Abstract
CXC chemokine ligand 13 (CXCL13), CC chemokine ligand 21 (CCL21), and CCL19 are constitutively expressed in secondary lymphoid organs, where they control the placement of lymphocytes and dendritic cells. However, these chemokines are also inducibly expressed in the lung after influenza infection. Here we show that, in the absence of spleen and lymph nodes, the expression of homeostatic chemokines in the lung is essential for local B and T cell responses to influenza and for the development and organization of inducible bronchus-associated lymphoid tissue (iBALT). Surprisingly, despite the association between local CXCL13 expression and the formation of ectopic lymphoid tissues, the loss of CXCL13 in the lung had minimal impact on either the development or function of iBALT. In contrast, the loss of CCL19 and CCL21 impaired iBALT formation as well as B and T cell responses. These results demonstrate that the local expression of homeostatic chemokines in nonlymphoid organs, such as the lung, plays an important role in protective immune responses.
Keywords: inducible bronchus, associated lymphoid tissue, ectopic lymphoid follicle, pulmonary inflammation, mucosal immunity
Chemokines regulate immunity and inflammation (1) and are often divided into inflammatory chemokines, which are inducibly expressed at sites of inflammation or infection, and homeostatic chemokines, which are constitutively expressed in secondary lymphoid organs (2). Inflammatory chemokines are expressed in the lung on viral infection (3–6) and contribute to both innate and adaptive immune responses. For example, macrophage-inflammatory protein-1α is important for pulmonary inflammation after influenza infection and for viral clearance (7). In addition, CC chemokine ligand 5 (CCL5) is induced in airway epithelial cells after viral infection and promotes macrophage survival and trafficking to the airways (8). In contrast, homeostatic chemokines, such as CXC chemokine ligand 13 (CXCL13), CCL19, and CCL21, are normally expressed in secondary lymphoid organs, where they direct the steady-state recruitment and placement of lymphocytes and dendritic cells (DCs) (9, 10). Homeostatic chemokines can also be inducibly expressed in nonlymphoid tissues after inflammation or infection and may help to recruit lymphocytes to these tissues during local immune responses (11). For example, CXCL13, CCL19, and CCL21 are expressed in the lung after influenza infection in areas of B and T cell accumulation (12). Some of these lymphoid accumulations resemble ectopic lymphoid follicles, with separated B and T cell zones, well developed germinal centers, and high endothelial venules (HEVs). We have termed these areas inducible bronchus-associated lymphoid tissue (iBALT) (12–14).
Ectopic lymphoid tissues are often associated with chronic diseases, such as rheumatoid arthritis, diabetes, and thyroiditis, and are thought to be organized by the local expression of CXCL13 and CCL21 (14–17). Ectopic lymphoid tissues support local immune responses (18, 19). However, their formation does not always reflect immune activity, because transgenic mice expressing CXCL13, CCL19, or CCL21 under the ectopic promoters all develop well formed ectopic lymphoid follicles, yet do not develop autoimmunity (20–23). Despite the clear association of homeostatic chemokines, particularly CXCL13, with the formation of ectopic lymphoid follicles, it is difficult to distinguish the potential roles of these chemokines in the development and function of ectopic lymphoid tissues from their established functions in secondary lymphoid organs. Thus, although it is clear that Cxcl13−/− and plt/plt mice make impaired immune responses (24–26), it is less clear whether these defects are due to impaired function of conventional lymphoid organs or are additionally due to the failure of local immunity.
Results
Pulmonary Expression of CCR7 Ligands Is Important for Local Immune Responses to Influenza.
