Abstract
Blooms of the phytoplankton Phaeocystis can comprise 85% of total production and generate major biogeochemical signals across broad oceanic regions. The success of Phaeocystis may result from its ability to change size by many orders of magnitude when it shifts from small cells of 4–6 μm to large colonies of up to 30,000 μm in diameter. Single cells are consumed by ciliates but not copepods, whereas colonies are consumed by copepods but not ciliates. We demonstrate that chemical cues associated with each of these grazers induce consumer-specific, but opposing, morphological transformations in the bloom-forming species Phaeocystis globosa. Chemical cues from grazing copepods suppress colony formation by a significant 60–90%, a response that should be adaptive because copepods feed four times more on colonies versus solitary cells. In contrast, chemical cues from grazing ciliates enhance colony formation by >25%, a response that should be adaptive because ciliates grow three times faster when fed solitary cells versus colonies. Because size-selective predation fundamentally alters community structure and ecosystem function, this chemically cued shift may redirect energy and nutrients from food webs supporting fisheries to those fueling detrital pathways, thus potentially altering ecosystem-level processes such as productivity, carbon storage, and nutrient release.
Keywords: chemical signaling, consumer–prey interaction, inducible defense, Phaeocystis, size-selective predation
Species of Phaeocystis commonly dominate phytoplankton blooms in portions of the world's oceans (1), sequester large amounts of carbon (2, 3), and thus produce the major biogeochemical signals within these communities (4). This large pulse of production is sometimes described as a palatable input that drives classical food webs but at other times as a resource avoided by consumers, thus going primarily through the microbial food web, with this production being recycled by pelagic microbes (5, 6). The effects of this variability on fisheries and local community structure can be considerable and could be generated by grazer-induced transformations between the two primary forms of most Phaeocystis species: solitary cells of 4–6 μm and colonies that can reach up to 30,000 μm in diameter (7). Although these transformations can represent a biovolume change of >10 orders of magnitude and may affect bloom initiation, the cues affecting colony formation are inadequately understood (8, 9). Given that size-specific feeding is common in planktonic consumers (10, 11), detecting the threat of grazing and responding by switching to a less susceptible phenotype could decrease losses of Phaeocystis to consumers as do the induced morphological and chemical defenses of some terrestrial plants, seaweeds, and freshwater zooplankton (12–14). Furthermore, because Phaeocystis is responsible for the majority of production in some ecosystems and because size-selective feeding can affect communities and ecosystem-level processes such as productivity, response to nutrient pulses, and carbon storage and release (10, 15, 16), shifts in morphology that affect consumer acceptance of Phaeocystis could alter fundamental patterns of production, biomass, species composition, and potentially whether production supports fisheries or the microbial food web. In general, too little is known about induced responses in marine planktonic systems where chemical signaling is rarely investigated but is hypothesized to have strong, ecosystem-wide effects (17).
Phaeocystis globosa might change morphology in response to grazer-associated cues by transforming between solitary cells and colonies, or, once colonial, by growing larger and beyond the size that consumers will accept (18–20). Because different zooplankton grazers feed more efficiently on only one of P. globosa's primary morphologies [either solitary cells or colonies, not both (21, 22)] or consume colonies only within specific size ranges (23), either response could provide a refuge from grazers. Previous studies reported colony enlargement but not alterations in colony formation rates after P. globosa encountered cues from heterotrophic dinoflagellates and calanoid copepods (19, 20). However, the relative proportion of cells in the colonial versus solitary form was not measured, and effects of cues from an initial few days of grazing (no copepods survived at the end of the experiment) were sometimes assessed 10–15 days later, leaving the role of cues from feeding copepods versus cues released from dying copepods open to question.
By measuring both colony formation and colony size, we examined the ability of a clone of P. globosa (CCMP 627) solitary cells to detect and discriminate between grazer-associated signals from consumers that eat solitary cells versus those that eat colonies. We compared colony formation when P. globosa was exposed to only the chemical signals from feeding ciliates (which consume small cells) versus mesozooplankton such as copepods (which consume primarily intermediate sized colonies). Enhanced colony formation was expected to be a useful defense against ciliates but not copepods because intermediate-sized colonies are too large to be consumed by ciliates but are preferred by copepods. We also assessed nutrient and pH changes in these experiments because these factors have been suggested to affect colony formation (24, 25). When grazer-associated cues affected colony formation, we tested the potential adaptive value of the response for P. globosa exposed to that consumer type.
