Abstract
Social status impacts reproductive behavior in diverse vertebrate species, but little is known about how it affects brain morphology. We explore this in the naked mole-rat, a species with the most rigidly organized reproductive hierarchy among mammals. Naked mole-rats live in large, subterranean colonies where breeding is restricted to a single female and small number of males. All other members of the colony, known as subordinates, are reproductively suppressed. Subordinates can become breeders if removed from the colony and placed with an opposite sex partner, but in nature most individuals never attain reproductive status. We examined the brains of breeding and subordinate naked mole-rats of both sexes, including several regions linked to reproduction and shown to be sexually dimorphic in other mammals. Stereological analyses revealed that neural morphology depends on status, such that breeders, regardless of sex, had more cells than subordinates in the ventromedial nucleus of the hypothalamus and a larger volume of the bed nucleus of the stria terminalis, paraventricular nucleus, and medial amygdala. Several other brain regions examined were unaffected. Surprisingly, males and females did not differ on any measure. These findings provide evidence that a change in social status triggers considerable neural remodeling and indicate that status, rather than sex, has a predominant role in determining neural structure in this remarkably social mammal.
Keywords: eusociality, neuroplasticity, reproductive strategy, sex difference, social status
Sexual dimorphisms have been identified in the nervous systems of all vertebrate classes (1) and presumably arise and persist because they contribute in some way to the reproductive success of the organism. Males and females evolve different reproductive strategies, necessitating different morphologies and behaviors (2). In mammals, sex differences in the central nervous system often can be traced to developmental actions of gonadal steroid hormones (1). However, the study of sexual differentiation of the mammalian nervous system has focused on a limited number of relatively nonsocial species in which reproductive success is largely obtained through direct reproductive efforts.
In social species, reproduction may depend on status within the group, with members ranking lower in the hierarchy forgoing reproduction as well as sex-specific roles associated with reproduction (3). Naked mole-rats (Heterocephalus glaber) exhibit the strictest reproductive hierarchy known to mammals and the closest mammalian equivalent of eusociality. These small rodents, native to Africa, live in underground colonies averaging 60–80 individuals, including a single breeding female (the queen), one to three breeding males, and numerous nonreproductive adults, known as subordinates (4). Subordinates exhibit no mating behavior but assist in foraging, colony defense, maintenance of the tunnel system, and care of the young (4–7). Subordinates can become breeders if a breeding member of the colony dies or if they are removed from their colony and housed with a mate (5, 8, 9). Once established, however, the breeding pair is rarely overthrown, and it is estimated that in nature <5% of all naked mole-rats ever attain reproductive status (10). These animals therefore provide a rare opportunity for testing hypotheses about how social status affects the mammalian brain, particularly regions involved in reproduction.
A change in status within a reproductive hierarchy alters neuronal gene expression, activity, and cell size in an African cichlid fish (11–16). It is not known whether the transition from subordinate to breeder similarly affects the brains of naked mole-rats. Studies of the naked mole-rat brain have been conducted only very recently and have focused on the organization of somatosensory cortex and the visual system (e.g., 17, 18). We recently described the distribution of vasopressin, a neuropeptide associated with social behaviors, in the naked mole-rat brain (19). Morphometric comparisons between the brains of breeders and subordinates, however, have not been made.
The degree to which neural sex differences are present in naked mole-rats also is not known, although subordinates display a relative lack of sex differences in anatomy and behavior. Mean body size does not differ, and the external genitalia and anogenital distance are very similar in male and female subordinates (7, 20, 21). Subordinates of both sexes participate equally in vocalizing, food retrieval, dominance behaviors, colony defense, and pup care (6, 22). Perhaps most significant, we previously found no sex differences in the morphology of the perineal muscles (which attach to the phallus and control penile reflexes in other mammals) or in cell size or number of the innervating motoneurons in the spinal cord (21, 23). Naked mole-rats are, to date, the only mammal that fails to exhibit a sex difference in morphology of this neuromuscular system.
