Abstract
Recent fermentation studies have identified actinomycetes of the marine-dwelling genus Salinispora as prolific natural product producers. To further evaluate their biosynthetic potential, we sequenced the 5,183,331-bp S. tropica CNB-440 circular genome and analyzed all identifiable secondary natural product gene clusters. Our analysis shows that S. tropica dedicates a large percentage of its genome (≈9.9%) to natural product assembly, which is greater than previous Streptomyces genome sequences as well as other natural product-producing actinomycetes. The S. tropica genome features polyketide synthase systems of every known formally classified family, nonribosomal peptide synthetases, and several hybrid clusters. Although a few clusters appear to encode molecules previously identified in Streptomyces species, the majority of the 17 biosynthetic loci are novel. Specific chemical information about putative and observed natural product molecules is presented and discussed. In addition, our bioinformatic analysis not only was critical for the structure elucidation of the polyene macrolactam salinilactam A, but its structural analysis aided the genome assembly of the highly repetitive slm loci. This study firmly establishes the genus Salinispora as a rich source of drug-like molecules and importantly reveals the powerful interplay between genomic analysis and traditional natural product isolation studies.
Keywords: genome mining, marine bacteria, natural products, polyketide synthase
Actinomycetes are an exceptionally prolific source of secondary metabolites, accounting for more than half of all microbial antibiotics discovered to date (1). Remarkably, the vast majority of these compounds are derived from the single actinomycete genus Streptomyces, raising the intriguing possibility that additional chemically prolific taxa await discovery. Further incentive to explore actinomycetes as a source of novel secondary metabolites comes from the genome sequences of Streptomyces coelicolor (2) and Streptomyces avermitilis (3), both of which revealed many unanticipated biosynthetic gene clusters, thus demonstrating that even well studied taxa have the potential to yield new metabolites. Such genomic-based information has been used not only to predict the chemical structures of previously unobserved metabolites but also to develop fermentation methods that enhance their production (4–7). Bioinformatics-based approaches to natural product discovery have also been used successfully at the industrial level, where genome scanning has led to the discovery of significant new chemical entities (8, 9). These methods have great potential to eliminate the redundant isolation of previously described compounds while allowing detailed fermentation studies or molecular cloning experiments to be focused on strains that possess a high probability of producing new chemical structures.
Genomics has already been particularly useful to microbial natural products studies because actinomycete secondary metabolites such as polyketides, nonribosomal peptides, and hybrids thereof are often biosynthesized by large, multifunctional synthases that in an assembly line process sequentially assemble small carboxylic acid and amino acid building blocks into their products (10). The biosynthetic genes responsible for the production of these metabolites are almost invariably tightly packaged into operon-like clusters that include regulatory elements and resistance mechanisms (11). In the case of modular polyketide synthase (PKS) and nonribosomal peptide synthetase (NRPS) systems, the repetitive domain structures associated with these megasynthases generally follow a colinearity rule (12) that, when combined with bio informatics and biosynthetic precedence, can be used to predict the chemical structures of new polyketide and peptide-based metabolites.
Marine-derived actinomycetes have become a focus in the search for novel secondary metabolites (13). Among the strains cultured from marine samples is the genus Salinispora, which was recently described as the first seawater-requiring marine actinomycete (14). This genus is widely distributed in tropical and subtropical ocean sediments and currently comprises the two formally described species, Salinispora tropica and Salinispora arenicola, and a third species for which the name Salinispora pacifica has been proposed (15). These actinomycetes are proving to be an exceptionally rich source of structurally diverse secondary metabolites, which are produced in species-specific patterns (16). In the case of S. tropica, the compounds observed to date from this bacterium include the potent proteasome inhibitor salinosporamide A (17), which is currently in phase I human clinical trials for the treatment of cancer; the unprecedented halogenated macrolides sporolides A and B (18); lymphostin, which was observed by scientists at Nereus Pharmaceuticals (San Diego, CA) during salinosporamide A fermentation development (R. Lam, personal communication) and was first reported from a Streptomyces species (19); and salinilactam, the structure of which was solved as part of the present study.
Here we report all identified secondary metabolic biosynthetic gene clusters derived from the complete genome sequence of the marine actinomycete S. tropica strain CNB-440. To our knowledge, this strain is now found to hold the most diverse assemblage of polyketide biosynthetic mechanisms observed in a single organism as well as the largest percentage of a genome devoted to natural product biosynthesis. Bioinformatic analysis was used to facilitate the structure elucidation of the polyene macrolactam salinilactam A. Sequence analyses further revealed that many of these clusters are likely to have been introduced into this genome as a result of recent horizontal gene transfer, which has important implications for the origin of this organism's secondary metabolome.
Results and Discussion
General Features of the Genome and Associated Secondary Metabolome.
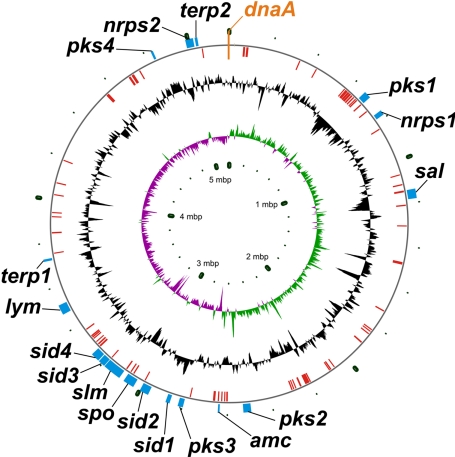
The complete genome sequence of S. tropica strain CNB-440 revealed a single circular chromosome composed of 5,183,331 bp, with no plasmids, and an average G+C content of 69.5% (Fig. 1). S. tropica has 4,536 predicted protein-coding sequences and is similar in size to other actinomycetes that harbor circular chromosomes [Mycobacterium tuberculosis (20), Frankia sp. CcI3 (GenBank accession CP000249), and Nocardia farcinica (21)] yet is substantially smaller than those with linear chromosomes [S. coelicolor (11) and Rhodococcus sp. RHA1 (22)] (Table 1).
