Abstract
The anterior cingulate cortex (ACC) has been suggested as a monitoring center that is responsible for online detection of response conflicts. In this view, the conflict signal detected by the ACC is transmitted to other brain regions, such as the dorsal part of the lateral prefrontal cortex (lPFC), to increase the level of cognitive control. In this functional MRI (fMRI) study, we examined the conflict resolution that goes beyond online detection of response conflicts. Participants learned pseudoarithmetic problem-solving tasks that involve stimulus–response mapping rules with high or low conflicts. On half of the trials, participants had a preview of the upcoming operator that allowed advance preparation for the mapping rules. The preview significantly reduced the conflict effects on latency. During the preview, both the ACC and lPFC were activated in anticipation of conflict, and this anticipatory activation was highly predictive of the subsequent latency. These results suggest that the ACC and lPFC are responsible for both anticipatory preparation and online adjustment in response to conflicts. The results also confirm the roles of the lPFC and ACC in managing conflict during problem solving and extend these roles to include responding to anticipation of conflicts that may arise between incompatible stimulus–response mappings maintained in working memory during preparation.
Keywords: lateral prefrontal cortex, preparation, problem solving
Effective task execution requires a mixture of bottom–up and top–down control. Although it is preferable to stay focused on the task-relevant information, it is not efficient to constantly rely on executive mechanisms. Executive mechanisms take a substantial toll on working memory to maintain task-relevant information. Moreover, a strict top–down approach impedes flexible responses to unexpected, yet meaningful, events. In contrast, when stimulus-driven processing dominates, the course of action digresses constantly, interfering with goal-directed behavior.
Cognitive neuroimaging and neuropsychology studies have consistently shown that the lateral prefrontal cortex (lPFC), especially its dorsal stream, and the anterior cingulate cortex (ACC) are critically active when participants are engaged in cognitively demanding tasks. Although there is a consensus that lPFC is responsible for executive functions, such as maintaining and manipulating task-relevant information, the role of the ACC in cognitive control, although generally accepted, is still controversial. Some theories have argued that the ACC detects conflicting response tendencies in the environment, and others have suggested that the ACC may respond to cognitive complexity more generally.
The conflict-monitoring model (1) has proposed that the ACC monitors competing response tendencies elicited by stimuli. According to this model, the conflict signal detected by the ACC is transmitted to a high-level control mechanism such as the lPFC, thereby increasing the extent of conscious control (2). The response conflict may arise when the task-irrelevant information (i.e., distractor) is associated with a response that is incongruent with the response associated with the task-relevant information (i.e., target). The distractor is more likely processed when the level of the executive control is relatively low and therefore relaxes the filtering of task-irrelevant stimuli (3). Thus, ACC activation should be observed when lPFC activation level is relatively low. Once conflict is detected, the executive control mechanism may strengthen the level of attentional focus or filtering so that distractors are not processed any further than the perceptual level. This reciprocal interaction between the lPFC and the ACC has been supported by sequential increases and decreases in their activation levels. For example, ACC activation was observed when subjects encountered occasional instances of response conflict embedded in predominantly congruent trials, in which the level of top–down control (i.e., lPFC) is relatively low. In contrast, ACC activation was not evident when measured among predominantly incongruent trials presumably because the lPFC is already fully engaged (4). The involvement of the ACC in conflict monitoring also has been demonstrated with respect to semantic conflict between stimulus elements in the absence of response conflict (5) and conflict because of memory retrieval failure (6).
One implication of the conflict-monitoring model is that lPFC and ACC activations may reflect different sources of cognitive challenges. Reflecting the extent of executive control, the lPFC responds to the level of task difficulty detected in a predictive manner. In contrast, the ACC responds to response conflict detected online, when the control level engaged by the lPFC is relatively low. Consistent with this implication, lPFC activation was higher when an advance task cue indicated that the upcoming task would be more cognitively demanding, therefore increasing the amount of anticipatory task preparation, whereas ACC activation did not reflect the processing of an advance task cue (2). Instead, the increased ACC activation in response to the target–distractor incongruence was observed during task performance, whereas lPFC activation did not reflect the incongruence.
