Abstract
Bmi-1 and SALL4 are putative oncogenes that modulate stem cell pluripotency and play a role in leukemogenesis. Murine Sall4 also has been shown to play an essential role in maintaining the properties of ES cells and governing the fate of the primitive inner cell mass. Here, we demonstrate that transcription from the Bmi-1 promoter is strikingly activated by SALL4 in a dose-dependent manner by using a luciferase reporter gene assay. Both promoter deletion construct studies and ChIP from a myeloid stem cell line, 32D, demonstrate that SALL4 binds to a specific region of the Bmi-1 promoter. Deletion of one copy of Sall4 by gene targeting in mouse bone marrow significantly reduced Bmi-1 expression. Reducing SALL4 expression by siRNA in the HL-60 leukemia cell line also results in significant down-regulation of Bmi-1. Furthermore, Bmi-1 expression is up-regulated in transgenic mice that constitutively overexpress human SALL4, and the levels of Bmi-1 in these mice increase as they progress from normal to preleukemic (myelodysplastic syndrome) and leukemic (acute myeloid leukemia) stages. High levels of H3–K4 trimethylation and H3–K79 dimethylation were observed in the SALL4 binding region of the Bmi-1 promoter. These findings suggest a novel link between SALL4 and Bmi-1 in regulating self-renewal of normal and leukemic stem cells. An increase in histone H3–K4 and H3–K79 methylation within the Bmi-1 promoter provides an epigenetic mechanism for histone modifications in SALL4-mediated Bmi-1 gene deregulation.
Keywords: leukemia, methylation, stem cells
The SALL4 zinc-finger transcription factor, which is a member of the SALL gene family, was originally cloned based on sequence homolog to Drosophila spalt (sal) (1–3). In Drosophila, sal is a homeotic gene and essential in the development of posterior head and anterior tail segments (4). Human SALL4 mutations are associated with the Duane radial ray syndrome (also called Okihiro syndrome), a human autosomal-dominant syndrome involving multiple organ defects (3, 5, 6). Alternative splicing generates two variant forms of human SALL4 mRNA: SALL4A and SALL4B, each having a different tissue distribution (7). We demonstrated previously that SALL4 is expressed constitutively in human leukemia cell lines and primary acute myeloid leukemia (AML) cells (7). Transgenic mice that overexpress SALL4B, one of the SALL4 isoforms, exhibit myelodysplastic syndrome (MDS)-like symptoms and subsequently develop AML that is transplantable (7). Recently, SALL4 has been shown to play an important role in maintaining ES cell (ESC) pluripotency and self-renewal properties. Our group and others (8, 9) have shown that murine Sall4 plays an essential role in maintaining the properties of ESCs and governing the fate of the primitive inner cell mass by interacting with Oct4 and Nanog. Sall4+/− ESCs have significantly reduced levels of pluripotency markers, Oct4 and Nanog, as compared with WT cells. Similar to Oct4, reduction of Sall4 in murine ESCs results in respecification of ESCs to the trophoblast lineage.
Bmi-1 is a member of the polycomb group of proteins initially identified in Drosophila as a repressor of homeotic genes (10–12). In humans, the polycomb gene Bmi-1 plays an essential role in regulating adult, self-renewing hematopoietic stem cells (HSCs) and leukemia stem cells (13–19). Bmi-1 is expressed highly in purified HSCs, and its expression declines with differentiation (14). Knockout of the Bmi-1 gene in mice results in a progressive loss of all hematopoietic lineages (17). This loss results from the inability of the Bmi-1 (−/−) stem cells to self-renew. In addition, Bmi-1 (−/−) cells display altered expression of the cell cycle inhibitor genes p16INK4a and p19ARF (20). The expression of Bmi-1 appears to be important in the accumulation of leukemic cells. Interestingly, inhibiting self-renewal in tumor stem cells after deleting Bmi-1 can prevent leukemic recurrence. Recently, Bmi-1 expression has been used as an important marker for predicting the development of MDS and disease progression to AML (21).
The identification of downstream targets of SALL4 or factors that regulate Bmi-1 in leukemogenesis is of significant interest. We demonstrate here that Bmi-1 is a direct target gene of SALL4. Induction of SALL4 expression is associated with increased levels of histone H3–K4 and H3–K79 methylation in the Bmi-1 promoter. Our data provide a novel connection between SALL4 and polycomb group proteins in leukemogenesis and a mechanism whereby aberrant expression of SALL4 can alter Bmi-1 expression directly.
Results
Dose-Dependent Activation of the Bmi-1 Promoter by SALL4 Isoforms.
