation but require appropriate intracellular compartmentalization strongly suggests that a specific protein interacting structural domain of TC10 is responsible. In this regard, overexpression of lipid raft microdomain targeted TC10 was recently observed to disrupt adipocyte cortical actin and several studies have demonstrated that actin structure is necessary for insulin-stimulated GLUT4 translocation (Tsakiridis et al., 1998; Wang et al., 1998; Omata et al., 2000; Kanzaki et al., 2002). In this study, we have identified and characterized a novel amino terminal structural motif in TC10 that is responsible for the disruption of adipocyte cortical actin and inhibition of insulin-stimulated GLUT4 translocation when specifically targeted to plasma membrane lipid raft microdomains.
MATERIALS AND METHODS
Materials
The mammalian expression plasmid pKH3-TC10/WT encoding for the human TC10, mouse TC10α and TC10β isoforms were prepared as described previously (Chiang et al., 2001, 2002). R-Ras1 and R-Ras2 were subcloned into pKH3 vector from pCGN vector and pZip vector, respectively. Murine 3T3L1 preadipocytes were purchased from the American Type Tissue Culture repository and differentiated as described by Min et al. (1999). Monoclonal anti-HA and anti-Myc antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescent secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Rhodamine-phalloidin was purchased from Sigma (St. Louis, MO).
Cell Culture and Transient Transfection of 3T3L1 Adipocytes
Murine 3T3L1 preadipocytes were cultured in DMEM supplemented with 25 mM glucose and 10% calf serum at 37°C with 8% CO2. Cells were then differentiated into adipocytes as previously described (Olson et al., 1997). Differentiated adipocytes were electroporated using the Gene Pulsar II (Bio-Rad, Hercules, CA) with settings of 0.16 kV and 950 μF (Min et al., 1999). After electroporation, cells were plated on 6-well plates and allowed to recover in adipocytes medium for ∼16–18 h. The next day the cells were starved with serum-free DMEM for 2–3 h and then stimulated with 100 nM insulin for 30 min.
Immunofluorescence and Image Analysis
Transfected adipocytes were washed in phosphate-buffered saline (PBS) and fixed for 15 min in 4% paraformaldehyde containing 0.2% Triton X-100. Cells were washed briefly in PBS and then blocked in 5% donkey serum (Sigma) plus 1% bovine serum albumin (BSA; Sigma) for 1 h. Primary and secondary antibodies were used at 1:100 dilutions in blocking solution, and samples were mounted on glass slides with Vectashield (Vector Labs, Burlingame, CA). Cells were imaged using confocal fluorescence microscopy (LSM 510; Zeiss, Thornwood, NY). Images were then imported into Adobe Photoshop (Adobe Systems, Inc., San Jose, CA) for processing and composite files were generated.
Preparation of TC10 Amino Terminal Deletion, Substitutions, and Chimeras
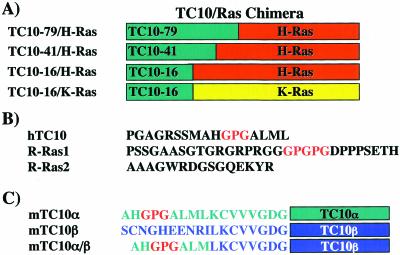
Amino terminal deletions PGAGRSSMAHGPGALML (ΔNT-TC10/WT) and substitutions for amino acids GAG at positions 3–5 to ASA (TC10/GAG-ASA) and GPG at position 12–14 to ASA (TC10/GPG-ASA) were generated from the human TC10/WT cDNA using the PCR with oligonucleotide primers containing the appropriate base-substitutions. The amino terminal extension of TC10 (amino acids 1–16, 1–41, and 1–79), R-Ras1 and R-Ras2 (amino acids 1–16) were fused to both H-Ras and K-Ras as described previously (Horton et al., 1993).
Single-Cell Microinjection
The microinjection and visualization of single 3T3L1 adipocytes were performed as described previously (Baumann et al., 2000). Briefly, the cells were grown on coverslips, and the medium was changed to Lebovitz's L-15 medium containing 0.1% BSA before microinjection. Differentiated 3T3L1 adipocyte nuclei were injected with 200 μg/ml cDNAs in microinjection buffer containing 100 mM KCl, 5 mM Na2PO4, pH 7.2, with Eppendorf model 5171 micromanipulator. The cells were allowed to recover for 16–18 h and cells were fixed for 15 min in 4% paraformaldehyde containing 0.2% Triton X-100. Cells were washed briefly in PBS and then blocked in 5% donkey serum (Sigma) plus 1% BSA (Sigma) for 1 h. Primary antibodies was used at 1:100 dilutions in blocking solution and rhodamine-phalloidin staining for actin structure at 1: 2500 dilutions in blocking solution. Samples were mounted on glass slides with Vectashield (Vector Labs). Cells were imaged using confocal fluorescence microscopy (Zeiss LSM 510). Images were then imported into Adobe Photoshop (Adobe Systems, Inc.) for processing and composite files were generated.
RESULTS
Lipid Raft Compartmentalization of the TC10 Amino Terminal Domain Inhibits Insulin-stimulated GLUT4 Translocation
Recently, we have observed that overexpression of wild-type TC10 isoform (TC10/WT), a constitutively active GTP-bound mutant (TC10/Q75L) and constitutively inactive GDP-bound mutant (TC10/T31N), all inhibited insulin-stimulated GLUT4 translocation (Chiang et al., 2001). These data suggest that neither GTP binding nor effective domain function is responsible for this effect. Because overexpression of H-Ras or K-Ras does not have any significant effect on insulin-stimulated GLUT4 translocation in adipocytes (Watson et al., 2001), we utilized both H-Ras and K-Ras backbone sequences to identify the structural domain in TC10 responsible for this inhibitory action.
Sequence alignment of Rho family members revealed that TC10 is most divergent from most of these family members at the amino and carboxyl termini. The TC10 carboxy terminus is related to the H-Ras carboxy terminus in that they both have an appropriate context of cysteine residues necessary for both farnesylation and dual palmitoylation (Hancock et al., 1989; Seabra, 1998; Choy et al., 1999; Resh, 1999; Apolloni et al., 2000; Watson et al., 2001). These posttranslational modifications result in the lipid raft microdomain compartmentalization of both H-Ras and TC10 (Dupree et al., 1993; Li et al., 1996; Song et al., 1996; Watson et al., 2001). Therefore, we initially prepared three amino terminal TC10/H-Ras fusion proteins that contain the TC10 amino terminal 79 residues (TC10–79/H-Ras), 41 residues (TC10–41/H-Ras), and 16 residues (TC10–16/H-Ras) fused to H-Ras (Figure 1). After expression by electroporation, confocal immunofluorescence localization demonstrated that all three TC10/H-Ras chimera distributed in a manner indistinguishable from that of TC10/WT or H-Ras (Figure 2A, panels 1–5). These proteins were localized to the peri-nuclear secretory membrane compartments and the plasma membrane. In contrast, the K-Ras carboxyl terminal domain is not palmitoylated and only contains a single cysteine residue in the appropriate context for farnesylation (Hancock et al., 1990; Choy et al., 1999; Resh, 1999). This results in the localization of K-Ras to nonlipid raft microdomains of the plasma membrane and exclusion from the endomembrane system (Apolloni et al., 2000). Consistent with these data, overexpression of both K-Ras and TC10–16/K-Ras chimera was exclusively plasma membrane associated with little if any protein localized to the secretory membrane compartments (Figure 2A, panels 6 and 7).
Figure 1.