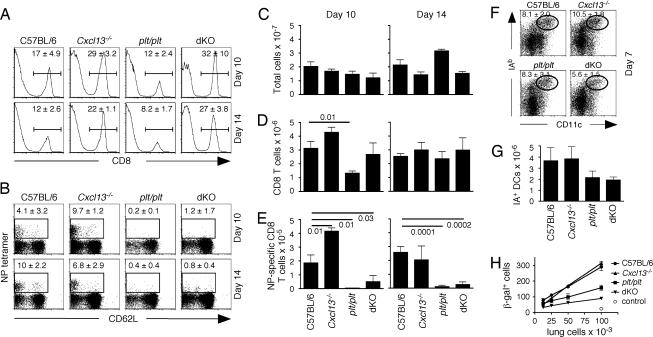
We wanted to evaluate the importance of CXCL13, CCL19, and CCL21 in pulmonary immune responses to influenza by using chemokine-deficient mice. However, because these chemokines are critical for the development, organization, and function of secondary lymphoid organs, it is difficult to distinguish their potential role in the lung from their established roles in spleen and lymph nodes (LNs). Because we previously showed that mice lacking spleen and LNs make immune responses to influenza and clear virus with no adverse effects (12), we decided to eliminate spleen and LNs in chemokine-deficient mice and evaluate the role of CXCL13, CCL19, and CCL21 only at the local level in the lung. To do this, we treated pregnant C57BL/6, Cxcl13−/−, plt/plt, and Cxcl13−/− × plt/plt [double knockout (dKO)] mice with soluble lymphotoxin β receptor (LTβR)-Ig and TNFR1-Ig to block LN development (27). We splenectomized the LN-deficient mice at 6 weeks of age, infected them with influenza 4 weeks later, and assayed local immune responses in the lung. As shown in Fig. 1A, the frequency of CD8+ T cells recruited to the lung was somewhat reduced in plt/plt mice, but was relatively high in Cxcl13−/− and dKO mice. However, the ability of mice to generate nucleoprotein (NP)-specific CD8 T cells was impaired in plt/plt and in dKO mice, but was minimally affected in Cxcl13−/− mice (Fig. 1B). Although the numbers of total cells and the numbers of CD8 T cells in the lungs were similar in all groups at both time points (despite a drop in the number of CD8 T cells in the lungs of plt/plt mice on day 10) (Fig. 1 C and D), the numbers of NP-specific CD8 T cells were reduced in the lungs of plt/plt and dKO mice (Fig. 1E). Poor influenza-specific T cell responses in plt/plt and dKO mice correlated with reduced numbers of DCs in the lungs (Fig. 1 F and G) and with impaired antigen-presenting capacity of lung leukocytes (Fig. 1H).
Fig. 1.
Influenza-specific CD8 responses are impaired in spleen- and LN-deficient plt/plt mice. (A and B) Spleen- and LN-deficient mice were infected with influenza, and the frequencies of CD8 T cells (A) and NP-specific CD8 T cells (B) in the lungs were evaluated by flow cytometry. The data shown are gated on live lymphocytes (A) or live CD8 T cells (B). (C–E) Total leukocytes were enumerated in the lungs of infected mice by counting (C), and the total numbers of CD8 T cells (D) and NP-specific CD8 T cells (E) were calculated. There were four to five mice per group at each time. Statistical significance was evaluated by using an unpaired t test. (F and G) The frequency of activated DCs in the lungs was determined 7 days after infection by flow cytometry (F), and the total number of CD11c+IAb+ cells in the lungs was calculated (G). (H) Serial dilutions of total leukocytes from the lungs of infected mice were cultured overnight with NP-specific T cell hybridoma cells that contained an IL-2–β-galactosidase reporter construct, and blue cells were enumerated.
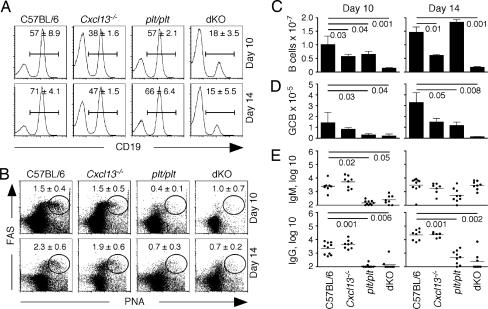
We next examined B cell responses to influenza. Cxcl13−/− mice had relatively normal frequencies of B cells (Fig. 2A), whereas the frequency of B cells in the lungs of dKO mice was reduced. Germinal center B cells (GCB) were easily detected in the lungs of C57BL/6 and Cxcl13−/− mice on days 10 and 14 (Fig. 2B). However, well defined populations of GCB were difficult to find in either plt/plt mice or in dKO mice at either time (Fig. 2B). The number of B cells in the lungs of Cxcl13−/− mice was modestly reduced relative to the number of B cells in the lungs of C57BL/6 mice at both day 10 and day 14 after infection (Fig. 2C). Moreover, the number of B cells in the lungs of dKO mice was dramatically lower than the number of B cells in the lungs of C57BL/6 mice at both day 10 and day 14 after infection (Fig. 2C). Similarly, the number of GCB was most reduced in dKO mice compared with the lungs of C57BL/6 mice (Fig. 2D). The low frequencies of GCB in plt/plt and dKO mice correlated with impaired antibody responses (Fig. 2E) because IgM responses were delayed and IgG responses were severely impaired in plt/plt and dKO mice, but were not affected in Cxcl13−/− mice (Fig. 2E).
