Abstract
Fission yeast Cdc42p, a small GTPase of the Rho family, is essential for cell proliferation and maintenance of the rod-like cell morphology. Scd1/Ral1p is a GDP-GTP exchange factor (GEF) for Cdc42p. This study and a parallel study by others establish that Gef1p is another GEF for Cdc42p. Deletions of gef1 and scd1 are synthetically lethal, generating round dead cells, and hence mimic the phenotype of cdc42 deletion. Gef1p is localized mainly to the cell division site. Scd1p is also there, but it is also detectable in other parts of the cell, including the nucleus, growing ends, and the tips of conjugation tubes. Gef1p and Scd1p form a ring structure at the cell division site, which shrinks during cytokinesis following the contraction of the actomyosin ring. Formation of the Gef1p/Scd1p ring apparently depends on the integrity of the actomyosin ring. In turn, recruitment of Cdc42p to the cell division site follows the shrinking Gef1p/Scd1p ring; the Cdc42p accumulates like a closing iris. These observations suggest that Gef1p and Scd1p may have a role in mediating between contraction of the actomyosin ring and formation of the septum, by recruiting active Cdc42p to the septation site.
INTRODUCTION
Cdc42p, a Rho-family GTPase expressed ubiquitously in eukaryotes, is known to play a crucial role in establishment of the cell polarity (Johnson, 1999). It acts as a binary switch, being active in the GTP-bound state and inactive in the GDP-bound state. Conversion between these two states is regulated by two accessory factors. One is the GDP-GTP exchange factor (GEF), which activates Cdc42p via an exchange of GDP with GTP. The other is the GTPase activating protein (GAP), which enhances the intrinsic GTPase activity of Cdc42p, causing its inactivation (Narumiya, 1996).
In the fission yeast Schizosaccharomyces pombe, Cdc42p is essential for cell proliferation and also for maintenance of the rod-like cell morphology (Miller and Johnson, 1994). Cdc42p is localized to the medial region of the cell in early cytokinesis and remains at the cell-division site until cell separation; it is also localized to the cell periphery and internal membranes (Merla and Johnson, 2000). Fission yeast Cdc42p is activated by a GEF called Scd1p or Ral1p (Chang et al., 1994; Fukui and Yamamoto, 1988), which is activated in turn by the single Ras homologue in this organism, Ras1p (Chang et al., 1994). Scd1p has been shown to localize in the nucleus, spindle, cell ends, and septum, and it has been suggested to be essential for the proper formation of the spindle (Li et al., 2000). Scd1p has been the only known Cdc42p GEF, but the presence of another Cdc42p GEF has been suspected, because deletion of cdc42 results in inviability in addition to deformed cell morphology (Miller and Johnson, 1994), whereas the scd1Δ mutant is misshapen and mating-defective but fully viable (Chang et al., 1994; Fukui and Yamamoto, 1988).
Through this study and an independent study by Coll and colleagues (Coll et al., 2003), it is now clear that Gef1p is a second GEF for Cdc42p in fission yeast. In this article, we first describe briefly our identification and characterization of Gef1p and our comparison of its function with that of Scd1p. We then demonstrate an unexpected behavior of Gef1p and Scd1p, that is, their formation of a ring structure that shrinks during cytokinesis
MATERIALS AND METHODS
Fission Yeast Strains, Genetic Methods, and Media
S. pombe strains used in this study are listed in Table 1. General genetic procedures for S. pombe were carried out as described by Gutz et al. (1974). Complete medium YE and minimal medium SD (Sherman et al., 1986) were used for the routine culture of S. pombe. Sporulation medium SPA (Gutz et al., 1974) was used to observe the conjugation process under the microscope. Minimal medium MM (Moreno et al., 1990) was used to induce transcription from the nmt1 promoter. Transformation of S. pombe cells was done by the lithium acetate method (Okazaki et al., 1990). To generate diploid strains from mating-defective scd1 haploid strains, we used a cell fusion technique as described previously (Sipiczki and Ferenczy, 1977), with minor modifications (Matsuyama et al., 2000).
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| JY450 | h90 ade6-M216 leu1 | Lab stock (M.Y.) |
| JY803 | h90 ade6-M210 leu1 ura4-D18 scd1::LEU2 | See text |
| JX124 | h90 ade6-M216 leu1 ura4-D18 scd1::ura4+ | See text |
| JX348 | h90 ade6-M216 leu1 ura4-D18 gef1::ura4+ | See text |
| JX349 | h90 ade6-M210 leu1 ura4-D18 gef1::ura4+ | See text |
| JX351 | h90/h90 ade6-M216/ade6-M210 leu1/leu1 ura4-D18/ura4-D18 gef1::ura4+/gef1+ scd1::ura4+/scd1+ | JX124 X JX349 |
| JZ175 | h90 ade6-M216 leu1 cdc3-6 | M. Balasubramanian et al. (1994) |
| JZ489 | h90/h90 ade6-M216/ade6-M210 leu1/leu1 ura4-D18/ura4-D18 | Lab stock (M.Y.) |
| K40 | h+ ade6-469 leu1 ura4-D18 | Lab stock (K.O.) |
| K193 | h- ura4-D18 leu1 | Lab stock (K.O.) |
| D11 | h+/h- ade6-M469/ade6+ his3-D1/his3+ leu1/leu1 ura4-D18/ura4-D18 gef1::KanR/gef1+ scd1::KanR/scd1+ | See text |
| D6A | h90 ade6 leu1 ura4-D18 gef1::ura4+ scd1::ura4+ (pREP3-cdc42-G12V) | See text |
Cloning and Sequencing of gef1
The original gef1 clone was isolated as a high-copy-number suppressor of the map3-dn9 strain. This strain carries a mutation in the map3 gene, which encodes the M-factor receptor, and hence is defective in mating (Hirota et al., 2001). The cDNA clone, termed FT4–17, appeared to lack the first methionine codon. To obtain a full-length gef1 cDNA, the missing 5′-terminal region was isolated by 5′-RACE, using a kit from Clontech (Palo Alto, CA) and following the manufacturer's instructions. We used 1 μg polyadenylated RNA prepared from S. pombe cells and gef1-specific primers. Products of the nested PCR were gel-purified (Geneclean II; Q-biogene, Carlsbad, CA) and cloned into pBluescript SK+ (Stratagene, LaJolla, CA). A genomic clone that covered the entire gef1 ORF and the 2.5-kb 5′-flanking region was isolated from a pDB248′-based library (Maeda et al., 1994) using FT4–17 as a probe. DNA sequences of the cDNA and genomic clones were determined using a DNA sequencer model 4000L (LI-COR, Lincoln, NE). Comparison of the sequences confirmed the presence of an intron between codons 14 and 15, as predicted by the fission yeast genome sequence project at the Sanger Centre (Wood et al., 2002).
