Abstract
Vessel loss precipitates many diseases. In particular, vessel loss resulting in hypoxia induces retinal neovascularization in diabetic retinopathy and in retinopathy of prematurity (ROP), major causes of blindness. Here we define insulin-like growth factor binding protein-3 (IGFBP3) as a new modulator of vascular survival and regrowth in oxygen-induced retinopathy. In IGFBP3-deficient mice, there was a dose-dependent increase in oxygen-induced retinal vessel loss. Subsequent to oxygen-induced retinal vessel loss, Igfbp3−/− mice had a 31% decrease in retinal vessel regrowth versus controls after returning to room air. No difference in serum insulin-like growth factor 1 (IGF1) levels was observed among groups. Wild-type mice treated with exogenous IGFBP3 had a significant increase in vessel regrowth. This correlated with a 30% increase in endothelial progenitor cells in the retina at postnatal day 15, indicating that IGFBP3 could be serving as a progenitor cell chemoattractant. In a prospective clinical study, we measured IGFBP3 (and IGF1) plasma levels weekly and examined retinas in all premature infants born at gestational ages <32 weeks at high risk for ROP. The mean level of IGFBP3 at 30–35 weeks postmenstrual age (PMA) for infants with proliferative ROP (ROP stages 3>, n = 13) was 802 μg/liter, and for infants with no ROP (ROP stage 0, n = 38) the mean level was 974 μg/liter (P < 0.03). These results suggest that IGFBP3, acting independently of IGF1, helps to prevent oxygen-induced vessel loss and to promote vascular regrowth after vascular destruction in vivo in a dose-dependent manner, resulting in less retinal neovascularization.
Keywords: angiogenesis, insulin, like growth factor 1, insulin, like growth factor binding protein, 3, stem cells, retinopathy of prematurity
Insulin-like growth factor 1 (IGF1) is an important modulator of retinopathy in premature infants and has been implicated in diabetic retinopathy (1). However, the role of a major serum binding protein, IGF binding protein-3 (IGFBP3) in angiogenesis in vivo, is not yet well defined. How IGFBP3 modulates angiogenesis is important because the IGF1/IGFBP3 combination is now available for clinical use. Premature infants and diabetic individuals are at risk for vascular loss as well as proliferative retinopathy. In diabetes, loss of insulin and hyperglycemia over time precipitates vessel loss and leads to retinal ischemia. In retinopathy of prematurity, loss of maternal growth factors required for normal vascular development and normally provided in utero in the third trimester (such as IGF1) are lost after premature birth. In addition, the relatively hyperoxic external environment compared to that in utero suppresses oxygen-regulated factors, such as VEGF, required for normal vascular development. Loss of these growth factors in retinopathy of prematurity (ROP) leads again to retinal ischemia. Hypoxia induces the formation of morphologically abnormal neovascularization (NV), as well as apoptotic neuronal cell death in the retina, causing retinal degeneration. If vascular loss can be prevented, proliferative retinopathy, driven by the resultant hypoxia, will also be suppressed. In addition, many complications of diabetes, such as ischemic heart disease, are based on vascular loss. Therefore, preventing vessel loss would be of great clinical benefit.
IGFBP3 regulates the promitogenic and antiapoptotic functions of the IGFs, but also has independent functions (2, 3). The role of IGFBP3 in controlling cell growth is ambiguous. IGFBP3 can enhance the proliferative effects of IGFs or inhibit IGF actions (4–6). High-serum IGFBP3 levels decrease the risk of breast cancer (7), whereas a high tissue level of IGFBP3 is associated with the formation of large, highly proliferative tumors (8–11). In cell culture, IGFBP3 has also been shown to promote both apoptosis and survival (3, 12–15). These data imply that IGFBP3 may enhance or suppress cell growth depending on specific conditions.
In vivo, IGFBP3 is highly expressed in vascular endothelial cells during angiogenesis of the corpus luteum (16) and in breast tumor vessels (17). These data suggest that IGFBP3 may be associated with the regulation of physiologic as well as pathologic angiogenesis. To date, however, in vivo studies blocking or enhancing IGFBP3 to define its role in vascular survival and growth have been lacking.