Fig. 2.
The formation of germinal centers and the production of antibodies are impaired in spleen- and LN-deficient plt/plt mice. (A and B) Spleen- and LN-deficient mice were infected with influenza, and the frequencies of CD19 B cells (A) and GCB (B) in the lungs were evaluated by flow cytometry. PNA, peanut agglutinin. The data shown are gated on live lymphocytes (A) or live CD19 B cells (B). (C and D) The total numbers of CD19 B cells (C) or GCB (D) in the lungs of infected mice were calculated. (E) Serum titers of influenza-specific IgM and IgG were determined by ELISA. Flow cytometry was performed by using four to five mice per group, and antibody titers were determined in 7 to 10 mice per group. Statistical significance was evaluated by using an unpaired t test.
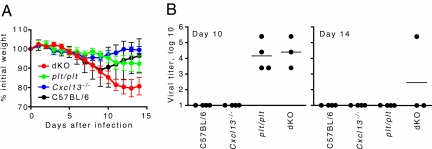
We also determined whether the impaired local B and T cell responses in plt/plt and dKO mice resulted in impaired viral clearance or exacerbated morbidity. As shown in Fig. 3A, C57BL/6 mice lost weight over the first 8 days of infection and then rapidly recovered. Cxcl13−/− mice exhibited a similar pattern of weight loss and recovery, although the weight loss was less severe (Fig. 3A). In contrast, plt/plt and dKO exhibited a progressive decline in body weight and never showed signs of recovery (Fig. 3A). Although one might expect weight loss to correlate with impaired immunity, poor T cell responses often lead to reduced weight loss (12, 28), even though viral clearance may also be impaired. We also found high viral titers in plt/plt and dKO mice on day 10, when C57BL/6 and Cxcl13−/− mice had cleared virus (Fig. 3B). Moreover, virus was detectable in some dKO mice on day 14 (Fig. 3B). These findings suggest that local expression of homeostatic chemokines, particularly CCR7 ligands, play an important role in generating effective immune responses.
Fig. 3.
Prolonged morbidity and a delay in viral clearance correlate with the inability to express homeostatic chemokines in the lung. (A) Spleen- and LN-deficient mice were infected with influenza and weighed daily. (B) Viral titers in the lungs were assayed in embryonated eggs.
Both CXCL13 and CCR7 Ligands Are Essential for iBALT Formation.