Plasmids
We constructed a full-length gef1 cDNA by connecting FT4–17 and the 5′-terminal region, obtained as described above at the SalI site at gef1 codon 372, and used it as the template for further PCR amplification. Full-length cDNAs for scd1 and cdc42 were amplified by PCR using an S. pombe cDNA library (Iino and Yamamoto, 1997) as the template. The following sets of primers were used in PCR: 1.
gef1–5Nde: GTTTTCCATATGGAGACTTTAAAAGCAGATC and gef1–3BH: GTTGCGGATCCACCCTCGCAGCTAAAGAGACAC
scd1–5Sal: CCGTCGACATGAAACTTAGGCTTTTACAG and scd1–3BglII: CCAGATCTAAATAAACAACAACGTGCAAAG
cdc42–5BglII: CCAGATCTTATGCCCACCATTAAGTGTG and cdc42–3′: GGGTTACAGTACCAAACACTTTG
cdc42–5Nde: ATCATATGCCCACCATTAAGTGTG and cdc42–3BH: GGGGATCCAGTACCAAACACTTTGAC
Each PCR-amplified fragment was cloned into vector pCR2.1-TOPO (Invitrogen, Carlsbad, CA) using the procedure termed “TOPO TA Cloning” by the manufacturer. The resultant plasmids were respectively named pCR2.1-GEF, pCR2.1-SCD, pCR2.1-CDC42–1, and pCR2.1-CDC42–2. They were sequenced to confirm that no mutations had been introduced during the isolation process. Using these primers, the termination codons in pCR2.1-GEF and pCR2.1-CDC42–2 were replaced by the BamHI site, and that in pCR2.1-SCD was replaced by the BglII site, so that the gene products could be fused with a tag at their C terminus. Similarly, a BglII site that could be used for tagging was generated just upstream of the first methionine codon in pCR2.1-CDC42–1.
All the plasmids used for tagging target proteins with GFP, CFP, or YFP and expressing the resultant fusion proteins were derived from pREP41 or pREP81, which contain the attenuated nmt1 promoter of medium (pREP41) or weak (pREP81) strength (Maundrell, 1993; Basi et al., 1993), or from pREP42, which is similar to pREP41, except that it carries ura4+ instead of LEU2 as selective maker for transformation. pRGT41, which is a derivative of pREP41-based pGFT41 (Yamashita et al., 1998) that carries no NdeI site in the GFP ORF (A. Matsuyama and M.Yamamoto, unpublished results), was used to attach GFP to the C-terminus of gef1 and cdc42, using the NdeI-BamHI fragments from pCR2.1-GEF and pCR2.1-CDC42–2. Two modified versions of pRGT41, namely pRCT41 and pRYT41 (K.Hirota and M.Yamamoto, unpublished results), were used to attach CFP and YFP, respectively. Similarly, the SalI-BglII fragment from pCR2.1-SCD was cloned in pRGT41 to generate pREP41-scd1-GFP. pREP81-GFP-myo2 was described previously as pGFT81-myo2 (Kitayama et al., 1997), and pREP42-GFP-cdc4 was provided by M. Toya (EMBL, Heidelberg, Germany). The YFP-(Ala8) construct was used to tag the N-terminus of Cdc42p as described by Merla and Johnson (2000), using the BglII-EcoRV fragment from pCR2.1-CDC42–1 and vector pREP81. The levels of expression of the fusion proteins were controlled by the strength of the nmt1 promoter and the amount of thiamine in the medium.
To construct a plasmid expressing gef1-Flag, the NdeI-BamHI fragment from pCR2.1-GEF was fused in-frame to the Flag ORF in pREP42F (H. Tanaka and M.Yamamoto, unpublished results), a derivative of pREP42 that carries the Flag sequence at the multicloning site. To construct a plasmid expressing cdc42-HA, the NdeI-BamHI fragment from pCR2.1-CDC42–2 was fused in-frame to the 3-HA ORF in pREP41H (H. Tanaka and M.Yamamoto, unpublished results), a derivative of pREP41 that carries the 3-HA sequence at the multicloning site. The resultant plasmids were named pREP42-gef1F and pREP41-cdc42H, respectively.
Disruption of gef1 and scd1
A 0.5-kb EcoRI fragment (codons 204–367) of the cloned gef1 cDNA FT4–17 was replaced by a 1.8-kb S. pombe ura4+ cassette (Grimm et al., 1988). A HindIII fragment carrying the disrupted gene was used to transform diploid ura4 strain JZ489. Stable Ura+ transformants were selected, and proper replacement of a wild-type gef1 allele with the disrupted construct was confirmed by PCR and Southern blot analysis. Disruption of gef1 was also carried out by insertion of a KanR cassette (Bähler et al., 1998) at the SalI site (codon 372) within the conserved GEF domain in pCR2.1-GEF. A XhoI-BamHI fragment carrying the disrupted ORF was used to transform the heterothallic haploid strain K40. Geneticin (G418)-resistant transformants were selected, and disruption of gef1 was confirmed by PCR using an appropriate set of primers.
Three types of scd1 disruption alleles were used in this study. One of these carried an S. cerevisiae LEU2 cassette at the HindIII site (codon 808) within the scd1 ORF (Y. Fukui and M.Y., unpublished results). The second was constructed by replacing a 1.2-kb BclI fragment within the ORF (codons 406–792) with a 1.8-kb S. pombe ura4+ cassette (C. Kitayama and M.Yamamoto., unpublished results). The third was constructed by inserting a KanR cassette (Bähler et al., 1998) at the SnaBI site (codon 589) within the scd1 ORF.
To confirm the presence of the disrupted gef1 and scd1 alleles in the putative double mutant D6A (gef1::ura4+ scd1::ura4+), a 0.5-kb EcoT14I fragment in gef1 and a 0.7-kb XhoI-SacI fragment in the scd1 5′ flanking region were used as the probes in Southern blotting. Genomic DNA isolated from either D6A or the wild-type strain JY450 was digested by HincII for analysis of gef1 or by EcoRI/EcoRV for analysis of scd1; these enzymes cut neither the ura4+ cassette nor the fragments that had been replaced. The disruptions of gef1 and scd1 yielded band shifts from 1.0 to 2.3 kb and from 2.0 to 2.6 kb, respectively, as expected from the structures of the replacements.