Fig. 1.
Circular chromosome of S. tropica CNB-440, oriented to the dnaA gene. The outside outer ring shows the locations of secondary metabolic gene clusters. The inside outer ring shows the locations of putative mobile genetic elements. The center ring shows a normalized plot of GC content (maximum, 75.5%; minimum, 60.3%; average, 69.5%). The inner ring shows a normalized plot of GC skew (maximum, 0.2346; minimum, −0.2504; average, −0.0020).
Table 1.
S. tropica CNB-440 genome data in comparison to other actinomycete natural product producers
| Organism | Size, Mb | Chr. org. | %G+C content | %GDSM | Major NP clusters, n |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PKS |
Mixed PKS/NRPS | NRPS | Non-NRPS siderophore | ||||||||
| Modular type I | Enediyne | Type II | Type III | ||||||||
| S. tropica CNB-440 | 5.18 | C | 69.5 | ≈9.9 | 1 | 2 | 2 | 1 | 4 | 3 | 1 |
| S. coelicolor A3(2) | 8.72 | L | 72 | ≈8 | 2 | — | 2 | 3 | — | 3 | 1 |
| M. tuberculosis H5N1 | 4.41 | C | 67 | ND | 7 | — | 1 | 3* | — | 2 | — |
| Frankiasp. CcI3 | 5.43 | C | 70 | ND | 4 | — | 2 | — | 1 | 3 | 1 |
| N. farcinica IFM 10152 | 6.01 | C | 70 | ND | 4 | — | 1 | 1 | 1 | 7 | — |
NP, nonribosomal peptide; Mb, megabases; Chr. org., chromosome organization; %GDSM, percentage of the genome dedicated to secondary metabolism; C, circular; L, linear; ND, not determined; —, not applicable.
*Two type III PKS enzymes are associated with the modular type I PKSs pks7, pks8, pks9, and pks17.
Biosynthetic gene clusters were initially identified from a draft genome sequence provided by the Joint Genome Institute and checked against a single contiguous sequence provided in advance of public release. Each putative ORF was compared against a representative library of all known PKS and NRPS domains as well as known biosynthetic accessory-type genes [including, but not limited to, cytochrome P450s, terpene synthetases and cyclases, prenyl transferases, methyl transferases, NAD(P)H-dependent oxidoreductases, and CoA/AMP ligases].
These analyses revealed 17 secondary metabolic biosynthesis gene clusters that we predict to be involved in siderophore, melanin, polyketide, nonribosomal peptide, terpenoid, and aminocyclitol production (Table 1). The combined length of these gene clusters is estimated at 518 kb, establishing that, among bacteria sequenced to date, S. tropica devotes the largest percentage of its genome (≈9.9%) to natural product assembly (Table 1). These analyses also confirm that the biosynthetic potential of this strain is considerably greater than that observed by fermentation, as was previously reported in analyses of the S. coelicolor and S. avermitilis genome sequences (2, 23).
The majority of the clusters are concentrated in a single quadrant of the chromosome nearly antipodal to the replication-controlling dnaA gene (Fig. 1). This section of the genome also harbors a significant proportion of the approximate 128 chromosomal mobile genetic elements, such as transposases, integrases, resolvases, and phage-related ORFs, many of which reside in close proximity to secondary metabolic clusters (pks1, sal, spo, amc, sid3, and slm). Several clusters also have strong similarity in ORF and domain organization to existing gene clusters (sid1, sid2, sid4, and pks3), strongly suggesting that many of these clusters may have originated from donor genomes via horizontal gene transfer.
Biosynthetic Capabilities of S. tropica.
The majority of identified S. tropica secondary metabolite gene clusters use carrier protein-based biosynthetic logic in the assembly of their products. Hence, biosynthetic precursors and intermediates are expected to be largely protein-bound during the assembly process and are not likely to cross-talk with other primary or secondary metabolic pathways. S. tropica harbors five 4′-phosphopantetheinyl transferase-encoding genes (24) whose products transfer the 4′-phosphopantetheine moiety from CoA to the approximate 35 conserved serine residues in fatty acid synthase-, PKS-, and NRPS-associated carrier proteins. Three transferases are found in identified carrier protein-containing biosynthetic clusters (Stro0685 with nrps1, Stro2496 with pks3, and Stro2822 with sid4) and thus may be specific to their pathways.