We speculated that sexual differentiation might be “on hold” in subordinate naked mole-rats, and that sex differences might emerge only in those few individuals that become breeders. Alternatively, naked mole-rats might stand out from other mammals in having a sexually monomorphic brain throughout life, irrespective of reproductive status. To address these questions, we performed a stereological analysis of volume, cell number, and cell size in the naked mole-rat brain, using age-matched animals randomly assigned to become breeders or to remain subordinate. All breeders had been paired for at least 4 years and produced at least one litter. We selected brain regions that are related to reproduction and display robust sex differences in other animals (e.g., 24–27): the principal nucleus of the bed nucleus of the stria terminalis (BSTp), the paraventricular nucleus of the hypothalamus (PVN), the medial nucleus of the amygdala (MeA), and the ventromedial nucleus of the hypothalamus (VMH). To test the specificity of effects on hypothalamic, limbic, or general neural morphology, we also examined the suprachiasmatic nucleus (SCN), a hypothalamic region controlling circadian rhythms, the cell dense region of the anterior cortical amygdaloid nucleus (ACo), a limbic region adjacent to MeA that receives olfactory input, and the cortex.
Results
Summary.
The design and major outcomes of the study are depicted schematically in Fig. 1. Significant main effects of breeding status were found, such that breeders had larger volumes or more cells than did subordinates in several brain regions (Figs. 1–3). In contrast, no sex differences were detected on any measure. Statistically significant effects are detailed below (see also supporting information (SI) Table 1 for the presentation of all statistical analyses).
Fig. 1.
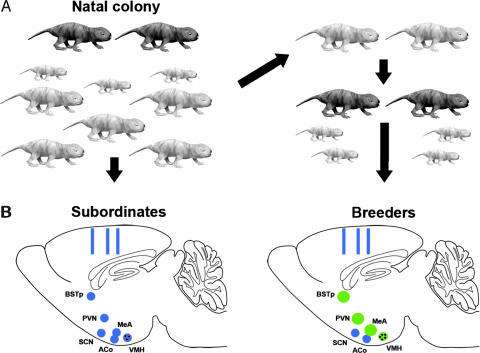
Social status affects reproductive brain nuclei in naked mole-rats. (A) All subjects initially were adult subordinates (light gray) within the natal colony, which also contains breeders (dark gray) and pups of various ages. One-half of the animals were randomly assigned to remain as colony subordinates (Left). The other one-half were removed from the colony and housed with a subordinate of the opposite sex (Right). These paired animals were defined as breeders when they had produced at least one litter. (B Left) Schematic of the sagittal plane of the brain of a subordinate naked mole-rat illustrating the approximate location of the brain regions examined. Hypothalamic and limbic nuclei are indicated by blue circles; rectangles indicate sites of cortical thickness measurements. (Right) The brain of an animal that has transitioned to breeding status. Green circles indicate regions that are either significantly larger (BSTp, PVN, and MeA) or have more cells (black dots, VMH) in breeders. Regions shaded blue did not differ between subordinates and breeders (SCN, ACo, and cortical thickness).
Fig. 2.
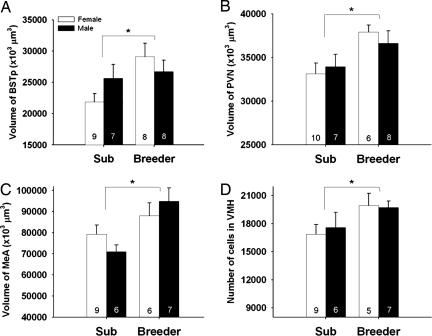
(A–C) Mean (± SEM) regional volume of the BSTp, PVN, and MeA and (D) mean cell number in the VMH of subordinate (Sub) and breeder naked mole-rats. Number of animals per group is noted at the base of each bar. An asterisk indicates a significant main effect of social status. No main effects of sex or status-by-sex interactions were detected for any measure.
