Abstract
The centromere is the DNA region of the eukaryotic chromosome that determines kinetochore formation and sister chromatid cohesion. Centromeres interact with spindle microtubules to ensure the segregation of chromatids during mitosis and of homologous chromosomes in meiosis. The origin of centromeres, therefore, is inseparable from the evolution of cytoskeletal components that distribute chromosomes to offspring cells. Although the origin of the nucleus has been debated, no explanation for the evolutionary appearance of centromeres is available. We propose an evolutionary scenario: The centromeres originated from telomeres. The breakage of the ancestral circular genophore activated the transposition of retroelements at DNA ends that allowed the formation of telomeres by a recombination-dependent replication mechanism. Afterward, the modification of the tubulin-based cytoskeleton that allowed specific subtelomeric repeats to be recognized as new cargo gave rise to the first centromere. This switch from actin-based genophore partition to a tubulin-based mechanism generated a transition period during which both types of cytoskeleton contributed to fidelity of chromosome segregation. During the transition, pseudodicentric chromosomes increased the tendency toward chromosomal breakage and instability. This instability generated multiple telocentric chromosomes that eventually evolved into metacentric or holocentric chromosomes.
Centromeres are typically composed of rapidly evolving satellite DNA sequences; therefore, centromeric DNA is not broadly conserved throughout evolution. However, in agreement with the conserved centromeric function, many centromere/kinetochore proteins are highly conserved. This apparent paradox can be explained by the coevolution of kinetochore proteins and centromeric DNA sequences as is apparent with the centromere-specific histone H3 variant CENP-A in Drosophila and Arabidopsis (1, 2). The periodic homogenization/amplification undergone by any tandemly repeated DNA sequence via unequal crossing over (3, 4) provides a mechanism for new sequences to be amplified that are specific for novel kinetochore protein variants. Such a progressive substitution of tandemly repeated DNA sequences would explain the lack of discernible homology between centromeric sequences in different organisms. Nevertheless, it is also possible that a detailed study of centromeric sequences could lead to the identification of a common sequence-independent structural recognition determinant within centromeric DNA. Although such a conserved structural motif might direct the formation of centromeric chromatin on its own, the episodic occurrence of centromere activity associated with noncentromeric sequences, neocentromeres (5, 6), and the frequent inactivation/activation of centromeric structures (7–10) indicate that centromere specification involves an epigenetic mechanism that recognizes some characteristic of centromeric DNA (11).
The comparative analysis of the centromeres from Drosophila melanogaster, vertebrates, and plants (12–14) shows a common genome organization: islands of transposons embedded in large regions of simple and/or complex sequence repeats. The subtelomeric regions of all eukaryotic chromosomes consist of mosaics of repeats and retrotransposons structured in a remarkably similar way (15).
The centromeric DNA, RNA, and protein components have been analyzed in recent years. Although the overall organization of centromeres in several eukaryotic species has been described, our understanding of centromere function remains elusive. Understanding the evolutionary origin of the centromere may well elucidate aspects of its function. The conspicuous similarities of the kinetochore proteins among eukaryotes led Tyler-Smith and Floridia (16) to propose a single evolutionary origin of the centromere in an early eukaryotic ancestor. Recently, a systematic phylogenetic analysis of kinetochore proteins has shown that both simple centromeres (Saccharomyces cerevisiae; 125 bp) and complex centromeres (fungi, plants, and animals; up to several megabases) seem to derive from a common ancestor that had complex centromeres containing repetitive DNA (17). Surprisingly, nothing else has been proposed concerning the origin of the centromere. In this article, we will first review ideas about the genome organization and the cytoskeleton of the protoeukaryote and then propose a centromeres-from-telomeres hypothesis for the origin of centromeres.
Genome Organization and Cytoskeleton of the Protoeukaryote
The consensus theory for the evolution of eukaryotes proposes that a symbiosis of bacterial and archaeal cells gave birth to a protoeukaryote containing a bacterial endosymbiont (18–20). Afterward, repeated transfer of genes from the bacterial endosymbiont to the chromosome of the protoeukaryotic cell took place. Recently, comprehensive analyses involving large numbers of genomes have confirmed that the nucleus of the eukaryotic cell contains genes derived from a fusion of archaeal and bacterial genomes (21, 22). During the transition of a presumed ancestral circular genophore to the multiple linear chromosomes found in modern eukaryotes, the structure of the protoeukaryotic genome had to undergo radical changes including linearization, fragmentation, and an increase in genome complexity. The size of prokaryotic genomes is maintained by the rate of gene gain (gene duplication and horizontal gene transfer) being balanced by gene loss (23), and the average genome size is fairly constant. Prokaryotes readily generate tandem DNA repeats, but rapidly delete them again by the same mechanism (24). In sharp contrast, the size of eukaryotic genomes varies by several orders of magnitude. The changes in genome complexity are principally due to a massive proliferation of retrotransposons, tandem repeats, and polyploidization. An additional increase in complexity and size results from spliceosomal introns. It is believed that type II introns began as retroelements that invaded the nuclear genome of the protoeukaryote, from the eubacterial endosymbiont, and subsequently evolved into spliceosomal introns (25, 26). The presence of numerous spliceosomal introns in the same positions in orthologous genes from distant eukaryotes strongly suggests that the first introns arose at the earliest stages of eukaryotic evolution (27–29). A massive mobilization of type II introns might have been activated as a genomic response to the Archaea–Bacteria symbiosis. Such a catastrophic intron invasion might have led to a large reduction in population size, leaving a permissive environment for the fixation of a new genomic organization (30).