Results
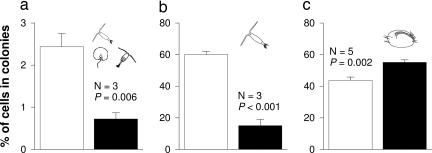
P. globosa responded to grazer-associated chemical cues by altering the proportion of cells allocated to the colonial phenotype (Fig. 1). Chemical cues from a natural, mixed-species collection of feeding mesozooplankton (>100 μm) dominated by copepods suppressed P. globosa colony formation by 70% (Fig. 1a, P = 0.006) even though rates of colony formation were low overall compared with other experiments (note y axis for Fig. 1 a versus b and c). Similarly, when receiving chemical signals from neighbors being grazed only by the copepod Acartia tonsa, P. globosa suppressed colony formation by 75% compared with control filtrates (Fig. 1b, P < 0.001). Because copepod grazing on colonies was ≈300% higher than on solitary cells (Fig. 2a, P = 0.012; also see ref. 26) and because solitary and colonial forms of P. globosa grow at equivalent rates (27), suppression of colony formation in the presence of copepods should slow Phaeocystis loss to grazing copepods. In addition to altering P. globosa morphology, cues from grazers might also alter rates of growth. We did not detect such growth rate changes in the experiment with the mixed assemblage of zooplankton (Fig. 1a); total cell densities for treatments and controls were 730,960 ± 60,000 and 723,050 ± 43,010 cells per ml; mean ± SEM, respectively, and did not differ significantly (P = 0.920, ANOVA). However, cues from A. tonsa alone (Fig. 1b) strongly stimulated growth of P. globosa; total cell densities were 359,200 ± 21,910 cells per ml for the treatment receiving copepod cues but only 119,550 ± 7,190 for the controls; P < 0.001, ANOVA).
Fig. 1.
Percentage of total P. globosa cells within colonies after incubations with chemical cues from grazer-free (open bars) or grazer-containing P. globosa cultures (filled bars). Grazers were either a natural mixture of mesozooplankton (collected in plankton tows in coastal Georgia) (a), the copepod A. tonsa alone (b), or the ciliate Euplotes sp. alone (c). P values designate differences between treatments (ANOVA). Values are means ± 1 SEM.
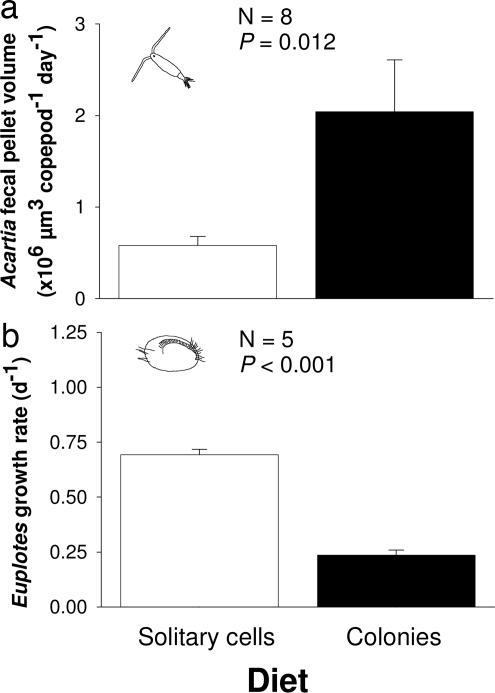
Fig. 2.
Feeding and performance of grazers on P. globosa solitary cells (open bars) or colonies (filled bars). (a) Fecal pellet production of A. tonsa. (b) Euplotes sp. growth rates. P values designate differences between treatments (ANOVA). Values are means ± 1 SEM.
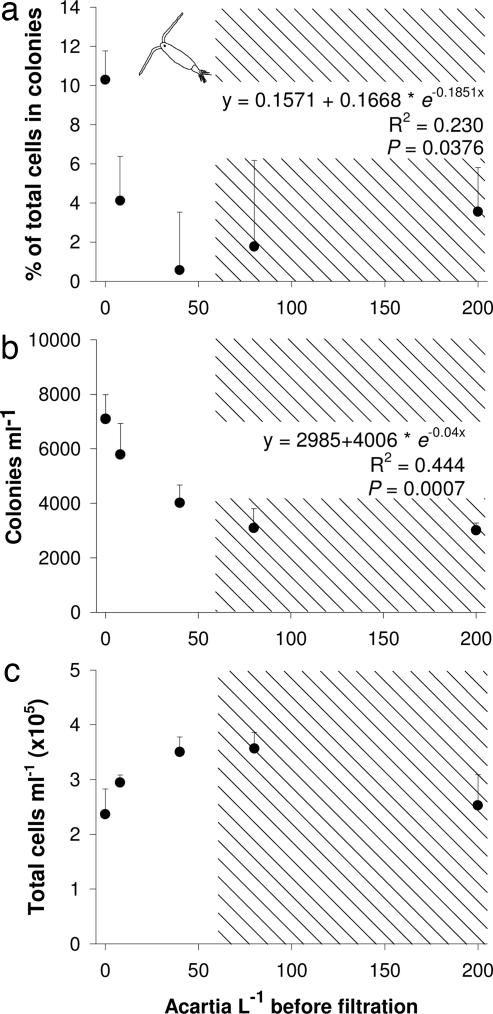
Because the above assays were conducted with copepod densities above natural levels, we conducted similar assays using a range of copepod densities that bracketed the observed natural densities of copepods co-occurring with Phaeocystis in the field [maximal mean densities of 17–19 liter−1 (28, 29)]. We also included high-density treatments (80 and 200 liter−1) to examine how the effects of high densities used in previous experiments compared with the effects of natural densities. We also reasoned that mean natural densities of 20 copepods per liter might include localized patches with twice this density, and so we included a 40-liter−1 treatment. The response of P. globosa was rapid and strong at even low densities of copepods. When P. globosa received signals from conspecifics being grazed by 8 A. tonsa per liter, allocation to the colony growth form declined by ≈60%; this decline grew to 90% when signals were from copepods at densities of 40 liter−1 (Fig. 3a). Additional declines were not apparent at copepod densities of 80 and 200 liter−1. This decline in relative allocation to colonies also produced a trend for a decline in absolute colony concentration (Fig. 3b) despite signals from attacked conspecifics appearing to produce some increase in Phaeocystis growth rate (Fig. 3c), as had occurred in the previous experiment with A. tonsa grazing. In the field, copepods reach mean densities of 17–19 liter−1 in areas with P. globosa blooms (28, 29), so the strong effects we detected at 8–40 copepods per liter are ecologically relevant. Given that the response of P. globosa was produced by only a single input of chemical signal at day 0 of the 3-day experiment, our measured responses may be conservative. We attempted to determine the compound cueing colony suppression so that we could evaluate its concentration, persistence, and rate of degradation. We found that the cue associated with