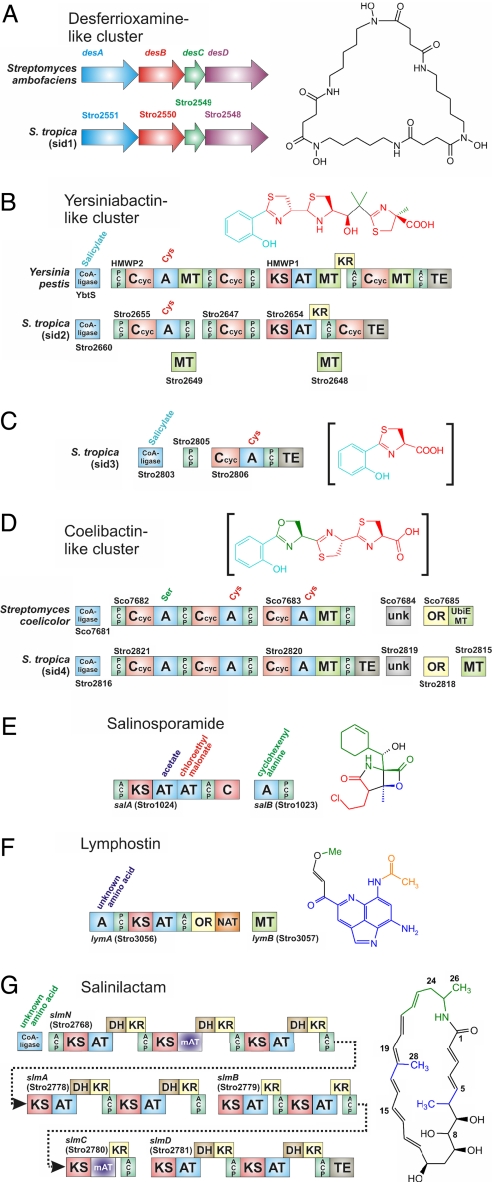
A striking feature of the S. tropica genome is its remarkable diversity of polyketide biosynthetic pathways, which is greater in variety than in other sequenced bacterial genomes (Table 1). Pathways include those for modular type I PKSs (slm), iterative enediyne type I PKSs (spo and pks1), hybrid type I PKS–NRPSs (sal, sid2, sid3, and lym), heterodimeric type II PKSs (pks2 and pks3), and a homodimeric type III PKS (pks4) (Fig. 1). Interestingly, none of the 15 type I PKS-associated modules contains the full set of reductive domains required to completely reduce the transient β-carbonyl group to a saturated methylene carbon during the polyketide elongation process. Hence, S. tropica PKS-derived products are expected to be highly oxidized, as is the case for the characterized metabolites salinosporamide A (sal), sporolide A (spo), lymphostin (lym), and salinilactam A (slm) (Fig. 2).
Fig. 2.
Selected genes from S. tropica modular biosynthetic enzyme systems and their associated natural products. (A–D) Putative siderophore clusters. (A) sid1 cluster in comparison to the desferrioxamine cluster from Streptomyces coelicolor A3 (2). (B) sid2 cluster in comparison to the yersiniabactin cluster from Y. pestis. (C) sid3 cluster with putative product intermediate. (D) sid4 cluster in comparison to proposed S. coelicolor‘coelibactin' cluster (unconfirmed structure). (E–G) Novel modular enzyme systems. (E) salAB from proposed salinosporamide cluster. (F) lymAB from proposed lymphostin cluster. (G) slmNABCD modular PKS system from proposed salinilactam cluster (stereo chemistry proposed, green portion is derived from the proposed lysine-based starter unit). Domain notation: A, adenylation (amino acid substrate noted); C, condensation; Ccyc, condensation with cyclization; PCP, peptidyl carrier protein; KS, β-ketoacyl synthase; AT, acyl-CoA/ACP transacylase (activates malonyl-CoA unless otherwise noted); mAT, methylmalonyl-CoA/ACP transacylase; DH, dehydratase; ACP, acyl carrier protein; TE, thioesterase; OR, oxidoreductase; MT, SAM-dependent methyltransferase; NAT, N-acetyl transferase; unk, unknown.
A primary motivation for the sequencing of the CNB-440 strain of S. tropica was that it is a producer of the potent anticancer agent salinosporamide A (17). Its biosynthetic gene cluster (sal) encodes a 29-ORF, 41-kb hybrid PKS–NRPS pathway (Fig. 2E) that involves mechanisms of chlorination and β-lactone synthesis as well as 20S proteasome resistance (A. S. Eustáquio, L. Beer, D.W.U., and B.S.M., unpublished data). In addition to the sal cluster, S. tropica harbors two additional hybrid PKS–NRPS pathways. The lym cluster is proposed to synthesize the lymphocyte kinase inhibitor lymphostin, which was first isolated from Streptomyces sp. KY11783 (19). We propose that the uniquely organized two-module synthetase LymA (Stro3056) catalyzes a single polyketide extension followed by reductive offloading and N-acetylation of a nonproteinogenic tricyclic planar amino acid derived from tryptophan, with subsequent O-methylation catalyzed by the adjacent SAM-dependent methyl transferase LymB (Stro3057) (Fig. 2F). A set of four genes with homology to a domain of unknown function (DUF611) may play a role in the synthesis of the tryptophan-derived primer unit. The sid2 cluster bears strong domain organization (although lacks direct sequence similarity) to the yersiniabactin cluster (ybt) from Yersinia pestis (25) and other organisms (Fig. 2B). Although no siderophore molecules have yet been isolated from Salinispora species, the notable similarity in domain structure very strongly suggests that the resulting molecule will have the yersiniabactin polyketide/nonribosomal peptide core with possible alterations in sites of methylation and/or oxidation.