We have previously shown that transgenic mice that constitutively overexpress human SALL4B, one of the SALL4 isoforms, progress from normal through preleukemic stages (MDS) to AML (7). To search for specific gene targets of SALL4 in leukemogenesis, we performed microarray hybridization (Affymetrix, Santa Clara, CA) (using U133 chips) of SALL4B preleukemic bone marrow mRNA and compared the data with that of control bone marrow. Bmi-1 was identified as one of the genes whose expression was significantly increased (data not shown).
To examine the correlation between Bmi-1 expression and SALL4 expression, we first analyzed the mouse Bmi-1 promoter activity. A ≈2.1-kb sequence upstream of the translation start site was subcloned into the 5′ end of the promoterless pGL3-basic luciferase reporter plasmid. The SALL4 responsiveness of the Bmi-1 promoter then was evaluated through cotransfection of 0.25 μg of the Bmi-1 promoter construct and 0.04 μg of Renilla luciferase plasmid together with increasing ratios of the SALL4A or SALL4B expression constructs relative to the Bmi-1 promoter construct (0–2 ratios). As one increased the molar excess of the SALL4A or SALL4B construct, the Bmi-1 promoter was activated in a dose-dependent manner (Fig. 1).
Fig. 1.
Dose-dependent activation of Bmi-1 promoter by SALL4 in HEK-293 cells. Bmi-1-Luc construct (0.25 μg) was cotransfected with 0.04 μg of Renilla luciferase plasmid and increasing ratios of either the SALL4A or SALL4B expressing construct. pcDNA3 was used as the control. Data represent the mean of three individual experiments. HEK-293 cells, rather than 32D or HL60 cells, were used in these transfection experiments as these hematopoietic cells exhibit low transfection efficiency.
Mapping of the SALL4 Functional Site Within the Bmi-1 Promoter Region by a Luciferase Reporter Gene Assay.
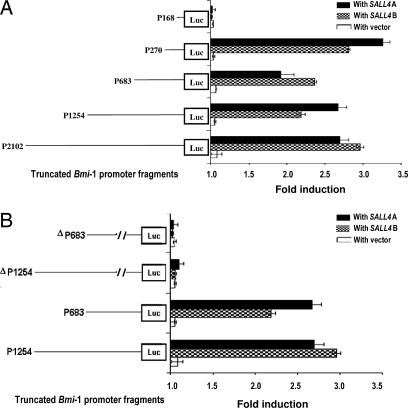
To define the minimal promoter sequence required to activate Bmi-1 by SALL4, transient cotransfection of SALL4 was performed with a series of deleted DNA fragments encompassing the Bmi-1 promoter fused to the luciferase reporter gene. The series of deleted promoter fragments used in the transfection is depicted in Fig. 2A. Each promoter reporter construct of Bmi-1 was transiently cotransfected with the SALL4 isoforms into HEK-293 cells. High levels of activation by both SALL4 isoforms were seen with constructs containing promoter sequences from 0 to −2102, 0 to −1254, 0 to −683, and 0 to −270. Removal of the upstream region between −270 and −168 leads to the inability of SALL4 isoforms to activate the Bmi-1 promoter, indicating the presence of a strong SALL4 activation site in this region. The SALL4 binding region (−270 to −168) then was deleted from the 0 to −1254 and 0 to −683 promoter fragments, and two new Bmi-1 promoter constructs were created. The luciferase activity of the resulting constructs (P1254 and ΔP683) was compared with activity in the WT promoter constructs with or without cotransfection of SALL4A or SALL4B in HEK-293 cells. There was no significant difference in luciferase activity between the Bmi-1 promoter mutants P1254 and ΔP683 and the WT promoter constructs in HEK-293 cells in the absence of SALL4. However, deletion of the −270 to −168 region abolished the activation of Bmi-1 by SALL4 when compared with that of the WT promoter constructs (Fig. 2B). These results indicate that the −270 to −168 region contains a functional site within the Bmi-1 promoter that is activated by the SALL4 oncogene.
Fig. 2.
Mapping of the SALL4 functional site within the Bmi-1 promoter region by a luciferase reporter gene assay. In HEK-293 cells, 0.3 μg of different length Bmi-1-Luc constructs was cotransfected with 0.04 μg of Renilla luciferase plasmid and 0.9 μg of either SALL4A or SALL4B plasmid. P1254 and ΔP683 refer to Bmi-1 mutant promoter constructs, −1254 or −683, in which the −270 to −168 sequence was deleted. (A) Deletion constructs of the Bmi-1 promoter and their corresponding promoter activity stimulated by either SALL4A or SALL4B. (B) SALL4A and SALL4B stimulation of −1254 and −683 or P1254 and ΔP683 Bmi-1 promoter constructs.