Yeast Two-Hybrid Interaction Assay
We performed yeast two-hybrid assays essentially as described by Durfee et al. (1993). To create a restriction fragment carrying the entire gef1 ORF with the termination codon, plasmids pCR2.1-GEF and FT4–17 (see above) were fused at their SalI sites. Then the gef1 ORF carried on a 2.4-kb NdeI-SmaI fragment was fused in-frame to the GAL4-DNA binding domain in pAS2. The cdc42 ORF was amplified by PCR so that it was carried on a 0.6-kb BamHI-EcoRI fragment and was then fused in-frame to the GAL4-activation domain in pACT2. The ORFs of ras1, scd1, and scd2 were also fused in-frame to the GAL4-activation domain in pACT2 after amplification by PCR based on their previously determined sequences (Fukui et al., 1986; Fukui and Yamamoto, 1988; Wood et al., 2002). We used the commercially available (Clontech) combination of pVA3 (pGBT9-p53(mouse)) and pTD1 (pGAD3F-largeT-antigen(SV40)) as a control that exhibits positive interaction in the two-hybrid system. S. cerevisiae strain Y190, which carries the GAL4-recognition sequence and the reporter genes lacZ and HIS3, was transformed with various combinations of plasmids. The β-galactosidase activity was assayed by the color reaction, and expression of the HIS3 reporter gene was examined by growth of the host on a -His plate containing 40 mM 3-aminotriazole.
Immunoprecipitation
For Gef1p-Cdc42p immunoprecipitation experiments, we expressed Gef1p-Flag and Cdc42p-HA in S. pombe using strain K193 transformed with plasmids pREP42-gef1F and pREP41-cdc42H. We performed immunoprecipitation experiments using extracts of the transformed and control strains essentially as described previously (Shinozaki-Yabana et al., 2000). Briefly, cells were harvested and washed once with STOP buffer (150 mM NaCl, 50 mM NaF, 10 mM EDTA, 1 mM NaN3 [pH 8.0]). Then 100 μl of HBIP buffer (25 mM morpholinepropane sulfonic acid [MOPS], 5 mM EGTA, 15 mM MgCl2, 50 mM β-glycerophosphate, 15 mM p-nitrophenylphosphate, 1 mM dithiothreitol, 0.1 mM sodium vanadate, 10% glycerol, 150 mM KCl, 1 mM Pefabloc SC [Roche Diagnostics, Indianapolis, IN], 40 μg of Aprotinin per ml, 20 μg of leupeptin per ml, 1 protease inhibitor cocktail tablet [Complete Mini; Roche Diagnostics] per ml) was added to the cell pellet. Cells were resuspended and then disrupted by glass beads, and 400 μl of HBIP buffer containing 2 mg/ml the nondenaturing detergent n-dodecyl-β-d-maltoside (Yesilaltay and Jenness, 2000) was added to the cell extract. Proteins in the membrane fraction were then solubilized by keeping the cell extract on ice for 2 h. After centrifugation, immunoprecipitation was performed by incubating the supernatant on ice for 40 min with mouse anti-Flag mAb M2 (Sigma, St. Louis, MO) and with 100 μl Dynabeads protein G (DYNAL, Oslo, Norway).
Fluorescence Microscopy and Inhibition Studies
Fluorescence images of living cells were taken by a cooled CCD camera (Hamamatsu Photonics, Hamamatsu, Japan) attached to a Zeiss Axiophot microscope and stored digitally using the Fish Imaging Software program (Hamamatsu Photonics). For time-lapse recording, live cells of strain K193 expressing Gef1p-YFP, Gef1p-CFP, Scd1p-GFP, YFP-A8-Cdc42p, GFP-Cdc4p, or GFP-Myo2p were observed under the fluorescence microscope (Olympus IX71). The images were taken by a cooled CCD camera (Hamamatsu Photonics) and stored digitally using MetaMorph software (Universal Imaging, Downingtown, PA). For three-dimensional deconvolution microscopy (Figures 3B and 5B), the image of each focal plane was taken and stored digitally as described above, and each image was cleaned by removing blurs from other focal planes using the Auto-Deblur program (Datacell, Finchampstead, UK). 3-D pictures were reconstructed from these cleaned images using the MetaMorph software.
Figure 3.
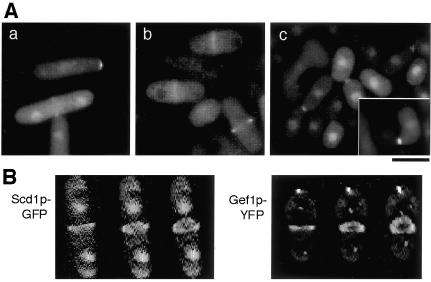
Subcellular localization of Scd1p and Gef1p. (A) Fluorescence microscopy of scd1Δ strain JX124 carrying pREP41-scd1-GFP (a and c) and of gef1Δ strain JX348 carrying pREP41-gef1-GFP (b). (a and b) Cells growing mitotically in SD medium at 30°C. (c) Cells spotted on SPA sporulation medium supplemented with 2 μM thiamine and incubated for 4 h at 30°C. Bar, 10 μm. (B) Reconstructed 3-D images (see MATERIALS AND METHODS) of Scd1p-GFP and Gef1p-YFP ring structures in strains JX124 [pREP41-scd1-GFP] and JX348 [pREP41-gef1-GFP]. Three gradually tilted images of a cell at an early stage of septation are shown for each strain.
Figure 5.
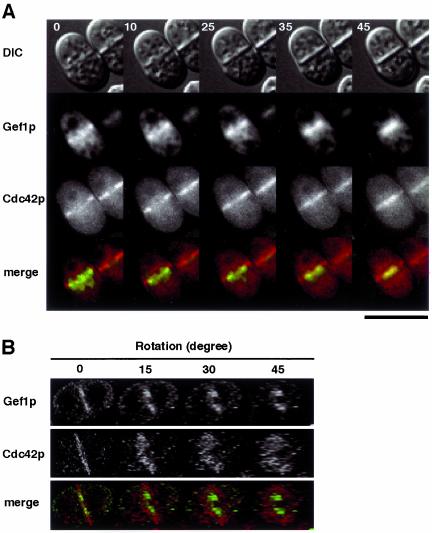
Images of single K193 cells expressing both Gef1p-CFP and YFP-A8-Cdc42p. K193 cells transformed with both pREP42-gef1-CFP and pREP81-YFP-(Ala8)-cdc42 were cultured in liquid MM and analyzed as described in Figure 4. Green represents Gef1p-CFP and red represents YFP-A8-Cdc42p. The region of overlap appears yellow. (A) Time-lapse images (times in minutes). (B) Reconstructed and rotated 3-D images of a cell undergoing septation. Bar, 10 μm.