Recent evidence suggests that the ACC may more directly reflect task difficulty or cognitive demands. For example, mathematical problem-solving studies showed that ACC activation gradually increased with problem complexity, just as lPFC activation did (7, 8). Problem complexity involves the number of mathematical steps necessary to solve a problem and is not correlated with response conflicts. In an episodic memory-retrieval study, both ACC and lPFC activations increased when the target information was weakly associated with a memory probe (9). The weak association requires a relatively greater amount of cognitive effort for the association to be available to consciousness. Another study manipulated task cues so that advance preparation of the upcoming task could be triggered on some trials (10). In this study, both ACC and lPFC activations were higher with an informative task cue than with a neutral cue. All these results are consistent with the recent proposals regarding the ACC that do not limit its role to online detection of response conflict. For example, the ACC has been suggested as part of a decision-making mechanism in which the ACC is sensitive to expected outcome of a particular action response (11). Further, the ACC may be activated when the task implies a higher level of difficulty, such as error likelihood, rather than response conflicts with environmental stimuli (12).
In summary, although the ACC is sensitive to various types of conflict information (13), it may respond to cognitive complexity more generally. The evidence of ACC involvement in response conflict has been acquired in experimental manipulations in which the conflict was manipulated online with task performance but in which cognitive complexity was not otherwise manipulated (2, 4, 14). Studies showing effects of cognitive complexity, however, did not directly manipulate response conflict (7, 8, 9). In the current study, we manipulated both response conflict and cognitive complexity.
To better understand what determines ACC activation, we had participants perform Boolean arithmetic tasks, in which both input and output have binary values (I or B). A Boolean operator specifies a relationship between an input pattern and an output value. We used six rules (Identity, Reverse, AND, NAND, OR, and NOR). Table 1 presents complete sets of stimulus–response mappings for each rule. Half of these rules (Identity, AND, and OR) implied low-level conflict, in the sense that they affirmatively associated input and output. The other half (Reverse, NAND, and NOR) implied high-level conflict, specifying negative relationships between input and output. For example, the NAND rule is “If both input values are Bs, then the output is I.” The high-conflict rules, therefore, create a conflict in which the target stimulus and the appropriate response are not compatible. The high-conflict rules are also cognitively more complex because they involve processing a negation. We used a paradigm that assessed the effect of the greater cognitive complexity of these rules versus their greater response conflict.
Table 1.
Pseudoarithmetic Boolean rules used in the current study
| Input | Complex |
Simple |
||||
|---|---|---|---|---|---|---|
| Positive |
Negative |
Positive |
Negative |
|||
| #: AND | $: OR | %: NAND | ^: NOR | !: Identify | @: Reverse | |
| (I, I) | I | I | B | B | I | B |
| (I, B) | I | B | B | I | I | B |
| (B, I) | I | B | B | I | B | I |
| (B, B) | B | B | I | I | B | I |
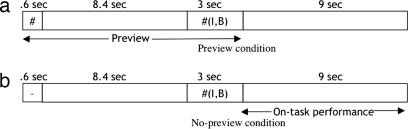
In a functional MRI (fMRI) study, we contrasted two ways of testing these rules, as illustrated in Fig. 1. In the preview condition, the Boolean operator symbol was previewed 9 sec before the entire problem (i.e., the operator and the input pattern) so that participants could prepare the relevant rule implied by the operator. With the preview of high-conflict rules, participants may prepare for the upcoming trial, and they have more to prepare for in the case of complex rules. However, because they do not know what the operators will be, they cannot respond, and there cannot be response conflict. Therefore, the brain activity during the preview interval in the preview condition allows for investigation of the effect of cognitive complexity in the absence of response conflict. In the no-preview condition, a noninformative symbol was presented instead of an operator symbol. Therefore, in the no-preview condition, conflicts may be detected online once the entire problem is presented. The task performance interval in the no-preview condition allows for investigation of the effect of the conflict detected online.