Martin and Koonin (31) have recently hypothesized that the nuclear membrane evolved to separate unspliced transcripts from ribosomes; nevertheless, a protoeukaryotic cell could not have evolved a nucleus without concurrently evolving a new machinery of chromosome segregation: the microtubule-based mitotic spindle (32). Long-distance microtubule-based transport would have evolved through a series of progressive changes to become the principal transport mechanism for chromosome segregation. It is probable that microtubules were selected in the protoeukaryotic cell because the microtubule lattice provided a much larger contact surface for the initial centromere/kinetochore capture. The discovery of the bacterial ancestors of tubulin and actin, FtsZ and MreB, has revealed the prokaryotic origin of the cytoskeleton (33). Recent studies have shown that, whereas eukaryotes use microtubules for chromosome segregation, prokaryotes seem to use helical filaments made of actin-like proteins for the same purpose (34, 35). However, in eukaryotes, it is becoming apparent that there is cooperation between the actin- and tubulin-based cytoskeletons at several cell division stages. For instance, the spindle microtubules are required to promote the reorganization of actin filaments into the cleavage furrow during cytokinesis (36), and actin filaments are required to deliver chromosomes to the microtubule spindle during chromosome congression (37, 38).
The accurate partitioning/segregation of genophores and low-copy number plasmids into the daughter cells is often achieved via the action of a pair of partitioning proteins on a palindromic binding site, the parS sequence. Plasmids have only one parS site, but bacterial chromosomes have several parS sequences scattered around the origin of replication (oriC). The parS sequence is a 16-bp palindrome that is bound by a member of the ParB family of partitioning proteins, the Spo0J. Both the parS sequence and the Spo0J protein are conserved in a wide range of bacterial species (39), but the number and distribution of parS sequences vary between species. In Bacillus subtilis, Spo0J binds to eight parS sites scattered within an 850-kb region around the oriC (39); whereas Caulobacter crescentus carries six parS sequences near the oriC (40). Streptomyces coelicolor carries a linear chromosome with 21 parS sites within a 400-kb oriC proximal region (41). In addition, during sporulation in B. subtilis, the RacA protein (for remodeling and anchoring of the chromosome) binds preferentially to a 14-bp GC-rich palindromic sequence and these 25 palindromic sites detected in the chromosome spread, asymmetrically, 612 kb across the oriC (42). Therefore, to ensure proper chromosome segregation during sporulation, the newly replicated copies of the oriC region bind ParB and RacA proteins and rapidly move apart toward opposite poles of the cell. In this sense, the proximal region of the prokaryotic origin of replication (partitioning locus) has been considered as the counterpart of the centromere. However, in eukaryotes, the centromere is often composed of highly repetitive sequences: this represents a major evolutionary innovation, which could not be maintained in prokaryotes, which rapidly eliminate tandem repeat sequences (24). The repetitive nature of centromeres might have been selected because it allowed a rapid karyotype evolution (43).
A Hypothesis for the Origin of the Centromere
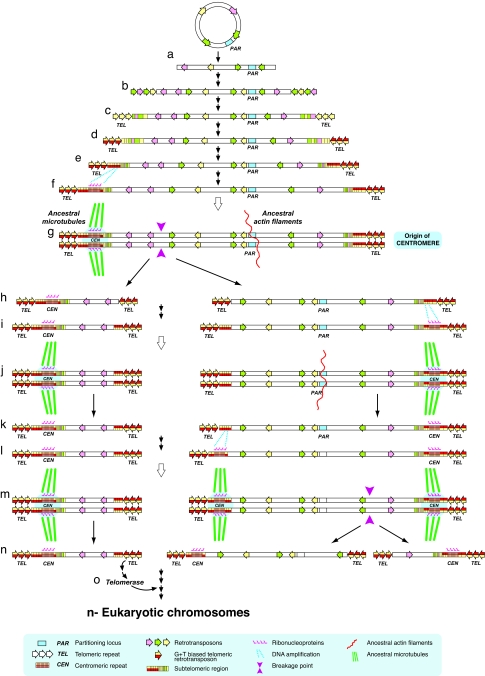
To explain the origin of the centromere, we propose the following parsimonious scenario: to initiate the generation of the first linear chromosome, the breakage of the ancestral circular genophore (Fig. 1a) activated the mobilization of retroelements as a cellular response to the resulting stress (44). The capture of those retrotransposons at the broken DNA ends (45, 46) would eventually heal the exposed ends (47) (Fig. 1b). Later, the retroelement that was most effective for telomere-specific transposition was selected (Fig. 1c). A model for this scenario occurs now in D. melanogaster where three non-LTR retrotransposons, HeT-A, TART, and TAHRE (48–51), maintain telomeres by occasional transposition to the chromosome ends. Moreover, these telomere-specific retrotransposons derived from an ancestral element that was recruited to replace the simple telomerase-generated G-rich repeats (52).
Fig. 1.