Fig. 3.
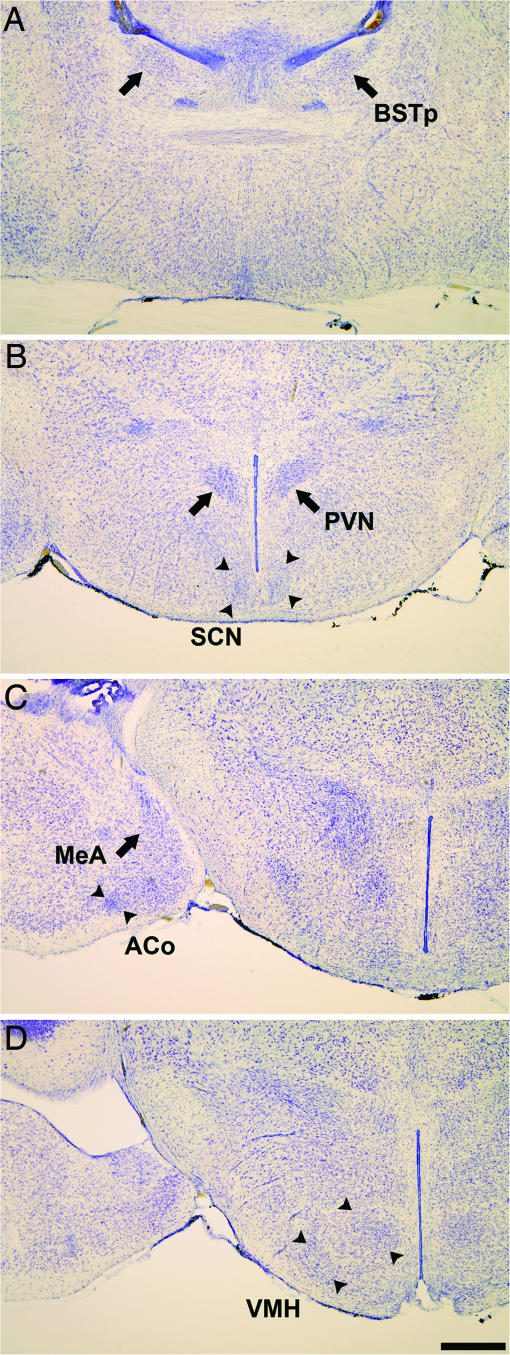
Photomicrographs illustrate the location of nuclei in thionin-stained coronal sections of the naked mole-rat brain. Shown are BSTp (A); the PVN and SCN (B); MeA and ACo (C); and VMH (D). (Scale bar: 500 μm.)
Regional Volume.
Social status significantly affected overall volume of the BSTp (P = 0.034), PVN (P = 0.009), and MeA (P = 0.005), with breeders having larger volumes than subordinates in all cases (Figs. 1 and 2 A–C). Status did not affect volume of the VMH, SCN, or ACo. We did not find a significant main effect of sex on volume in any brain region. Similarly, no sex-by-status interactions were detected.
Cell Number.
Breeding naked mole-rats, regardless of sex, had more cells in the VMH than subordinates (P = 0.04; Figs. 1 and 2D). This trend was seen in both major subdivisions of the nucleus: the dorsomedial and ventrolateral VMH. There was no effect of breeding status on cell number in any other region examined. In addition, no significant effects of sex or sex-by-status interactions on cell number were detected.
Cell Size and Cortical Thickness.
Cell size (cross-sectional area) did not vary significantly by sex or status, and there were no sex-by-status interactions on cell size in any brain region. However, the effect of status on cell size in the PVN approached statistical significance (larger in breeders; P = 0.057). As a measure of general brain size, we also examined cortical thickness in sections containing the reproductive nuclei described above. Cortical thickness did not vary by sex or status, nor was there a sex-by-status interaction on this measure (all P values >0.60).