Binding of SALL4 Proteins to the Bmi-1 Promoter in Vivo.
The myeloid stem cell line 32D expresses Bmi-1 but has very low levels of endogenous SALL4 (data not shown). Binding of SALL4 proteins to the Bmi-1 promoter in 32D cells was analyzed by using ChiP assays. 32D cells were transfected with SALL4A, and SALL4B cDNA constructs were tagged with HA. Chromatin was then extracted, sonicated, and immunoprecipitated by using rabbit polyclonal antibodies against HA. The forward and reverse primer sets (7 + 8 and 9 + 10) amplified strong 225-bp amplicons from the input sample (Fig. 3B, input lane) and immunoprecipiates (Fig. 3B, + lane). Immunoprecipitation reactions using preimmune serum show very little amplification of the Bmi-1 promoter in the immunoprecipitated DNA (Fig. 3B, − lane). All ChIP samples were tested for false-positive PCR amplification by sequencing amplicon DNAs to ascertain the specificity of the SALL4 that bound to the cis-regulatory elements. The intensity of each PCR amplicon was also normalized against the ChIP input band to show the relative abundance of SALL4A that bound to the Bmi-1 promoter (Fig. 3C) by quantitative real-time PCR (QRT-PCR). The observed binding was specific, as essentially no signal was observed in parallel ChIP experiments using cells transfected by an empty vector (pcDNA3). This study indicated that a region between −450 to −1 of the Bmi-1 promoter could be a binding site for SALL4A, consistent with the previous luciferase promoter deletion experiments. As expected, SALL4B also demonstrated a similar binding distribution on the Bmi-1 promoter. These studies indicate that the −450 to −1 region of the Bmi-1 promoter has a functional site for activation by both SALL4 isoforms (Fig. 3C). We also demonstrated that SALL4 was able to bind the cis-regulatory elements of Bmi-1 in ESCs, HEK 293 cells, an acute leukemic cell line (NB4), and two AML human samples including M0 (French–American–British classification) and AML transformed from chronic myeloid leukemia by using ChIP-on-ChIP assays (data not shown).
Fig. 3.
SALL4 specifically binds to the endogenous mouse Bmi-1 promoter (−450 to +1) with ChIP assays. (A) Schematic representation of the primer sets specific for Bmi-1 promoter. (B) ChIP assays were performed by using an antibody against HA (lane +) or preimmune sera (lane −); enriched chromatin was analyzed by PCR with primers as shown in A. (C) Relative enrichment of Bmi-1 promoter regions in 32D cells that were transfected with SALL4 isoforms tagged with HA or the control pcDNA3. ChIP assays were performed with HA antibody. Amplicons were quantitated by Q-PCR. Endogenous SALL4 also bound to the human Bmi-1 promoter at the same position as seen in the human HEK-293 cells, leukemia cell lines, and NB4 with SALL4 antibodies (data not shown).
SALL4 Is Able to Affect the Levels of Endogenous Bmi-1 Expression.
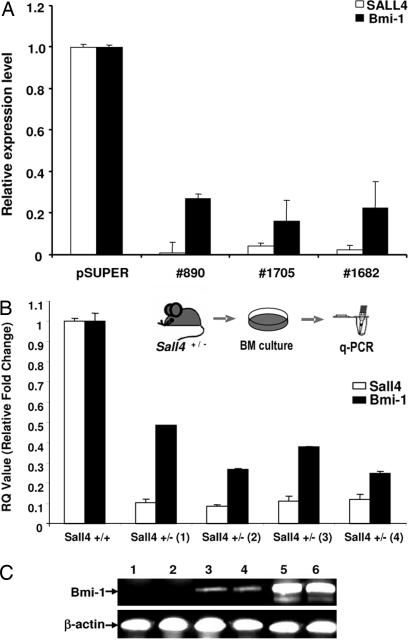
To verify regulation of Bmi-1 by SALL4, we attenuated SALL4 expression in a leukemic cell line, HL60, by using siRNA-mediated knockdown. Three siRNA retroviral constructs that target different regions of the SALL4 mRNA were made, and their ability to knock down SALL4 mRNA in HL60 cells was confirmed by QRT-PCR. Cells from the HL-60 leukemia cell line were infected with the virus collected after 48 h of transduction. Stable infected cells were identified under G418 selection. In all three SALL4 siRNA constructs, down-regulation of SALL4 significantly reduced Bmi-1 levels (Fig. 4A). SALL4 mRNA levels were knocked down by >90%, and Bmi-1 expression was reduced by 75–85%.