Schematic and sequence relationship between TC10, H-Ras, K-Ras, R-Ras1, and R-Ras2. (A) Chimera between the TC10α amino terminal 79-, 41-, and 16-amino acid residues were fused to either H-Ras or K-Ras. (B) The amino terminal amino acid sequences of TC10α are compared with that of R-Ras1 and R-Ras2, all of which contain similar carboxyl terminal targeting motifs. (C) The amino terminal amino acid sequences of TC10α, TC10β, and the TC10α/β chimera are shown.
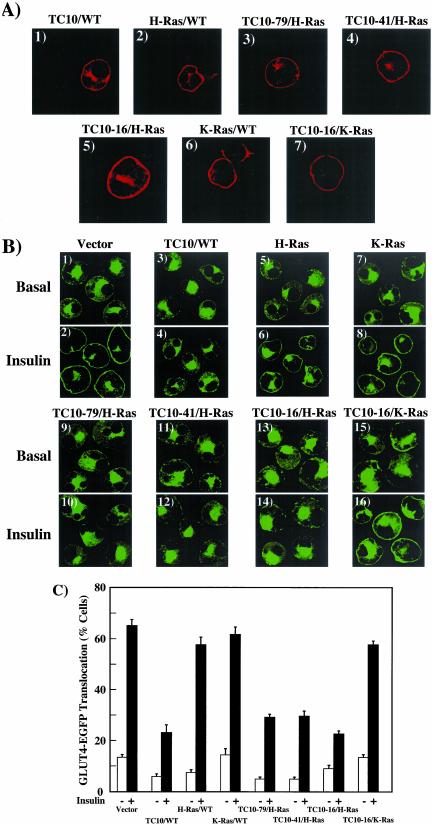
Figure 2.
Targeting of the amino terminal TC10 extension to lipid raft microdomains inhibits insulin-stimulated GLUT4 translocation. (A) Differentiated 3T3L1 adipocytes were electroporated with 50 μg of the HA epitope–tagged TC10/WT (panel 1), H-Ras/WT (panel 2), TC10–79/H-Ras (panel 3), TC10–41/H-Ras (panel 4), TC10–16/H-Ras (panel 5), K-Ras/WT (panel 6), and TC10–16/K-Ras (panel 7) chimera cDNAs as described in MATERIALS AND METHODS. Eighteen hours later, the cells were fixed and the subcellular localization was determined by confocal fluorescent microscopy. (B) Differentiated 3T3L1 adipocytes were coelectroporated with 50 μg of GLUT4-EGFP plus 200 μg of the empty vector (panels 1 and 2), TC10/WT (panels 3 and 4), H-Ras/WT (panels 5 and 6), K-Ras/WT (panels 7 and 8), TC10–79/H-Ras (panels 9 and 10), TC10–41/H-Ras (panels 11 and 12), TC10–16/H-Ras (panels 13 and 14), and TC10–16/K-Ras (panels 15 and 16) cDNAs. Eighteen hours later, the cells were then treated without (Basal, panels 1, 3, 5, 7, 9, 11, 13, and 15) or with 100 nM insulin (Insulin, panels 2, 4, 6, 8, 10, 12, 14, and 16) for 30 min. The cells were then fixed and the subcellular localization of GLUT4-EGFP was assessed by confocal fluorescent microscopy. (C) GLUT4 translocation was quantified by determining the number of cells displaying a continuous plasma membrane GLUT4-EGFP fluorescent signal from the counting of 50 cells per experiment. These data were obtained from the average of four independent experiments (200 cells total).
As typically observed in cells expressing the GLUT4-EGFP fusion protein, insulin stimulation resulted in a marked translocation of GLUT4 from intracellular storage sites to the plasma membrane (Figure 2B, panels 1 and 2). This is readily detected by the appearance of a strong plasma membrane rim of fluorescence (Figure 2B, panel 2). As previously reported (Watson et al., 2001), expression of TC10/WT but not H-Ras inhibited insulin-stimulated GLUT4-EGFP translocation (Figure 2B, panels 3–6). Similar to TC10/WT, expression of TC10–79/H-Ras, TC10–41/H-Ras, and TC10–16/H-Ras all inhibited insulin-stimulated GLUT4 translocation (Figure 2B, panels 9–14). In contrast, neither K-Ras (Figure 2B, panels 7 and 8) nor the TC10–16/K-Ras chimera (Figure 2B, panels 15 and 16) had any significant effect on insulin-stimulated GLUT4 translocation.
These data were quantified by determining the number of cells displaying a continuous cell surface GLUT4-EGFP fluorescence (Figure 2C). In control vector-transfected cells, insulin stimulated GLUT4 translocation in 65 ± 2.4% of the adipocyte population and was not significantly different in cells coexpressing H-Ras/WT (58 ± 3.0%) or K-Ras/WT (62 ± 3.0%). Similarly, coexpression with TC10–16/K-Ras resulted in a comparable degree of insulin-stimulated GLUT4 translocation (58 ± 1.5%). In contrast, there was a marked inhibition of insulin-stimulated GLUT4 translocation in adipocytes expressing TC10/WT (23 ± 3.1%), TC10–79/H-Ras (29 ± 1.3%), TC10–41/H-Ras (30 ± 2.1%), and TC10–16/H-Ras (23 ± 1.3%). Together, these data demonstrated that the amino terminal 16 amino acids of TC10 contain the necessary information to inhibit insulin-stimulated GLUT4 translocation independent of effector binding function. Importantly, this property of TC10 is only manifested when the amino terminal domain is targeted to lipid raft microdomains.
Amino Terminal Extension of TC10 Disrupts Cortical Actin Structure
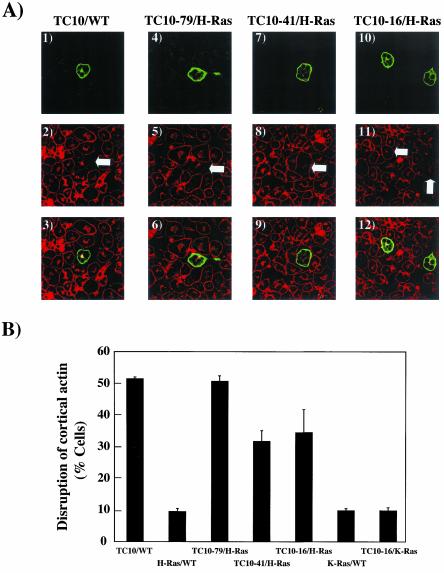
Several recent studies have reported that the cortical actin structures in muscle and adipocytes play an essential role in the insulin-stimulated GLUT4 translocation process (Tsakiridis et al., 1998; Wang et al., 1998; Omata et al., 2000; Kanzaki et al., 2001; Tong et al., 2001; Jiang et al., 2002). Because TC10 has been observed to alter actin polymerization both in vitro and in vivo (Kanzaki et al., 2002), we next assessed the effect of the various chimeras on cortical actin organization by single cell microinjection (Figure 3). Phalloidin staining of adipocytes demonstrated the presence of a thick filamentous actin network surrounding the plasma membrane as well as polymerized actin in the peri-nuclear region (Figure 3A, panels 2, 5, 8, and 11). In cells expressing TC10/WT (Figure 3A, panels 1–3), there was a near complete disruption of the cortical network with little effect on peri-nuclear actin structure. This is in marked contrast to cells expressing H-Ras, which had little, if any effect on cortical actin labeling (our unpublished results). Similar to TC10/WT, adipocytes expressing TC10–79/H-Ras had a complete loss of cortical actin organization (Figure 3A, panels 4–6). Expression of TC10–41/H-Ras and TC10–16/H-Ras also resulted in a loss of cortical actin structure (Figure 3A, panels 7–12). Although the loss of cortical actin structure was readily apparent, TC10–41/H-Ras and TC10–16/H-Ras were somewhat less effective than TC10/WT and TC10–79/H-Ras (Figure 3B). In any case, expression of neither K-Ras nor TC10–16/K-Ras had any significant effect on cortical actin organization (our unpublished results). Quantification of the number of cells displaying a disruption of cortical actin is presented in Figure 3B. Cortical actin was disorganized in 51 ± 0.5%, 51 ± 1.7%, 31.8 ± 3.3%, and 34.5 ± 7.2% of cells expressing TC10/WT, TC10–79/H-Ras, TC10–41/H-Ras, and TC10–16/H-Ras, respectively. In contrast cells expressing H-Ras, K-Ras, or TC10–16/K-Ras had 9.5 ± 0.9%, 9.8 ± 0.6%, and 9.8 ± 1.0% disrupted cortical actin, respectively. These data demonstrate that the amino terminal TC10 domain disrupts adipocyte cortical actin organization when targeted to lipid raft microdomains.