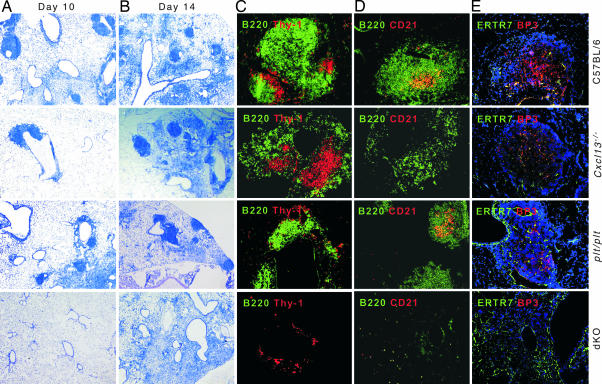
We next examined whether iBALT was formed in the lungs of chemokine-deficient mice. As shown in Fig. 4 A and B, the lungs of C57BL/6 and Cxcl13−/− mice had well defined lymphoid structures at both day 10 and 14 after infection. The lungs of plt/plt mice also had numerous lymphoid structures; however, the lungs of dKO mice had no lymphoid follicles on day 10 after infection and exhibited only diffuse inflammatory areas on day 14 [Fig. 4 A and B and supporting information (SI) Fig. 6A]. In fact, the lungs of C57BL/6 mice already contained well organized iBALT on day 10, with tightly packed B cell follicles separated by interfollicular T cell zones (Fig. 4C). The B cell follicles contained CD21+ follicular DCs (FDCs) (Fig. 4D) and BP3-expressing stromal cells (Fig. 4E). Although B and T cell areas were segregated in Cxcl13−/− mice, T cell areas appeared larger and were surrounded by loosely organized B cell zones (Fig. 4C) that lacked FDCs (Fig. 4D), although they did form BP3+ stromal cell networks (Fig. 4E). Conversely, plt/plt mice exhibited dense B cell follicles with FDCs and BP3+ stromal cells, but had poorly organized T cell areas. Finally, the lungs of dKO mice contained only a few small clusters of B and T cells with no perceptible organization (Fig. 4 C–E and SI Fig. 6B).
Fig. 4.
The formation of iBALT requires both CXCL13 and CCL19/CCL21. (A and B) Spleen- and LN-deficient mice were infected with influenza, and frozen sections of lungs were stained with MacNeal's tetrachrome. (C–E) Sections were probed with anti-B220 and anti-Thy-1.2 to visualize B and T cells (C), with anti-B220 and anti-CD21 to show B cells and FDCs (D), or with anti-ERTR7 and anti-BP3 to identify stromal cells (E). (Magnification: A and B, ×25; C–E, ×100.)
Endothelial and Stromal Cells Express Homeostatic Chemokines in the Lungs of Influenza-Infected Mice.
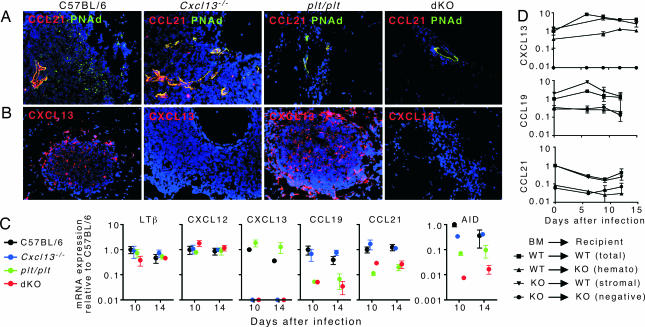
We also wanted to evaluate where homeostatic chemokines were expressed. We detected high levels of CCL21 that colocalized with peripheral node addressin-positive (PNAd+) HEVs in C57BL/6 and Cxcl13−/− mice (Fig. 5A). The HEVs in the lungs of C57BL/6 mice were located mainly around the edge of iBALT, whereas the HEVs in the lungs of Cxcl13−/− mice were in the center of these structures, consistent with the location of T cell zones in both groups. We also detected lower levels of CCL21 on PNAd+ HEVs in plt/plt and dKO mice (Fig. 5A). In addition, the HEVs in these mice were much less numerous than in C57BL/6 and Cxcl13−/− mice (SI Fig. 7). We did not observe CXCL13 expression associated with HEVs in any group, although it was easily detected in the B cell follicles of C57BL/6 and plt/plt mice, but not in Cxcl13−/− or dKO mice (Fig. 5B).
Fig. 5.
The expression of homeostatic chemokines in iBALT. Spleen- and LN-deficient mice were infected with influenza. (A and B) Frozen sections of infected lungs were probed with anti-CCL21 and anti-PNAd to visualize HEVs (A) and with anti-CXCL13 to visualize B cell follicles (B). (Magnification: ×200.) (C) RNA was extracted from frozen sections that contained areas of iBALT, and quantitative PCR was performed to evaluate the expression of LTβ, CXCL12, CXCL13, CCL19, CCL21, and AID. Expression of mRNA was normalized to the levels of mRNA in C57BL/6 mice on day 10, which was set at 1. Reciprocal BM chimeras were generated by using C57BL/6 and Cxcl13−/− mice as well as C57BL/6 and plt/plt mice. (D) Mice were allowed to reconstitute for 8 weeks and were infected with influenza. RNA was extracted from the lungs and the expression of CXCL13, CCL21, and CCL19 was assayed by quantitative PCR.