Fig. 4.
Effects of endogenous Bmi-1 expression levels. (A) siRNA mediated SALL4 suppression in leukemia cells: Three siRNA oligonucleotides, targeting the SALL4 gene at positions 890, 1682, and 1705, respectively, were cloned into a pSUPER retrovirus vector; PT67 packaging cells were transfected and HL-60 cells were infected with the virus collected after 48 h of infection. Stable infected cells were collected under G418 selection. Total RNA was extracted by TRIzol, RT-PCR was performed, and the relative amount of target gene mRNA was analyzed. The SALL4/GAPDH ratio in noninfected cells was set at 1; values are the mean of duplicate reactions. Bars indicate SD. (B) Sall4+/− heterozygous bone marrow cells showed decreased levels of Bmi-1 expression. Bone marrow cells from SALL4+/− and SALL4+/+ mice were isolated. QRT-PCR was performed to analyze expression levels of SALL4 and Bmi-1. Values are the mean of duplicate reactions. (C) Up-regulation of Bmi-1 in SALL4B transgenic mice associated with disease progression. RT-PCR analysis was performed on total bone marrow cells from two WT control mice (lanes 1 and 2) and two preleukemic transgenic mice (lanes 3 and 4) and leukemic bone marrow cells from two leukemic transgenic SALL4B mice (lanes 5 and 6).
To gain further supporting evidence of Bmi-1 regulation by SALL4, we analyzed Sall4+/− mice (8). Homozygous Sall4 mutant embryos die at very early gestation. Approximately 50% of heterozygous Sall4 knockout mice (Sall4+/−) survive despite the defect at the ESC level (data not shown). Bone marrow cells from mutant Sall4+/− and WT Sall4+/+ mice were isolated. QRT-PCR was performed to compare expression levels of Sall4 and Bmi-1. The heterozygous Sall4+/− bone marrow cells had reduced SALL4 expression as expected. In addition, these heterozygous cells had significantly reduced expression levels of Bmi-1 as compared with normal mouse bone marrow cells (Fig. 4B).
Increased Expression of Bmi-1 in SALL4B Transgenic Mice Associated with Disease Progression.
Previously, we demonstrated that transgenic mice that overexpress one of the SALL4 isoforms, SALL4B, exhibited MDS-like features and, subsequently, also exhibited AML transformation (7). Here, we report the effect of SALL4B overexpression on Bmi-1 gene expression in SALL4B transgenic mice. We found that, in contrast to WT control mice, the mRNA expression for Bmi-1 was up-regulated significantly in preleukemic bone marrows and leukemic blasts from SALL4B transgenic mice (Fig. 4C). Events associated with the progression of MDS and MDS transformation in SALL4B transgenic mice were associated with the up-regulation of Bmi-1. HSCs and granulocyte macrophage progenitor cells (GMPs) were isolated from three leukemic SALL4 transgenic mice and three nonleukemic SALL4 transgenic mice. We observed that both leukemic HSCs and GMPs had much higher levels of Bmi-1 expression than observed in normal HSCs and GMPs by QRT-PCR. These values range from a 2- to a 20-fold increase. Variable SALL4B expression levels were observed in different founder mice but in each case the expression levels of Bmi-1 were correlated with the SALL4B expression levels in the HSC and GMP populations (data not shown). In addition, SALL4 expression levels consistently increased as leukemia progresses because of expansion of HSCs and hematopoietic progenitor cells (data not shown).
Expression of High Levels of SALL4 Expression in Human AML Is Associated with the Expression of High Levels of Bmi-1.
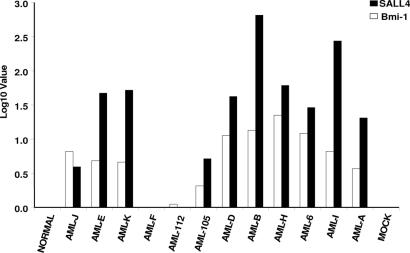
Previously, we had shown that SALL4 was widely expressed in human leukemia cell lines and primary AML cells (7), which led us to investigate the correlation of SALL4 expression with the expression of Bmi-1. We analyzed 12 random clinical AML samples from bone marrows using QRT-PCR to quantify relative mRNA expression of SALL4 and Bmi-1 (Fig. 5). Ten of 12 AML samples showed significant SALL4 expression ranging from a 3.93- to 653-fold increase relative to the averaged normal controls. These results were consistent with SALL4 protein expression as demonstrated by immunostaining with a SALL4 antibody (22). Interestingly, the same 10 of 12 AML samples showed high levels of Bmi-1 expression ranging from a 1.10- to 22-fold increase. There was a strong correlation between the SALL4 and Bmi-1 expression in the AML samples that were examined.