F-actin was visualized by staining with BODIPY-FL-phallacidin (Molecular Probes, Eugene, OR). To disintegrate F-actin, latrunculin-A (Wako Pure Chemical, Osaka, Japan), dissolved in DMSO at 20 mM, was added to the S. pombe culture to a final concentration of 10 μM (Petersen et al., 1998). Septa were visualized by staining with Calcofluor White (Sigma).
RESULTS
Identification of gef1
We originally isolated gef1 from an S. pombe cDNA library as a weak high-copy-number suppressor of a map3 mutant defective in the M-factor receptor, as described in MATERIALS AND METHODS. However, subsequent analysis indicated that the major function of Gef1p was not pertinent to mating (see below). The original clone FT4–17 was found to contain a truncated ORF, which corresponded to SPAC24H6.09 denoted by the S. pombe genome project (Wood et al., 2002).
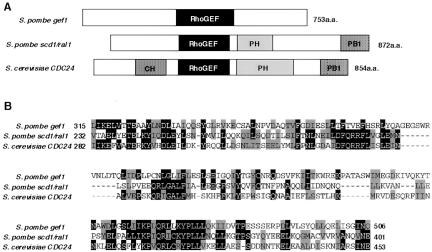
The deduced product of SPAC24H6.09, which we call Gef1p hereafter, consists of 753 amino acids and carries a conserved GEF domain. Comparison of Gef1p with Scd1/Ral1p and the S. cerevisiae GEF Cdc24p is shown schematically in Figure 1. As the protein product expressed from FT4–17 would lack the N-terminal 198 amino acid residues, we isolated a full-length gef1 cDNA, as described in MATERIALS AND METHODS. This clone turned out to be less effective than FT4–17 in suppressing the mating defect of the map3 mutant. Overexpression of the full-length gef1 cDNA converted cells to spherical morphology (our unpublished results), as has been reported for the mutational activation of cdc42 (cdc42-G12V) (Miller and Johnson, 1994). Interestingly, this morphological effect of overexpression was again greater with the 5′-truncated clone FT4–17 than with the full-length clone, especially under conditions of nutrient starvation (our unpublished results), implying that the N-terminal region of Gef1p may have a role in regulating the activity of Gef1p.
Figure 1.
Sequence comparison of Gef1p with Scd1/Ral1p and S. cerevisiae Cdc24p. (A) Schematic illustration of structural features analyzed by the SMART program (Letunic et al., 2002; http://smart.embl-heidelberg.de/). Domains are indicated: CH, calponin homology domain; RhoGEF, domain conserved among GEFs for Rho/Rac/Cdc42-like GT-Pases; PH, Pleckstrin homology domain; and PB1, Phox and Bem1p domain conserved in many eukaryotic cytoplasmic signaling proteins. (B) Alignment of the RhoGEF domains by ClustalW (Thompson et al., 1994; http://www.ebi.ac.uk/clustalw/). Amino acid residues are shaded according to BOXSHADE (http://www.ch.embnet.org/software/BOX-form.html).
Although it is not clear why our screening system using the M-factor receptor mutant could pick up gef1, we isolated a scd1/ral1 cDNA also in the same screening. This implies that the isolation of gef1 may not be totally fortuitous.
Synthetic Lethality of gef1 and scd1 Disruptions
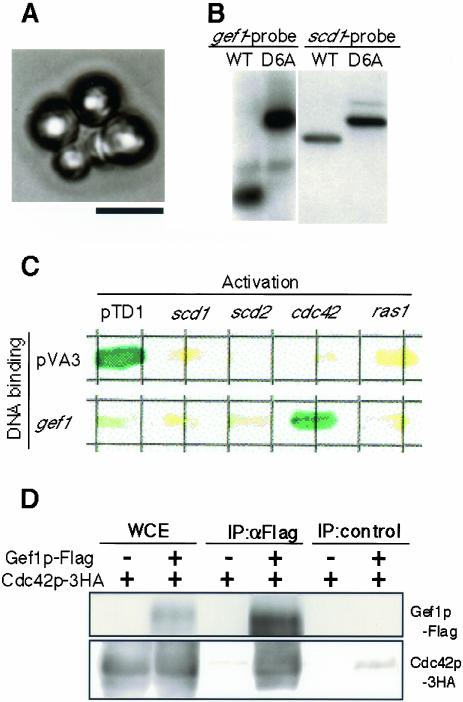
We constructed a strain defective in gef1 by replacing the central one-third of the gef1 ORF with the ura4+ maker, as detailed in MATERIALS AND METHODS. The resulting gef1Δ strain showed no obvious defect in viability or fertility. We used this deletion construct in subsequent experiments, while we later confirmed that deletion of the entire gef1 ORF also resulted in no obvious defect (our unpublished results). To produce scd1– gef1Δ double mutants, JX348 (gef1::ura4+) was cross-ed with JY803 (scd1::LEU2) and subjected to tetrad analysis. As summarized in Table 2, no Leu+ Ura+ progeny were recovered, suggesting that scd1– gef1Δ cells were inviable. Microscopic observation indicated that the presumed scd1– gef1Δ spores could germinate but were arrested after one or two rounds of cell division. The arrested cells displayed a round shape (Figure 2A), as has been found with cdc42Δ cells. Essentially the same results were obtained when we analyzed a heterozygous diploid strain (D11) in which both scd1 and gef1 were disrupted by a Kanr cassette (our unpublished results).
Table 2.
Phenotypes of ascospores generated from the cross of JX348 (gef1::ura4+) and JY803 (scd1::LEU2)
| Ascus Type | Ura+ Leu- | Ura- Leu+ | Ura+ Leu+ | Ura- Leu- | Viable spores/ascus | No. of asci/total |
|---|---|---|---|---|---|---|
| PD | 2 | 2 | 0 | 0 | 4 | 8/24 |
| T | 1 | 1 | 0 | 1 | 3 | 11/24 |
| NPD | 0 | 0 | 0 | 2 | 2 | 4/24 |
| Aberrant | 0 | 1 | 0 | 1 | 2 | 1/24 |
PD, parental ditype; T, tetratype; NPD, nonparental ditype.
Figure 2.