A. tonsa grazing was lipid-soluble and nonvolatile because we could retain it on columns packed with a hydrophobic resin and could concentrate it in vacuo (Fig. 4). We also found that this cue alone could suppress colony formation independent of other changes in the physical environment [supporting information (SI) Table 1]; however, we were unable to identify the chemical structure of the compound involved. As we further purified and separated the lipid extract retained on the hydrophobic resin, our bioassays became more variable, with significant colony suppression being lost, possibly because of compound degradation. Similar problems have been experienced by researchers investigating chemical signals among other phytoplankton genera (J. Kubanek, personal communication).
Fig. 3.
Effects of filtrates from P. globosa cultures with increasing A. tonsa densities on P. globosa. (a) Percentage of total cells within colonies. (b) Colony concentration. (c) Total cell concentration. The shaded region of the graph represents copepod densities far above natural maximal densities. Density-dependent effects on colony formation were analyzed with a curvilinear regression fitted to an exponential decay curve. Percentages were arcsine-transformed before analysis, and reported equations are those using the transformed data. Values are means ± 1 SEM.
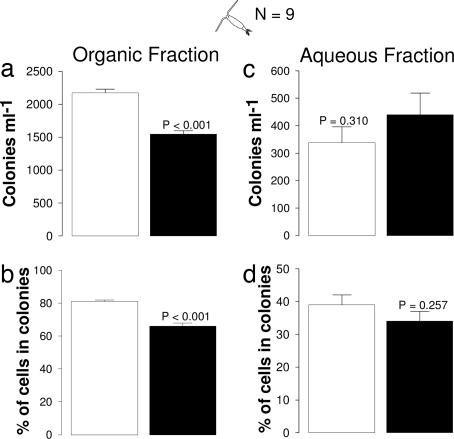
Fig. 4.
Effects of organic or aqueous fractions of filtrates on P. globosa colony concentration (a and c) and percentage of total cells within colonies (b and d). Filtrates sources were either P. globosa cultures (open bars) or P. globosa cultures with A. tonsa (filled bars). P values designate differences between filtrate source (ANOVA). Values are means ± 1 SEM.
In contrast to the colony suppression detected when Phaeocystis was receiving chemical signals from grazing mesozooplankton or A. tonsa that consume larger particles, chemical signals from the grazing ciliate Euplotes sp., which selectively grazes smaller particles, enhanced colony formation by Phaeocystis (Fig. 1c, P = 0.002). Enhancing colony formation in the presence of Euplotes is likely adaptive for P. globosa because Euplotes grew three times faster on a diet composed primarily of solitary cells versus a diet composed primarily of colonies (Fig. 2b, P < 0.001). In contrast to the growth stimulation seen in experiments with A. tonsa, cues from grazing Euplotes slightly (9%), but significantly, suppressed growth of P. globosa (122,840 ± 2,680 and 135,190 ± 4,460 total cells per ml, treatments and controls, respectively; P = 0.045, ANOVA).
Similar to other studies (A. Jacobsen and V. Rousseau, personal communication), we observed large between-experiment variation in the proportion of total cells allocated to colonies, even among our controls (Figs. 1, 3, and 4). Thus, there can be considerable variance in the ratio of solitary to colonial cells among cultures started at different times (even under similar conditions). Despite this unexplained variance, chemical signals from feeding consumers significantly affected colony formation in each of our experiments.
The reduced proportion of cells in colonies after exposure to copepod cues was driven primarily by suppression of colony formation rather than by changes in cell densities within colonies. For the assay with the natural mixture of mesozooplankton, mean colony concentrations ± SEM were: 947 ± 29 and 197 ± 35 colonies per ml for cultures receiving control and mesozooplankton cues, respectively (P = 0.006; see Fig. 3b for the assay with A. tonsa only), but cells were not packaged differently within colonies in the different treatments (e.g., the density of cells on the colony surface was 0.003 ± <0.001 cells per μm for both controls and treatments). Cell densities within colonies of P. globosa cultures grown elsewhere are similar, or lower, than our cultures (0.0013 cells per μm; ref. 24). The tighter packing of cells in colonies within our experiments suggests that the cultures were growing rapidly and were thus healthy. In contrast to previous reports (19, 20), we found no evidence of colony enlargement in treatments receiving grazer cues; the mean colony diameter ± SEM in the mesozooplankton cue experiment was 46 ± 6 μm and 54 ± 5 μm for control and mesozooplankton cue treatments, respectively (P = 0.87).