Figure 3.
The amino terminal TC10 extension disrupts adipocyte cortical actin when targeted to lipid raft microdomains. (A) Differentiated 3T3L1 adipocyte nuclei were microinjected with 200 μg/ml HA-tagged TC10/WT (panels 1–3), TC10–79/H-Ras (panels 4–6), TC10–41/H-Ras (panels 7–9), and TC10–16/H-Ras (panels 10–12) cDNAs. Eighteen hours later, the cells were fixed and the localization of the expressed proteins was determined by confocal fluorescent microscopy using an FITC-labeled anti-HA antibody (panels 1, 4, 7, and 10). The cells were also colabeled with rhodamine-phalloidin to observe the distribution of filamentous actin (panels 2, 5, 8, and 11). The merged images are presented in panels 3, 6, 9, and 12. (B) Quantification of the number of cells displaying a disrupted cortical actin structure was determined from the counting of more than 50 cells per experiment were that expressed TC10/WT, TC10–79/H-Ras, TC10–41/H-Ras, TC10–16/H-Ras, and TC10–16/K-Ras proteins. These data were obtained from the average of four independent experiments (>200 cells total).
Amino Terminus Mutations Inactivate the Inhibitory Properties of TC10
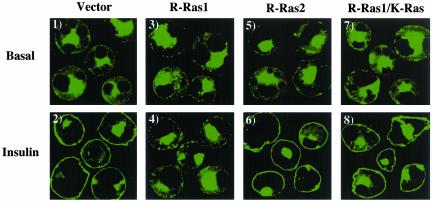
Comparison of the amino terminal domains of all small GTP binding proteins that are predicted to undergo carboxyl terminal farnesylation and palmitoylation indicated that R-Ras1 has an amino terminal extension that exhibits some similarity with TC10 (Figure 1B). As observed for TC10, coexpression of R-Ras1 with GLUT4-EGFP resulted in an inhibition of insulin-stimulated GLUT4 translocation compared with empty vector-transfected cells (Figure 4, panels 1–4). As additional controls, R-Ras2 has an identical effecter and carboxyl terminal targeting domains as R-Ras1 but with an unrelated amino terminal extension. Coexpression of R-Ras2 with GLUT4-EGFP had no significant effect on insulin-stimulated GLUT4 translocation (Figure 4, panels 5 and 6). Furthermore, replacement of the R-Ras1 carboxyl terminal targeting domain with the K-Ras carboxy terminus resulted in a chimera that was unable to inhibit insulin-stimulated GLUT4 translocation (Figure 4, panels 7 and 8). These data demonstrate that both the R-Ras1 and TC10 amino terminal extensions have similar functions when targeted to lipid raft microdomains.
Figure 4.
Lipid raft microdomain targeting of R-Ras1 inhibits insulin-stimulated GLUT4 translocation. Differentiated 3T3L1 adipocytes were coelectroporated with 50 μg of GLUT4-EGFP plus 200 μg of the empty vector (panels 1 and 2), HA-tagged R-Ras1 (panels 3 and 4), R-Ras2 (panels 5 and 6), and R-Ras1/K-Ras (panels 7 and 8) cDNAs. Eighteen hours later, the cells were then treated without (Basal, panels 1, 3, 5, and 7) or with 100 nM insulin (Insulin, panels 2, 4, 6, and 8) for 30 min. The cells were then fixed and the subcellular localization of GLUT4-EGFP was assessed by confocal fluorescent microscopy. These are representative images of cells obtained from three to four independent determinations.
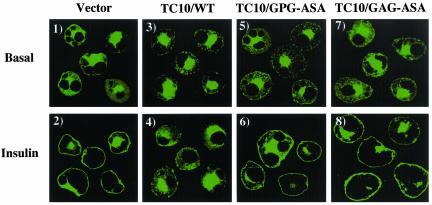
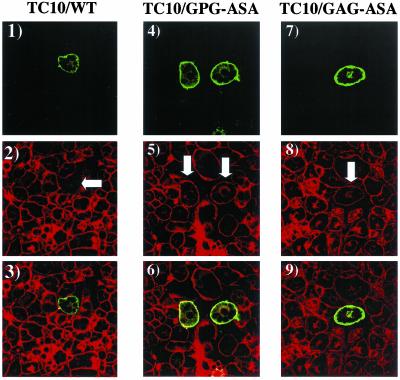
TC10 and R-Ras both have a conserved GPG sequence located within their respective amino terminal extensions (Figure 1B). To evaluate the potential role of this sequence within TC10, we substituted the GPG sequence at position 12–14 with ASA (TC10/GPG-ASA). We also replaced the GAG sequence at residues 3–5 with ASA TC10/GAG-ASA). Although expression of TC10/WT (Figure 5, panels 3 and 4) effectively inhibited insulin-stimulated GLUT4 translocation, expression of these mutants TC10/GPG-ASA and TC10/GAG-ASA were unable to block the insulin stimulation of GLUT4 translocation (Figure 5, panels 5–8). In parallel, TC10/WT (Figure 6, panels 1–3) disrupted cortical actin, whereas TC10/GPG-ASA and TC10/GAG-ASA had no significant effect (Figure 6, panels 4–9). The finding that inactivating point mutations occur at the very amino terminal (amino acids 1–3) and carboxyl end of this extension (amino acids 12–14) suggests that the entire structure (residues 1–16) of this extension is required.
Figure 5.
Point mutations in the TC10 amino terminal extension neutralize the inhibitory effects on insulin-stimulated GLUT4 translocation. Differentiated 3T3L1 adipocytes were coelectroporated with 50 μg of GLUT4-EGFP plus 200 μg of the empty vector (panels 1 and 2), TC10/WT (panels 3 and 4), TC10/GPG-ASA (panels 5 and 6), or TC10/GAG-ASA (panels 7 and 8) cDNAs. Eighteen hours later, the cells were then treated without (Basal, panels 1, 3, 5, and 7) or with 100 nM insulin (Insulin, panels 2, 4, 6, and 8) for 30 min. The cells were then fixed and the subcellular localization of GLUT4-EGFP was assessed by confocal fluorescent microscopy. These are representative images of cells obtained from three to four independent determinations.
Figure 6.
Point mutations in the TC10 amino terminal extension neutralize the inhibitory effects on cortical actin organization. Differentiated 3T3L1 adipocyte nuclei were microinjected with 200 μg/ml HA-tagged TC10/WT (panels 1–3), TC10/GPG-ASA (panels 4–6), or TC10/GAG-ASA (panels 7–9) cDNAs. Eighteen hours later, the cells were fixed and the localization of the expressed proteins was determined by confocal fluorescent microscopy using a FITC-labeled anti-HA antibody (panels 1, 4, and 7). The cells were also colabeled with rhodamine-phalloidin to observe the distribution of filamentous actin (panels 2, 5, and 8). The merged images are presented in panels 3, 6, and 9. These are representative fields of cells observed in four independent experiments.