Fig. 1.
Experimental paradigm. In the preview condition (a), an operator symbol (e.g., “#” in this example) was previewed before a problem was presented. In the no-preview condition (b), a noninformative symbol was presented instead of an operator symbol.
Results
Behavioral Performance.
Accuracy and latency for the scanning session are presented in Table 2. The results were substantially different for simple and complex rules, so we analyzed the behavioral data separately. With respect to accuracy, there were no significant effects of preparation or conflict on the simple rules data (P > 0.20). In contrast, for the complex rules, there were significant effects of both factors [F(1, 12) = 7.6, MSE = .006, P < 0.05 for preparation and F(1, 12) = 8.65, MSE = .007, P < 0.05 for conflict], and a significant interaction such that accuracy was much lower in the no-preview, conflict condition [F(1, 12) = 22.29, MSE = .002, P < 0.0001]. With respect to latency, for the simple rules there were significant effects of conflict [F(1, 12) = 99.56, MSE = 2606, P < 0.0001] and preparation [F(1, 12) = 529.22, MSE = 4366, P < 0.0001], but no significant interaction (P > 0.10). This lack of interaction means that preparation did not reduce the size of the conflict effect. In contrast, for complex rules, there was a significant interaction in addition to the main effects [F(1, 12) = 38.17, MSE = 14033, P < 0.0001]. The conflict effect was reduced from 487 msec without preparation to 70 msec with preparation [t(12) = 6.18, P < 0.0001].
Table 2.
Behavioral results: Latency in each condition
| Condition | Simple |
Complex |
||
|---|---|---|---|---|
| Low conflict | High conflict | Low conflict | High conflict | |
| Preview | 740 msec (0.96) | 908 msec (0.96) | 693 msec (0.96) | 763 msec (0.95) |
| No preview | 1,188 msec (0.93) | 1,303 msec (0.96) | 1,306 msec (0.97) | 1,793 msec (0.83) |
Accuracy in parentheses.
It is not entirely clear why preparation did not reduce the conflict effect for simple rules. Perhaps they were sufficiently easy that participants did not put much effort into preparation beyond saving the cost of perceptually encoding the operator. Because we did not get an impact of preparation on reducing conflict for simple rules, we decided to focus the analysis of the imaging data on the complex rules, for which all of the effects are large and for which we had twice as many observations.
Imaging Results.
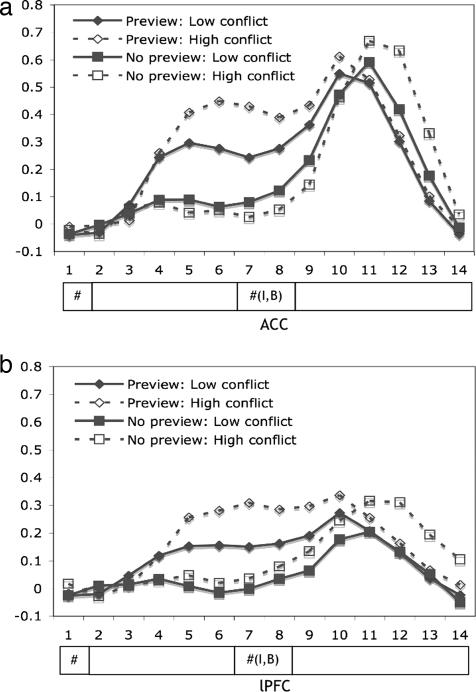
Imaging data were analyzed in two ways. The anticipatory preparation of conflict was examined in the preview interval (the first eight scans). The effect of conflict detected online was analyzed in the on-task performance interval (the last six scans). Two a priori regions of interest, the ACC and lPFC, were defined on the basis of previous studies of mathematical problem solving and memory retrieval (7, 8, 9). Each region was 5 voxels wide, 5 long, and 4 high, for a total of 100 voxels per region. The voxel dimension was 3.125 × 3.125 × 3.2 mm3. The majority of the ACC region is in the left rostral cingulate motor areas (15), with its central voxel located at Talairach coordinates –6, 9, 40 [Brodmann's areas (BA) 32 and 24], close to the regions where conflict-related activation has been frequently reported (2, 14). The majority of the lPFC region was in the inferior dorsolateral and superior ventrolateral prefrontal cortex, with its central voxel located at Talairach coordinates –44, 21, 21 (BA 9, 44, 45, and 46), close to the regions where higher prefrontal activation was reported during memory retrieval than during working memory maintenance (16, 17), as well as during the preparation-induced control activity (2, 10). Fig. 2 presents mean activation changes in the ACC and lPFC. Only correct trials were included in the analyses of imaging results. For each region, the preview and the performance intervals were subjected to a three-way ANOVA with preparation, conflict, and scan as variables.