Both pH and nutrient levels have been suggested to affect colony formation in Phaeocystis (24, 25); these factors rarely appear to affect the patterns reported above (SI Table 1). Neither pH nor ammonium, nitrate, or phosphate levels differed between the control and treatment containers where A. tonsa (Fig. 1b) suppressed colony formation. For the assays with mixed species of mesozooplankton (Fig. 1a), nitrate (P = 0.002), but not any of the other parameters, did differ significantly between treatments (SI Table 1), but the similar effects of copepods on colony formation in Fig. 1 a and b, where nitrate levels did and did not differ, suggest that suppression of colony formation was driven by grazing signals, rather than by nitrate levels. Had differences in pH or inorganic nutrients been responsible for colony suppression, we would have seen these differences in the more water-soluble fractions of filtrates, and such differences did not occur (Fig. 4 c and d).
Discussion
P. globosa senses grazer-associated chemical cues, senses the type of consumer attacking conspecifics, and responds adaptively to these grazer-specific cues with opposing shifts in phenotype. Copepod-associated cues suppressed, whereas ciliate-associated cues enhanced, colony formation. These consumer-specific responses should be adaptive given that copepods feed preferentially on colonies and ciliates grow faster on diets of solitary cells. Although extrapolating laboratory results to the field can be challenging, patterns from field studies are consistent with our laboratory experiments. For example, blooms of Phaeocystis colonies frequently start during periods of low copepod abundance, and thus low grazing, when cues suppressing colony formation should also be low (30, 31). In addition, the maximum concentration of Phaeocystis colonies reportedly occurs during, or near, maximum microzooplankton densities when colony-enhancing cues should be most abundant (32, 33). We demonstrate here that a simple phytoplankton prey can chemically detect and respond, appropriately and opposingly, to different species of consumers.
The opposing nature of the consumer-specific responses presents a potential conflict for Phaeocystis when they encounter a mixture of ciliate and copepod grazers (6). Resolution of this conflict may depend on the relative densities of these two grazer types and the availability of alternate prey. For example, copepods typically prefer ciliates and dinoflagellates over Phaeocystis (34). Thus, copepods may not present a significant threat to Phaeocystis when alternate prey are available. In these situations, Phaeocystis could escape ciliate grazing by enhancing colony formation but without suffering much loss to copepods, so long as ciliates or dinoflagellates were common and copepods focused their attacks on these alternative prey. While copepods were preferentially consuming microzooplankton, Phaeocystis colonies could grow through the intermediate-size range where they are most susceptible to copepod grazing [30- to 100-μm diameter (23)] and reach an escape in colony size (19). Phaeocystis colonies can grow to diameters of 30,000 μm, thus exceeding by orders of magnitude the size range preferred by copepods. By the time copepods have depleted microzooplankton populations, Phaeocystis may have reached a size that is beyond the largest size that copepods can efficiently consume. Although we did not measure such large colonies during our short-term experiments, our stock cultures regularly formed colonies of several millimeters in diameter over longer incubations.
Several alternative hypotheses fail to account for the altered colony formation in the presence of grazers. First, the hypotheses of inorganic nutrients and pH suppressing colony formation were not supported; treatments and controls did not differ significantly in these variables in six of seven analyses (SI Table 1). Furthermore, the Acartia cue was present in organic extracts, not in aqueous fractions where differences of inorganic nutrients or pH would occur, indicating that there is a lipid-soluble signal being released and that this signal can produce colony suppression independently of other changes in the physical environment. Second, in longer-term experiments investigating copepod effects on Phaeocystis morphology, the poor food quality of Phaeocystis leaves most copepods dead within a few days (19, 20, 35); our procedures avoided the possibility that Phaeocystis responses were to chemicals released by dead copepods rather than to signals produced by copepod grazing. The high survivorship of copepods in filtrate experiments eliminated this potential problem and provided only cues produced by live grazers.