Relationships among gef1, scd1, and cdc42. (A) Synthetic lethality of the gef1Δ scd1Δ double mutant. A double mutant was identified from the nonparental ditype (NPD) pattern in tetrad dissection, and its terminal phenotype was photographed after germination on a YE plate. Bar, 10 μm. (B) Suppression of the lethality of the gef1Δ scd1Δ double mutant by the activated cdc42 allele cdc42-G12V. Southern blot analysis to confirm that D6A, a putative gef1Δ scd1Δ strain carrying pREP3-cdc42-G12V, indeed bears the disrupted alleles for gef1 and scd1 (see MATERIALS AND METHODS). The wild-type strain JY450 was analyzed as a control. (C) Two-hybrid interaction between Gef1p and Cdc42p. The Gal4 activation domain was fused to each of Scd1p, Scd2p, Cdc42p, and Ras1p. These fusion proteins were coexpressed with Gef1p fused with the Gal4 DNA-binding domain in the reporter strain S. cerevisiae Y190. The combination of pTD1 and pVA3 was included in the analysis as a positive control. Three transformants were examined by coloring assay for each combination of bait and prey; those shown are representative. (D) Coimmunoprecipitation of Gef1p and Cdc42p. S. pombe wild-type strain K193 was doubly transformed with pREP41-cdc42–3HA and pREP42-gef1-Flag, and cultured to the midlog phase in MM medium. K193 transformed with pREP41-cdc42–3HA and pREP42-Flag was also examined as a control. A whole cell extract (WCE) was prepared from each culture and subjected to immunoprecipitation using either α-Flag antibody or α-GFP antibody as a control. The precipitates were separated by SDS-PAGE and examined by Western blot using α-HA and α-Flag antibody as probes.
Suppression of the scd1Δ gef1Δ Double Mutant by Activated Cdc42p
The scd1– gef1Δ double mutant phenotype suggested that Scd1p and Gef1p are both involved in the activation of Cdc42p but are redundant for cell viability. Consistent with this idea, cdc42-G12V, a cdc42 allele encoding constitutively active Cdc42p with no GTPase activity (Miller and Johnson, 1994), could suppress the lethality of the scd1Δ gef1Δ strain. This was shown by cross-ing JX349 (gef1::ura4+) with JX124 (scd1::ura4+) and transforming the resultant diploid strain with plasmid pREP3-cdc42-G12V (our unpublished results), which expresses the Cdc42p variant from the normal nmt1 promoter (Maundrell, 1990, 1993). Tetrads derived from transformed diploid cells were dissected, and most had four viable spores. We could identify scd1Δ gef1Δ progeny on the basis of a nonparental ditype distribution of the ura4+ maker in an ascus. One such progeny, named D6A, was analyzed further. We confirmed by Southern blotting that D6A indeed carried the disrupted scd1 and gef1 alleles (Figure 2B). D6A exhibited a round cell shape, as expected from the phenotype of the cdc42-G12V strain, and it grew more slowly than the wild-type, scd1Δ, or gef1Δ strains, often formed aggregates, and sometimes showed aberrant septum formation (our unpublished results). It should be noted that these results are not sufficient to prove that Cdc42p is a target of Gef1p, because, as disruption of gef1 is not lethal, recovery of cell growth will be expected even if cdc42-G12V suppresses only scd1Δ.
Physical Interaction of Gef1p with Cdc42p
To test for physical interaction of Gef1p with Cdc42p, we carried out a yeast two-hybrid assay. The results shown in Figure 2C indicate that Gef1p and Cdc42p interact with each other in this system. Their physical interaction was further confirmed by coimmunoprecipitation assay. Cdc42p tagged with 3HA and Gef1p tagged with Flag were expressed simultaneously in S. pombe cells. As shown in Figure 2D, Cdc42p-3HA could be coimmunoprecipitated by Gef1p-Flag. These results strongly support the hypothesis that Gef1p, like Scd1p, is involved in the GDP-GTP exchange reaction to activate Cdc42p.
In an independent study, Coll et al. (2003) have also demonstrated that Gef1p physically interacts with Cdc42p and observed that the GDP-bound form of Cdc42p has stronger affinity for Gef1p than the GTP-bound form. Furthermore, they have demonstrated that Gef1p can increase the GTP-bound form of Cdc42p overthe GDP-bound form both in vivo and in vitro. Altogether, it appears unequivocal that Gef1p is a second GEF for fission yeast Cdc42p.
Both Gef1p and Scd1p Are Localized to the Cell Division Site
To ask how Gef1p and Scd1p might be used differentially in activation of Cdc42p, we visualized their subcellular localizations by tagging them with fluorescent proteins. We constructed high-copy-number plasmids pREP41-scd1-GFP and pREP41-gef1-GFP, which express the GFP-tagged proteins from the thiamine-regulated promoter of medium strength (Maundrell, 1993; Basi et al., 1993). JX124 (scd1::ura4+) was transformed with pREP41-scd1-GFP, and JX348 (gef1::ura4+) was transformed with pREP41-gef1-GFP. The transformants were cultured in SD medium, which contains 1.3 μM thiamine, and then spotted on SPA plates supplemented with 2 μM thiamine. In the presence of ample thiamine, as in these cases, the expression from the modified nmt1 promoter used here is expected to be only 1/200 of the maximally derepressed expression from the normal nmt1 promoter (Maundrell, 1990, 1993; Basi et al., 1993). We adopted these conditions to avoid artificial localization of the proteins due to excessive overproduction.
In cells growing vegetatively, we observed fluorescence of Scd1p-GFP within the nucleus, at the cell division site, and at growing ends (Figure 3A-a). In cells undergoing conjugation, Scd1p-GFP was localized in the nucleus, on the cell surface facing the mating partner, and at the apical tip of conjugation tubes (Figure 3A-c). In contrast, vegetative cells expressing gef1-GFP emitted fluorescence mainly from the division site (Figure 3A-b), and we could not detect any particular localization of Gef1p-GFP in mating cells (our unpublished results). These results suggest that Scd1p is likely to have a wider cellular function than Gef1p, although both of them may be involved in septation. It is probable, for instance, that Scd1p plays a role in the cell periphery to stimulate elongation of a conjugation tube toward the mating partner, whereas Gef1p does not.
Gef1p and Scd1p Form a Shrinking Ring during Cytokinesis
To clarify the precise localizations of Gef1p and Scd1p during cytokinesis, we reconstructed the 3-D geometry of Gef1p tagged with YFP and Scd1p tagged with GFP from twenty images taken at different focal planes in each case. An unexpected view emerged in each case, in which both Gef1p-YFP and Scd1p-GFP were located in a ring structure (Figure 3B). The two GEFs appeared to occupy a similar space, suggesting that Scd1p and Gef1p might function redundantly in the activation of Cdc42p necessary to evoke septum formation and cell separation.