Fig. 2.
Percent activation charge of the ACC (a) and IPFC (b).
ACC.
Fig. 2 shows that, in the preview interval, the preview condition elicited different responses to conflicts, whereas the no-preview condition hardly produced any ACC responses. For the preparation interval, the preview condition elicited greater activation change compared with the no-preview condition [F(7, 84) = 19.96, MSE = .0127, P < 0.0001]. In contrast, in the performance interval, the preview condition produced no difference for low conflict versus high conflict, whereas the no-preview condition yielded a significant conflict effect [F(5, 60) = 8.14, MSE = .0007, P < 0.0001]. The patterns of ACC activation reveal that the ACC is engaged when processing conflict information, regardless of whether the conflict is anticipated or detected online.
lPFC.
Similar to ACC activation, during the preview interval, lPFC activation revealed conflict effect only in the preview condition, whereas the lPFC hardly responded in this interval in the no-preview condition [F(7, 84) = 2.77, MSE = .004, P < 0.05]. In contrast, during the performance interval, the conflict effect was detected only in the no-preview condition [F(5, 60) = 8.14, MSE = .007, P < 0.0001]. Consistent with ACC activation, the lPFC also responds to conflict information, regardless of whether the conflict is anticipated or detected online.
Behavioral Correlates.
If the conflict effects revealed by the ACC and lPFC during the preview interval truly reflect the level of anticipatory preparation, the subsequent problem solving should be more efficient at higher activations during the preview interval. To test this prediction, we separately median-split trials with low- and high-conflict rules of the preview condition in terms of the total activation change in the ACC and lPFC during the preview interval and compared the subsequent latencies. The subsequent latency was shorter with greater activation changes (704 msec) in the ACC during the preparation interval than with fewer activation changes (751 msec) [t(12) = 2.46, P < 0.05]. The subsequent latency was also faster with greater activation changes (716 msec) in the lPFC during the preparation interval than with fewer activation changes (739 msec) [t(12) = 2.55, P < 0.05].
If the conflict effects revealed by the ACC and lPFC during the performance interval reflect the amount of online adjustment due to conflict detection, the concurrent problem solving should be delayed more when there is higher activation. Trials in the no-preview condition were median-split in terms of the total activation change in the ACC and lPFC during task performance. The concurrent response latencies were longer when ACC activation changes were relatively high (1,619 msec) than when they were relatively low (1,477 msec) [t(12) = 6.77, P < 0.0001]. This pattern did not hold when trials were median-split in terms of lPFC activation changes (P > 0.20). However, the lPFC result seemed to have been driven by two participants who produced a negative activation effect. Excluding these two, the concurrent response latencies were slower when lPFC activation changes were relatively high (1,473 msec) and faster when they were relatively low (1,402 msec) [t(10) = 2.62, P < 0.05].
Discussion
To investigate the processing of conflict, we used a task-cueing paradigm, in which a task cue may or may not provide information about the upcoming task. Participants performed a pseudoarithmetic task in which they associated an input pattern with an output value. When an advance operator cue was available, ACC and lPFC activations during the preview interval were higher with operators that imply higher conflict. Without advance operator information, a similar pattern was observed during the on-task performance interval.