To more quantitatively measure the local expression of homeostatic chemokines and other molecules involved in iBALT organization and function, we sectioned through the lungs until we found areas containing lymphoid infiltrates. We then cut thick sections and extracted RNA, which was used for quantitative PCR analysis. As shown in Fig. 5C, both LTβ and CXCL12 were detected in all groups of mice at similar levels, regardless of whether organized iBALT was present. In contrast, the levels of CXCL13 mRNA were widely divergent between groups, with CXCL13 expressed at highest levels in C57BL/6 and plt/plt mice and undetectable in Cxcl13−/− and dKO mice, consistent with the targeted mutation in Cxcl13−/− mice. In addition, levels of CCL19 and CCL21 mRNAs were highest in C57BL/6 and Cxcl13−/− mice and were lowest in plt/plt and dKO mice, consistent with the genetic deletion in plt/plt mice. Finally, we observed that activation-induced cytidine deaminase (AID) mRNA was produced at highest levels in C57BL/6 and Cxcl13−/− mice, at lower levels in plt/plt mice, and at very reduced levels in dKO mice, consistent with the ability of this molecule to facilitate isotype switching (29).
To test whether CXCL13 was predominantly expressed in the lung by hematopoietic or nonhematopoietic cells, we generated reciprocal bone marrow (BM) chimeras by using C57BL/6 mice and Cxcl13−/− mice, infected these animals with influenza, and measured the expression of CXCL13 mRNA by quantitative PCR. As shown in Fig. 5D, the expression of CXCL13 was nearly identical in WT mice reconstituted with WT BM and in WT mice reconstituted with Cxcl13−/− BM. In contrast, the expression of CXCL13 mRNA was reduced 3- to 4-fold in Cxcl13−/− mice reconstituted with WT BM and was reduced to undetectable levels in Cxcl13−/− mice reconstituted with Cxcl13−/− BM. To test whether CCL19 or CCL21 were expressed in the lung by hematopoietic or nonhematopoietic cells, we generated similar BM chimeras by using C57BL/6 mice and plt/plt mice, infected them with influenza, and measured the expression of CCL19 and CCL21 mRNAs by quantitative PCR. As shown in Fig. 5D, the expression of both CCL19 and CCL21 was nearly identical in WT mice reconstituted with WT BM and in WT mice reconstituted with plt/plt BM. In contrast, the expression of CCL19 and CCL21 mRNA was reduced 4- to 10-fold in plt/plt mice reconstituted with WT BM and in plt/plt mice reconstituted with plt/plt BM. Together these results indicate that the majority of homeostatic chemokine expression in the lung is by nonhematopoietic cells. However, significant levels of CXCL13 mRNA are detectable in undefined BM-derived cells.
Discussion
Despite the common perception that local ectopic lymphoid tissues, such as those in the diabetic pancreas or rheumatoid joints, are prominently involved in local immune responses, most studies are unable to separate the relative contributions of local and systemic tissues. In contrast, we were able to distinguish the role of chemokines in the lung from their established roles in spleen and LNs by using chemokine-deficient mice that also lacked conventional lymphoid organs. Our results clearly demonstrate that pulmonary expression of CXCL13 and CCL19/21 is important for the formation of iBALT and for generation of local respiratory immune responses to influenza infection. Similar to their role in conventional lymphoid organs, CXCL13 and CCL19/21 are important for the development and organization of iBALT. However, these chemokines do not initiate iBALT formation by attracting lymphoid tissue inducer cells (30), because lymphoid tissue inducer cells are not required for iBALT formation (D. M. Carragher and T.D.R., unpublished data). Thus, iBALT development is probably initiated directly by activated B and T cells that are attracted to the lung by the expression of CXCL13 and CCL19/21.