Fig. 5.
mRNA expression of Bmi-1 and SALL4 in human AML blast samples showed a strong correlation between Bmi-1 and SALL4 expression. Twelve randomly selected blastic AML samples were analyzed by RT-PCR for relative mRNA expression of Bmi-1 and SALL4 genes. Ten of 12 AML samples showed significant Bmi-1 gene amplification ranging from 1.10- to 22.32-fold increases relative to the averaged normal controls. Interestingly, the same 10 of 12 AML samples also showed elevated SALL4 gene expression amplification, ranging from a 3.93- to 653.03-fold increase relative to the averaged normal controls. The Log10 scale represents the relative quantification of genes of interest. Using data for the 12 AML samples, we performed a statistical analysis and determined the correlation coefficient to be 0.703 with a P value of 0.0159.
Epigenetic Alterations at Bmi-1 Gene Promoter Induced by SALL4 Protein.
As shown above, SALL4 binds to the Bmi-1 promoter and the regulation of Bmi-1 by SALL4 has been noted in both in vitro and in vivo models of SALL4. We further explored a mechanism to explain this up-regulation by SALL4. H3–K4 trimethylation and H3–K79 methylation have been reported to couple directly to the transcriptional activation (22–26). Abnormal H3–K4 trimethylation and H3–K79 are also associated with leukemogenesis (27). We performed dimethylation ChIP on the 32D cells, which express no detectable endogenous SALL4 (data not shown), to analyze histone marks present on chromatin before SALL4 binds to the Bmi-1 promoter. We then performed ChIP on 32D cells that had been transfected with SALL4A constructs tagged with HA, or a control vector, and then immunoprecipitated through ChIP using antibodies specific for histone H3–K4 trimethylation and H3–K79 dimethylation. DNAs recovered from these ChIP experiments were amplified by QRT-PCR using primers that covered 1.5 kb of the Bmi-1 promoter. Consistent with binding of SALL4 to Bmi-1 promoter sites in the 32D cells transfected with SALL4A or SALL4B constructs, H3–K4 trimethylation was detected and increased ≈2- to 3-fold as compared with a vector control (Fig. 6). Similar analysis with H3–K79 methylation revealed robust methylation at SALL4 binding sites and closely paralleled the pattern of H3–K4 trimethylation in the presence of SALL4.
Fig. 6.

SALL4 specifically binds to the endogenous mouse Bmi-1 promoter (−450 to +1), resulting in H3–K4 and K79 methylation using ChIP assays. Enriched chromatin was analyzed by PCR with the primers shown in Fig. 3A. Shown are distributions of the H3 trimethylation levels of H3–K4 and H3–K79 on the Bmi-1 promoter regions, respectively, in 32D cells that were transfected with SALL4A tagged with HA or control DNA, pcDNA3. ChIP assays were performed by using histone H3–K4 trimethylation antibody (A) and histone H3–K79 methylation antibody (B). Amplicons were quantitated by Q-PCR. Experiments were repeated three times with similar results (data not shown).
Discussion
The polycomb gene family is essential for the self-renewal of both hematopoietic and neuronal stem cells, as well as cancer stem cells, particularly the protein encoded by the polycomb gene Bmi-1. The β-catenin-signaling pathway proteins constitute another family of proteins related to self-renewal that has been extensively studied. However, there is little known about the transcriptional regulation of the polycomb genes. We have show here that the stem cell gene transcription factor SALL4 directly regulates Bmi-1. This conclusion is supported by several lines of evidence: (i) Transcription from the Bmi-1 promoter is strikingly activated by SALL4, and the SALL4 responsiveness of the Bmi-1 promoter exhibits dose-dependent activation in a luciferase reporter gene assay. (ii) SALL4 binds to the Bmi-1 promoter in a region (−270 to −168) involving SALL4 stimulation. (iii) Deleting one copy of the SALL4 oncogene by gene targeting in mouse bone marrow significantly reduces Bmi-1 expression. (iv) SALL4 up-regulates Bmi-1 expression in transgenic mice that constitutively overexpress SALL4, and the up-regulated levels of Bmi-1 in SALL4 transgenic mice correlates to disease progression from normal to preleukemic and leukemic stages. (v) Consistent with the role of Bmi-1 in leukemogenesis, endogenous SALL4 expression correlates with Bmi-1 expression in human AML cells. These studies, when taken together, indicate that SALL4 positively regulates Bmi-1 expression by binding to the Bmi-1 promoter. It is, however, unclear at this time whether SALL4 binds directly to promoter DNA sequences or mediates its effects via intermediate protein interactions.