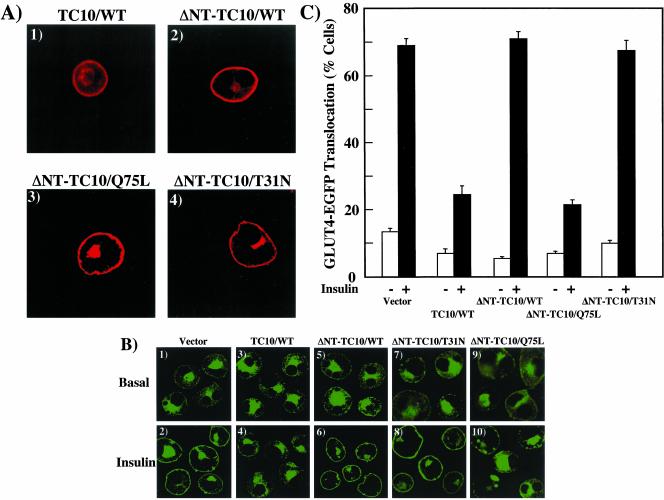
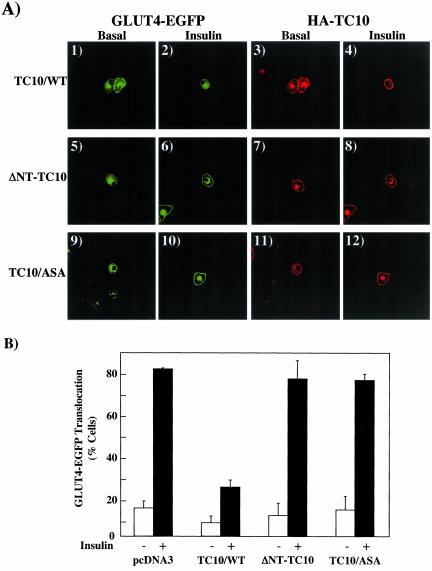
To further examine the properties of the TC10 amino terminal domain, we deleted the amino terminal 16 amino acids of TC10 (ΔNT) in the context of the wild-type TC10 (ΔNT-TC10/WT), constitutively inactive GDP-bound TC10 mutant (ΔNT-TC10/T31N), and constitutively active GTP-bound TC10 mutant (ΔNT-TC10/Q75L). Because the carboxyl terminal posttranslational modification and targeting domain was unaffected in these amino terminal TC10 deletions, overexpression of these constructs resulted in an identical intracellular distribution as TC10/WT with localization to both the plasma membrane and the peri-nuclear endomembrane system (Figure 7A, panels 1–4). Coexpression of GLUT4-EGFP with TC10/WT resulted in the typical inhibition of insulin-stimulated GLUT4 translocation compared with control empty vector-transfected adipocytes (Figure 7B, panels 1–4). As expected, coexpression of ΔNT-TC10/WT and ΔNT-TC10/T31N had no significant effect on insulin-stimulated GLUT4 translocation (Figure 7B, panels 5–8). Surprisingly, however, coexpression of ΔNT-TC10/Q75L resulted in a marked inhibition of insulin-stimulated GLUT4 translocation (Figure 7B, panels 9 and 10). Quantification of the above results showed that insulin-stimulated GLUT4-EGFP translocation in 69 ± 2.0% in empty vector control-transfected cells, 71 ± 2.1% in of ΔNT-TC10/WT-transfected, and 68 ± 2.9% in ΔNT-TC10/T31N-transfected cells. In contrast, insulin-stimulated GLUT4 translocation was only 24 ± 2.6% and 21 ± 1.5% in cells expressing TC10/WT and ΔNT-TC10/Q75L (Figure 7C).
Figure 7.
Effects of TC10 amino terminal deletions on GLUT4 translocation. (A) Differentiated 3T3L1 adipocytes were electroporated with 50 μg of the HA epitope-tagged TC10/WT (panel 1), HA epitope–tagged ΔNT-TC10/WT (panel 2), Myc-tagged ΔNT-TC10/Q75L (panel 3), and Myc-tagged ΔNT-TC10/T31N (panel 4) cDNAs as described in MATERIALS AND METHODS. Eighteen hours later, the cells were fixed and the subcellular localization was determined by confocal fluorescent microscopy. (B) Differentiated 3T3L1 adipocytes were coelectroporated with 50 μg of GLUT4-EGFP plus 200 μg of the empty vector (panels 1 and 2), TC10/WT (panels 3 and 4), ΔNT-TC10/WT (panels 5 and 6), ΔNT-TC10/T31N (panels 7 and 8), and ΔNT-TC10/Q75L (panels 9 and 10) cDNAs. Eighteen hours later, the cells were then treated without (Basal, panels 1, 3, 5, 7, and 9) or with 100 nM insulin (Insulin, panels 2, 4, 6, 8, and 10) for 30 min. The cells were then fixed and the subcellular localization of GLUT4-EGFP was assessed by confocal fluorescent microscopy. These are representative images of cells obtained from three to four independent determinations. (C) GLUT4 translocation was quantified by determining the number of cells displaying a continuous plasma membrane GLUT4-EGFP fluorescent signal from the counting of 50 cells per experiment. These data were obtained from the average of four independent experiments (200 cells total).
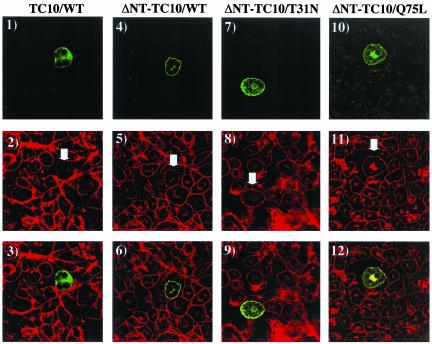
Analysis of cortical actin organization again demonstrated that the expression of TC10/WT resulted in the disruption of adipocyte cortical actin compared with the surrounding nonmicroinjected control cells (Figure 8, panels 1–3). Consistent with the lack of inhibition of insulin-stimulated GLUT4 translocation, expression of ΔNT-TC10/WT and ΔNT-TC10/T31N did not affect cortical actin organization (Figure 8, panels 4–9). It should be noted, however, that expression of ΔNT-TC10/T31N did appear to reduce the extent of perinuclear actin (Figure 8, panels 7–9). In any case, although expression of in ΔNT-TC10/Q75L inhibited insulin-stimulated GLUT4 translocation, this construct also did not appear to alter cortical actin organization (Figure 8, panels 10–12). Instead, this mutant markedly increased the amount of polymerized actin localized to the peri-nuclear endomembrane compartments. In any case, data further demonstrate that the amino terminus of TC10 is responsible for the disruption of cortical actin.
Figure 8.
Effects of TC10 amino terminal deletions on adipocyte cortical actin organization. Differentiated 3T3L1 adipocyte nuclei were microinjected with 200 μg/ml HA-tagged TC10/WT (panels 1–3), HA-tagged ΔNT-TC10/WT (panels 4–6), Myc-tagged ΔNT-TC10/T31N (panels 7–9), and Myc-tagged ΔNT-TC10/Q75L (panels 10–12) cD-NAs. Eighteen hours later, the cells were fixed and the localization of the expressed proteins was determined by confocal fluorescent microscopy using a FITC-labeled anti-HA or anti-Myc antibody (panels 1, 4, 7, and 10). The cells were also colabeled with rhodamine-phalloidin to observe the distribution of filamentous actin (panels 2, 5, 8, and 11). The merged images are presented in panels 3, 6, 9, and 12. These are representative fields of cells observed in four independent experiments.