Discussion
Taken together, these results suggest that in naked mole-rats, social status has greater influence on brain morphology than does sex. We found no sex difference in any measure. We also found no sex-by-status interactions, which might be predicted if a brain region that was monomorphic in subordinates became sexually dimorphic in breeders. We did, however, find several significant main effects of breeding status: breeders had a larger BSTp, PVN, and MeA and more cells in the VMH than did subordinates. There were no effects of sex or status on the SCN, ACo, or cortical thickness, demonstrating that not all brain regions exhibited differences as a function of status. Because animals were randomly assigned to become a breeder or remain subordinate, we conclude that the change in status caused the neural modifications.
The brain regions affected by status in naked mole-rats (BSTp, PVN, MeA, and VMH) comprise key nodes of a circuit consistently linked to neuroendocrine function and reproductive behaviors in other rodents (28, 29). These same regions are also sexually dimorphic in other species [refs. 24–27; the SCN, which was not affected by status, may be sexually dimorphic (30), but see ref. 31]. We did not find sex differences in any area, nor did we find a clearly discernable nucleus that might be homologous to the sexually dimorphic nuclei of the medial preoptic area of other mammals (e.g., 32, 33). We do not conclude, however, that there are no morphological sex differences in the naked mole-rat brain. Sample sizes were relatively small, and many more brain regions and other levels of analysis would have to be considered before one reached such a conclusion. If sexual dimorphisms do exist in the naked mole-rat brain, however, they appear to be more subtle than the effects of social status, which are detectable at a gross level using even small sample sizes. Taken together with reports on spinal motoneurons innervating perineal muscles (21, 23), and vasopressin innervation of the naked mole-rat brain (19), in which no sex differences were found, these data suggest that neural sex differences are attenuated in naked mole-rats compared with other mammals.
We speculate that the relative lack of sexual differentiation in naked mole-rats may be related to their unusual mating system. Although environmental or social cues can alter the timing of puberty and reproductive behaviors in many mammals (34, 35), the large majority of individuals in most species become reproductively mature and exhibit sex-specific behaviors by the time that they approach full adult body size. By contrast, >95% of naked mole-rats will remain subordinate throughout life (10). It seems likely that some of the typical specializations for male or female roles are unnecessary and may even be detrimental to success as helpers in a eusocial species. Naked mole-rats belong to the family Bathyergidae, which includes ≈15 species ranging in social organization from solitary to highly social (36). It would be of interest to determine whether Damaraland mole-rats (Cryptomys damarensis), which exhibit less reproductive skew than naked mole-rats but may also be considered eusocial (36), exhibit a comparable absence of neural sex differences and whether such differences are more prominent in solitary bathyergids. An attenuation of sex differences in naked mole-rats also agrees with sexual selection theory, which predicts that dimorphisms in secondary sex characteristics will be more pronounced in polygamous than in monogamous species (e.g., 37, 38). Although naked mole-rats are not strictly monogamous, they do form long term, stable reproductive bonds.
In the most extensively studied rodent model systems, gonadal hormones organize the nervous system as male or female during perinatal life (39). Nothing is known about the perinatal endocrinology of naked mole-rats, but the available evidence suggests that few permanent sex differences are established at this time. Once this developmental period has passed, there may be constraints on sex-related brain plasticity. For example, several of the best known neural sex differences are in cell number and depend on differential cell death in males and females during development (40). After the developmental cell death period, cell number may be relatively immutable. Here, we observed one change in cell number associated with a change in status: an increase in the number of cells in the VMH of breeders. The addition of new cells in adulthood has rarely been reported in the rodent hypothalamus. Interestingly, however, in one of the few such reports a social manipulation (exposure to a male) increased the number of newly generated cells in the VMH of female prairie voles (41).