Model for the origin and evolution of eukaryotic chromosomes. PAR, TEL, and CEN indicate partitioning locus, telomeric repeat, and centromeric repeat, respectively. A blue square represents the partitioning locus. Transposable elements are shown as plain colored arrows. Double-colored arrows (red and yellow) represent the telomere-specific retrotransposons with a G+T-rich strand bias. Purple arrowheads indicate breakage points. Fuchsia symbols over the centomeres indicate the presence of ribonucleoproteins. Ancestral cytoskeleton is represented with straight green lines for microtubules and with a twisted red line for actin-like filaments. Blue dotted lines represent a DNA amplification. In between steps, a single black arrow indicates a single event, two consecutive black arrows indicate a longer period of evolutionary time, and a big empty arrow indicates just one cell division. (a) The breakage of the ancestral circular genophore leads to the first linear genome. (b) As a cellular response to stress, retroelements are mobilized to heal the DNA ends. (c) A most effective telomeric-specific retrotransposon is selected. (d) A retrotransposon with a more effective capping capability (G+T-rich strand bias) is selected. (c–e) Subtelomeric repeats appear. (f) A specific subtelomeric repeat is amplified. (g) The amplified region is recognized by the tubulin-based cytoskeleton. The coexistence of the centromere and the partitioning locus (each locus recognized by different transport mechanism) produced a pseudodicentric chromosome that might or might not break the chromosome depending on their orientation during anaphase and their differential strength. (h) A breakage of the pseudodicentric occurs, giving rise to the first eukaryotic chromosome. (i) Again, an amplification of the subtelomeric region occurs. (j) A centromere appears, generating a new pseudodicentric. (k) Because of the greater strength of the newly formed centromere, the later pseudodicentric segregates via spindle microtubules without breaking. (l) The partitioning locus would eventually decay. A new amplification of the subtelomeric region occurs at the telomeric end of the telocentric chromosome. (m) The first real dicentric chromosome appears. (n) The breakage of the dicentric chromosome would lead to the formation of two new eukaryotic chromosomes. (o) The appearance of the telomerase to accomplish the maintenance of telomeres was crucial for the stabilization of genome fragmentation.
Some modern bacteria also have linear genomes, and we note that Bao and Cohen (53) have found a reverse transcriptase activity in a Streptomyces telomere-bound topoisomerase. Therefore, the mechanisms that stabilize chromosomes in eukaryotes and linear genophores in some prokaryotes seem to have significant similarities because both use reverse transcriptases.
De Lange (54) has recently hypothesized that telomere function was originally mediated by telomeric loop formation by using terminal repeats. The telomeric loop is proposed to be formed by the insertion of the 3′ overhang into the double-stranded region of telomeric repeats. Therefore, the occurrence of transposon-derived arrays at the DNA ends (Fig. 1c) could allow the formation of telomeric loops. This telomeric configuration would resolve the end-replication problem and would provide the sequestration of the end. In this scenario, a recombination-dependent replication mechanism would allow telomere maintenance.
On the other hand, the conservation of G-rich telomeric sequences in the majority of eukaryotes suggests that telomere capping depends on the inherent ability of G-tracts to form G-quadruplex DNA structures in vitro (55) and in vivo (56). Although the unexpected complexity of the Drosophila telomeric sequences seems to challenge the requirement for the conventional telomerase-synthesized repeats with G-tracts, Drosophila's telomeric retrotransposon arrays have the same strong strand bias: the strand running 5′–3′ toward the end of the chromosome is G+T rich (57). Moreover, the 3′ noncoding region of the abundant HeT-A element contains sequences with the propensity to form G-quadruplex DNA structures (58). Therefore, the mechanism which generated this telomeric G+T strand-biased retrotransposon repeat in the Drosophila lineage might have also acted earlier in eukaryotic evolution (Fig. 1d). This mechanism would have been selected because the resulting strand bias allows the formation of G-quadruplexes, which improve the previous capping by telomeric loops.
The concomitant recombination-based mechanisms during telomere maintenance led inevitably to the rapid divergence of the internal repeats, giving rise to subtelomeric regions (Fig. 1 c–e). In this evolutionary scenario, we propose that, subsequent to the amplification of specific subtelomeric repeats containing G/C tracts (Fig. 1f), these regions became the first centromeres after their recognition as new cargo by the tubulin-based cytoskeleton (Fig. 1g). To envision how microtubules could interact with the first centromeric region, it should be borne in mind that the transcription of centromeric repeats recruits the RNAi machinery to centromeres (59–61); therefore, the interaction between microtubules and the protocentromere could occur through ribonucleoprotein complexes. Several recent findings in Drosophila are in agreement with this suggestion: (i) Argonaute-2 and dFMR1, two components of the RNAi machinery, are required for the assembly of functional centromeres (62, 63); (ii) the FMR1, an RNA binding protein that recognizes G-quadruplex RNA structures (64), appears as a component of both the ribonucleoprotein granules transported along microtubules by dynein and kinesin-1 (65) and the RNAi apparatus (66, 67); and (iii) the transcription and retrotransposition of specific telomere repeats are also under control of the RNAi machinery in the Drosophila germline (68, 69). The RNAi machinery seems to have had a role in centromere function since the beginning of eukaryotic chromosomes because it was responsible for two key processes: the recognition and sequestration of repetitive sequences through repressive chromatin structures (70) and the preferential recruitment of cohesin between sister centromeres (71, 72). In addition, it has been recently shown that ribonucleoprotein complexes are required for mitotic spindle assembly (73).
In an evolving linear chromosome, the rapid turnover of subtelomeric regions could have provided the variability that led to the innovation of a centromere, whereas the partitioning locus (PAR) continued to provide the required segregation function. Once the microtubule-based machinery started to recognize the progenitor centromeric repeats as a novel cargo, the coexistence of a functional centromere with the PAR would have generated pseudodicentrics chromosomes. By analogy to the breakage–fusion cycle of dicentric chromosomes in Zea mays (74), we would expect this chromosomal structure to be unstable and give an increased rate of breakage. A breakage event in which the broken ends became healed by retrotransposition of telomere-specific elements could have generated the first eukaryotic chromosome; whereas the remaining fragment would retain its PAR site (Fig. 1h). The process can be repeated when amplification of the subtelomeric region in the PAR-carrying fragment would occur again (Fig. 1i Right), giving rise to the formation of another centromere recognized by the spindle microtubules (Fig. 1j Right). The studies of Novitski (75) and Hays and Salmon (76) show that the outcome of segregation of eukaryotic chromosomes carrying partially duplicated centromeric regions depend on the relative “strengths” of the centromeric regions. If the strength of the new centromeric region in an ancestral eukaryotic pseudodicentric chromosome were greater than the strength of the PAR site, breakage would not occur and the opposing homologous would be pulled toward the spindle pole by the interaction between the centromere and the spindle microtubules (Fig. 1k Right). A pseudodicentric with a centromere and a PAR site of similar strengths would give an increase in chromosomal breakage during segregation. We cannot know now the order of these evolutionary events, but in a chromosome with a functional centromere, the partitioning locus tends to be lost by sequence decay. Subsequently, the appearance of another centromeric structure at the other end of a telocentric chromosome could generate a dicentric eukaryotic chromosome (Fig. 1 l and m Right) that would eventually break (Fig. 1n Right). The resulting burst of chromosomal breakages might well explain the fragmentation of the early eukaryotic genomes. This early fragmentation of the genome was a major innovation for the cell because it helped to establish highly ordered arrangements of chromatin throughout interphase. Additionally, the frequent nonhomologous recombination of subtelomeric regions (77, 78) produces interchromosomal segmental duplications at the ends of the chromosomes (79). The resultant evolutionary plasticity of these regions has provided an important source of gene invention during the evolution of eukaryotes (80, 81).