A previous investigation by Tang (19) reported that chemical cues from grazers caused significant enlargement of P. globosa colonies. In our experiments, this effect occurred only when P. globosa was in direct contact with mesozooplankton (35); when exposed to only chemical cues, we detected no change in colony size, but we did observe changes in colony density. We present five hypotheses to explain this discrepancy between our study and the experiments of Tang. First, interstrain differences could produce the differing responses; Tang's P. globosa strain (CCMP 1528) was isolated from the Galapagos Islands, whereas ours (CCMP 627) was isolated from the Gulf of Mexico. Strains may respond differently to grazers given the recent discoveries that P. globosa is a multispecies complex (36) and that inducible responses to grazers can be population- or strain-specific (37, 38). Second, our design prevented new cue production after filtrate collection, whereas Tang's design allowed for continuous cue production. Third, Phaeocystis may use multiple strategies to escape grazing, including adjusting both colony formation and colony size. Colony formation appears to be an ineffective defensive strategy in the presence of mesozooplankton, but colonies that grow rapidly through a susceptible, intermediate-size range may escape mesozooplankton grazing once they become too large to handle. Fourth, significant differences in colony size were not evident until at least 9 days after the start of Tang's experiments. We limited our experimental duration to only 3–6 days to avoid the confounding factor of copepod death. However, a preliminary study revealed no evidence of colony enlargement after 12 days with filtrates from either Acartia or Euplotes (J.D.L. and G.W.S., unpublished observation). Fifth, Tang found significant grazer death in four of five experiments documenting colony enlargement, and therefore Phaeocystis were exposed to signals from copepod grazing in the early stages of the experiment and also to signals of copepod death throughout the latter portion of the experiment, thus, possibly confounding signals of grazing with those of copepod death. We observed similar mortality during incubations longer than 3 days, which is why we switched to shorter bioassays that assured cues from living, as opposed to dead, grazers.
Ultimately, colony formation during Phaeocystis blooms may be a function of multiple factors (25, 39), including the relative concentration of these as yet unidentified chemical signals. The 60–90% reduction in colony formation generated by chemical signals from copepod grazing and the 25% increase in colony formation caused by signals from ciliate grazing are similar to changes in colony formation generated by different aeration (80%; ref. 24) and light regimes (33–75%; ref. 40). Our initial chemical assays indicated that the grazer cues were lipid-soluble and nonvolatile and that the signal suppressed colony formation independent of other changes in the physical environment (Fig. 4 a and b and SI Table 1). As we subjected the chemical cue that suppressed colony formation to additional manipulations involved with further purification, we lost consistent bioactivity, which may have occurred because of the compound being relatively unstable. A compound that was too stable would make a poor signaling molecule because it would persist for too long in the environment and produce a signal that was predictive of pervious, but not present, threats to the receiving organism. Organisms relying on chemical cues to induce defenses should evolve to sense cues whose persistence and stability have time courses similar to those of the consumers generating the signal.
Consumer-specific responses to grazer-associated chemical cues represent a sophisticated defense in marine phytoplankton. The previous findings that P. globosa changed colony size in response to grazer signals (19) and our findings that it changed colony initiation rates (but not size) suggest that this genus may have multiple ways of shape-shifting to lessen loss to consumers and that these shifts may be differentially responsive to specific consumers or to the environmental context within which Phaeocystis senses the presence of the consumers. Given the demonstrated importance of size-selective predation in structuring pelagic food webs (10, 15, 16), species that can significantly alter their sizes should be selected to use chemical information from consumers or attacked conspecifics to induce phenotypes with reduced susceptibility to locally abundant consumers. Such critical chemical cues could be common, but generally overlooked, drivers of ecological patterns and interactions in these systems. Induced responses to grazer cues in pelagic systems could affect food web structure, the timing of phytoplankton blooms, and how energy and nutrients move through pelagic ecosystems (17). Understanding these interactions may be critical to our ability to predict the timing and consequences of phytoplankton blooms, including harmful and nuisance species whose bloom magnitude and frequency of occurrence are increasing at alarming rates (41).
Materials and Methods
Culture Conditions.
A xenic clone of P. globosa (CCMP 627) was grown in L1-Si medium at 20°C under a light/dark cycle of 14 h/10 h at 100–150 microeinsteins (μE) m−2 s−1. Copepods and the Euplotes sp. culture isolate were collected from the Wilmington River Estuary, Savannah, GA. Euplotes sp. cultures were fed Isochrysis galbana with f/2 vitamins and trace metals added and were grown at 25 μE m−2 s−1. Our experiments were set up with solitary cells or colonies as needed for the specific experiments, but it is common for field samples to vary from extremes of most cells occurring as solitary cells to most occurring within colonies and for these conditions to shift with time and location (31, 42, 43). Thus, all of our ratios of solitary cells to colonies may have real-world parallels in some locations or times.
Effects of Mixed Mesozooplankton-Associated Cues.
To examine whether chemical cues from a natural mixture of grazing mesozooplankton affected colony formation, we fitted 750-ml plastic bottles with sides made from 260 cm2 of 1-μm mesh, and we partially submerged the bottles inside 2-liter beakers. Doing so should have permitted chemical exchange between the bottle and beaker but prevented organismal movement between compartments. Equal concentrations of solitary P. globosa cells (50,000 cells per ml) were added to the bottle and beaker, whereas mesozooplankton (>100 μm), dominated by adult copepods (overall density in the sum of the bottle and beaker volumes = 105 ± 8 liter−1), were added to the bottles only. Dominant grazers were the copepods A. tonsa and Pseudodiaptomus pelagicus and the heterotrophic dinoflagellate Noctiluca scintillans. Mesozooplankton were collected by sieving the contents of plankton tows. This process excluded microzooplankton from these experiments. Chambers (n = 3 treatments and 3 controls) were mixed by rotating them on orbital shakers and by lifting each bottle twice daily until half of its volume was displaced into the 2-liter beaker holding the bottle (the grazer-free side for the treatment beakers). This lifting procedure was necessitated by preliminary studies with water-soluble dye, indicating that only 12% of the dye placed in the bottles moved through the screen to the beaker after 24 h when this process relied on diffusion alone. Each lifting process resulted in 50% of the water from the bottles being dispersed into the surrounding beakers; although some of the water would have flowed back into the bottles when they were again submerged, ≈50% of the copepod grazing signal should have moved from the bottles into the beakers. After 6 days, P. globosa cell, colony, and nutrient concentrations in treatment chambers were compared with equivalently treated, grazer-free, controls. During follow-up experiments using these chambers, we found that a few cells passed through the mesh during the slow lifting of the bottles. To assure that changes in treatments were caused by chemical cues alone and not by a small number of cells having experienced direct contact, the remaining experiments were conducted by using cell-free filtrates from containers with versus without feeding grazers.