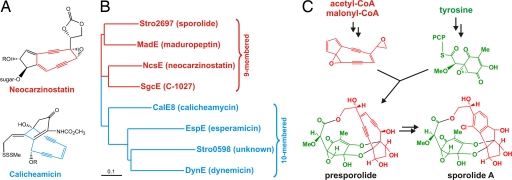
Two independent enediyne PKS biosynthetic gene clusters (spo and pks1) are found in the S. tropica genome. Enediyne natural products, such as the potent DNA cleaving agents calicheamicin, dynemicin, C-1027, and neocarzinostatin are polyketide secondary metabolites produced by members of the order Actinomycetales, many of which (like the genus Salinispora) belong to the family Micromonosporaceae (26). The enediyne polyketide structural motif falls into two classes that possess either nine- or 10-membered rings, and their associated PKSs are phylogenetically distinct. Phylogenetic analysis of the S. tropica enediyne PKSs associated with the spo and pks1 clusters revealed that they are putatively involved in the biosynthesis of distinct nine- and 10-membered enediyne polyketides, respectively (Fig. 3). Although no enediyne natural products have yet been directly characterized from S. tropica, sporolides A and B (18) are putatively derived from a nine-membered enediyne PKS product that is uniquely halogenated with chloride during the aromatization of the enediyne unit to yield the tricyclic hydrocarbon nucleus, as proposed for the related cyanosporasides from “S. pacifica” (27) and recently evaluated synthetically with model substrates (28). The ≈50-kb spo cluster harbors as many as 39 ORFs, which include other conserved enediyne PKS-associated genes, as well as those that encode the postulated biosynthesis and coupling of the sporolide cyclohexenone unit derived from the amino acid tyrosine (Fig. 3). The 10-membered enediyne PKS in gene cluster pks1 also bears the canonical enediyne PKS domain architecture, and further analysis of its 30-kb gene cluster did not reveal accessory genes involved in deoxyhexose biosynthesis and attachment or additional polyketide pathways typically associated with 10-membered enediyne polyketide biosynthesis (i.e., calicheamicin and dynemicin), suggesting that this orphan pathway codes for the biosynthesis of a novel natural product.
Fig. 3.
Enediyne polyketide biochemistry in S. tropica. (A) Representative enediyne structures. (B) Phylogenetic tree of representative enediyne PKSs. GenBank accession nos.: MadE, AAQ17110; NcsE, AAM78012; SgcE, AAL06699; CalE8, AAM94794; EspE, AAP92148; DynE, AAN79725. (C) Proposed biosynthesis of sporolide A.
In addition to the abundance of diverse PKS pathways, S. tropica harbors four NRPS operons (nrps1, nrps2, sid3, and sid4) (Fig. 1). Although the products of the dimodular nrps1 and tetramodular nrps2 gene clusters are unknown and could not be accurately predicted on the basis of bioinformatics alone (29, 30), sid3 and sid4 are homologous to gene sets that encode known siderophore pathway biosynthetic enzymes. When combined with the highly homologous S. coelicolor NRPS-independent siderophore desferrioxamine (31) cluster sid1 (Fig. 2A) and the ybt homologous PKS–NRPS hybrid cluster sid2 (Fig. 2B), a total of four identifiable siderophore-like clusters reside in S. tropica. The three assembly line siderophore synthetases encoded by gene sets sid2–sid4 each have related priming mechanisms involving homologous ATP-dependent aryl-CoA ligases, which typically incorporate aromatic residues, such as salicylate or 2,3-dihydroxybenzoate, into their NRP siderophore products, such as yersiniabactin, enterobactin, and pyochelin (32). The NRPS from the sid4 gene cluster shares unmistakable domain organization with a biosynthetic cluster for an as-yet-unisolated siderophore (“coelibactin”) from S. coelicolor (Fig. 2D) (2). The S. tropica sequence, however, differs from that in S. coelicolor in that it is flanked by an additional transport system typical of siderophore export and iron-bound uptake. The smaller sid3 cluster is adjacent to the sid4 cluster and encodes a highly unusual type II PKS and a single NRPS module that we propose extends and cyclizes an aromatic acid with cysteine, tentatively producing a siderophore-like intermediate (Fig. 2C). It is unclear whether sid3 is fully functional or is instead biosynthetically linked with sid4. Although the latter three gene clusters are traditionally found in γ-proteobacteria, these S. tropica gene clusters bear %G+C values similar to the S. tropica total genome percentage and therefore do not suggest a very recent assimilation, despite similarities to existing clusters.
Genome-Guided Natural Product Discovery of Salinilactam A.
The slm cluster is the largest S. tropica biosynthetic cluster and consists of six genes that encode a 10-module PKS with 49 domains. Because of the highly repetitive nature of the DNA sequence associated with the slm loci and an extremely high level of sequence identity (>99% in many regions), assembly of the cluster and, as a consequence, closure of the genome was problematic. Initial inspection of the partial slm gene cluster from an early draft genome sequence suggested that it coded for a novel lysine-primed polyene macrolactam polyketide devoid of sugar or other appendages often associated with polyketide natural products. A fermentation broth of S. tropica CNB-440 was inspected for compounds with characteristic UV chromophores associated with polyene units, which led to the isolation of a series of polyene macrolactams exemplified by salinilactam A (Fig. 2G). NMR and MS characterization of salinilactam A quickly revealed a polyene macrolactam framework that was consistent only with the slm cluster. Structural features included two isolated polyene fragments, a 1,2,3,5-tetrahydroxy alkane moiety, three methyl groups, and an amide functionality. However, completion of the structure was initially hindered by the chemical instability of the compound and the presence of eight conjugated olefins with similar NMR properties, which impeded their accurate assignment.