Previously, we have demonstrated that both isoforms of SALL4 were able to bind to β-catenin and synergistically enhance the Wnt/β-catenin-signaling pathway (7). Now, we have shown that SALL4 positively regulates Bmi-1 expression by binding to the Bmi-1 locus. Bmi-1 is expressed highly in purified HSCs, and Bmi-1 expression declines with differentiation (14). Knockout of the polycomb gene Bmi-1 in mice results in a progressive loss of all hematopoietic lineages (17). This loss results from the inability of Bmi-1 (−/−) stem cells to self-renew. Thus, expression of Bmi-1 appears to be important in the accumulation of leukemic cells. Recently, Bmi-1 expression has been used as an important marker for predicting MDS disease progression to AML (21). Similar to Bmi-1, SALL4B is expressed highly in HSCs and is down-regulated as differentiation proceeds (data not shown). As a result of the constitutive expression of SALL4B in mice, we have demonstrated that SALL4B transgenic mice reveal distinct developmental abnormalities in the stem compartment with selective expansion of the hematopoietic progenitor cell (HPC) population (L.C. and Y.M., unpublished data). The expansion of HPCs is accompanied by MDS and progression of MDS to AML in SALL4 transgenic mice and is associated with up-regulation of Bmi-1 expression. In addition, our studies have shown that SALL4B is able to transactivate Bmi-1 promoter by binding, further indicating that Bmi-1 is a downstream target of SALL4B that mediates leukemic stem cell self-renewal.
Epigenetic modifications play an important role in human cancer, including leukemia. Histone methylation is one of such modifications contributing to deregulation of cancer-relevant genes. Histone lysines can be monomethylated, dimethylated, and trimethylated by a group of enzymes called histone methyltransferases. It has been shown recently that methylation of histone H3–K4 and H3–K79 residues are associated with gene activation and involved in leukemogenesis (27). Here, we demonstrate, using ChiP experiments, that H3–K4 and H3–K79 sites on histones associated with the SALL4 binding region of the Bmi-1 promoter are hypermethylated in the presence of SALL4. There also appears to be a correlation between the induction of Bmi-1 expression and the levels of histone H3–K4 methylation. This result provides at least one possible mechanism whereby SALL4 mediates the overexpression of Bmi-1.
Although it is not known exactly how the associated aberrant epigenetic modifications of Bmi-1 occur in the presence of SALL4, it is possible that the binding of SALL4 to the Bmi-1 promoter interferes with the recruitment of the repression machinery. Alternatively, SALL4 may be, or become, associated with a methyltransferase and that this enzyme is recruited to this target gene through the binding of SALL4 to the Bmi-1 promoter. Further studies are needed to explore the mechanistic possibilities. It is interesting to note, however, that SALL1, another member of the SALL gene family, has recently been shown to interact with chromatin remodeling complexes and recruit histone deacetylase involving gene regulation (28).
In summary, we have demonstrated that the oncogene Bmi-1 is a direct target gene of SALL4. We also have shown that SALL4 expression strongly correlates with Bmi-1 in primary AML. These data provide a novel link between SALL4 and polycomb group proteins and suggest a role for a SALL4/Bmi-1 network in leukemogenesis. Furthermore, we provide in vivo evidence for a mechanism whereby the aberrant expression of SALL4 can alter Bmi-1 gene expression via epigenetic modulation.
Materials and Methods
Cell Culture and Transfection.
All cell cultures were maintained at 37°C with 5% CO2. HEK-293 (ATCC CRL-11268) cells were cultured in DMEM supplemented with 10% heat-inactivated FBS and penicillin/streptomycin (P/S). The HL60 cell line was cultured in RPMI medium 1640 supplemented with 10% FBS and P/S. A murine hemopoietic multipotential cell line, 32D (ATCC CRL-1821), was maintained in RPMI 1640 supplemented with 10% FBS, P/S, and mouse leukemia inhibitory factor (1 × 103 units/ml, Chemicon, Pittsburgh, PA). Transfection of plasmids into HEK-293, mouse 32D cells, and HL60 cells was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations.
Bmi-1 Promoter Constructs and Site-Directed Mutagenesis.