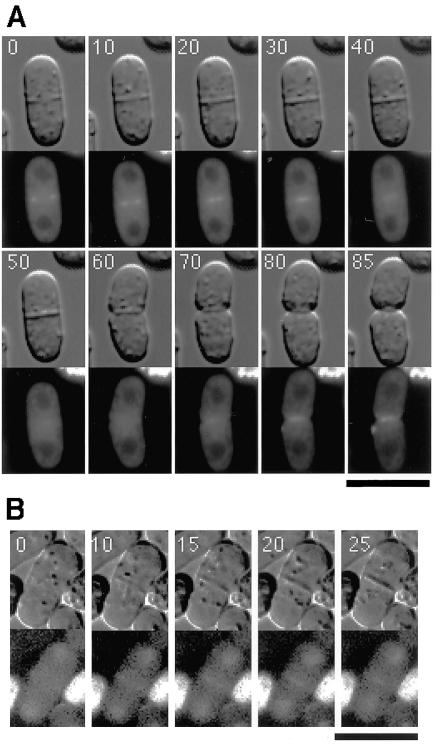
To examine the dynamics of the Gef1p/Scd1p ring during cytokinesis, we monitored the behavior of Gef1p-YFP and Scd1p-GFP by time-lapse recording. Cells of the wild-type strain K193 were transformed with pREP42-gef1-YFP or pREP41-scd1-GFP and cultured in thiamine-free MM medium or SD medium, respectively, to midlog phase. Under these culture conditions, expression of gef1-YFP was derepressed, and we could detect fluorescence of the fusion protein with a short exposure time suitable for time-lapse recording (100 ms). However, some transformed cells became roundish in shape with a strong, uniform fluorescence, indicating that they were physiologically disturbed by the overproduction of Gef1p-YFP. Therefore, we chose cells showing relatively normal shape for subsequent analyses. Gef1p-YFP and Scd1p-GFP gave essentially the same localization pattern in this time-lapse experiment, as described below.
Gef1p-YFP was first detected as a pair of short bars extending from the cortex toward the center at the division site, when a cell was viewed perpendicularly to its axis (Figure 4A, 0 min). At this stage, a partially formed septum was detected by DIC microscopy. As septum formation proceeded, Gef1p-YFP appeared as short bars that shifted toward the center of the cell (Figure 4A, 10–40 min), suggesting that a ring structure formed by Gef1p was shrinking during septation. The Gef1p-YFP signal eventually reached the center of the cell and then faded away (Figure 4A, 50 min). Later, however, when the daughter cells began to separate, Gef1p-YFP reappeared at the separating ends (Figure 4A, 70–85 min). Similarly, the ring structure formed by Scd1p-GFP also underwent shrinkage during septation (Figure 4B).
Figure 4.
Time-lapse images of single K193 cells expressing either Gef1p-YFP (A) or Scd1p-GFP (B). Cells were cultured in liquid MM (A) or SD (B) medium to midlog-phase and then mounted on an agarose slab containing the same medium. DIC images (top panels) and fluorescence images (bottom panels) were recorded. Each number indicates the time in minutes from the start of observation. Bar, 10 μm.
Gef1p Localizes between the Contractile Ring and the Ring of Cdc42p
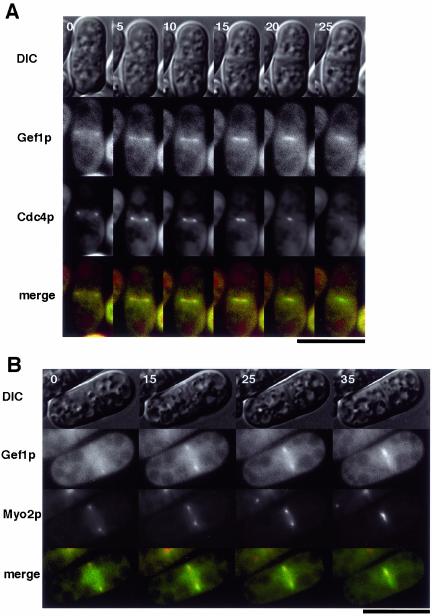
We next compared the localization of Gef1p with those of Cdc42p and components of the contractile ring. First, wild-type cells expressing both Gef1p-CFP and YFP-A8-Cdc42p were analyzed by time-lapse photography. Like Gef1p, YFP-(Ala8)-Cdc42p was first visible as short bars extending from the cortex at the division site (Figure 5A, 0 min). At this stage, Gef1p-CFP appeared to locate at the inner edge of these bars. Whereas Gef1p-CFP moved toward the center as a pair of short bars during septation, YFP-(Ala8)-Cdc42p appeared to simply expand the length of its bars toward the center, until it formed a single continuous line bridging the sides of the cell, as viewed perpendicularly (Figure 5A, 10–45 min). This suggested that in the three-dimensional view, Cdc42p expanded its area like a closing iris, led by a shrinking Gef1p ring located at the inner edge of the iris. The relative localization of Gef1p and Cdc42p was confirmed by construction of a double-labeled 3-D image. As shown in Figure 5B, the Gef1p ring was indeed located at the inner edge of the broader ring formed by Cdc42p.
In fission yeast, the F-actin contractile ring appears to provide the primary force to constrict the cytoplasm during cytokinesis. Components of this ring include the myosin light chain Cdc4p (McCollum et al., 1995) and the type II myosin heavy chain Myo2p (Kitayama et al., 1997; May et al., 1997). Thus, we transformed wild-type cells with plasmids expressing Gef1p-CFP and either GFP-Cdc4p or GFP-Myo2p and examined them by time-lapse photography. As expected, the contractile-ring components were visualized as rings that constricted toward the middle of the cell during cytokinesis (Figure 6, A and B). In both cases, the Gef1p-CFP signal followed just after the contractile-ring components.
Figure 6.
Time-lapse images (times in minutes) of single K193 cells transformed with pREP-gef1-CFP and either pREP42-GFP-cdc4 (A) or pREP81-GFP-myo2 (B). Green represents Gef1p-CFP and red represents the GFP-labeled protein. Bar, 10 μm.