Inspection of the structure fragments, together with the molecular formula C28H39NO5, revealed by high-resolution MS, suggested that salinilactam A was derived from a PKS with at least 10 extension modules. This information was useful to help resolve and properly assemble the repetitive DNA sequences associated with the slm PKS into two operons (Stro2778–2781 and Stro2768) separated by nine accessory genes presumed to be involved in starter unit biosynthesis and macrolactam hydroxylation (Fig. 2G). On the basis of the colinearity rule of modular polyketide biosynthesis, the relationship of slm to the terminal region of the vicenistatin PKS (33), and partial NMR-based structural fragments we were able to accurately predict the gross chemical structure of salinilactam A. Initial assignment of the C-28 methyl group in the middle of the hexaene unit at C-18 was complicated by overlapping olefinic NMR signals and was guided by bioinformatics based on the associated methylmalonyl-CoA specific acyl transferase (AT) domain in SlmN (Stro2768) and the absence of β-methylation machinery (34). The natural product would thus be produced by chain extension of the lysine-derived PKS starter unit 5-aminohex-2-enoate with eight malonate and two methylmalonate units, followed by macrolactamization and cytochrome P450 hydroxylation at C-8.
The salinilactam A structure was verified by comprehensive NMR analyses of the purified natural product to confirm the bioinformatics-based total structure assignment. Although the relative and absolute stereochemistry of salinilactam A was not determined spectroscopically, the stereochemistry of the C-7, C-9, and C-11 hydroxyl groups was predicted to be R, S, and R, respectively, on the basis of the strong homology of all slm ketoreductase (KR) domains to other “A-type” KRs (35). The intimate interplay between microbial genomics, biosynthetic logic, and natural product chemistry was critical not only in the structural elucidation of this new chemical entity but also in the final closure of the genome sequence.
Conclusions
There is an ongoing resurgence in natural product drug discovery research (36). This renewed interest is driven by the inherent structural diversity and biological activity associated with secondary metabolites, the low productivity of alternative drug discovery strategies, and the application of improved analytical methods that make it possible to solve structures by using small quantities of material. A relatively new concept that can be added to this list is the molecular genetics of natural product biosynthesis (37). Advances in this field, in combination with increased access to DNA sequencing, are providing a wealth of information about how natural products are assembled, mechanisms by which natural product gene clusters can be manipulated to yield new product diversity, and the genetic potential of individual organisms. Concerning the latter, complete genome sequencing provides unparalleled access to the genes involved in secondary metabolism; the means of their assembly; and, in some cases, what products they may yield (2, 3). Armed with these data, it becomes possible to compare the compounds observed by using traditional fermentation procedures with those predicted from gene sequences, design fermentation methods that may activate or enhance the production of predicted products, and assess the evolutionary history of individual gene clusters (8, 9). The genus Salinispora has become a model organism by which to address questions about species-specific patterns of natural product production (16) and, as described in the present study, the application of whole genome sequencing to the process of natural product discovery and structure elucidation.
Genome sequencing of S. tropica revealed an abundance of novel biosynthetic gene clusters, the majority of which were unexpected on the basis of previous fermentation analyses of this and closely related species and strains. With this information now available, there is a clear need for further genome-guided fermentation studies, because our analysis clearly confirms the value of the Salinispora genus as a source of novel drug-like molecules. In the absence of other Micromonsporacea genome sequences, it is unclear whether this natural product diversity is general to this family or whether the diversity in Salinispora derives from environmental novelty. Sequencing of a related species, Salinispora arenicola CNS-205, already underway, should shed further light on the metabolic capabilities of this marine actinomycete genus and address marine adaptation features in these sediment-dwelling actinomycetes.
Outside of the exploratory aspects of this project, our bioinformatic analysis was of broad practical utility in conjunction with natural product isolation. The isolation of a large polyene molecule led to identification and resolution of a large, repetitive PKS sequence, the analysis of which spurred further refinement and identification of salinilactam A as a polyene. We expect that the genome information will facilitate similar studies in the future, and this result demonstrates the benefits that can result from greater interplay between information technology and natural product structure elucidation. We recently developed a genetics system in S. tropica for rapid gene knockouts via PCR targeting that will further facilitate the genome mining of this metabolically rich bacterium (A. S. Eustáquio, L. Beer, D.W.U., and B.S.M., unpublished data).
Currently, the implications for secondary metabolism due to the circular nature of the S. tropica chromosome are unclear. A mechanism for the biogenesis of novel secondary metabolic clusters in linear chromosomes was recently reported (38) and makes clear that the instability found at of the terminal ends of Streptomyces linear chromosomes has been used to provide rapid evolutionary adaptability to the organism. By contrast, no particular region of the circular S. tropica chromosome seems unstable to the same degree observed in the chromosomal ends of Streptomyces as pseudogenes are largely absent and secondary metabolic clusters and transposable elements are more dispersed throughout the chromosome. This may indicate that Salinispora species will have more frequently acquired their secondary metabolic systems horizontally from other species rather than evolving most themselves. It will be appealing to put this hypothesis to the test when the S. arenicola CNS-205 genome is completed and directly compared with S. tropica CNB-440.
Materials and Methods
Genome Sequencing, Annotation, and Analysis.