Because the visualization of actin structure was performed in microinjected cells whereas GLUT4 translocation was assessed in electroporated cells, we wanted to ensure that the effects observed were directly comparable by the same technique. We therefore microinjected 3T3L1 adipocytes with GLUT4-EGFP plus several of the germane TC10 constructs (Figure 9). As previously observed by electroporation, microinjection of TC10/WT resulted in a marked inhibition of insulin-stimulated GLUT4 translocation (Figure 9A, panels 1–4). In contrast, adipocytes microinjected with GLUT4-EGFP plus either ΔNT-TC10 or TC10/ASA had no significant effect on insulin-stimulated GLUT4 translocation (Figure 9A, panels 5–12). Quantification of these data are presented in Figure 9B. These data confirm that the disruption of cortical actin by the amino terminal domain of TC10 directly correlates with the loss of insulin-stimulated GLUT4 translocation in adipocytes.
Figure 9.
Examination of GLUT4 translocation by microinjection of TC10 mutants. (A) Differentiated 3T3L1 adipocyte nuclei were microinjected with GLUT4-EGFP plus HA-tagged TC10/WT (panels 1–4), ΔNT-TC10 (panels 5–8), or TC10/GAG-ASA (panels 9–12) cDNAs. Eighteen hours later, the cells were either left untreated or incubated with 100 nM insulin for 30 min. The cells were then fixed and the subcellular localization of GLUT4-EGFP (panels 1, 2, 5, 6, 9, and 10) and HA-TC10 (panels 3, 4, 7, 8, 11, and 12) was assessed by confocal fluorescent microscopy. These are representative images of cells obtained from three to four independent determinations. (B) GLUT4 translocation was quantified by determining the number of cells displaying a continuous plasma membrane GLUT4-EGFP fluorescent signal from the counting of 30–40 cells per experiment. These data were obtained from the average of three to four independent experiments (90–120 cells total).
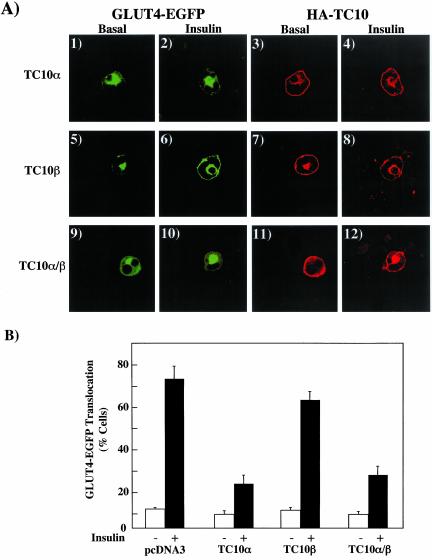
The Amino Terminus of Mouse TC10α in the Context of Mouse TC10β Confers Inhibition of GLUT4 Translocation and Disruption of Cortical Actin
In addition to human TC10, we and others have identified three related mouse TC10 isoforms, termed TC10α, TC10β, and TC10β-long and demonstrated that only expression of mouse TC10α inhibited insulin-stimulated GLUT4 translocation and disrupted cortical actin organization (Chiang et al., 2002). Mouse TC10α is highly related to human TC10 and shares the identical amino terminal domain with the exception of an 8-amino acid extension in the human TC10 (Figure 1). Because both mouse TC10α and TC10β containing the same carboxyl terminal targeting sequences but primarily differ at the amino terminus, we next prepared a chimera replacing the mouse TC10β amino termini with that of mouse TC10α (Figure 1C). As expected, microinjection of TC10α inhibited insulin-stimulated GLUT4 translocation, whereas expression of TC10β had no significant effect (Figure 10A, panels 1–8). However, the TC10α/β chimera was as effective as TC10α in inhibiting insulin-stimulated GLUT4 translocation (Figure 10A, panels 9–12). These data are quantified and presented in Figure 10B. Thus, these data further demonstrate that the mouse TC10α amino terminal domain when overexpressed in the context of heterologous proteins containing the appropriate targeting domains (H-Ras and TC10β) functions as a potent inhibitor of insulin-stimulated GLUT4 translocation.
Figure 10.
Comparison of mouse TC10α and TC10β on insulin-stimulated GLUT4 translocation. (A) Differentiated 3T3L1 adipocyte nuclei were microinjected with GLUT4-EGFP plus HA-tagged mouse TC10α (panels 1–4), TC10β (panels 5–8), or TC10α/β (panels 9–12) cDNAs. Eighteen hours later, the cells were either left untreated or incubated with 100 nM insulin for 30 min. The cells were then fixed and the subcellular localization of GLUT4-EGFP (panels 1, 2, 5, 6, 9, and 10) and HA-TC10 (panels 3, 4, 7, 8, 11, and 12) was assessed by confocal fluorescent microscopy. These are representative images of cells obtained from three to four independent determinations. (B) GLUT4 translocation was quantified by determining the number of cells displaying a continuous plasma membrane GLUT4-EGFP fluorescent signal from the counting of 30–40 cells per experiment. These data were obtained from the average of three to four independent experiments (90–120 cells total).
DISCUSSION
We have recently observed that insulin can stimulate a signaling pathway leading to the activation of TC10α in adipocytes (Baumann et al., 2000; Chiang et al., 2001). This apparently results from the insulin-dependent tyrosine phosphorylation of the Cbl proto-oncogene product and its recruitment to a lipid raft plasma membrane microdomain via the adapter proteins APS and CAP (Ribon et al., 1998; Ahmed et al., 2000; Baumann et al., 2000). Importantly, overexpression of TC10α was found to specifically inhibit insulin-stimulated GLUT4 translocation. The inhibitory function of TC10 occurred not only when an inactive constitutively GDP-bound mutant form of TC10 was expressed but also for an active constitutively GTP-bound mutant and more surprisingly when the wild-type TC10 was expressed. Furthermore, expression of mouse TC10β had no significant effect on insulin-stimulated GLUT4 translocation despite sharing an identical effecter and GTP binding domain with mouse TC10α (Chiang et al., 2002). These data suggest that a structural domain independent of GTP or effecter binding was responsible for this inhibition of insulin signaling. Moreover, the ability of TC10 to inhibit insulin-stimulated GLUT4 translocation was also dependent on the intracellular targeting information provided by posttranslational modification of the carboxy terminus (Watson et al., 2001).
A specific role for the carboxyl-terminal domains of the small GTP binding proteins of the Ras family in directing different intracellular trafficking routes and plasma membrane subdomain compartmentalization have been well established (Choy et al., 1999; Roy et al., 1999; Apolloni et al., 2000; Michaelson et al., 2001). For example, farnesylation and palmitoylation of the H-Ras carboxyl terminal CxCxxCaax sequence results in the biosynthetic trafficking and localization of H-Ras to caveolin-enriched plasma membrane microdomains (Dupree et al., 1993; Li et al., 1996; Song et al., 1996). In contrast, the K-Ras carboxyl terminal sequence KKKKKKxxCaax is only subjected to farnesylation and is excluded from the secretory membrane system, resulting in its localization to nonlipid raft domains of the plasma membrane (Hancock et al., 1990; Choy et al., 1999; Apolloni et al., 2000). Using these trafficking domains, we previously demonstrated that the inhibitory function of TC10 only occurred when localized to lipid raft microdomains but not to nonlipid raft domains of the plasma membrane (Watson et al., 2001).