Effects of Acartia- and Euplotes-Associated Cues.
We also examined how chemical cues from the copepod A. tonsa (n = 3) feeding on P. globosa or the ciliate Euplotes sp. (n = 5) feeding on P. globosa affected colony formation. A. tonsa co-occurs with P. globosa (29), and ciliates can be important grazers during Phaeocystis blooms (44). A single P. globosa culture containing both solitary cells and colonies was inoculated at a concentration of 20,000 solitary cells per ml (A. tonsa experiments) or 100,000 cells per ml (Euplotes sp. experiments) into both treatment and control replicates. For the Euplotes sp. experiment, colonial cells represented an additional 20,000 cells per ml. Adult female Acartia (final density = 200 liter−1) or Euplotes (200 cells per ml) were added to treatment cultures. Natural concentrations of microzooplankton like Euplotes sp. can reach 118 ml−1 during Phaeocystis blooms (32), with densities of copepods being up to ≈20 liter−1 (28, 29). Filtrates were collected 2–2.5 days later by passing cultures through a GF/F filter under low vacuum (≤200 mmHg). Each filtrate was spiked with L1-Si nutrients, restocked with solitary cells at 20,000 cells per ml, and divided among flasks that were rotated on a plankton wheel at 0.5 rpm. After 3 days, P. globosa cell, colony, and nutrient concentrations were determined. Filtration assured that cells would not move across barriers but also resulted in one initial input of chemical signal with no continuous production of grazer-associated cues over the next 3 days. This single-cue input could bias against detecting an effect of grazer-associated cues; our elevated grazer densities producing the initial cues should compensate for this decreased cue production. Given the poor transmission of chemical cues across 1-μm mesh (12% in 24 h) and the passage of a few 20-μm diameter microzooplankton (Oxyrrhis marina) through the 1-μm mesh in preliminary experiments, shifting to filtrates was judged to be the best procedure to avoid the above problems.
Cell Count and Colony Measurements.
Containers were sampled after 3 (Acartia and Euplotes) or 6 days (mesozooplankton). For each replicate, two samples were preserved in Lugol's solution for enumerating either solitary or total cells. Because preserved colonies can release cells, thereby inflating solitary cell counts, colonies were removed and discarded from solitary cell samples by filtering through a 10-μm mesh before preservation. For enumeration, a subsample was settled in Palmer–Maloney chambers (0.1 ml). We counted cells in randomly selected fields of view using a compound microscope at ×100–400 until 1,000 cells were counted per chamber. Samples for total cell counts were agitated before enumeration to break apart colonies for a more even cell distribution in chambers. The colonial cell concentration was determined by subtracting the solitary cell concentration from the total cell concentration. To determine colony concentration, 1 ml from each experimental container was settled for 1 h and counted with an inverted microscope. A colony was defined as a group of four or more cells whose colony matrix was at least partially visible. We measured the diameters of at least 10 randomly selected colonies per replicate.
Effects of Grazer Density.
To assess the response of Phaeocystis to chemical signals from differing densities of grazing copepods (and thus whether our initial high densities may have biased our results), triplicate cultures of Phaeocystis (20,000 solitary cells per ml; 5,000 colony cells per ml) were incubated with A. tonsa at densities of 0, 8, 40, 80, or 200 liter−1. After 2 days, each culture was passed through a GF/F filter. Filtrates were spiked with L1-Si nutrients and restocked at 20,000 solitary cells per ml. The filtrate from each density treatment was divided into three flasks, systematically interspersed on a plankton wheel, and incubated for 3 days.
Adaptive Value of Colony Suppression.
We measured fecal pellet production of A. tonsa when fed either solitary cells or colonies (22,000 cells per ml; n = 8). Fecal pellet production is used as a proxy for ingestion rates for copepods, including A. tonsa (45) and avoids overestimating grazing based on colony or colony cell counts when grazers physically or chemically affect colony formation (46). Had we used direct measures of apparent cell removal, cues from copepods would have suppressed colony formation thereby leading us to overestimate colonies lost to grazing and underestimate grazing on solitary cells (26). Additionally, the colony mucous forms a thin layer on the colony periphery (47) so it is unlikely to represent a significant biomass that would change fecal pellet production relative to copepods feeding on solitary cells. Solitary cells and colonies were separated by reverse filtration. For each replicate, 20 adult female Acartia were incubated with Phaeocystis. All jars were systematically interspersed on a plankton wheel and rotated at 0.5 rpm, and grazing was allowed overnight. Fecal pellets were then collected by gently sieving each jar's contents through 25-μm mesh; retained pellets were counted with a dissecting microscope. Fecal pellet production rates were corrected for surviving copepods and for fecal pellet size using a diet-specific fecal pellet volume, determined by measuring the lengths and widths of at least 50 randomly selected fecal pellets from each diet (see ref. 26).