After the first steps of this evolutionary process, telomerase evolved from a non-LTR retrotransposon reverse transcriptase (82) that had already acquired introns. The relatively late appearance of telomerase to accomplish the maintenance of the telomeres was nevertheless crucial for the stabilization of chromosome fragmentation (Fig. 1o). Thus, both the origin of the centromere and the origin telomerases (83) appear to correlate with or predate the origin of eukaryotes as linear chromosomes with short GT-rich telomeric repeats are found in the oldest known eukaryotes.
The centromeres-from-telomeres hypothesis is based on considerable cellular, molecular, and structural work on centromeres and telomeres from several groups. The genomic and cytological analyses of the Y centromere of D. melanogaster have shown that a HeT-A/TART-related array, typically found at telomeric ends, is now found at this centromere (10, 84, 85). Recently, the sequence of a clone from this centromeric region has confirmed its telomeric origin (M.M.-L., unpublished work). Furthermore, Berloco et al. (86) have also shown the presence of HeT-A-related sequences in the centromeric region of the Y chromosome of all of the Drosophila species analyzed independent of the centromere position. These results support our proposition that telomere-derived sequences may be involved in centromeric functions (84), and suggest that telomeres and centromeres might have common structural motifs. In fact, it has been shown that telomeric-like sequences are present in centromeric heterochromatin in many vertebrate species (87–89), Arabidopsis thaliana (90), maize (91), and potato (92). Many centromeric satellites from ruminants, birds, plants, flies, and humans share with the telomeric repeats a clear G/C strand asymmetry and may have arisen independently by similar pathways of divergence from the widespread telomeric sequence. In particular, the structural analysis has shown that the centromeric human 5-bp satellite 3, the CENP-B box of the human α satellite, and the D. melanogaster centromeric dodeca satellite form fold-back structures (93–96), a feature previously reported for telomeric sequences. Thus, these studies are compatible with the existence of a common telomeric-like secondary structure in centromeric DNA (97). However, the recent detection of transcripts derived from centromeric tandem repeats would allow the existence of a conserved structural determinant made of RNA. If such a key structural determinant exists, it should also be possible to identify it in neocentromeres. Choo and collaborators (98) have found a significant enrichment of L1 retroelements within a functional human neocentromere and have suggested that these elements may adopt a structural configuration that is more suitable for centromeric chromatin formation. Importantly, Howell and Usdin (99) had shown that the 3′ end of L1 retrotransposons contain a G-rich polypurine tract of variable length and sequence that can form intrastrand quadruplexes. This ability is an evolutionarily conserved characteristic of L1 retrotransposons.
Also noteworthy is that in Shizosaccharomyces pombe, the conserved meiotic telomere clustering to the spindle pole body (the yeast counterpart of the centrosome) during the bouquet stage is likely mediated by cytoplasmic microtubules (100), and the movements for centromere reclustering to the spindle pole body seem to be also controlled by the cytoplasmic microtubular system (101). Additionally, one of the two proteins that connect telomeres to the spindle pole body, Bqt1, shares weak sequence similarity with the centromere protein Dam1, which plays a role in mitotic spindle attachment (100, 102). Interestingly, in fission yeast circular chromosomes that lack telomeric repeats, the telomere–spindle pole body clustering is maintained through the subtelomeric repeat region (103) and this region contains sequences homologous to the S. pombe dh centromere repeats (104).
Most centromere studies have been performed on monocentric chromosomes; however, a large number of eukaryotes have holocentric chromosomes that are characterized by the presence of a diffuse or nonlocalized centromere during mitosis (105). Cytological observations have shown that the ends of holocentric chromosomes attach, randomly, to microtubules to ensure chromosome segregation during meiosis (106–108). Thus, holocentric chromosomes behave as monocentric (telocentric) during the first and second meiotic anaphase. In the nematode Parascaris univalens, the meiotic sites with centromeric activity are terminal heterochromatic blocks (109) that originated by segmental amplification of two simple repeats that are themselves derived from telomerase-generated repeats (110). Recently, in Caenorhabditis elegans, Monen et al. (111) have found that CENP-A, an essential histone required for centromeric chromatin assembly during mitosis, is dispensable for chromosome segregation during meiosis. It is possible that the particular behavior of C. elegans holocentric chromosomes during meiosis could be due to the presence of common structural motifs in telomeres and centromeres.
There is controversy about the evolutionary relationship between holocentric and monocentric chromosomes (112, 113). However, a continuous spreading of end sequences throughout the chromosome could explain a monocentric to holocentric transition during evolution. In agreement with this, the holocentric chromosomes of C. elegans have many tandem repeats (including the telomeric repeat TTAGGC) at multiple internal sites, although they are more abundant at the ends (114, 115).