Perhaps the most interesting question raised by the data presented here is: What are the social cues responsible for the neural changes observed in breeders? We presume that such cues are related to the signals that normally suppress reproduction in subordinates and that trigger a change in neuroendocrine function and behavior when a subordinate is removed from its colony. A role for pheromones in the reproductive suppression of subordinate naked mole-rats has so far not been substantiated: exposing newly isolated subordinates to soiled bedding from their former colony does not prevent reproductive activation (42, 43). However, the breeding female frequently shoves other individuals and exhibits several other forms of behavioral dominance (4, 8, 44, 45). Thus, it has been suggested, albeit without direct evidence, that the queen exerts reproductive inhibition via dominance behaviors (42, 43). An important cue triggering changes in brain morphology in new breeders may therefore be the removal of such suppressive signals from the queen. Alternatively, or in addition, the introduction of “positive” social cues arising from the new mate may also play a role.
Effects of social cues and reproductive status on the brain have been particularly well studied in fish. For example, changes in the social environment trigger changes in reproductive status and alterations in neural morphology and neuropeptide gene expression in males of the species Astatotilapia burtoni (11–15). The behavioral and neural changes are reversible, as A. burtoni males may switch status both rapidly and repeatedly (46). Increases in immediate-early gene expression are observed in gonadotrophin-releasing hormone (GnRH) neurons within minutes of a relevant social stimulus, suggesting that this is a very early event in a molecular cascade presumably leading to longer-term changes (16). Breeding status is also reflected in altered neuropeptide expression in naked mole-rats, with subordinates exhibiting less vasopressin in the dorsomedial hypothalamus (19). GnRH neurons have not yet been examined in naked mole-rats, but subordinates release less pituitary luteinizing hormone in response to a GnRH pulse than do breeders (47).
However, in contrast to the changes in A. burtoni, the rise to breeding status of a former subordinate in a naked mole-rat colony is a protracted process; many months may elapse before a new breeding pair is established after the death or removal of the former breeders (7). The transition to breeding status also does not appear to be reversible; naked mole-rats are the longest-lived rodents, routinely surviving >20 years in captivity, and breeders generally maintain their status until death (20, 48). Although we do not know how long an animal must be a breeder before measurable neural changes occur, we suggest that the increases in neuron number and overall size of reproductive brain regions seen here may represent a relatively stable, long-term outcome of events occurring much more rapidly. Currently, the earliest physiological marker heralding a change in status in naked mole-rats is the increase in reproductive hormones seen 5–8 days after separating a subordinate from its colony (8, 42, 49).
The endocrine changes that accompany a change in breeding status (50) suggest that hormones must be considered as possible factors mediating effects of social status on brain morphology. Each of the reproductive brain regions we examined expresses gonadal steroid hormone receptors in other rodents (51, 52), and our own preliminary observations confirm that the four regions showing effects of status also exhibit androgen receptor immunoreactivity in naked mole-rats (M.M.H., unpublished work). Behavioral observations, however, suggest that some effects of breeding status on neural morphology may prove to be independent of the gonads in naked mole-rats. In a colony setting, only the breeders display genital nuzzling and they do so at all times of the queen's ovulatory cycle, during pregnancies, and after gonadectomy of both members of the breeding pair (6, 53). Similarly, breeding pairs maintain their status and continue to suppress reproduction of other colony members over long periods of time, even when not producing offspring and in the absence of gonads (53). Some effects of social status on the brain and behavior are also likely to be independent of the gonads in fish (16, 54).
We do not know whether the brain changes seen here would be maintained following gonadectomy of the breeders. If so, then gonadal steroids could be ruled out as necessary for maintaining these neural differences. It would remain possible that gonadal steroids are required for initiating the changes or that steroids from a nongonadal source (e.g., neurosteroids or adrenal steroids) are responsible. Adrenal glucocorticoids are associated with social stress and/or social rank and also can have effects on neural morphology (55), but evidence as to whether cortisol levels relate to social status in naked mole-rat colonies has been contradictory (45, 56). The recent demonstration that steroid hormone receptors can be activated by neurotransmitters, in the absence of hormone (so-called ligand-independent activation of receptors), suggests another pathway by which the environment may influence reproductive brain regions (57). More generally, the activation of neural gene expression either by hormones or neurotransmitters provides a framework for understanding how social stimuli may affect brain morphology.