Adaptive Value of Colony Enhancement.
We measured growth rates of Euplotes feeding on either solitary cells or colonies for 3 days (n = 5). Euplotes (200 cells per ml) and algal prey (250,000 cells per ml) were incubated at 25 μE m−2 s−1. Solitary cells and colonies were separated by reverse filtration, but separation was not completely effective. Initially, solitary cells comprised 60 ± 2% and 11 ± 1% (mean ± SEM) of the total cell concentration in the solitary cell and colony treatment, respectively. Euplotes growth rates on each diet were calculated during exponential growth.
Chemical Investigation of the A. tonsa Cue.
Filtrates from P. globosa cultures (20,000 solitary cells per ml and 5,000 colony cells per ml) with and without Acartia (200 liter−1) that had incubated for 2 days were separated into organic and aqueous fractions by using 2.5-cm (inner diameter) glass columns packed with 20 g of a hydrophobic polymer-based resin (Diaion HP-20; Supelco; Bellefonte, PA). Before use, columns were rinsed with methanol followed by deionized water and then equilibrated with autoclaved seawater. Filtrates from three cultures within each treatment were combined to produce one filtrate that previously held only Phaeocystis and one that held both Phaeocystis and Acartia. Each filtrate was passed repeatedly through an HP-20 column to retain lipid-soluble metabolites (i.e., organic fraction). The liquid passing through these columns retained the more water-soluble compounds (i.e., aqueous fraction). The HP-20 columns were flushed with acetone to retrieve lipid-soluble metabolites, and the solvent was removed by rotary evaporating and freeze-drying. L1-Si medium was added to both the lipid- and water-soluble partitions, dissolution was ensured by sonication, and solitary cells of P. globosa were added at 20,000 cells per ml to the media containing each fraction. Because saturating nutrients were added to both fractions, differences of inorganic nutrients between fractions could not account for changes in colony formation. Each fraction was divided among nine 70-ml culture flasks and systematically interspersed on a plankton wheel that rotated at 0.5 rpm in a 20°C incubator at a light level of ≈100–150 μE m−2 s−1. Phaeocystis cells and colonies and nutrients were sampled after 3 days.
Data Analysis.
Two-tailed ANOVA determined differences in percentages of total cells in colonies (after arcsine transformation), fecal pellet production rates, colony diameter, and Euplotes growth rates. Density-dependent effects were tested by using curvilinear regression.
Supplementary Material
Acknowledgments
We thank J. Kubanek and anonymous reviewers for comments on the manuscript and G.-A. Paffenhöfer and J. Nejstgaard for teaching us copepodology. This work was supported by National Science Foundation Biocomplexity Grant 00P 0083381 (to P. Verity, M. Frischer, M.E.H., and B. Patten), Environmental Protection Agency Science to Achieve Results Fellowship U-91599501-0 (to J.D.L.), and the Teasley Endowment to Georgia Institute of Technology.
Abbreviation
- μE
microeinsteins.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611600104/DC1.
References
- 1.Irigoien X, Huisman J, Harris RP. Nature. 2004;429:863–867. doi: 10.1038/nature02593. [DOI] [PubMed] [Google Scholar]
- 2.Verity PG, Villareal TA, Smayda TJ. J Plankton Res. 1988;10:749–766. [Google Scholar]
- 3.Arrigo KR, Robinson DH, Worthen DL, Dunbar RB, DiTullio GR, VanWoert M, Lizotte MP. Science. 1999;283:365–367. doi: 10.1126/science.283.5400.365. [DOI] [PubMed] [Google Scholar]
- 4.Smith WO, Codispoti LA, Nelson DM, Manley T, Buskey EJ, Niebauer HJ, Cota GF. Nature. 1991;352:514–516. [Google Scholar]
- 5.Weisse T, Tande K, Verity P, Hansen F, Gieskes W. J Marine Syst. 1994;5:67–79. [Google Scholar]
- 6.van Boekel WHM, Hansen FC, Riegman R, Bak RPM. Mar Ecol Prog Ser. 1992;81:269–276. [Google Scholar]