Finally, a recent cytogenetic analysis has shown that the maize knob satellite, first described as neocentromeres by Rhoades (116), is a subtelomeric element (117).
Any theory regarding the origin of the centromere can be supported only by indirect evidence. As it has been previously described, one basic kind of structural organization appears to underlie the telomeric and centromeric heterochromatin and telomeres behave like centromeres under certain circumstances. According to these facts, it would not be obvious to determine which came first: centromeres or telomeres. However, the alternative scenario where telomeres derived from centromeres was discarded based on the fact that only linear chromosomes have allowed the evolution of complex centromeres containing repetitive sequences. The apparent necessity of linear chromosomes implied that telomeres had to preexist, to establish the ends of these linear chromosomes. Therefore, the origin of telomeres has to have preceded the origin of centromeres. In accordance with this directionality, it is important to highlight that the recurrent appearance of unstable dicentric chromosomes, through the formation of new centromeres (from telomeres), triggered the fragmentation of the genomes, giving rise to karyotypes with multiple chromosomes, as we know them nowadays. Thus, the hypothesis presented on the origin of centromeres not only explains that centromeres derived from telomeres during the evolution of the eukaryotic chromosome but also provides an evolutionary mechanism for the fragmentation of the eukaryotic genomes.
In support of our model, it would be particularly valuable to document the presence of retrotransposons, with G+T strand asymmetry, at the chromosome ends of a number of species that have lost telomerase. To gain new insights into the involvement of common structural determinants in the function of centromeres and telomeres, it would be necessary to investigate the structural behavior of DNA and RNA oligonucleotides from telomeric-like centromeric repeats and the interaction of centromere-associated proteins with the resulting DNA, RNA, or DNA–RNA secondary structures.
Acknowledgments
We dedicate this article to the memory of Antonia Martín-Gallardo, with whom we discussed the early stages of this hypothesis. We thank Lynn Margulis for encouraging us to write this article; C. Tyler-Smith, J. Haber, M. J. Puertas, D. Gubb, C. Goday, I. Sandoval, R. Giraldo, and C. Sentis for helpful criticism of the manuscript; and the reviewers for comments and suggestions. This work was supported by Ministerio de Educación y Ciencia Grant BFU2005-07690-C02-01 (to A.V.) and an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa.”
Abbreviations
- PAR
partitioning locus
- TEL
telomeric repeat
- CEN
centromeric repeat.
Footnotes
The authors declare no conflict of interest.
References
- 1.Malik HS, Henikoff S. Genetics. 2001;157:1293–1298. doi: 10.1093/genetics/157.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talbert PB, Masuelli R, Tyagi AP, Comai L, Henikoff S. Plant Cell. 2002;14:1053–1066. doi: 10.1105/tpc.010425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southern EM. J Mol Biol. 1975;94:51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- 4.Roizes G. Nucleic Acids Res. 2006;34:1912–1924. doi: 10.1093/nar/gkl137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.du Sart D, Cancilla MR, Earle E, Mao JI, Saffery R, Tainton KM, Kalitsis P, Martyn J, Barry AE, Choo KH. Nat Genet. 1997;16:144–153. doi: 10.1038/ng0697-144. [DOI] [PubMed] [Google Scholar]
- 6.Williams BC, Murphy TD, Goldberg ML, Karpen GH. Nat Genet. 1998;18:30–37. doi: 10.1038/ng0198-30. [DOI] [PubMed] [Google Scholar]
- 7.Steiner NC, Clarke LA. Cell. 1994;79:865–874. doi: 10.1016/0092-8674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 8.Fisher AM, Al-Gazali L, Pramathan T, Quaife R, Cockwell AE, Barber JC, Earnshaw WC, Axelman J, Migeon BR, Tyler-Smith C. Chromosoma. 1997;106:199–206. doi: 10.1007/s004120050240. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan BA, Willard HF. Nat Genet. 1998;20:227–228. doi: 10.1038/3024. [DOI] [PubMed] [Google Scholar]
- 10.Agudo M, Abad JP, Molina I, Losada A, Ripoll P, Villasante A. Chromosoma. 2000;109:190–196. doi: 10.1007/s004120050427. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan BA, Blower MD, Karpen GH. Nat Rev Genet. 2001;2:584–596. doi: 10.1038/35084512. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Le HD, Wahlstrom JM, Karpen GH. Genome Res. 2003;13:182–194. doi: 10.1101/gr.681703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schueler MG, Higgins AW, Rudd MK, Gustashaw K, Willard HF. Science. 2001;294:109–115. doi: 10.1126/science.1065042. [DOI] [PubMed] [Google Scholar]