Regarding the ACC, the result that the ACC responded to conflicting response tendencies during the task performance is consistent with the proposal that the ACC is triggered by online detection of response conflict as implied by the conflict-monitoring model. This result is also in line with previous studies (2, 4, 14), in which ACC activation was observed with unexpected response conflict. Perhaps the response-conflict interpretation can be extended to the anticipatory results by a broader application of the conflict-monitoring model. Given the operator in advance, participants may retrieve and rehearse the stimulus–response mappings implied by that operator. Conflict may arise when the operator specifies incompatible stimulus–response mappings. The retrieval and, especially, the maintenance of a high-conflict rule must have increased PFC activation in the preview interval, as we observed. ACC activation during the preview interval may have been a response to the internally represented conflict as a result of memory retrieval.
To some extent, the increased ACC activation in response to the cues for high-conflict rules seems to be consistent with the error-likelihood model (12), which suggests that the ACC is responsible for more general learning of the likelihood of making errors in a specific context and that response conflict is associated with an increased likelihood of errors. As evidence, when the level of conflict was controlled, ACC activation was greater with a higher error probability than with a lower probability (12). In our study, it is possible that, through behavioral practice before the scanning session, participants may have acquired associations between task cues and different levels of error likelihood. However, the empirical evidence for the error-likelihood model has yet to be established in wider varieties of cognitive tasks (18). Moreover, our data do not allow the analysis of ACC activation over a period of initial learning of the error likelihood. Therefore, the extent to which our data lend support to the error-likelihood model is limited to the idea that the ACC is sensitive to internally generated conflict.
ACC activation in response to anticipatory preparation has not always been observed in the current literature. For example, MacDonald and his colleagues (2) used the Stroop paradigm, in which participants are presented with a color word (e.g., RED or GREEN), written in either a congruent or an incongruent color, and are asked to either name the ink color or read the word. Because of extensive practice of reading, the word task is dominant (e.g., shorter latency and higher accuracy than the color task). In their results, ACC activation did not differ when an advance task cue indicated which task was to be performed. However, advance warning was reflected in the dorsolateral prefrontal cortex (dlPFC) activation. In contrast, the current study showed that both the ACC and lPFC did respond to anticipated conflict that correlates with the task difficulty. The critical difference is that in the current study conflict information in terms of response selection may have been more explicit than when a task cue conveyed only task dominance information. In the Stroop task, although the cue for the color task may indicate that stimulus selection for this task would be challenging, the task dominance information per se does not clearly indicate which responses would be in contention for selection. The Boolean rules of the current study, however, are more explicit about which responses should be filtered out in relation to which input patterns. Interestingly in the MacDonald et al. study (2), dlPFC activation during task performance was prominent, whereas its activation did not differ by response conflict. It is possible that participants had already engaged dlPFC after the cue and may not have needed to engage it further while responding to the probe because they were “prepared.”
lPFC activation showed very much the same patterns as the ACC to both the anticipation of conflict as well as and online conflict, and the behavioral consequence of lPFC activation was similar to those of ACC activation. As previously reported (2), both ACC and lPFC activations during the preview interval were associated with the subsequent latency, and both activations during task performance were associated with the concurrent behavior. It has been well established that the lPFC is activated in anticipation of a difficult task (2) or transition (18). Like the current studies, these studies established behavioral ties between advance lPFC activation and the subsequent latency. We obtained the result that ACC activation was tightly related to behavior in both cases. It seems that both lPFC and ACC activations are critical during anticipatory preparation as well as during actual task performance.
In summary, the current study provides evidence that the ACC responds more strongly to a more complex task cue even when there is no response cue, which may reflect a response to anticipation of response conflict. This result extends the previous finding that the ACC is responsible for monitoring for conflict or competition during stimulus processing and response selection (1, 2, 4, 14) and is consistent with the notion that the ACC is responsible for learning the likelihood of making errors (12). Further, our study provides evidence that the ACC may monitor conflict regardless of whether the source is online or anticipatory, and this pattern is similar to that of lPFC activation.
Methods
Behavioral Protocol.