- 7.Chen Y-Q, Wang N, Zhang P, Zhou H, Qu L-H. Biochem Syst Ecol. 2002;30:15–22. [Google Scholar]
- 8.Hamm CE. J Sea Res. 2000;43:307–315. [Google Scholar]
- 9.Rousseau V, Vaulot D, Casotti R, Cariou V, Lenz J, Gunkel J, Baumann M. J Marine Syst. 1994;5:23–39. [Google Scholar]
- 10.Brooks JL, Dodson SI. Science. 1965;150:28–35. doi: 10.1126/science.150.3692.28. [DOI] [PubMed] [Google Scholar]
- 11.Hansen H, Bjornsen PK, Hansen PJ. Limnol Oceanogr. 1994;39:395–403. [Google Scholar]
- 12.Karban R, Baldwin LT. Induced Responses to Herbivory. Chicago: Chicago Univ Press; 1997. [Google Scholar]
- 13.Tollrian R, Harvell CD, editors. The Ecology and Evolution of Inducible Defenses. Princeton: Princeton Univ Press; 1999. [Google Scholar]
- 14.Toth GB, Pavia H. Proc Natl Acad Sci USA. 2000;97:14418–14420. doi: 10.1073/pnas.250226997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE. Ecol Monogr. 2001;71:163–186. [Google Scholar]
- 16.Cottingham KL, Schindler D. Ecology. 2000;81:183–200. [Google Scholar]
- 17.Hay ME, Kubanek J. J Chem Ecol. 2002;28:2001–2016. doi: 10.1023/a:1020797827806. [DOI] [PubMed] [Google Scholar]
- 18.Turner JT, Ianora A, Esposito F, Carotenuto Y, Miralto A. J Plankton Res. 2002;24:1185–1195. [Google Scholar]
- 19.Tang KW. J Plankton Res. 2003;25:831–842. [Google Scholar]
- 20.Jakobsen HH, Tang KW. Aquat Microb Ecol. 2002;27:261–273. [Google Scholar]
- 21.Huntley M, Tande K, Eilertsen HC. J Exp Mar Biol Ecol. 1987;110:197–212. [Google Scholar]
- 22.Tande KS, Bamstedt U. Sarsia. 1987;72:313–320. [Google Scholar]
- 23.Hansen B, Tande KS, Berggreen UC. J Plankton Res. 1990;12:1173–1187. [Google Scholar]
- 24.Peperzak L. PhD dissertation. Groningen: Rijksuniversiteit; 2002. [Google Scholar]
- 25.Cariou V, Casotti R, Birrien JL, Vaulot D. J Plankton Res. 1994;16:457–470. [Google Scholar]
- 26.Long JD, Hay ME. Limnol Oceanogr. 2006;51:988–996. [Google Scholar]
- 27.Rousseau V, Mathot S, Lancelot C. Mar Biol. 1990;107:305–314. [Google Scholar]
- 28.Martens P. Kieler Meeresforsch. 1981;5:153–163. [Google Scholar]
- 29.Weisse T. Mar Biol. 1983;74:87–94. [Google Scholar]
- 30.Bautista B, Harris RP, Tranter PRG, Harbour D. J Plankton Res. 1992;14:691–703. [Google Scholar]
- 31.Davies AG, Demadariaga I, Bautista B, Fernandez F, Harbour DS, Serret P, Tranter PRG. J Mar Biol Assoc UK. 1992;72:691–708. [Google Scholar]
- 32.Admiraal W, Venekamp LAH. Neth J Sea Res. 1986;20:61–66. [Google Scholar]
- 33.Rousseau V, Becquevort S, Parent JY, Gasparini S, Daro MH, Tackx M, Lancelot C. J Sea Res. 2000;43:357–372. [Google Scholar]
- 34.Tang KW, Jakobsen HH, Visser A. Limnol Oceanogr. 2001;46:1860–1870. [Google Scholar]
- 35.Long JD. PhD dissertation. Atlanta: Georgia Institute of Technology; 2004. [Google Scholar]
- 36.Lange M, Chen YQ, Medlin LK. J Phycol. 2002;37:77–92. [Google Scholar]
- 37.Lurling M. J Phycol. 1999;35:19–23. [Google Scholar]
- 38.Long JD, Trussell GT. Mar Ecol Prog Ser. 2007;333:75–80. [Google Scholar]
- 39.Peperzak L. J Plankton Res. 1993;15:809–821. [Google Scholar]
- 40.Hegarty SG, Villareal TA. J Exp Mar Biol Ecol. 1998;226:241–258. [Google Scholar]
- 41.Anderson DM. In: Red Tides: Biology, Environmental Science and Toxicology. Okaichi T, Anderson DM, Nemoto T, editors. New York: Elsevier; 1989. pp. 11–16. [Google Scholar]
- 42.Hamm C, Reigstad M, Riser CW, Muhlebach A, Wassmann P. Mar Ecol Prog Ser. 2001;209:55–69. [Google Scholar]
- 43.Hansen FC, van Boekel WHM. Mar Ecol Prog Ser. 1991;78:123–129. [Google Scholar]
- 44.Weisse T, Scheffel-Moser U. Mar Biol. 1990;106:153–158. [Google Scholar]
- 45.Reeve MR, Walter MA. J Exp Mar Biol Ecol. 1977;29:211–221. [Google Scholar]
- 46.Hansen B, Verity P, Falkenhaug T, Tande KS, Norrbin F. J Plankton Res. 1994;16:487–511. [Google Scholar]
- 47.Hamm CE, Simson DA, Merkel R, Smetacek V. Mar Ecol Prog Ser. 1999;187:101–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.