On the basis of this information, we speculated that unusual amino terminal extension of TC10α, when coupled with a lipid raft targeting carboxyl terminal domain, was responsible for the inhibition of insulin-stimulated GLUT4 translocation. We specifically addressed this hypothesis by expressing chimera proteins containing the amino terminal domain of TC10 fused to full-length H-Ras and K-Ras. The amino terminal TC10–16/H-Ras but not the TC10–16/K-Ras fusion protein inhibited insulin-stimulated GLUT4 translocation. In addition, TC10β had no effect on insulin-stimulated GLUT4 translocation, whereas substitution of the TC10α amino termini on TC10β resulted in a marked inhibition of GLUT4 translocation. Similarly, R-Ras1 also contains a related amino terminal extension but has a completely distinct effecter domain. Nevertheless, expression of R-Ras1 also inhibited insulin-stimulated GLUT4 translocation, whereas R-Ras2, which has an unrelated amino terminus, was unable to alter insulin action. Furthermore, TC10 inhibitory function was completely abrogated by selective point mutations within the amino terminal extension and deletion of this domain in the context of TC10/WT or TC10/T31N. These data clearly demonstrate that the amino terminal domain provides an inhibitory function but only when localized to lipid raft microdomains by the appropriate carboxyl terminal targeting motif.
Rho-family GTPases play critical roles in cytoskeleton organization, proliferation, differentiation, and apoptosis (Neudauer et al., 1998). Although the members of this family, such as RhoA, Cdc42, and Rac share many regulators and effectors, they apparently produce different phenotypes when expressed as gain-of-function mutants in cells (Neudauer et al., 1998). It has been recently documented that insulin stimulates dynamic actin remodeling at both the inner surface of the plasma membrane and in the perinuclear region that is sensitive to Clostridium difficile toxin B, a Rho family-specific toxin (Kanzaki and Pessin, 2001; Kanzaki et al., 2001). In parallel, TC10 overexpression has been observed to disrupt adipocyte cortical actin and inhibition of dynamic actin remodeling in vivo prevents insulin-stimulated GLUT4 translocation (Kanzaki et al., 2001). Similarly, TC10 amino terminal/H-Ras chimeras that inhibited insulin-stimulated GLUT4 translocation also resulted in a disruption of adipocyte cortical actin. Furthermore, mutations that prevent the inhibition of insulin-stimulated GLUT4 translocation had no effect on cortical actin integrity. As with the case of GLUT4 translocation, only those TC10 amino termini containing constructs that were targeted to lipid raft microdomains disrupted cortical actin. In this regard, recent studies have observed that caveolin forms large organized caveolae/lipid raft structures in the plasma membrane (Brown and London, 1998; Fujimoto et al., 1998; Baumann et al., 2000; Watson et al., 2001; Kanzaki and Pessin, 2002; Parton et al., 2002). These large organized caveolin rosette structures appear to be the sites of cortical actin anchoring and are consistent with only lipid raft targeted TC10 displaying cortical actin-depolymerizing properties.
To further gain insight into the function of the TC10 amino terminus to disrupt cortical actin and inhibit insulin-stimulated GLUT4 translocation, we examined the properties of amino terminal deletion mutants. As expected, expression of ΔNT-TC10/WT and ΔNT-TC10/T31N lost their ability to inhibit insulin-stimulated GLUT4 translocation. In parallel these mutants were unable to disrupt cortical actin. Surprisingly however, expression of ΔNT-TC10/Q75L appeared to uncouple cortical actin from GLUT4 translocation. That is, expression of ΔNT-TC10/Q75L retained the ability to inhibit insulin-stimulated GLUT4 translocation but had no effect on cortical actin structure. There are several possible explanations for this observation. First, although cortical actin structure may remain intact, cortical actin remodeling may be prevented by expression of ΔNT-TC10/Q75L. It is also possible that inhibitory effect of TC10/Q75L may be unrelated to changes in cortical actin and reflects a requirement for TC10 GTP/GDP cycling. In this regard, the lack of effect of ΔNT-TC10/T31N may be that this mutant does not function as a dominant-interfering mutant. Alternatively, the inhibitory action of ΔNT-TC10/Q75L could be related to the large increase in peri-nuclear actin polymerization because this is the apparent storage site for the insulin-responsive GLUT4 storage compartment. Clearly, further studies are necessary to resolve these issues and to further elucidate the function of the endogenous TC10 protein and the mechanisms by which the amino terminal domain inhibits cortical F-actin.
In any case, we have previously postulated that TC10 plays an important regulatory role in the insulin signaling pathway involved in GLUT4 translocation (Chiang et al., 2001). This was based on the ability of insulin to activate TC10 and that expression of upstream TC10 regulators had dramatic effects on insulin-stimulated GLUT4 translocation. Furthermore, overexpression of TC10 was found to inhibit insulin-stimulated GLUT4 translocation. Based on our current data, the TC10 inhibitory function is a direct structural property of its unusual amino terminal extension and is apparently independent of GTP binding or effecter function. One interpretation of these data is that TC10 is not directly involved an insulin-signaling pathway regulating GLUT4 translocation but reflects the requirement for cortical actin. Alternatively, it also remains possible that TC10 has both positive and negative regulator functions that balance the rate of actin polymerization/depolymerization. However, when overexpressed and targeted to lipid raft domains, the negative activity (disruption of cortical actin) becomes predominant, thereby masking the function of the endogenous TC10. Further studies will be necessary to resolve these issues and to determine the specific role that TC10 may be playing in insulin signaling.
In summary, we have identified an unusual amino terminal extension present in TC10 and R-Ras1 that interacts with a component(s) necessary for the maintenance of adipocyte cortical actin structure. This domain functions in a dominant-negative manner disrupting cortical actin but only when targeted to lipid raft microdomains of the plasma membrane. The targeting of this motif corresponds to precise localization of the F-actin attachment sites, termed Cav-actin. Importantly, the ability of overexpressed TC10/WT and TC10/T31N to inhibit insulin-stimulated GLUT4 translocation appears to be independent of GTP binding and effecter domain function but is dependent on the disruption of cortical actin. In contrast, overexpression of a constitutively active TC10 mutant (TC10/Q75L) inhibited of insulin-stimulated GLUT4 translocation and enhanced peri-nuclear actin polymerization but without any significant affect on cortical actin. This latter finding directly demonstrates that the TC10 activation state has dramatic effects on actin polymerization and insulin-stimulated GLUT4 translocation.
Acknowledgments
We thank Diana Boeglin and Amanda Kalen for their care and maintenance of the 3T3L1 adipocytes and Drs. Makoto Kanzaki and Robert T. Watson for helpful discussions during the course of this study. This study was supported by Grants DK55811 and DK33823 from the National Institutes of Health.
DOI:10.1091/mbc.E03-01-0012.