Draft sequencing and automated annotation were provided by the Department of Energy, Joint Genome Institute, under the Community Sequencing Program, and completed sequence and annotations have been deposited with GenBank (accession no. CP000667). Automated gene prediction and functional annotation was performed by the Genome Analysis Pipeline (39). Automated prediction of protein-coding genes was based on the output of CRITICA (40) complemented with the output of Glimmer (41). Initial functional annotation was performed by means of similarity searches against the TIGRFAM (42), PRIAM (43), Pfam (44), SMART (45), COGs (46), SwissProt/TrEMBL (47), and KEGG (48) databases by using a set of rules for assigning a specific product description depending on the combination of the search results; product descriptions were further manually refined. Annotated genome sequences are also available at the Microbial Portals (http://genome.ornl.gov/microbial) and through the Integrated Microbial Genomes system (http://img.jgi.doe.gov) (49).
To more specifically identify and categorize natural product biosynthetic gene clusters, we wrote a custom Perl script to sequentially search each translated protein sequence via BLASTP alignment against a hand-constructed and expandable library of model natural product domains and genes. Hits were grouped by physical proximity within the chromosome, and putative clusters were further examined with software available at the National Center for Biotechnology Information web site and visualized with Vector NTI (Invitrogen). Transposons and other mobile genetic element ORFs were identified in a similar manner. Suggested boundaries of all gene clusters discussed are cataloged in Table 2.
Table 2.
S. tropica CNB-440 secondary metabolite biosynthetic gene clusters
| Cluster designation | Actual (*) or predicted product | Type | %G+C | Size, kb | Gene cluster location |
|---|---|---|---|---|---|
| pks1 | 10-membered enediyne | Enediyne PKS | 67 | 30 | Stro0586–0610 |
| pks2 | Glycosylated decaketide | Type II PKS | 69 | 35 | Stro2204–2235 |
| pks3 | Spore pigment | Type II PKS | 71 | 23 | Stro2486–2509 |
| spo | Sporolide* | Enediyne PKS | 68 | 50 | Stro2695–2733 |
| slm | Salinilactam* | Type I PKS | 70 | 80 | Stro2757–2781 |
| pks4 | Aromatic polyketide | Type III PKS | 73 | 10 | Stro4262–4271 |
| nrps1 | Unknown dipeptide | NRPS | 68 | 31 | Stro0672–0699 |
| nrps2 | Reductively offloaded tetrapeptide | NRPS | 70 | 33 | Stro4409–4429 |
| sal | Salinosporamide* | PKS–NRPS | 67 | 41 | Stro1012–1043 |
| lym | Lymphostin* | PKS–NRPS | 71 | 33 | Stro3042–3066 |
| sid1 | Desferrioxamine-like siderophore | NRPS-independent | 72 | 18 | Stro2539–2554 |
| sid2 | Yersiniabactin-like siderophore | PKS–NRPS | 71 | 28 | Stro2636–2660 |
| sid3 | Unknown siderophore | PKS–NRPS | 72 | 30 | Stro2786–2813 |
| sid4 | ″Coelibactin″-like siderophore | NRPS | 70 | 44 | Stro2814–2842 |
| amc | Unknown aminocyclitol | Aminocyclitol | 70 | 8 | Stro2340–2346 |
| terp1 | Unknown terpene | Terpene | 72 | 10 | Stro3244–3253 |
| terp2 | Unknown carotenoid | Terpene | 70 | 12 | Stro4437–4445 |
Salinilactam A Isolation and Characterization.
Strain CNB-440/ΔsalD was cultured in nine 1-liter Fernbach flasks containing A1bFe+C medium [10 g of starch/4 g of yeast extract/2 g of peptone/1 g of CaCO3/5 ml of 8 g/liter Fe2(SO4)3·4H2O/5 ml of 20 g/liter KBr/1 liter of seawater] at 27°C with shaking at 230 rpm. XAD-7 resin (20 g) was added to each flask after 24 h, and the fermentation was continued for another 6 days. The resin was filtered, washed with water, and then extracted with acetone to afford 1.1 g of crude extract, which was fractionated by reversed-phase C18 vacuum liquid chromatography eluting with increasing amounts of methanol in water. The third fraction that eluted with 60% methanol (105 mg), was purified by reversed phase-HPLC (Prep Nova-Pak HR C18; 6 μm; 60 Å 300 mm × 40 mm; flow rate of 10 ml/min; detection at 210 nm; 25% for 10 min then a linear gradient up to 40% CH3CN over 15 min and linear gradient to 100% over another 25 min) to afford salinilactam A [(tR = 25 min, 1.5 mg): light yellow solid; [α]D −28.8 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 250 (4.01), 305 (4.51), 335 (4.26), 355 (4.18) nm; IR (NaCl) λmax 3,464, 2,930, 1,739, 1,547, 1,414, 1,279, 1,135, 1,074, 998, and 743 cm−1; 1H NMR (500 MHz, CD3OD) δ 7.02 (1H, dd, J = 15.6, 10.7 Hz, H-3), 6.76 (1H, d, J = 14.6 Hz, H-17), 6.51 (1H, dd, J = 15.0, 10.2 Hz, H-20), 6.49 (1H, dd, J = 14.8, 10.3 Hz, H-14), 6.30 (1H, dd, J = 15.0, 10.2 Hz, H-16), 6.29 (1H, dd, J = 14.8, 10.2 Hz, H-15), 6.27 (1H, dd, J = 14.9, 10.5 Hz, H-13), 6.25 (1H, dd, J = 15.1, 10.7 Hz, H-4), 6.10 (1H, dd, J = 14.6, 10.5 Hz, H-21), 6.05 (1H, dd, J = 15.0, 10.0 Hz, H-22), 6.02 (1H, dd, J = 15.1, 10.1 Hz, H-5), 5.95 (1H, d, J = 15.1 Hz, H-19), 5.87 (1H, d, J = 15.2 Hz, H-2), 5.77 (1H, dd, J = 14.8, 10.5 Hz, H-12), 5.67 (1H, ddd, J = 15.0, 10.1, 7.0 Hz, H-23), 4.36 (1H, dd, J = 11.2, 4.1 Hz, H-11), 3.97 (1H, ddd, J = 11.2, 6.8, 4.1 Hz, H-9), 3.86 (1H, m, H-25), 3.60 (1H, dd, J = 7.7, 4.1 Hz, H-8), 3.50 (1H, dd, J = 7.7, 5.0 Hz, H-7), 2.59 (1H, m, H-6), 2.33 (1H, m, H-24a), 2.19 (1H, ddd, J = 14.1, 7.0, 6.5 Hz, H-24b), 1.98 (1H, m, H-10a), 1.84 (3H, s, H3-28), 1.78 (1H, dd, J = 14.8, 6.8 Hz, H-10b), 1.27 (3H, d, J = 7.1 Hz, H3-26), 1.08 (3H, d, J = 7.2 Hz, H3-27) [coupling constants derived from analysis of homo-J-resolved 1H NMR spectral data]; 13C NMR (125 MHz, CD3OD) δ 168.2 (C-1), 142.1 (C-5), 139.4 (C-3), 138.9 (C-20), 136.9 (C-12), 134.4 (C-22), 133.1 (C-18), 131.2 (C-15), 130.5 (C-19), 130.3 (C-23), 129.6 (C-17), 129.4 (C-21), 128.9 (C-16), 128.8 (C-14), 128.7 (C-4), 128.6 (C-13), 123.1 (C-3), 75.6 (C-7), 75.2 (C-8), 71.3 (C-11), 69.3 (C-9), 46.7 (C-25), 39.5 (C-10), 39.4 (C-24), 38.7 (C-6), 19.7 (C-25), 19.4 (C-28), 13.8 (C-27); ESIMS m/z 452 (M – H2O + H), 470 (M + H), 492 (M + Na), 961 (2M + Na); HRESITOFMS m/z 492.2720 [calculated for C28H39NO5Na+, 492.2726].
Acknowledgments
We thank G. L. Challis (University of Warwick) and R. P. McGlinchey for valuable discussions, R. Lam and G. Tsueng (Nereus Pharmaceuticals) for providing the initial material that was used for the preliminary isolation and structural elucidation of salinilactam A, D. C. Oh for preliminary characterization of salinilactam A, and A. S. Eustáquio (University of California at San Diego) for strain CNB-440/ΔsalD. This work was supported by the National Oceanic and Atmospheric Administration through Oceans and Human Health Initiative Grant NA05NOS4781249 (to B.S.M. and P.R.J.) and National Institutes of Health Grants R01 CA127622 (to B.S.M.) and R37 CA44848 (to W.F.). Genome sequencing was provided through a grant from the Community Sequencing Program of the Joint Genome Institute (to P.R.J. and B.S.M.).
Abbreviations
- KR
ketoreductase
- NPRS
nonribosomal peptide synthetase
- PKS
polyketide synthase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence described in this paper has been deposited in the GenBank database (accession no. CP000667).
References
- 1.Berdy J. J Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 2.Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, et al. Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 3.Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, et al. Proc Natl Acad Sci USA. 2001;98:12215–12220. doi: 10.1073/pnas.211433198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lautru S, Deeth RJ, Bailey LM, Challis GL. Nat Chem Biol. 2005;1:244–245. [Google Scholar]
- 5.Song L, Barona-Gomez F, Corre C, Xiang L, Udwary DW, Austin MB, Noel JP, Moore BS, Challis GL. J Am Chem Soc. 2006;128:14754–14755. doi: 10.1021/ja065247w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bok JW, Hoffmeister D, Maggio-Hall LA, Murillo R, Glasner JD, Keller NP. Chem Biol. 2006;13:31–37. doi: 10.1016/j.chembiol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Gross H, Stockwell VO, Henkels MD, Nowak-Thompson B, Loper JE, Gerwick WH. Chem Biol. 2007;14:53–63. doi: 10.1016/j.chembiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Zazopoulos E, Huang K, Staffa A, Liu W, Bachmann BO, Nonaka K, Ahlert J, Thorson JS, Shen B, Farnet CM. Nat Biotechnol. 2003;21:187–190. doi: 10.1038/nbt784. [DOI] [PubMed] [Google Scholar]
- 9.McAlpine JB, Bachmann BO, Piraee M, Tremblay S, Alarco AM, Zazopoulos E, Farnet CM. J Nat Prod. 2005;68:493–496. doi: 10.1021/np0401664. [DOI] [PubMed] [Google Scholar]
- 10.Fischbach MA, Walsh CT. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 11.Martin JF. J Ind Microbiol. 1992;9:73–90. doi: 10.1007/BF01569737. [DOI] [PubMed] [Google Scholar]
- 12.Staunton J, Weissman KJ. Nat Prod Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- 13.Fenical W, Jensen PR. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado L, Fenical W, Goodfellow M, Jensen PR, Ward AC. Int J System Appl Microbiol. 2005;55:1759–1766. doi: 10.1099/ijs.0.63625-0. [DOI] [PubMed] [Google Scholar]
- 15.Jensen PR, Mafnas C. Environ Microbiol. 2006;8:1881–1888. doi: 10.1111/j.1462-2920.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- 16.Jensen PR, Williams PG, Oh D-C, Zeigler L, Fenical W. Appl Environ Microbiol. 2007;73:1146–1152. doi: 10.1128/AEM.01891-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Angew Chem Int Ed. 2003;115:369–371. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan GO, Williams PG, Feling RH, Kauffman CA, Jensen PR, Fenical W. Org Lett. 2005;7:2731–2734. doi: 10.1021/ol050901i. [DOI] [PubMed] [Google Scholar]