- 14.Nagaki K, Cheng Z, Ouyang S, Talbert PB, Kim M, Jones KM, Henikoff S, Buell CR, Jiang J. Nat Genet. 2004;36:138–145. doi: 10.1038/ng1289. [DOI] [PubMed] [Google Scholar]
- 15.Pryde FE, Gorham HC, Louis EJ. Curr Opin Genet Dev. 1997;7:822–828. doi: 10.1016/s0959-437x(97)80046-9. [DOI] [PubMed] [Google Scholar]
- 16.Tyler-Smith C, Floridia G. Cell. 2000;102:5–8. doi: 10.1016/s0092-8674(00)00004-0. [DOI] [PubMed] [Google Scholar]
- 17.Meraldi P, McAinsh AD, Rheinbay E, Sorger PK. Genome Biol. 2006;7:R23. doi: 10.1186/gb-2006-7-3-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margulis L. Origin of Eucaryotic Cells. New Haven, CT: Yale Univ Press; 1970. [Google Scholar]
- 19.Lake JA, Rivera MC. Proc Natl Acad Sci USA. 1994;91:2880–2881. doi: 10.1073/pnas.91.8.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira D, Lopéz-García P. J Mol Evol. 1998;47:517–530. doi: 10.1007/pl00006408. [DOI] [PubMed] [Google Scholar]
- 21.Horiike T, Hamada K, Kanaya S, Shinozawa T. Nat Cell Biol. 2001;3:210–214. doi: 10.1038/35055129. [DOI] [PubMed] [Google Scholar]
- 22.Rivera MC, Lake JA. Nature. 2004;431:152–155. doi: 10.1038/nature02848. [DOI] [PubMed] [Google Scholar]
- 23.Kunin V, Ouzounis CA. Genome Res. 2003;13:1589–1594. doi: 10.1101/gr.1092603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achaz G, Rocha EP, Netter P, Coissac E. Nucleic Acids Res. 2002;30:2987–2994. doi: 10.1093/nar/gkf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eickbush TH. In: The Evolutionary Biology of Viruses. Morse SS, editor. New York: Raven; 1994. pp. 121–157. [Google Scholar]
- 26.Lambowitz AM, Zimmerly S. Annu Rev Genet. 2004;38:1–35. doi: 10.1146/annurev.genet.38.072902.091600. [DOI] [PubMed] [Google Scholar]
- 27.Roy SW, Gilbert W. Proc Natl Acad Sci USA. 2005;102:1986–1991. doi: 10.1073/pnas.0408355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy SW, Gilbert W. Proc Natl Acad Sci USA. 2005;102:5773–5778. doi: 10.1073/pnas.0500383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sverdlov AV, Rogozin IB, Babenko VN, Koonin EV. Nucleic Acids Res. 2005;33:1741–1748. doi: 10.1093/nar/gki316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch M, Conery JS. Science. 2003;302:1401–1404. doi: 10.1126/science.1089370. [DOI] [PubMed] [Google Scholar]
- 31.Martin W, Koonin EV. Nature. 2006;440:41–45. doi: 10.1038/nature04531. [DOI] [PubMed] [Google Scholar]
- 32.Pickett-Heaps J. BioSystems. 1974;6:37–48. doi: 10.1016/0303-2647(74)90009-4. [DOI] [PubMed] [Google Scholar]
- 33.van den Ent F, Amos L, Lowe J. Curr Opin Microbiol. 2001;4:634–638. doi: 10.1016/s1369-5274(01)00262-4. [DOI] [PubMed] [Google Scholar]
- 34.Moller-Jensen J, Jensen RB, Lowe J, Gerdes K. EMBO J. 2002;21:3119–3127. doi: 10.1093/emboj/cdf320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gitai Z, Dye NA, Reisenauer A, Wachi M, Shapiro L. Cell. 2005;120:329–341. doi: 10.1016/j.cell.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.D'Avino PP, Savoian MS, Glover DM. J Cell Sci. 2005;118:1549–1558. doi: 10.1242/jcs.02335. [DOI] [PubMed] [Google Scholar]
- 37.Forer A, Pickett-Heaps JD. Chromosome Res. 1998;6:533–549. doi: 10.1023/a:1009224322399. [DOI] [PubMed] [Google Scholar]
- 38.Lénárt P, Bacher CP, Daigle N, Hand AR, Eils R, Terasaki M, Ellenberg J. Nature. 2005;436:812–818. doi: 10.1038/nature03810. [DOI] [PubMed] [Google Scholar]
- 39.Lin DC, Grossman AD. Cell. 1998;92:675–685. doi: 10.1016/s0092-8674(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 40.Mohl DA, Easter J, Jr, Gober JW. Mol Microbiol. 2001;42:741–755. doi: 10.1046/j.1365-2958.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 41.Jakimowicz D, Chater K, Zakrzewska-Czerwinska J. J Mol Microbiol. 2002;45:1365–1377. doi: 10.1046/j.1365-2958.2002.03102.x. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Yehuda S, Fujita M, Liu XS, Gorbatyuk B, Skoko D, Yan J, Marko JF, Liu JS, Eichenberger P, Rudner DZ, et al. Mol Cell. 2005;17:773–782. doi: 10.1016/j.molcel.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Kolnicki RL. Proc Natl Acad Sci USA. 2000;97:9493–9497. doi: 10.1073/pnas.97.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClintock B. Stadler Genet Symp. 1978;10:25–47. [Google Scholar]
- 45.Moore JK, Haber JE. Nature. 1996;383:644–646. doi: 10.1038/383644a0. [DOI] [PubMed] [Google Scholar]
- 46.Teng SC, Kim B, Gabriel A. Nature. 1996;383:641–644. doi: 10.1038/383641a0. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y, Fujimoto Y, Arai R, Fujie M, Usami S, Yamada T. Nucleic Acids Res. 2003;31:4646–4653. doi: 10.1093/nar/gkg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason JM, Biessmann H. Trends Genet. 1995;11:58–62. doi: 10.1016/s0168-9525(00)88998-2. [DOI] [PubMed] [Google Scholar]