References
- Ahmed, Z., Smith, B.J., and Pillay, T.S. (2000). The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475, 31–34. [DOI] [PubMed] [Google Scholar]
- Alessi, D.R., and Downes, C.P. (1998). The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta 1436, 151–164. [DOI] [PubMed] [Google Scholar]
- Alessi, D.R., James, S.R., Downes, C.P., Holmes, A.B., Gaffney, P.R., Reese, C.B., and Cohen, P. (1997). Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7, 261–269. [DOI] [PubMed] [Google Scholar]
- Anderson, K.E., Coadwell, J., Stephens, L.R., and Hawkins, P.T. (1998). Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8, 684–691. [DOI] [PubMed] [Google Scholar]
- Apolloni, A., Prior, I.A., Lindsay, M., Parton, R.G., and Hancock, J.F. (2000). H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell. Biol. 20, 2475–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay, G., Standaert, M.L., Zhao, L., Yu, B., Avignon, A., Galloway, L., Karnam, P., Moscat, J., and Farese, R.V. (1997). Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 272, 2551–2558. [DOI] [PubMed] [Google Scholar]
- Baumann, C.A., Ribon, V., Kanzaki, M., Thurmond, D.C., Mora, S., Shigematsu, S., Bickel, P.E., Pessin, J.E., and Saltiel, A.R. (2000). CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207. [DOI] [PubMed] [Google Scholar]
- Brown, D.A., and London, E. (1998). Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136. [DOI] [PubMed] [Google Scholar]
- Bryant, N.J., Govers, R., and James, D.E. (2002). Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3, 267–277. [DOI] [PubMed] [Google Scholar]
- Cantley, L.C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655–1657. [DOI] [PubMed] [Google Scholar]
- Cheatham, B., Vlahos, C.J., Cheatham, L., Wang, L., Blenis, J., and Kahn, C.R. (1994). Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, D.N.A. synthesis, and glucose transporter translocation. Mol. Cell. Biol. 14, 4902–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, S.H., Baumann, C.A., Kanzaki, M., Thurmond, D.C., Watson, R.T., Neudauer, C.L., Macara, I.G., Pessin, J.E., and Saltiel, A.R. (2001). Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410, 944–948. [DOI] [PubMed] [Google Scholar]
- Chiang, S.H., Hou, J.C., Hwang, J., Pessin, J.E., and Saltiel, A.R. (2002). Cloning and functional characterization of related TC10 isoforms, a subfamily of Rho proteins involved in insulin-stimulated glucose transport. J. Biol. Chem. 277, 13067–13073. [DOI] [PubMed] [Google Scholar]
- Choy, E., Chiu, V.K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Ivanov, I.E., and Philips, M.R. (1999). Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98, 69–80. [DOI] [PubMed] [Google Scholar]
- Czech, M.P. (2000). PIP2 and PIP 3, complex roles at the cell surface. Cell 100, 603–606. [DOI] [PubMed] [Google Scholar]
- Ducluzeau, P.H., Fletcher, L.M., Vidal, H., Laville, M., and Tavare, J.M. (2002). Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diabetes Metab. 28, 85–92. [PubMed] [Google Scholar]
- Dupree, P., Parton, R.G., Raposo, G., and Kurzchalia, T.V. (1993). Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 12, 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa, K. et al. (2002). Membrane localization of 3-phosphoinositide-dependent protein kinase-1 stimulates activities of Akt and atypical protein kinase C but does not stimulate glucose transport and glycogen synthesis in 3T3–L1 adipocytes. J. Biol. Chem. 277, 38863–38869. [DOI] [PubMed] [Google Scholar]
- Fujimoto, T., Hagiwara, H., Aoki, T., Kogo, H., and Nomura, R. (1998). Caveolae: from a morphological point of view. J. Electron Microsc. 47, 451–460. [DOI] [PubMed] [Google Scholar]
- Giovannone, B. et al. (2000). Insulin receptor substrate (IRS) transduction system: distinct and overlapping signaling potential. Diabetes Metab. Res. Rev. 16, 434–441. [DOI] [PubMed] [Google Scholar]
- Guilherme, A., and Czech, M.P. (1998). Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J. Biol. Chem. 273, 33119–33122. [DOI] [PubMed] [Google Scholar]
- Hancock, J.F., Magee, A.I., Childs, J.E., and Marshall, C.J. (1989). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57, 1167–1177. [DOI] [PubMed] [Google Scholar]
- Hancock, J.F., Paterson, H., and Marshall, C.J. (1990). A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63, 133–139. [DOI] [PubMed] [Google Scholar]
- Holman, G.D., and Sandoval, I.V. (2001). Moving the insulin-regulated glucose transporter GLUT4 into and out of storage. Trends Cell Biol. 11, 173–179. [DOI] [PubMed] [Google Scholar]
- Horton, R.M., Ho, S.N., Pullen, J.K., Hunt, H.D., Cai, Z., and Pease, L.R. (1993). Gene splicing by overlap extension. Methods Enzymol. 217, 270–279. [DOI] [PubMed] [Google Scholar]
- Huang, C., Somwar, R., Patel, N., Niu, W., Torok, D., and Klip, A. (2002). Sustained exposure of L6 myotubes to high glucose and insulin decreases insulin-stimulated GLUT4 translocation but upregulates GLUT4 activity. Diabetes 51, 2090–2098. [DOI] [PubMed] [Google Scholar]
- Imamura, T., Ishibashi, K., Dalle, S., Ugi, S., and Olefsky, J.M. (1999). Endothelin-1-induced GLUT4 translocation is mediated via Galpha(q/11) protein and phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J. Biol. Chem. 274, 33691–33695. [DOI] [PubMed] [Google Scholar]
- Isakoff, S.J., Taha, C., Rose, E., Marcusohn, J., Klip, A., and Skolnik, E.Y. (1995). The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc. Natl. Acad. Sci. USA 92, 10247–10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z.Y., Chawla, A., Bose, A., Way, M., and Czech, M.P. (2002). A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J. Biol. Chem. 277, 509–515. [DOI] [PubMed] [Google Scholar]
- Kanzaki, M., and Pessin, J.E. (2001). Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 276, 42436–42444. [DOI] [PubMed] [Google Scholar]
- Kanzaki, M., and Pessin, J.E. (2002). Caveolin-associated filamentous actin (Cav-actin) defines a novel F-actin structure in adipocytes. J. Biol. Chem. 277, 25867–25869. [DOI] [PubMed] [Google Scholar]
- Kanzaki, M., Watson, R.T., Hou, J.C., Stamnes, M., Saltiel, A.R., and Pessin, J.E. (2002). Small GTP-binding protein TC10 differentially regulates two distinct Populations of filamentous actin in 3T3L1 adipocytes. Mol. Biol. Cell 13, 2334–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki, M., Watson, R.T., Khan, A.H., and Pessin, J.E. (2001). Insulin stimulates actin comet tails on intracellular GLUT4-containing compartments in differentiated 3T3L1 adipocytes. J. Biol. Chem. 276, 49331–49336. [DOI] [PubMed] [Google Scholar]
- Kohn, A.D., Summers, S.A., Birnbaum, M.J., and Roth, R.A. (1996). Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31372–31378. [DOI] [PubMed] [Google Scholar]
- Konrad, D., Somwar, R., Sweeney, G., Yaworsky, K., Hayashi, M., Ramlal, T., and Klip, A. (2001). The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 50, 1464–1471. [DOI] [PubMed] [Google Scholar]
- Kristiansen, S., Nielsen, J.N., Bourgoin, S., Klip, A., Franco, M., and Richter, E.A. (2001). GLUT-4 translocation in skeletal muscle studied with a cell-free assay: involvement of phospholipase D. Am. J. Physiol. Endocrinol. Metab. 281, E608–E618. [DOI] [PubMed] [Google Scholar]
- Krook, A. et al. (1997). Two naturally occurring insulin receptor tyrosine kinase domain mutants provide evidence that phosphoinositide 3-kinase activation alone is not sufficient for the mediation of insulin's metabolic and mitogenic effects. J. Biol. Chem. 272, 30208–30214. [DOI] [PubMed] [Google Scholar]
- Le Good, J.A., Ziegler, W.H., Parekh, D.B., Alessi, D.R., Cohen, P., and Parker, P.J. (1998). Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045. [DOI] [PubMed] [Google Scholar]