Naked mole-rats lie at one extreme of the spectrum of sociality displayed by mammals, with larger colony sizes, greater interrelatedness of colony members, and more marked behavioral specializations than seen in any other social mammal (38). Nonetheless, many mammalian species (particularly among the rodents, canids, and primates) exhibit a reproductive division of labor, in which some members of the social group reproduce whereas others are reproductively suppressed and assist in rearing young that are not their own (58). Although effects of social status on the brain may be more pronounced and therefore easier to detect in naked mole-rats, the phenomenon is likely to apply much more broadly.
Materials and Methods
Animals and Tissue Collection.
Housing conditions and animal history and care have been reported (23). Brains from 8 breeding females, 8 breeding males, 10 subordinate females, and 8 subordinate males were collected. Animals were anesthetized and rapidly decapitated, and brains were removed and immersion fixed in 5% acrolein in phosphate buffer for 4 h. Brains were then transferred to 30% sucrose in phosphate buffer and frozen-sectioned at 30 μm in the coronal plane. Alternate sections were mounted onto slides and stained with thionin. All procedures adhered to institutional and federal guidelines.
Tissue Analyses.
All measures were performed on slides coded to conceal the sex and status of the animals. Stereological analyses of the BSTp, SCN, PVN, ACo, MeA, and VMH (Fig. 3) were performed by using StereoInvestigator software (MicroBrightfield, Williston, VT).
The VMH was initially subdivided into three regions (dorsomedial, central, and ventrolateral), which were analyzed separately. Because VMH subregion did not significantly interact with either sex or status in statistical analyses, subregions were combined in the data presented here. To calculate overall volumes, outlines of each region were traced in each section, and the summed areas were multiplied by section thickness. Unbiased estimates of cell number within each region were obtained by using the optical disector method, with parameters adjusted for each brain region. Counting frames varied from 16 × 16 μm to 20 × 20 μm, and sampling grid size was varied from 80 × 80 μm to 150 × 150 μm depending on the size of the region analyzed. For a given measure, parameters were held constant for all animals. Mean cell size in each region was determined by randomly placing a grid in each section through the rostral-caudal extent of each brain region and tracing every in-focus cell with a neuronal morphology that fell within the grid. At least 50 cells per animal were traced for each region. All volume and cell count data reflect unilateral estimates.
For each animal, cortical thickness was measured in three sections chosen to correspond to the level of (i) the BSTp (most anterior), (ii) SCN and PVN, and (iii) ACo, MeA and VMH (most posterior). Three measurements were taken from each hemisphere in each of the chosen sections, resulting in an average cortical thickness based on a total of 18 measurements per animal. Measures were taken from the lower boundary of layer I to the beginning of the white matter below layer VI. The first measure was taken immediately lateral to the elevation of the corpus callosum, and second and third measures were taken 150 and 300 μm lateral, respectively (as in ref. 59).
Dependent variables were analyzed by using two-way ANOVAs (sex-by-status). Tissue artifact caused some animals to be removed from analyses for specific brain regions. There was always a minimum of five animals per group per brain region.
Supplementary Material
Acknowledgments
We thank Sharry Goldman for outstanding assistance with animal husbandry. We also thank Marc Breedlove, Ashley Monks, John Morris, and Marianne Seney for helpful discussions and advice. This work was funded by National Science Foundation Grant IOB-0344312 (to N.G.F., B.D.G., and G.J.d.V.), National Institutes of Health Grant K02 MH072825 (to N.G.F.), and a Canadian Institutes of Health Research postdoctoral fellowship (to M.M.H.).