- 19.Aotani Y, Nagata H, Yoshida M. J Antibiot. 1997;50:543–545. doi: 10.7164/antibiotics.50.543. [DOI] [PubMed] [Google Scholar]
- 20.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, et al. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa J, Yamashita A, Mikami Y, Hoshino Y, Kurita H, Hotta K, Shiba T, Hattori M. Proc Natl Acad Sci USA. 2004;101:14925–14930. doi: 10.1073/pnas.0406410101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLeod MP, Warren RL, Hsiano WWL, Araki N, Myhre M, Fernandes C, Miyazawa D, Wong W, Lillquist AL, Wang D, et al. Proc Natl Acad Sci USA. 2006;103:15582–15587. doi: 10.1073/pnas.0607048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, et al. Proc Natl Acad Sci USA. 2001;98:12215–12220. doi: 10.1073/pnas.211433198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT. Chem Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 25.Gehring AM, DeMoll E, Fetherston JD, Mori I, Mayhew GF, Blattner FR, Walsh CT, Perry RD. Chem Biol. 1998;5:573–586. doi: 10.1016/s1074-5521(98)90115-6. [DOI] [PubMed] [Google Scholar]
- 26.Shen B, Liu W, Nonaka K. Curr Med Chem. 2003;10:2317–2325. doi: 10.2174/0929867033456701. [DOI] [PubMed] [Google Scholar]
- 27.Oh DC, Williams PG, Kauffman CA, Jensen PR, Fenical W. Org Lett. 2006;8:1021–1024. doi: 10.1021/ol052686b. [DOI] [PubMed] [Google Scholar]
- 28.Perrin CL, Rodgers BL, O'Connor JM. J Am Chem Soc. 2007;129:4795–4799. doi: 10.1021/ja070023e. [DOI] [PubMed] [Google Scholar]
- 29.Stachelhaus T, Mootz HD, Marahiel MA. Chem Biol. 1999;6:493–505. doi: 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 30.Challis GL, Ravel J, Townsend CA. Chem Biol. 2000;7:211–224. doi: 10.1016/s1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 31.Barona-Gomez F, Wong U, Giannakopulos AE, Derrick PJ, Challis GL. J Am Chem Soc. 2004;126:16282–16283. doi: 10.1021/ja045774k. [DOI] [PubMed] [Google Scholar]
- 32.Crosa JH, Walsh CT. Microbiol Mol Biol Rev. 2002;66:223–249. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogasawara Y, Katayama K, Minami A, Otsuka M, Eguchi T, Kakinuma K. Chem Biol. 2004;11:79–86. doi: 10.1016/j.chembiol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Calderone CT, Kowtoniuk WE, Kelleher NL, Walsh CT, Dorrestein PC. Proc Natl Acad Sci USA. 2006;103:8977–8982. doi: 10.1073/pnas.0603148103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siskos AP, Baerga-Ortiz A, Bali S, Stein V, Mamdani H, Spiteller D, Popovic B, Spencer JB, Staunton J, Weissman KJ, et al. Chem Biol. 2005;12:1145–1153. doi: 10.1016/j.chembiol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Clardy J, Walsh C. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 37.Fischbach MA, Walsh CT. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 38.Choulet F, Aigle B, Gallois A, Mangenot S, Gerbaud C, Truong C, Francou FX, Fourrier C, Guerineau M, Decaris B, et al. Mol Biol Evol. 2006;23:2361–2369. doi: 10.1093/molbev/msl108. [DOI] [PubMed] [Google Scholar]
- 39.Hauser L, Larimer F, Land M, Shah M, Uberbacher E. Gen Engineer. 2004;26:225–238. doi: 10.1007/978-0-306-48573-2_11. [DOI] [PubMed] [Google Scholar]
- 40.Badger JH, Olsen GJ. Mol Biol Evol. 1999;16:512–524. doi: 10.1093/oxfordjournals.molbev.a026133. [DOI] [PubMed] [Google Scholar]
- 41.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Nucleic Acids Res. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haft DH, Loftus BJ, Richardson DL, Yang F, Eisen JA, Paulsen IT, White O. Nucleic Acids Res. 2001;29:41–43. doi: 10.1093/nar/29.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claudel-Renard C, Chevalet C, Faraut T, Kahn D. Nucleic Acids Res. 2003;31:6633–6639. doi: 10.1093/nar/gkg847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL. Nucleic Acids Res. 2002;30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P. Nucleic Acids Res. 2006;34:D257–D260. doi: 10.1093/nar/gkj079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatusov RL, Galperin MY, Natale DA, Koonin EV. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O'Donovan C, Phan I, et al. Nucleic Acids Res. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markowitz VM, Korzeniewski F, Palaniappan K, Szeto E, Werner G, Padki A, Zhao X, Dubchak I, Hugenholtz P, Anderson I, et al. Nucleic Acids Res. 2006;34:D344–D348. doi: 10.1093/nar/gkj024. [DOI] [PMC free article] [PubMed] [Google Scholar]