The retinal disease that develops in the mouse model used in this paper allows for examination of vessel loss (vasoobliteration), NV, and neuronal apoptosis, all features that characterize both ROP and diabetic retinopathy as well as other diseases (18). In this model, we used exogenous delivery of IGFBP3 as well as Igfbp3+/− and Igfbp3+/+ transgenic mice (19), which we have found to have various levels of IGFBP3 expression, to examine the effect of modulation of IGFBP3 on retinopathy in vivo. In the mouse model of ROP, we found that increasing levels of IGFBP3 are associated with increased vessel survival in hyperoxia-induced vascular loss and increased vessel regrowth and repair during the hypoxic phase of oxygen-induced retinopathy (OIR) resulting in decreased retinopathy. We then translated this work to the clinical setting. In a prospective clinical study of premature infants, we also found that increased levels of IGFBP3 are associated with reduced risk of ROP.
Results
Serum IGF1 Levels Are Unchanged from Wild Type in Igfbp3 Transgenic Mice.
The mean serum IGF1 levels as measured at postnatal day 5 (P5) in Igfbp3−/− (n = 8), Igfbp3+/− (n = 11), and Igfbp3+/+ (n = 10) sibling mice were 89 ± 19, 88 ± 8, and 95 ± 25 μg/liter, respectively, indicating that there was no significant difference in IGF1 in transgenic mice compared to controls. There was also no difference in weight between Igfbp3−/− mice and Igfbp3+/+ mice during development (19).
IGFBP3 Protects Against Oxygen-Induced Retinal Vessel Loss P8.
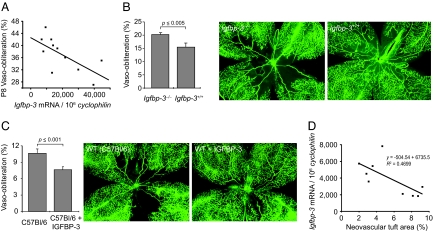
To evaluate the effect of low IGFBP3 on vessel survival in oxygen, we examined the degree of vessel loss in whole-mounted retinas of Igfbp3+/+ or Igfbp3+/− mice at P8 after 17 h of oxygen-induced vasoobliteration (n = 11 mice; each data point is the mean of right and left eyes of one mouse). The degree of vessel loss was compared to Igfbp3 mRNA expression in tail snips in wild-type and heterozygote mice (Fig. 1A). There was a significant increase in vessel survival with increasing levels of Igfbp3 mRNA expression (Fig. 1A) (P ≤ 0.006, r2 = −0.70).
Fig. 1.
IGFBP3 protects against oxygen-induced vessel loss. (A) Igfbp3 mRNA expression is associated with protection against oxygen-induced retinal vessel loss in a dose-dependent manner at P8 after 18 h of 75% oxygen in Igfbp3+/− mice (n = 11 mice, with mean of two retinas at each point) (r2 = −0.70; P ≤ 0.006). (B) (Center and Right) Loss of IGFBP3 decreases retinal vessel regrowth after oxygen-induced loss (P17). IGFBP3 protection after oxygen-induced vessel loss persists as shown at P17 in wild-type (n = 38 eyes) (Right) compared to Igfbp3−/− mice (n = 52 eyes) (Center). (Left) Fractional retinal area of vasoobliteration in Igfbp3−/− mice was 20.2 ± 0.1% (SEM) compared to wild-type mice with an area of 15.1 ± 0.5% (P ≤ 0.005) (Right). Representative retinal whole mounts show less vessel loss in wild-type than in Igfbp3−/− mice. (C) Exogenous IGFBP3 further increases vessel regrowth over baseline. (Center and Right) Whole-mounted retinas of C57BL/6 mice treated with saline after oxygen-induced vessel loss (Center) have decreased vessel regrowth centrally than littermates treated with i.p. IGFBP3 (Right) from P12 to P14. (Left) There is a 40% decrease in retinal area without vessels with IGFBP3 treatment compared to control (P ≤ 0.001) as seen in whole-mounted retinas. (D) Retinal NV decreases with increasing IGFBP3. Igfbp3 mRNA expression is associated with protection in a dose-dependent manner against retinal NV in the ROP mouse model at P17 in Igfbp3+/− and Igfbp3+/+ mice (n = 11 mice, with mean of two retinas at each point) (r2 = −0.47).