Although mice lacking spleen, LNs, and Peyer's patches are useful tools that allow us to distinguish the role of chemokines in the lung from their role in conventional secondary lymphoid organs, the lack of secondary lymphoid organs probably magnifies the importance of iBALT as an immune inductive site. In fact, we find that mice lacking spleen LNs and Peyer's patches form iBALT more easily than mice with a full complement of secondary lymphoid organs, probably because lymphocytes lack anywhere else to go. However, we also know that CXCL13, CCL19, and CCL21 are inducibly expressed in the lungs of normal mice after influenza infection and that lymphocytes accumulate in the lung at sites of chemokine expression (12). Therefore, in LN-sufficient animals, iBALT probably supports the recruitment and local proliferation of T and B cells that were first primed in draining LNs. This is consistent with other data showing that the local expression of CCR7 ligands facilitates the recruitment of activated T cells to the lung (11). Thus, we conclude that, even in normal animals, the influenza-induced expression of homeostatic chemokines triggers the local formation of iBALT, which plays a role in generating and maintaining local immune responses in the lung.
Given that CXCL13 is thought to be one of the most important molecules for the formation of ectopic lymphoid follicles because of its dramatically increased expression in nonlymphoid organs that contain ectopic follicles (14, 17), it was surprising that the formation of iBALT was only minimally affected by the loss of CXCL13. This suggests that other chemokines, such as CXCL12, CCL19/21, or even alternative ligands for CXCR5, recruit B cells to areas of iBALT. Although Cxcl13−/− mice were unable to generate FDC networks, consistent with findings in spleen, LNs, and nasal-associated lymphoid tissue of Cxcl13−/− mice (31), they were able to generate BP3+ stromal cell networks, suggesting that local interactions between lymphocytes and stromal cells were sufficient to trigger the differentiation of some types of stromal cells, possibly FDC precursors (32). In addition, numerous B cells were present in iBALT and were spatially separated from the T cell areas, again consistent with splenic structure in Cxcl13−/− mice (31). However, the loss of CXCL13 and FDCs did not prevent antigen-specific B cell responses to influenza. In fact, the production of class-switched antibody was normal in Cxcl13−/− mice, consistent with the nearly normal expression of AID and suggesting that isotype switching was occurring locally in the iBALT environment.
In contrast, plt/plt mice were unable to efficiently generate influenza-specific IgG and pulmonary expression of AID was reduced. This did not appear to be due to defects in B cell recruitment or organization, because B cell follicles in iBALT were tightly packed and contained both FDCs and BP3+ stromal cells. However, T cell areas were smaller in plt/plt mice and CCL21-expressing HEVs were less frequent and expressed lower levels of CCL21. Thus, we suspect that the defective B cell response in plt/plt mice reflects a lack of efficient T cell help. Although we did not examine CD4 T cell responses in plt/plt mice, the CD8 T cell response was clearly impaired and very few antigen-specific CD8 T cells were generated. Interestingly, the recruitment of B cells to the lung, the formation of iBALT, and the production of influenza-specific IgG and AID expression were most profoundly disrupted in dKO mice, which lack both CXCL13 and the CCR7 ligands. Together, these data suggest that CXCL13 and CCR7 ligands cooperate to promote local immune responses and generate iBALT.
Our data contrast with reports showing that CD8 T cell responses to other antigens are delayed, but ultimately enhanced in plt/plt mice (26). This delay is due to poor recruitment of naive T cells to LNs, which forces T cells to be primed in the spleen (33). Because the mice in our experiments completely lack spleen and LNs, T cells can only be primed locally in iBALT. However, the number of activated DCs is reduced in the lungs of plt/plt and dKO mice and the ability of these cells to present antigen is impaired. Thus, the inability of plt/plt mice to generate local T cell responses in iBALT is likely due to poor priming by local antigen-presenting cells. Although some reports have suggested that CCL21 and CCL19 are expressed by DCs that directly attract T cells (34, 35), we find that essentially all CCL19 and CCL21 mRNA expression can be accounted for in the nonhematopoietic compartment. Thus, CCL19 expression by stromal cells and CCL21 expression on HEVs appears to be most important for generating primary immune responses to influenza in the lung.