The 5′ flanking region of Bmi-1 was amplified with primers (5′ primer: 5′-CAT CCT CGA GGG CTG TTG ACA TCT GCA GAG ACT G-3′; 3′ primer: TCG TAG ATC TCA TTT CTG CTT GAT AAA AGA TCC TGG-3′) to generate a fragment from nucleotide −1 to nucleotide 2102 upstream of the starting codon ATG with XhoI and BglII sites at each end, respectively. Mouse genomic DNA isolated from ESCs was used as a template. The amplified PCR fragment was cloned into the promoterless pGL3-basic luciferase reporter plasmid (Promega, Madison, WI) to generate plasmid Bmi-1 (P2102) (i.e., nucleotides −1 to −2102; see Fig. 1). Promoter fusion reporter fragments from nucleotides −1 to −1254, −683, −270, and −168 (P1254, P683, P270, and P168) were created in the same manner as Bmi-1. The deletion mutant of the Bmi-1-Luc promoter constructs ΔP683 and ΔP1254, which lack the −168–270 sequence, was generated by using a QuikChange II mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol.
siRNA Constructs.
For down-regulation of SALL4, three different sets of 60-bp oligonucleotides targeting different regions of the human SALL4 sequence were synthesized. These fragments were cloned into the HindIII and BglII sites of pSuper-retro-puro (OligoEngine, Seattle, WA) to generate pSuper-retro/SALL4 siRNA constructs, designated: 890 (5′-gatcccccaacatcccttctgccaccttcaagagaggtggcagaagggatgttgtttttc-3′), 1682 (5′-gatcccccaccactgatcccaacgaattcaagagattcgttgggatcagtggtgtttttc-3′), and 1705 (5′-gatcccctcatttgccaccgagtcttttcaagagaaagactcggtggcaaatgatttttc-3′).
Generation of Retrovirus.
The Phoenix packaging cells (ATCC SD-3443) were grown in DMEM with 10% FBS in 5% CO2 at 37°C. Recombinant retroviruses were produced by using the Phoenix packaging cell line that was transfected with the pSuper construct containing the control RNAi sequence or sequence directed against SALL4. The viral supernatant was collected 48 h after transfection and filtered through a 0.45-μm filter.
Bmi-1 Promoter Assays.
Bmi-1 promoter luciferase assays were performed with the Dual-Luciferase Reporter Assay System (Promega). Twenty-four hours after transfection, HEK-293 cells were extracted with the use of a passive lysis buffer; a 20-μl aliquot was used for luminescence measurements with a luminometer. The data are represented as the ratio of firefly to Renilla luciferase activity. These experiments were performed in duplicate.
ChIP Assay.
HEK-293 32D cells (1 × 106 cells per well in 6-well plates), with or without transient transfection, were processed with a ChIP Assay Kit (Upstate, Charlottesville, VA) following the manufacturer's protocol. Briefly, cells were cross-linked by adding formaldehyde (27 μl of 37% formaldehyde/ml) and incubated for 10 min. Then, chromatin was sonicated to an average size of ≈500 bp and immunoprecipitated with SALL4 antibodies, preimmune serum, or anti-HA antibody. Antibodies for histone modifications, histone H3 trimethy K4 and histone H3 dimethy K79, were purchased from Abcam (Cambridge, MA). Histone–DNA cross-links were reversed by heating at 65°C followed by digestion with proteinase K (Invitrogen). DNA was recovered by using a PCR purification kit (Qiagen, Valencia, CA) and then used for PCR or QRT-PCR.
Total RNA Preparation and QRT-PCR Analysis.
Total RNA was extracted by using Trizol (Invitrogen) following the manufacturer's instructions. cDNA was generated by using 2 μg RNA and the Transcriptor First Strand cDNA Synthesis Kit (Roche, Palo Alto, CA). The cDNA was then analyzed by QRT-PCR using the Sybr Green RT-PCR Core Reagents kit (Applied Biosystems, Foster City, CA). The primer sequences of the oligonucleotides used for QRT-PCR are available on request.
Human Leukemia Samples and Sall4 Knockout.
Leukemia and normal samples frozen in DMSO were collected from the files of the University of Texas M. D. Anderson Cancer Center under approved Institutional Review Board protocols. The diagnosis of all tumors was based on morphologic and immunophenotypic criteria according to the French-American-British classification for hematopoietic neoplasms. The generation of Sall4 knockout mice was as described (8).
Acknowledgments
We thank Zaida Alipio for performing QRT-PCR experiments. This work is supported in part by National Institutes of Health Grants K08 CA097185 and P20 RR016464 (to Y.M.) and K08 DK063220 (to L.C.) and the National Blood Foundation (L.C.).