Thirteen right-handed college students (six females) participated for monetary compensation. Before the test, participants provided written informed consent in accordance with the policies of the Institutional Review Board of the University of Pittsburgh.
The E-Prime software package was used to present stimuli and collect behavioral performance. On the practice day, 1 day before fMRI scanning, participants were introduced to six Boolean rules. After mastering them perfectly, they solved 192 Boolean problems (32 problems for each rule), blocked in 24 trials. In the beginning of a trial, an asterisk was presented for 1.5 sec as a warning signal. On half of the trials, the warning signal was followed by the operator symbol of the trial for preview for 0.6 sec. This operator symbol was followed by an 8.4-sec interval before the actual problem was presented. During this preparation interval, participants were encouraged to rehearse the corresponding rule. On the other half of the trials, the preview was not allowed, and a minus sign was presented instead of the operator symbol. With no preview, participants simply waited until the problem was presented. The problem was presented for 3 sec, which was the response window. Answers were either “I”s or “B”s, and participants pressed their right index finger for the “I” response and their right middle finger for the “B” response. If no response was made, the trial was counted as incorrect. There was a 9-sec rest interval for before the next trials began. In the scanning session, participants solved another 192 problems in a scanner with the procedure just described.
Imaging Procedures.
Event-related fMRI data were collected with echo-planar imaging sequence on a Siemens (Malvern, PA) 3T Allegra head-only scanner (1,500 msec return time, 50 msec echo time, 70° flip angle, 20 cm field of view, 26 axial slices/scan with 3.2-mm thickness, 64 × 64 matrix, and with anterior commissure–posterior commissure at the 21st slice from the top). Images were motion corrected and cross-registered to a Montreal Neurological Institute brain by using the 12-parameter rigid body model of automatic image registration nonlinear warping procedure. Functional images were set to a standard mean intensity and smoothed (8-mm full width at half-maximum 3D Gaussian kernel).
Acknowledgments
We thank Matt Botvinick, Angus MacDonald, and Josh Brown for helpful comments on earlier drafts. This work was supported by National Institute of Mental Health Award MH068243 (to J.R.A.).
Abbreviations
- ACC
anterior cingulate cortex
- BA
Brodmann's area
- lPFC
lateral prefrontal cortex.
Footnotes
The authors declare no conflict of interest.
References
- 1.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Science. 2000;288:1835–1837. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 3.Lavie N. J Exp Psych Hum Perception Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- 4.Carter CS, MacDonald AM, Botvinick MM, Ross LL, Stenger VA, Noll D, Cohen JD. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Veen V, Carter CS. NeuroImage. 2005;27:497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Maril A, Wagner AD, Schachter DL. Neuron. 2001;31:653–660. doi: 10.1016/s0896-6273(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JR, Qin Y, Sohn M-H, Stenger VA, Carter CS. Psychonom Bull Rev. 2003;10:241–261. doi: 10.3758/bf03196490. [DOI] [PubMed] [Google Scholar]
- 8.Qin Y, Sohn M-H, Anderson JR, Stenger VA, Fissell K, Goode A, Carter CS. Proc Natl Acad Sci USA. 2003;100:4951–4956. doi: 10.1073/pnas.0431053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn M-H, Goode A, Stenger VA, Carter CS, Anderson JR. Proc Natl Acad Sci USA. 2003;100:7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luks TL, Simpson GV, Feiwell RJ, Miller WL. NeuroImage. 2002;17:792–802. [PubMed] [Google Scholar]
- 11.Holroyd CB, Coles MGH. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 12.Brown JW, Braver TS. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 13.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 14.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 15.Picard N, Strick PL. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 16.Cabeza R, Dolcos F, Graham R, Nyberg L. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- 17.Wagner AD, Maril AM, Bjork RA, Schacter DL. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwenhuis S, Schweizer TS, Mars RB, Botvinick MM, Hajcak G. Cereb Cortex. 2006 Sep 6; doi: 10.1093/cercor/bh1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn M-H, Ursu S, Anderson JR, Stenger VA, Carter CS. Proc Natl Acad Sci USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]