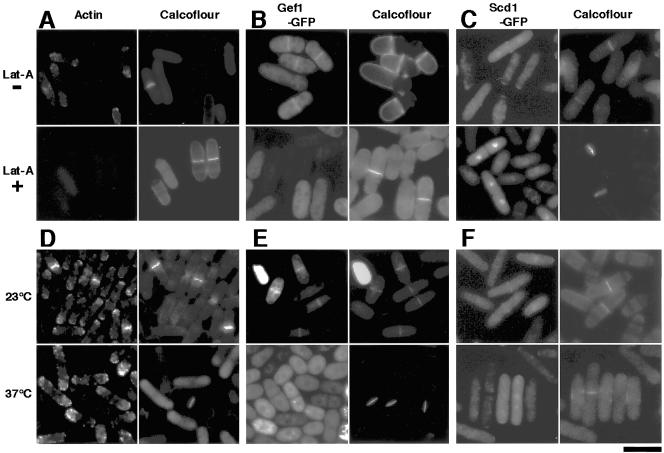
To examine whether the formation and/or maintenance of the Gef1p/Scd1p ring was dependent on the contractile ring, we performed two lines of experiments. First, we treated wild-type cells expressing Gef1p-GFP or Scd1p-GFP with the actin-depolymerizing drug latrunculin-A. The addition of the drug resulted in the disappearance of actin patches and the contractile ring (Figure 7A) and also in the complete disappearance of the Gef1p/Scd1p ring (Figure 7, B and C), whereas the control cells showed the Gef1p/Scd1p ring in 10–15% of the population, which matches the percentage of cells undergoing cytokinesis in an asynchronus culture. Interestingly, nuclear localization of Scd1p persisted even in the presence of latrunculin-A (Figure 7C). Second, we examined the Gef1p/Scd1p ring in a mutant temperature-sensitive for cdc3, which encodes profilin, an important component of the contractile ring (Balasubramanian et al., 1994). At permissive temperature, the cells displayed Gef1p/Scd1p rings at a frequency of 10–15%. However, they formed neither clear contractile rings (Figure 7D) nor Gef1p/Scd1p rings (Figure 7, E and F) at restrictive temperature, although actin patches and localization of Scd1p at cell tips (Figure 7F) remained detectable. These observations suggest that the generation of the Gef1p/Scd1p ring requires proper assembly of the contractile ring and that the Gef1p/Scd1p ring probably moves inward directed by the contractile ring.
Figure 7.
Requirement for the contractile ring for generation and maintenance of the Gef1p/Scd1p ring. (A-C) Wild-type cells (K193) carrying pREP41-gef1-GFP or pREP41-scd1-GFP were cultured in SD medium at 30°C and treated either with 10 μM latrunculin-A (Lat-A +) or with DMSO only as a control (Lat-A -) for 10 min. (D-F) Cells of the temperature-sensitive cdc3–6 mutant JZ175 carrying pREP41-gef1-GFP or pREP41-scd1-GFP were cultured in SD medium at permissive temperature (23°C), while a portion of each culture was shifted to restrictive temperature (37°C) and incubated for 2 h. In each culture, samples were observed for GFP fluorescence, stained with BODIPY-FL-phallacidin to visualize F-actin, and stained with Calcofluor White to confirm the presence of cells undergoing septation. Bar, 10 μm.
DISCUSSION
We have shown in this report that the fission yeast gef1 gene product, Gef1p, is likely to be a GEF for Cdc42p. This conclusion is also supported by recent work from others (Coll et al., 2003). Cdc42p has been suggested to play an important role in both polarized cell growth and septum formation in fission yeast (Merla and Johnson, 2000; Miller and Johnson, 1994; Ottilie et al., 1995). Whereas disruption of cdc42 is lethal (Miller and Johnson, 1994), disruption of gef1 causes no obvious phenotype. However, cells defective in both gef1 and scd1 are inviable. This indicates that Gef1p and Scd1p cooperate in activation of Cdc42p, performing a redundant function at least in part. Disruption of scd1 causes deformation of cell shape and inability to mate (Chang et al., 1994; Fukui and Yamamoto, 1988), but disruption of gef1 does not. In addition, overexpression of gef1 does not rescue the deformed cell shape and sterility of an scd1Δ strain (our unpublished results). Thus, it is likely that Scd1p, but not Gef1p, is involved in the establishment of cell polarity during both vegetative growth and mating. Consistent with this hypothesis, Scd1p is localized to growing ends in vegetative cells (Li et al., 2000; Figure 2A) and to the tip of conjugation tubes in mating cells (Figure 2A). In contrast, Gef1p was not detected in these regions but only at the site of septation, where Scd1p was also seen. Gef1p and Scd1p appear to have very similar localization and dynamics at this site. Therefore, we speculate that the function of Gef1p and Scd1p to activate Cdc42p at the septation site may be indispensable for cell proliferation.
Surprisingly, both Gef1p and Scd1p form a ring structure at the septation site, which undergoes shrinkage during cytokinesis. Precise analysis of its location indicates that the ring resides immediately outside of the contractile actomyosin ring, which is believed to generate the force to constrict the cytoplasm in cytokinesis. Formation and maintenance of the Gef1p/Scd1p ring depend on the integrity of the contractile ring, and the former apparently shrinks led by the latter. Cdc42p appears to be deposited at the shrinking Gef1p/Scd1p ring and left behind as if it fills the track of the ring (Figure 5). Cdc42p eventually forms a plaque structure in the position where the septum is being formed. We speculate that Gef1p and Scd1p may play a key role not only in activating Cdc42p through GDP-GTP exchange but also in recruiting it to the septation site through their affinity for it, although this remains to be proven.
Deposition of Gef1p and Scd1p to the septation site appears to be directed by the contractile ring, but the precise molecular mechanism of this linkage remains unclear. The overall scenario that we propose here extends the previous finding by Merla and Johnson (2000) that localization of Cdc42p to the division site depends on the formation and the proper contraction of the actomyosin ring. The relationship between the plaque structure of Cdc42p thus generated and the construction of a septum is also unclear. Cdc42p may activate vesicle transport to bring septum materials to the septation site, presumably through reorganization of cytoskeletal F-actin.
The counterpart of Scd1p in S. cerevisiae is Cdc24p. Cdc24p is apparently the single GEF for Cdc42p in S. cerevisiae, and disruption of CDC24 is lethal (Coleman et al., 1986; Zheng et al., 1994). An interesting speculation is that S. pombe has acquired the additional GEF during evolution in order to execute the more complicated cytokinetic process of separating daughter cells by fission.
Acknowledgments
We thank Dr. Mika Toya for her interest and generous supply of materials. We also thank Dr. Ken-ich Mizuno for the gift of S. pombe strains and Dr. Pilar Perez for communication during preparation of the manuscript. Thoughtful handling of the manuscript by the Monitoring Editor, Dr. John Pringle, is highly appreciated. This work was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–10–0665. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0665.