Together, our data alter our perceptions of how chemokines contribute to local immune responses in peripheral, nonlymphoid tissues, such as the lung. Although inflammatory chemokines are expressed at sites of infection and recruit inflammatory cells as well as activated lymphocytes, we show that homeostatic chemokines, including CXCL13, CCL19, and CCL21 are also expressed at sites of inflammation and contribute to the development of local lymphoid tissue and the initiation and expansion of adaptive immune responses. These data blur the distinction between lymphoid and nonlymphoid organs and suggest that, despite the association between ectopic lymphoid follicles and pathology, the formation of tertiary lymphoid structures is also a component of protective immunity.
Materials and Methods
Mice, Treatments, and Infection.
Cxcl13−/− and plt/plt mice on the C57BL/6 background were obtained from J. Cyster (University of California, San Francisco, CA) and bred at The Trudeau Institute. To prevent LN development, pregnant mice were treated with 100 μg of soluble LTβR-Ig and 100 μg of soluble TNFR1-Ig (both from Biogen-Idec, Cambridge, MA) on days 11, 14, and 17 of gestation (27). LN-ablated mice were splenectomized at 6 weeks of age and allowed to recover 4 weeks before analysis. BM chimeras were generated by reconstituting lethally irradiated recipients with 1 × 107 BM cells. Mice were allowed to reconstitute for 8 weeks before analysis. Mice were infected intranasally with 100 egg infectious units of influenza A/PR8/34 (PR8) in 100 μl. Viral titers were quantified as described (36). All animal procedures were approved by The Trudeau Institute Institutional Animal Care and Use Committee.
Isolation of RNA and Quantitative PCR.
RNA was either extracted from whole lungs by using an RNeasy kit (Qiagen, La Jolla, CA) or from 100-μm frozen sections first by using TRIzol (Invitrogen, Carlsbad, CA) with 20 μg of glycogen as a carrier and then by using an RNeasy kit to further purify the RNA. DNase-treated RNA (2 μg) was reverse transcribed with random hexamers and SuperScript II (Invitrogen). Quantitative PCR was performed as described (25).
Histology, Antibodies, and Immunofluorescence.
Frozen sections were stained with MacNeal's tetrachrome. Biotinylated antibodies (BD Biosciences, Mountain View, CA) were detected with streptavidin conjugated to Alexa 488 or Alexa 594 (Molecular Probes, Eugene, OR). Polyclonal goat anti-CXCL13 and anti-CCL21 (R&D Systems, Minneapolis, MN) were detected by using Alexa 594-conjugated donkey anti-goat IgG (Molecular Probes). Anti-BP3 (Research Diagnostics, Flanders, NJ) was biotinylated by using NHS-LC-biotin (Pierce, Rockford, IL). Anti-ERTR7 (Acris Antibodies, Hiddenhausen, Germany) was detected by using Alexa 488-conjugated donkey anti-rat IgG (Molecular Probes). All slides were viewed with a Zeiss (Oberkochen, Germany) Axioplan 2 microscope. Images were recorded with a Zeiss AxioCam digital camera. Images were saved as TIFF files.
Flow Cytometry.
Cells from collagenase-digested lungs were blocked with FcBlock, followed by fluorochrome-conjugated antibodies (BD Biosciences) or MHC class I tetramers. Peanut agglutinin was obtained from Vector Laboratories (Burlingame, CA). The H-2DbNP366–374 tetramer was generated by The Trudeau Institute Molecular Biology Core Facility.
Detection of Antigen-Presenting Cells.
Starting at 105 cells per well, 2-fold serial dilutions of total leukocytes from the lungs of influenza-infected mice were cultured overnight with 53-A8 hybridoma cells. Cultures were fixed with 2% formaldehyde and 0.2% glutaraldehyde for 5 min, washed with PBS, and incubated with 1 mg/ml X-Gal in 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 2 mM MgCl2 for 6 h at 37°C. Blue cells were counted in each well under a microscope.