- 49.Pardue M-L, Danilevskaya ON, Lowenhaupt K, Slot F, Traverse KL. Trends Genet. 1996;12:48–52. doi: 10.1016/0168-9525(96)81399-0. [DOI] [PubMed] [Google Scholar]
- 50.Levis RW, Ganesan R, Houtchens K, Tolar LA, Sheen F-M. Cell. 1993;75:1083–1093. doi: 10.1016/0092-8674(93)90318-k. [DOI] [PubMed] [Google Scholar]
- 51.Abad JP, de Pablos B, Osoegawa K, de Jong PJ, Martin-Gallardo A, Villasante A. Mol Biol Evol. 2004;21:1620–1624. doi: 10.1093/molbev/msh180. [DOI] [PubMed] [Google Scholar]
- 52.Villasante A, Abad JP, Planelló R, Méndez-Lago M, Celniker S, de Pablos B. Genome Res. 2007 doi: 10.1101/gr.6365107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao K, Cohen SN. Proc Natl Acad Sci USA. 2004;101:14361–14366. doi: 10.1073/pnas.0404386101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lange T. Nat Rev Mol Cell Biol. 2004;5:323–329. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- 55.Rhodes D, Giraldo R. Curr Opin Struct Biol. 1995;5:311–322. doi: 10.1016/0959-440x(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 56.Paeschke K, Simonsson T, Postberg J, Rhodes D, Lipps HJ. Nat Struct Mol Biol. 2005;12:847–854. doi: 10.1038/nsmb982. [DOI] [PubMed] [Google Scholar]
- 57.Danilevskaya ON, Lowenhaupt K, Pardue M-L. Genetics. 1998;148:233–242. doi: 10.1093/genetics/148.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abad JP, Villasante A. FEBS Lett. 1999;453:59–62. doi: 10.1016/s0014-5793(99)00695-x. [DOI] [PubMed] [Google Scholar]
- 59.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 60.Topp CN, Zhong C, Dawe RK. Proc Natl Acad Sci USA. 2004;101:15986–15991. doi: 10.1073/pnas.0407154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukagawa T, Nogami M, Yoshikawa M, Ikeno M, Okazaki T, Takami Y, Nakayama T, Oshimura M. Nat Cell Biol. 2004;6:784–791. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- 62.Deshpande G, Calhoun G, Schedl P. Genes Dev. 2005;19:1680–1685. doi: 10.1101/gad.1316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deshpande G, Calhoun G, Schedl P. Genetics. 2006;174:1287–1298. doi: 10.1534/genetics.106.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramos A, Hollingworth D, Pastore A. RNA. 2003;9:1198–1207. doi: 10.1261/rna.5960503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Proc Natl Acad Sci USA. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caudy AA, Myers M, Hannon GJ, Hammond SM. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishizuka A, Siomi MC, Siomi H. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiss D, Josse T, Anxolabehere D, Ronsseray S. Mol Genet Genomics. 2004;272:336–343. doi: 10.1007/s00438-004-1061-1. [DOI] [PubMed] [Google Scholar]
- 69.Savitsky M, Kwon D, Georgiev P, Kalmykova A, Gvozdev V. Genes Dev. 2006;20:345–354. doi: 10.1101/gad.370206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martienssen R, Volpe T, Lippman Z, Gendrel A-V, Kidner C, Rabinowicz P, Colot V. In: RNAi: A Guide to Gene Silencing. Hannon G, editor. Plainview, NY: Cold Spring Harbor Lab Press; 2003. pp. 129–148. [Google Scholar]
- 71.Hall IM, Noma K, Grewal SI. Proc Natl Acad Sci USA. 2003;100:193–198. doi: 10.1073/pnas.232688099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volpe T, Schramke V, Hamilton GL, White SA, Teng G, Martienssen RA, Allshire RC. Chromosome Res. 2003;11:137–146. doi: 10.1023/a:1022815931524. [DOI] [PubMed] [Google Scholar]
- 73.Blower MD, Nachury M, Heald R, Wels K. Cell. 2005;121:223–234. doi: 10.1016/j.cell.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 74.McClintock B. Mo Agric Exp Stn Res Bull. 1938;290:1–48. [Google Scholar]
- 75.Novitski E. J Cell Physiol. 1955;45(Suppl 2):151–169. doi: 10.1002/jcp.1030450509. [DOI] [PubMed] [Google Scholar]
- 76.Hays TS, Salmon ED. J Cell Biol. 1990;110:391–404. doi: 10.1083/jcb.110.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horowitz H, Thorburn P, Haber JE. Mol Cell Biol. 1984;4:2509–2517. doi: 10.1128/mcb.4.11.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Garcia M, Gonzalez-Sanchez M, Puertas MJ. Cytogenet Genome Res. 2006;115:179–185. doi: 10.1159/000095240. [DOI] [PubMed] [Google Scholar]
- 79.Linardopoulou EV, Williams EM, Fan Y, Friedman C, Young JM, Trask BJ. Nature. 2005;437:94–100. doi: 10.1038/nature04029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mefford HC, Trask BJ. Nat Rev Genet. 2002;3:91–102. doi: 10.1038/nrg727. [DOI] [PubMed] [Google Scholar]
- 81.Eichler EE, Sankoff D. Science. 2003;301:793–797. doi: 10.1126/science.1086132. [DOI] [PubMed] [Google Scholar]
- 82.Eickbush TH. Science. 1997;277:911–912. doi: 10.1126/science.277.5328.911. [DOI] [PubMed] [Google Scholar]
- 83.Malik HS, Burke WD, Eickbush TH. Gene. 2000;251:101–108. doi: 10.1016/s0378-1119(00)00207-9. [DOI] [PubMed] [Google Scholar]