References
- Bähler, J., Wu, J.-Q., Longtine, M.S., Shah, N.G., McKenzie, A., III, Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M.K., Hirani, B.R., Burke, J.D., and Gould, K.L. (1994). The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J. Cell Biol. 125, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi, G., Schmid, E., and Maundrell, K. (1993). TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123, 131–136. [DOI] [PubMed] [Google Scholar]
- Chang, E.C., Barr, M., Wang, Y., Jung, V., Xu, H.-P., and Wigler, M.H. (1994). Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79, 131–141. [DOI] [PubMed] [Google Scholar]
- Coleman, K.G., Steensma, H.Y., Kaback, D.B., and Pringle, J.R. (1986). Molecular cloning of chromosome I DNA from Saccharomyces cerevisiae: isolation and characterization of the CDC24 gene and adjacent regions of the chromosome. Mol. Cell. Biol. 6, 4516–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll, P., Trillo, Y., Ametzazurra, A., and Perez, P. (2003). Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe. Mol. Biol. Cell 14, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee, T., Becherer, K., Chen, P.L., Yeh, S.H., Yang, Y., Kilburn, A.E., Lee, W.H., and Elledge, S.J. (1993). The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7, 555–569. [DOI] [PubMed] [Google Scholar]
- Fukui, Y., Kozasa, T., Kaziro, Y., Takeda, T., and Yamamoto, M. (1986). Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell 44, 329–336. [DOI] [PubMed] [Google Scholar]
- Fukui, Y., and Yamamoto, M. (1988). Isolation and characterization of Schizosaccharomyces pombe mutants phenotypically similar to ras1-. Mol. Gen. Genet. 215, 26–31. [DOI] [PubMed] [Google Scholar]
- Grimm, C., Kohli, J., Murray, J., and Maundrell, K. (1988). Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol. Gen. Genet. 215, 81–86. [DOI] [PubMed] [Google Scholar]
- Gutz, H., Heslot, H., Leupold, U., and Loprieno, N. (1974). Schizosaccharomyces pombe. In: Handbook of Genetics, ed. R. D. King, New York, NY: Plenum Press.
- Hirota, K., Tanaka, K., Watanabe, Y., and Yamamoto, M. (2001). Functional analysis of the C-terminal cytoplasmic region of the M-factor receptor in fission yeast. Genes Cells 6, 201–214. [DOI] [PubMed] [Google Scholar]
- Iino, Y., and Yamamoto, M. (1997). The Schizosaccharomyces pombe cdc6 gene encodes the catalytic subunit of DNA polymerase δ. Mol. Gen. Genet. 254, 93–97. [DOI] [PubMed] [Google Scholar]
- Johnson, D.I. (1999). Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63, 54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, C., Sugimoto, A., and Yamamoto, M. (1997). Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 137, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic, I., Goodstadt, L., Dickens, N., Doerks, T., Schultz, J., Mott, R., Ciccarelli, F., Copley, R., Ponting, C., and Bork, P. (2002). Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 30, 242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.C., Chen, C.R., and Chang, E.C. (2000). Fission yeast Ras1 effector Scd1 interacts with the spindle and affects its proper formation. Genetics 156, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, T., Watanabe, Y., Kunitomo, H., and Yamamoto, M. (1994). Cloning of the pka1 gene encoding the catalytic subunit of the cAMP-dependent protein kinase in Schizosaccharomyces pombe. J. Biol. Chem. 269, 9632–9637. [PubMed] [Google Scholar]
- Matsuyama, A., Yabana, N., Watanabe, Y., and Yamamoto, M. (2000). Schizosaccharomyces pombe Ste7p is required for both promotion and withholding of the entry to meiosis. Genetics 155: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell, K. (1990). nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 265: 10857–10864. [PubMed] [Google Scholar]
- Maundrell, K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130. [DOI] [PubMed] [Google Scholar]
- May, K.M., Watts, F.Z., Jones, N., and Hyams, J.S. (1997). Type II myosin involved in cytokinesis in the fission yeast, Schizosaccharomyces pombe. Cell Motil. Cytoskeleton 38, 385–396. [DOI] [PubMed] [Google Scholar]
- McCollum, D., Balasubramanian, M.K., Pelcher, L.E., Hemmingsen, S.M., and Gould, K.L. (1995). Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 130, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla, A., and Johnson, D.I. (2000). The Cdc42p GTPase is targeted to the site of cell division in the fission yeast Schizosaccharomyces pombe. Eur. J. Cell Biol. 79, 469–477. [DOI] [PubMed] [Google Scholar]
- Miller, P.J., and Johnson, D.I. (1994). Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 14, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1990). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–826. [DOI] [PubMed] [Google Scholar]
- Narumiya, S. (1996). The small GTPase Rho: cellular functions and signal transduction. J. Biochem. (Tokyo) 120, 215–228. [DOI] [PubMed] [Google Scholar]
- Okazaki, K., Okazaki, N., Kume, K., Jinno, S., Tanaka, K., and Okayama, H. (1990). High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 18, 6485–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottilie, S., Miller, P.J., Johnson, D.I., Creasy, C.L., Sells, M.A., Bagrodia, S., Forsburg, S.L., and Chernoff, J. (1995). Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 14, 5908–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, J., Nielsen, O., Egel, R., and Hagan, I.M. (1998). F-actin distribution and function during sexual differentiation in Schizosaccharomyces pombe. J. Cell Sci. 111, 867–876. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Fink, G., and Hicks, J. (1986). Methods in yeast genetics: Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- Shinozaki-Yabana, S., Watanabe, Y., and Yamamoto, M. (2000). Novel WD-repeat protein Mip1p facilitates function of the meiotic regulator Mei2p in fission yeast. Mol. Cell. Biol. 20, 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki, M., and Ferenczy, L. (1977). Protoplast fusion of Schizosaccharomyces pombe auxotrophic mutants of identical mating-type. Mol. Gen. Genet. 151, 77–81. [DOI] [PubMed] [Google Scholar]
- Thompson, J., Higgins, D., and Gibson, T. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, V. et al. (2002). The genome sequence of Schizosaccharomyces pombe. Nature 415, 871–880. [DOI] [PubMed] [Google Scholar]
- Yamashita, A., Watanabe, Y., Nukina, N., and Yamamoto, M. (1998). RNA-assisted nuclear transport of the meiotic regulator Mei2p in fission yeast. Cell 95, 115–123. [DOI] [PubMed] [Google Scholar]
- Yesilaltay, A., and Jenness, D.D. (2000). Homo-oligomeric complexes of the yeast α-factor pheromone receptor are functional units of endocytosis. Mol. Biol. Cell 11, 2873–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Cerione, R., and Bender, A. (1994). Control of the yeast bud-site assembly GTPase Cdc42. J. Biol. Chem. 269, 2369–2372. [PubMed] [Google Scholar]