Abbreviations
- BSTp
principal nucleus of the bed nucleus of the stria terminalis
- PVN
paraventricular nucleus of the hypothalamus
- MeA
medial nucleus of the amygdala
- VMH
ventromedial nucleus of the hypothalamus
- SCN
suprachiasmatic nucleus
- ACo
anterior cortical amygdaloid nucleus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610344104/DC1.
References
- 1.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Front Neuroendo. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 2.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: Murray; 1871. [Google Scholar]
- 3.Abbot DH. J Zool. 1987;213:455–470. [Google Scholar]
- 4.Jarvis JUM. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 5.Brett RA. In: The Biology of the Naked Mole-Rat. Sherman PW, Jarvis JUM, Alexander RD, editors. Princeton: Princeton Univ Press; 1991. pp. 97–136. [Google Scholar]
- 6.Lacey EA, Alexander RD, Braude SH, Sherman PW, Jarvis JUM. In: The Biology of the Naked Mole-Rat. Sherman PW, Jarvis JUM, Alexander RD, editors. Princeton: Princeton Univ Press; 1991. pp. 209–242. [Google Scholar]
- 7.Lacey EA, Sherman PW. In: The Biology of the Naked Mole-Rat. Sherman PW, Jarvis JUM, Alexander RD, editors. Princeton: Princeton Univ Press; 1991. pp. 275–336. [Google Scholar]
- 8.Faulkes CG, Abbott DH, Jarvis JUM. J Reprod Fertil. 1990;88:559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- 9.Margulis SW, Saltzman W, Abbott DH. Horm Behav. 1995;29:227–247. doi: 10.1006/hbeh.1995.1017. [DOI] [PubMed] [Google Scholar]
- 10.Jarvis JUM, O'Riain MJ, Bennett NC, Sherman PW. Trends Ecol Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 11.Davis MR, Fernald RD. J Neurobiol. 1990;21:1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- 12.Francis RC, Soma K, Fernald RD. Proc Natl Acad Sci USA. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann HA, Fernald RD. J Neurosci. 2000;20:4740–4744. doi: 10.1523/JNEUROSCI.20-12-04740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White SA, Nguyen T, Fernald RD. J Exp Biol. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood AK, Fernald RD. Biol Reprod. 2004;71:909–918. doi: 10.1095/biolreprod.104.030072. [DOI] [PubMed] [Google Scholar]
- 16.Burmeister SS, Jarvis ED, Fernald RD. PLoS Biol. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catania KC, Remple MS. Proc Natl Acad Sci USA. 2002;99:5692–5697. doi: 10.1073/pnas.072097999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao J, Levitt JB, Buffenstein R. Brain Res. 2006;1077:81–89. doi: 10.1016/j.brainres.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. J Comp Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis JUM. In: The Biology of the Naked Mole-Rat. Sherman PW, Jarvis JUM, Alexander RD, editors. Princeton: Princeton Univ Press; 1991. pp. 384–425. [Google Scholar]
- 21.Peroulakis ME, Goldman B, Forger NG. J Neurobiol. 2002;51:33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- 22.Pepper JW, Braude SH, Lacey EA, Sherman PW. In: The Biology of the Naked Mole-Rat. Sherman PW, Jarvis JUM, Alexander RD, editors. Princeton: Princeton Univ Press; 1991. pp. 243–274. [Google Scholar]
- 23.Seney ML, Goldman BD, Forger NG. J Neurobiol. 2006;66:1354–1364. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto A, Arai Y. Endocrinol Jpn. 1983;30:277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- 25.Cooke BM, Tabibnia G, Breedlove SM. Proc Natl Acad Sci USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Proc Natl Acad Sci USA. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viau V, Bingham B, Davis J, Lee P, Wong M. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 28.De Vries GJ, Simerly RB. In: Hormones, Brain, and Behavior, Volume IV. Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Moss RL, Rubin RT, editors. San Diego: Academic; 2002. pp. 137–191. [Google Scholar]