Abbreviations
- AML
acute myeloid leukemia
- MDS
myelodysplastic syndrome
- ESC
ES cell
- HSC
hematopoietic stem cell
- QRT-PCR
quantitative real-time PCR.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kohlhase J, Schuh R, Dowe G, Kuhnlein R, Jackle H, Schroeder B, Schulz-Schaeffer W, Kretzchmar H, Kohler A, Muller U, et al. Genomics. 1996;38:291–298. doi: 10.1006/geno.1996.0631. [DOI] [PubMed] [Google Scholar]
- 2.Kohlhase J, Hausmann S, Stojmenovic G, Dixkens C, Bink K, Schulz-Schaeffer W, Altmann M, Engel W. Genomics. 1999;62:216–222. doi: 10.1006/geno.1999.6005. [DOI] [PubMed] [Google Scholar]
- 3.Al-Baradie R, Yamada K, St Hilaire C, Chan WM, Andrews C, McIntosh N, Nakano M, Martonyi EJ, Raymond WR, Okumura S, et al. Am J Hum Genet. 2002;71:1195–1199. doi: 10.1086/343821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhnlein RP, Schuh R. Development (Cambridge, UK) 1996;122:2215–2223. doi: 10.1242/dev.122.7.2215. [DOI] [PubMed] [Google Scholar]
- 5.Kohlhase J, Heinrich M, Schubert L, Liebers M, Kispert A, Laccone F, Turnpenny P, Winter RM, Reardon W. Hum Mol Genet. 2002;11:2979–2987. doi: 10.1093/hmg/11.23.2979. [DOI] [PubMed] [Google Scholar]
- 6.Borozdin W, Wright MJ, Hennekam RC, Hannibal MC, Crow YJ, Neumann TE, Kohlhase J. J Med Genet. 2004;41:e102. doi: 10.1136/jmg.2004.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Cui W, Yang J, Qu J, Di C, Amin HM, Lai R, Ritz J, Krause DS, Chai L. Blood. 2006;108:2726–2735. doi: 10.1182/blood-2006-02-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L, et al. Nat Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 9.Elling U, Klasen C, Eisenberger T, Anlag K, Treier M. Proc Natl Acad Sci USA. 2006;103:16319–16324. doi: 10.1073/pnas.0607884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zink D, Paro R. EMBO J. 1995;14:5660–5671. doi: 10.1002/j.1460-2075.1995.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennison JA. Annu Rev Genet. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- 12.Franke A, DeCamillis M, Zink D, Cheng N, Brock HW, Paro R. EMBO J. 1992;11:2941–2950. doi: 10.1002/j.1460-2075.1992.tb05364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raaphorst FM. Trends Immunol. 2003;24:522–524. doi: 10.1016/s1471-4906(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 14.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 15.Dick JE. Nature. 2003;423:231–233. doi: 10.1038/423231a. [DOI] [PubMed] [Google Scholar]
- 16.Ohta H, Sawada A, Kim JY, Tokimasa S, Nishiguchi S, Humphries RK, Hara J, Takihara Y. J Exp Med. 2002;195:759–770. doi: 10.1084/jem.20011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessard J, Sauvageau G. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 18.van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, et al. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 19.Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, Ema H, Kamijo T, Katoh-Fukui Y, Koseki H, et al. Immunity. 2004;21:843–851. doi: 10.1016/j.immuni.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 21.Mihara K, Chowdhury M, Nakaju N, Hidani S, Ihara A, Hyodo H, Yasunaga S, Takihara Y, Kimura A. Blood. 2006;107:305–308. doi: 10.1182/blood-2005-06-2393. [DOI] [PubMed] [Google Scholar]
- 22.Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Nat Cell Biol. 2004;6:73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- 23.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, III, Gingeras TR, et al. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Serville F, Lacombe D, Saura R, Billeaud C, Sergent MP. Genet Couns. 1993;4:109–112. [PubMed] [Google Scholar]
- 26.Im H, Park C, Feng Q, Johnson KD, Kiekhaefer CM, Choi K, Zhang Y, Bresnick EH. J Biol Chem. 2003;278:18346–18352. doi: 10.1074/jbc.M300890200. [DOI] [PubMed] [Google Scholar]
- 27.Okada Y, Jiang Q, Lemieux M, Jeannotte L, Su L, Zhang Y. Nat Cell Biol. 2006;8:1017–1024. doi: 10.1038/ncb1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiefer SM, McDill BW, Yang J, Rauchman M. J Biol Chem. 2002;277:14869–14876. doi: 10.1074/jbc.M200052200. [DOI] [PubMed] [Google Scholar]