- Li, S., Couet, J., and Lisanti, M.P. (1996). Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 271, 29182–29190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S.S., Haruta, T., Morris, A.J., Klippel, A., Williams, L.T., and Olefsky, J.M. (1996). Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3–L1 adipocytes. J. Biol. Chem. 271, 17605–17608. [DOI] [PubMed] [Google Scholar]
- Matsumoto, M., Ogawa, W., Hino, Y., Furukawa, K., Ono, Y., Takahashi, M., Ohba, M., Kuroki, T., and Kasuga, M. (2001). Inhibition of insulin-induced activation of Akt by a kinase-deficient mutant of the epsilon isozyme of protein kinase C. J. Biol. Chem. 276, 14400–14406. [DOI] [PubMed] [Google Scholar]
- Michaelson, D., Silletti, J., Murphy, G., D'Eustachio, P., Rush, M., and Philips, M.R. (2001). Differential localization of Rho GTPases in live cells. Regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, J., Okada, S., Coker, K., Ceresa, B.P., Elmendorf, J.S., Syu, L.-J., Noda, Y., Saltiel, A.R., and Pessin, J.E. (1999). Synip: a novel insulin-regulated syntaxin 4 binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell 3, 751–760. [DOI] [PubMed] [Google Scholar]
- Murphy, G.A., Jillian, S.A., Michaelson, D., Philips, M.R., D'Eustachio, P., and Rush, M.G. (2001). Signaling mediated by the closely related mammalian Rho family GTPases TC10 and Cdc42 suggests distinct functional pathways. Cell Growth Differ. 12, 157–167. [PubMed] [Google Scholar]
- Nakashima, N., Sharma, P.M., Imamura, T., Bookstein, R., and Olefsky, J.M. (2000). The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J. Biol. Chem. 275, 12889–12895. [DOI] [PubMed] [Google Scholar]
- Neudauer, C.L., Joberty, G., Tatsis, N., and Macara, I.G. (1998). Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr. Biol. 8, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Okada, T., Kawano, Y., Sakakibara, R., Hazeki, O., and Ui, M. (1994). Essential role of phophatidylinositol 3-kinase in insulin-induced glucose transport and antilypolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269, 3568–3573. [PubMed] [Google Scholar]
- Olson, A.L., Knight, J.B., and Pessin, J.E. (1997). Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 17, 2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata, W., Shibata, H., Li, L., Takata, K., and Kojima, I. (2000). Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem. J. 346(Pt 2), 321–328. [PMC free article] [PubMed] [Google Scholar]
- Parton, R.G., Molero, J.C., Floetenmeyer, M., Green, K.M., and James, D.E. (2002). Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J. Biol. Chem. 277, 46769–46778. [DOI] [PubMed] [Google Scholar]
- Ploug, T., and Ralston, E. (2002). Exploring the whereabouts of GLUT4 in skeletal muscle (review). Mol. Membr. Biol. 19, 39–49. [DOI] [PubMed] [Google Scholar]
- Resh, M.D. (1999). Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451, 1–16. [DOI] [PubMed] [Google Scholar]
- Ribon, V., Printen, J.A., Hoffman, N.G., Kay, B.K., and Saltiel, A.R. (1998). A novel, multifunctional c-Cbl binding protein in insulin receptor signaling in 3T3–L1 adipocytes. Mol. Cell. Biol. 18, 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribon, V., and Saltiel, A.R. (1997). Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3–L1 adipocytes. Biochem. J. 324, 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S., Luetterforst, R., Harding, A., Apolloni, A., Etheridge, M., Stang, E., Rolls, B., Hancock, J.F., and Parton, R. (1999). Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1, 98–105. [DOI] [PubMed] [Google Scholar]
- Saltiel, A.R., and Pessin, J.E. (2002). Insulin signaling pathways in time and space. Trends Cell Biol. 12, 65–71. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C. (1998). Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 10, 167–172. [DOI] [PubMed] [Google Scholar]
- Sharma, P.M., Egawa, K., Huang, Y., Martin, J.L., Huvar, I., Boss, G.R., and Olefsky, J.M. (1998). Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. 273, 18528–18537. [DOI] [PubMed] [Google Scholar]
- Simpson, F., Whitehead, J.P., and James, D.E. (2001). GLUT4 —at the cross roads between membrane trafficking and signal transduction. Traffic 2, 2–11. [DOI] [PubMed] [Google Scholar]
- Somwar, R., Kim, D.Y., Sweeney, G., Huang, C., Niu, W., Lador, C., Ramlal, T., and Klip, A. (2001). GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem. J. 359, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, K.S., Li, S., Okamoto, T., Quilliam, L.A., Sargiacomo, M., and Lisanti, M.P. (1996). Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271, 9690–9697. [DOI] [PubMed] [Google Scholar]
- Standaert, M.L., Bandyopadhyay, G., Sajan, M.P., Cong, L., Quon, M.J., and Farese, R.V. (1999). Okadaic acid activates atypical protein kinase C (zeta/lambda) in rat and 3T3/L1 adipocytes. An apparent requirement for activation of Glut4 translocation and glucose transport. J. Biol. Chem. 274, 14074–14078. [DOI] [PubMed] [Google Scholar]
- Stephens, L. et al. (1998). Protein kinase B kinases that mediate phosphatidylinositol 3, 4, 5-trisphosphate-dependent activation of protein kinase B. Science 279, 710–714. [DOI] [PubMed] [Google Scholar]
- Sweeney, G., Somwar, R., Ramlal, T., Volchuk, A., Ueyama, A., and Klip, A. (1999). An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3–L1 adipocytes and L6 myotubes. J. Biol. Chem. 274, 10071–10078. [DOI] [PubMed] [Google Scholar]
- Tong, P., Khayat, Z.A., Huang, C., Patel, N., Ueyama, A., and Klip, A. (2001). Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Invest. 108, 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridis, T., Bergman, A., Somwar, R., Taha, C., Aktories, K., Cruz, T.F., Klip, A., and Downey, G.P. (1998). Actin filaments facilitate insulin activation of the src and collagen homologous/mitogen-activated protein kinase pathway leading to DNA synthesis and c-fos expression. J. Biol. Chem. 273, 28322–28331. [DOI] [PubMed] [Google Scholar]
- Vollenweider, P., Clodi, M., Martin, S.S., Imamura, T., Kavanaugh, W.M., and Olefsky, J.M. (1999). An SH2 domain-containing 5′ inositolphosphatase inhibits insulin-induced GLUT4 translocation and growth factor-induced actin filament rearrangement. Mol. Cell. Biol. 19, 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Bilan, P.J., Tsakiridis, T., Hinek, A., and Klip, A. (1998). Actin filaments participate in the relocalization of phosphatidylinositol3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3–L1 adipocytes. Biochem. J. 331, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Somwar, R., Bilan, P.J., Liu, Z., Jin, J., Woodgett, J.R., and Klip, A. (1999). Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19, 4008–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, R.T., Furukawa, M., Chiang, S.H., Boeglin, D., Kanzaki, M., Saltiel, A.R., and Pessin, J.E. (2003). The exocytotic trafficking of TC10 occurs through both classical and non-classical secretory transport pathways in 3T3L1 adipocytes. Mol. Cell. Biol. 23, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, R.T., Shigematsu, S., Chiang, S.H., Mora, S., Kanzaki, M., Macara, I.G., Saltiel, A.R., and Pessin, J.E. (2001). Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 154, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, M.F. (1998). The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell Biochem. 182, 3–11. [PubMed] [Google Scholar]
- Wiese, R.J., Mastick, C.C., Lazar, D.F., and Saltiel, A.R. (1995). Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3–L1 adipocytes. J. Biol. Chem. 270, 3442–3446. [DOI] [PubMed] [Google Scholar]
- Yamada, T., Katagiri, H., Asano, T., Tsuru, M., Inukai, K., Ono, H., Kodama, T., Kikuchi, M., and Oka, Y. (2002). Role of PDK1 in insulin-signaling pathway for glucose metabolism in 3T3–L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 282, E1385–E1394. [DOI] [PubMed] [Google Scholar]