- 29.Simerly RB. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 30.Abizaid A, Mezei G, Sotonyi P, Horvath TL. Eur J Neurosci. 2004;19:2488–2496. doi: 10.1111/j.0953-816X.2004.03359.x. [DOI] [PubMed] [Google Scholar]
- 31.Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJG. J Comp Neurol. 1995;361:585–601. doi: 10.1002/cne.903610404. [DOI] [PubMed] [Google Scholar]
- 32.Gorski RA, Gordon JH, Shryne JE, Southam AM. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 33.Swaab DF, Hofman MA. Brain Res Dev Brain Res. 1988;44:314–318. doi: 10.1016/0165-3806(88)90231-3. [DOI] [PubMed] [Google Scholar]
- 34.Vandenbergh JG. Proc Life Sci. 1983:342–349. [Google Scholar]
- 35.Foster DL, Ebling FJ, Claypool LE. Reprod Nutr Dev. 1988;28:349–364. doi: 10.1051/rnd:19880302. [DOI] [PubMed] [Google Scholar]
- 36.Bennett NC, Faulkes CG. African Mole-Rats: Ecology and Eusociality. Cambridge, UK: Cambridge Univ Press; 2000. [Google Scholar]
- 37.Kleiman DG. Quart Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 38.Alexander RD, Hoogland JL, Howard RD, Noonan KM, Sherman PW. In: Evolutionary Biology and Human Social Behavior: An Anthropological Perspective. Chagnon NA, Irons W, editors. Belmont, CA: Duxbury; 1979. pp. 402–435. [Google Scholar]
- 39.Morris JA, Jordan CL, Breedlove SM. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 40.Forger NG. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Fowler CD, Liu Y, Ouimet C, Wang Z. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 42.Faulkes CG, Abbott DH. J Reprod Fertil. 1993;99:225–230. doi: 10.1530/jrf.0.0990225. [DOI] [PubMed] [Google Scholar]
- 43.Smith TE, Faulkes CG, Abbott DH. Horm Behav. 1997;31:277–288. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- 44.Reeve HK. Nature. 1992;358:147–149. doi: 10.1038/358147a0. [DOI] [PubMed] [Google Scholar]
- 45.Clarke FM, Faulkes CG. Proc R Soc Lond B. 1998;265:1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofmann HA, Benson ME, Fernald RD. Proc Natl Acad Sci. 1999;96:14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faulkes CG, Abbott DH, Jarvis JU, Sherriff FE. J Reprod Fertil. 1990;89:317–323. doi: 10.1530/jrf.0.0890317. [DOI] [PubMed] [Google Scholar]
- 48.Buffenstein R. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 49.Faulkes CG, Abbott DH. J Reprod Fertil. 1991;93:427–435. doi: 10.1530/jrf.0.0930427. [DOI] [PubMed] [Google Scholar]
- 50.Faulkes CG, Abbott DH, Jarvis JU. J Reprod Fertil. 1991;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- 51.Simerly RB, Chang C, Muramatsu M, Swanson LW. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 52.Shughrue PJ, Lane MV, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Goldman SL, Forger NG, Goldman BD. Horm Behav. 2006;50:77–84. doi: 10.1016/j.yhbeh.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Godwin J, Crews D, Warner RR. Proc R Soc Lond B. 1996;263:1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- 55.Tamashiro KL, Nguyen MM, Sakai RR. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Clarke FM, Faulkes CG. Proc R Soc Lond B. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blaustein JD. Ann N Y Acad Sci. 2003;1007:238–250. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 58.Solomon NG, French JA. In: Cooperative Breeding in Mammals. Solomon NG, French JA, editors. New York: Cambridge Univ Press; 1997. pp. 1–10. [Google Scholar]
- 59.Diamond MC, Krech D, Rosenzweig MR. J Comp Neurol. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.