Low IGFBP3 Is Associated with Persistent Vasoobliteration at P17.
To evaluate the effect of IGFBP3 on the persistence of vessel loss, we examined the area of vasoobliteration at P17 in whole-mounted retinas of Igfbp3−/− (n = 52 eyes) and sibling Igfbp3+/+ (n = 38 eyes) mice after oxygen-induction of vessel loss from P7 to P12. There was a 31% increase in the area of retinal vessel loss (P < 0.005) in the Igfbp3−/− mice compared to Igfbp3+/+, indicating persistence of vascular loss and/or lack of vessel regrowth with low IGFBP3 (Fig. 1B).
Exogenous IGFBP3 Increases Vessel Regrowth.
To evaluate the effect of elevated levels of serum IGFBP3 on vessel regrowth after oxygen-induced vasoobliteration, we examined the vascular area in whole-mounted retinas at P15 in C57BL/6 mice given three daily injections of IGFBP3 from P12 to P14 after vessel loss induced by oxygen. At P15, there was a 40% decrease in area of vessel loss in C57BL/6 mice (n = 8 mice) treated with IGFBP3 compared to vehicle control-treated mice (n = 7), indicating increased vessel regrowth with increased IGFBP3 (P ≤ 0.001) (Fig. 1C).
Increasing IGFBP3 Expression Is Associated with Decreasing Retinal Neovascularization in Mice.
To evaluate the association of Igfbp3 mRNA with retinal NV, Igfbp3−/+ and Igfbp3+/+ sibling mice (11 mice, 22 eyes) were evaluated for Igfbp3 mRNA expression levels in tail snips, and the degree of retinal NV was evaluated at P17 in whole-mounted retinas. There was decreased retinal NV with increasing Igfbp3 mRNA expression (Fig. 1D).
Retinal Igfbp3 mRNA Increases with Hypoxia.
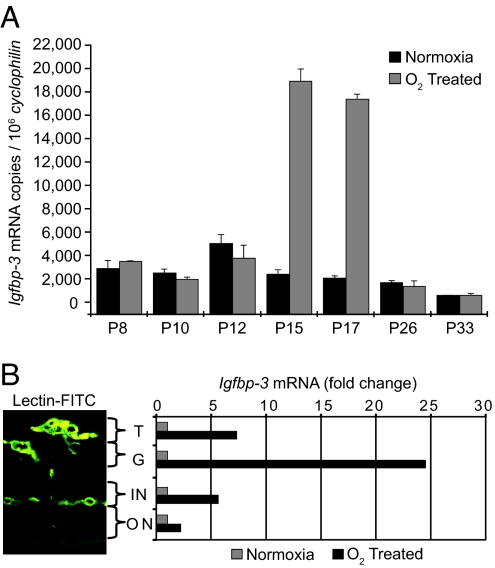
The onset of hypoxia occurs at P12 when mice are returned to room air after oxygen-induced retinal vessel loss. There is a 3- to >9-fold increase in Igfbp3 mRNA in whole retina between P12 and P15 persisting through P17 that decreases by P26 when hypoxia is relieved with revascularization (n = 12 retinas per data point) (Fig. 2A).
Fig. 2.
Igfbp3 mRNA in whole retina increases with hypoxia at P15 and P17 in whole retina. (A) Each bar represents Igfbp3 mRNA copy number normalized to 1 million copies of cyclophilin (an unchanging control gene). During hypoxia at P15 and P17 in O2-treated mice, Igfbp3 expression increased ≈9-fold over normoxic control (n = 12 for each group). (B) Igfbp3 mRNA is associated with retinal blood vessels and increases with hypoxia. Quantitative real-time RT-PCR analysis of Igfbp3 mRNA analyzed from laser-captured areas of retinal blood vessels shows a large increase in Igfbp3 mRNA in the superficial vascular layers at P17 with hypoxia, indicating that IGFBP3 is associated with retinal blood vessels and not with surrounding tissue. T, neovascular vessel tufts extending into the vitreous; G, ganglion cell layer; ON, outer nuclear or photoreceptor layer; IN, inner nuclear layer.