- 84.Agudo M, Losada A, Abad JP, Pimpinelli S, Ripoll P, Villasante A. Nucleic Acids Res. 1999;27:3318–3324. doi: 10.1093/nar/27.16.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abad JP, de Pablos B, Agudo M, Molina I, Giovinazzo G, Martin-Gallardo A, Villasante A. Chromosoma. 2004;113:295–304. doi: 10.1007/s00412-004-0318-0. [DOI] [PubMed] [Google Scholar]
- 86.Berloco M, Fanti L, Sheen F, Levis RW, Pimpinelli S. Cytogenet Genome Res. 2005;110:124–133. doi: 10.1159/000084944. [DOI] [PubMed] [Google Scholar]
- 87.Southern EM. Nature. 1970;227:794–798. doi: 10.1038/227794a0. [DOI] [PubMed] [Google Scholar]
- 88.Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OG, Wiley JE, Wurster-Hill DH, Yates TL, Moyzis RK. Chromosoma. 1990;99:3–10. doi: 10.1007/BF01737283. [DOI] [PubMed] [Google Scholar]
- 89.Zhdanova NS, Karamisheva TV, Minina J, Astakhova NM, Lansdorp P, Kammori M, Rubtsov NB, Searle JB. Chromosome Res. 2005;13:617–625. doi: 10.1007/s10577-005-0988-3. [DOI] [PubMed] [Google Scholar]
- 90.Richards EJ, Goodman HM, Ausubel FM. Nucleic Acids Res. 1991;19:3351–3357. doi: 10.1093/nar/19.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alfenito MR, Birchler JA. Genetics. 1993;135:589–597. doi: 10.1093/genetics/135.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tek AL, Jiang J. Chromosoma. 2004;113:77–83. doi: 10.1007/s00412-004-0297-1. [DOI] [PubMed] [Google Scholar]
- 93.Catasti P, Gupta G, Garcia AE, Ratliff R, Hong L, Yau P, Moyzis RK, Bradbury EM. Biochemistry. 1994;33:3819–3830. doi: 10.1021/bi00179a005. [DOI] [PubMed] [Google Scholar]
- 94.Gallego J, Golden EB, Stanley DE, Reid BR. J Mol Biol. 1999;285:1039–1052. doi: 10.1006/jmbi.1998.2334. [DOI] [PubMed] [Google Scholar]
- 95.Ferrer N, Azorín F, Villasante A, Gutierrez C, Abad JP. J Mol Biol. 1995;245:8–21. doi: 10.1016/s0022-2836(95)80034-4. [DOI] [PubMed] [Google Scholar]
- 96.Chou SH, Chin KH. J Mol Biol. 2001;314:139–152. doi: 10.1006/jmbi.2001.5131. [DOI] [PubMed] [Google Scholar]
- 97.Abad JP, Villasante A. Genetica. 2000;109:71–75. doi: 10.1023/a:1026546510127. [DOI] [PubMed] [Google Scholar]
- 98.Chueh AC, Wong LH, Wong N, Choo KH. Hum Mol Genet. 2005;14:85–93. doi: 10.1093/hmg/ddi008. [DOI] [PubMed] [Google Scholar]
- 99.Howell R, Usdin K. Mol Biol Evol. 1997;14:144–155. doi: 10.1093/oxfordjournals.molbev.a025747. [DOI] [PubMed] [Google Scholar]
- 100.Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 101.Goto B, Okazaki K, Niwa O. J Cell Sci. 2001;114:2427–2435. doi: 10.1242/jcs.114.13.2427. [DOI] [PubMed] [Google Scholar]
- 102.Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G. Mol Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 103.Sadaie M, Naito T, Ishikawa F. Genes Dev. 2003;17:2271–2282. doi: 10.1101/gad.1112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mandell JG, Bahler J, Volpe TA, Martienssen RA, Cech TR. Genome Biol. 2005;6:R1. doi: 10.1186/gb-2004-6-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maddox PS, Oegema K, Desai A, Cheeseman IM. Chromosome Res. 2004;12:641–653. doi: 10.1023/B:CHRO.0000036588.42225.2f. [DOI] [PubMed] [Google Scholar]
- 106.Goday C, Pimpinelli S. Chromosoma. 1989;98:160–166. doi: 10.1007/BF00329679. [DOI] [PubMed] [Google Scholar]
- 107.Albertson DG, Thomson JN. Chromosome Res. 1993;1:15–26. doi: 10.1007/BF00710603. [DOI] [PubMed] [Google Scholar]
- 108.Perez R, Panzera F, Page J, Suja JA, Rufas JS. Chromosome Res. 1997;5:47–56. doi: 10.1023/a:1018493419208. [DOI] [PubMed] [Google Scholar]
- 109.Goday C, Gonzalez-Garcia JM, Esteban MR, Giovinazzo G, Pimpinelli S. J Cell Biol. 1992;118:23–32. doi: 10.1083/jcb.118.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niedermaier J, Moritz KB. Chromosoma. 2000;109:439–452. doi: 10.1007/s004120000104. [DOI] [PubMed] [Google Scholar]
- 111.Monen J, Maddox PS, Hyndman F, Oegema K, Desai A. Nat Cell Biol. 2005;12:1148–1155. doi: 10.1038/ncb1331. [DOI] [PubMed] [Google Scholar]
- 112.Nagaki K, Kashihara K, Murata M. Plant Cell. 2005;17:1886–1893. doi: 10.1105/tpc.105.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moore G, Aragon-Alcaide L, Roberts M, Reader S, Miller T, Foote T. Plant Mol Biol. 1997;35:17–23. [PubMed] [Google Scholar]
- 114.Cangiano G, La Volpe A. Nucleic Acids Res. 1993;21:1133–1139. doi: 10.1093/nar/21.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Caenorhabditis elegans Sequencing Consortium. Science. 1998;282:2012–2018. [Google Scholar]
- 116.Rhoades MM, Vilkomerson H. Proc Natl Acad Sci USA. 1942;28:433–436. doi: 10.1073/pnas.28.10.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lamb JC, Meyer JM, Corcoran B, Kato A, Han F, Birchler JA. Chromosome Res. 2007;15:33–49. doi: 10.1007/s10577-006-1102-1. [DOI] [PubMed] [Google Scholar]