Supplementary Material
Acknowledgments
We thank The Trudeau Institute Animal Technical Services Core for setting up timed pregnancies, Dr. D. Woodland (The Trudeau Institute), for the 53A8 hybridoma, and Dr. J. Browning (Biogen-Idec, Cambridge, MA) for providing the LTβR-Ig and TNFR1-Ig and his critical evaluation of this manuscript. This work was supported by National Institutes of Health Grant HL69409 (to T.D.R.), the Sandler Program for Asthma Research, and The Trudeau Institute.
Abbreviations
- CCL
CC chemokine ligand
- CXCL
CXC chemokine ligand
- DC
dendritic cell
- FDC
follicular dendritic cell
- HEV
high endothelial venule
- iBALT
inducible bronchus-associated lymphoid tissue
- LN
lymph node
- dKO
double knockout
- LTβ
lymphotoxin β
- R
receptor
- NP
nucleoprotein
- GCB
germinal center B cell
- AID
activation-induced cytidine deaminase
- BM
bone marrow
- PNAd
peripheral node addressin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700591104/DC1.
References
- 1.Rossi D, Zlotnik A. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Moser B, Loetscher P. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 3.Sprenger H, Meyer RG, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. J Exp Med. 1996;184:1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matikainen S, Pirhonen J, Miettinen M, Lehtonen A, Govenius-Vintola C, Sareneva T, Julkunen I. Virology. 2000;276:138–147. doi: 10.1006/viro.2000.0542. [DOI] [PubMed] [Google Scholar]
- 5.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. J Leukoc Biol. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 6.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 8.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O'Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, et al. Nat Med. 2005;11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyster JG. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 10.Cyster JG. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo JC, Chin RK, Lee Y, Kang HS, Wang Y, Weinstock JV, Banks T, Ware CF, Franzoso G, Fu YX. J Clin Invest. 2003;112:1495–1505. doi: 10.1172/JCI19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 13.Woodland DL, Randall TD. Semin Immunol. 2004;16:163–170. doi: 10.1016/j.smim.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. J Clin Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, Chervonsky AV, Fu YX. Immunity. 2006;25:499–509. doi: 10.1016/j.immuni.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Aust G, Sittig D, Becherer L, Anderegg U, Schutz A, Lamesch P, Schmucking E. Eur J Endocrinol. 2004;150:225–234. doi: 10.1530/eje.0.1500225. [DOI] [PubMed] [Google Scholar]
- 17.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff C, Ruddle N. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krenn V, Konig A, Hensel F, Berek C, Souto Carneiro MM, Haedicke W, Wang Y, Vollmers H, Muller-Hermelink HK. Clin Exp Immunol. 1999;115:168–175. doi: 10.1046/j.1365-2249.1999.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berek C, Kim HJ. Semin Immunol. 1997;9:261–268. doi: 10.1006/smim.1997.0076. [DOI] [PubMed] [Google Scholar]
- 20.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 21.Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- 22.Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, Wiekowski MT, Romani N, Lira SA. J Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 23.Martin AP, Coronel EC, Sano G, Chen SC, Vassileva G, Canasto-Chibuque C, Sedgwick JD, Frenette PS, Lipp M, Furtado GC, et al. J Immunol. 2004;173:4791–4798. doi: 10.4049/jimmunol.173.8.4791. [DOI] [PubMed] [Google Scholar]
- 24.Ansel KM, Harris RB, Cyster JG. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 25.Rangel-Moreno J, Moyron-Quiroz J, Kusser K, Hartson L, Nakano H, Randall TD. J Immunol. 2005;175:4904–4913. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. J Exp Med. 2001;193:207–218. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rennert PD, Browning JL, Mebius RE, Mackay F, Hochman PS. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 29.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 30.Cupedo T, Mebius RE. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 31.Ansel KM, Ngo VN, Hayman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 32.Cyster JG, Ansel KM, Rief K, Hyman PL, Tang HL, Luther SA, Ngo VN. Immunol Rev. 2000;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 33.Junt T, Nakano H, Dumrese T, Kakiuchi T, Odermatt B, Zinkernagel RM, Hengartner H, Ludewig B. J Immunol. 2002;168:6032–6040. doi: 10.4049/jimmunol.168.12.6032. [DOI] [PubMed] [Google Scholar]
- 34.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngo VN, Tang HL, Cyster JG. J Exp Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. J Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.