Igfbp3 mRNA Is Localized to Retinal Vascular Areas.
Igfbp3 mRNA is localized to areas of retinal NV (tufts) extending into the vitreous at P17 and to the ganglion cell layer and inner nuclear layer where vessels are located. Within the local vascular areas, Igfbp3 mRNA increases between 5- and 25-fold with hypoxia at P17. In the avascular photoreceptor layer (i.e., outer nuclear layer), there is only a small increase in Igfbp3 mRNA with hypoxia at P17 compared to nonoxygen-treated controls (Fig. 2B).
Decreased Number of Endothelial Progenitor Cells (EPCs) in the Retina of Igfbp3−/− Mice.
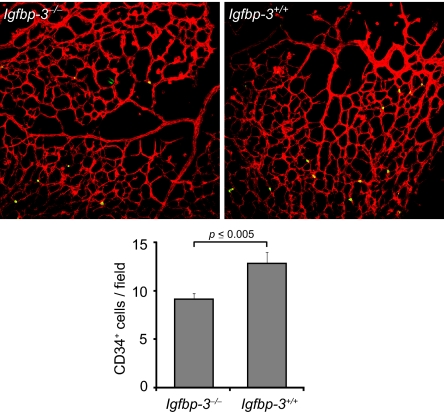
To evaluate the effect of IGFBP3 on EPC recruitment into retina, we examined at P15 retinas from Igfbp3−/− mice after OIR. EPCs are identified with CD34 antibody staining. In Igfbp3−/− mice (n = 8), there are ≈30% fewer CD34+ cells in the retina compared to wild-type animals (n = 7) (Fig. 3) (P = 0.003). This observation suggests that IGFBP3 may contribute to retinal vessel repair by recruiting bone marrow-derived EPCs into the site of injury.
Fig. 3.
Decreased number of EPCs in the retina of IGFBP3-null mice. Quantification of CD34+ cells in the retina of Igfbp3−/− mice (Upper Left) at P15 after OIR (n = 8) revealed a significantly decreased number of EPCs (Lower) compared to wild-type animal (n = 7; P = 0.003) (Upper Right). Red, lectin-Alexa 594 (endothelial cell-specific); green, CD34-FITC antibody.
In a Clinical Study, Increased Serum IGFBP3 Correlates with Less Severe ROP.
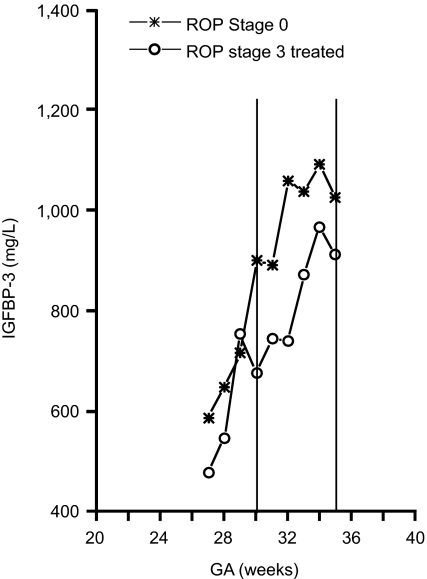
To test the hypothesis that increased IGFBP3 levels after birth protect against vessel loss, and therefore against proliferative ROP in premature infants, we prospectively measured IGFBP3 plasma levels weekly after birth and coordinately examined retinas in all premature infants born at gestational ages <32 weeks at high risk for ROP (n = 79). ROP stages 0 to 4 were defined according to the International Classification; for these studies, ROP stages 3 to 4 (n = 13) were defined as proliferative ROP and ROP stage 0 (n = 38) as no ROP. We confirmed that lack of vascular growth is associated with proliferative ROP. The normal immature retina has a gradual transition from translucent vascularized retina into gray, nonvascularized retina without a distinct border between the two. In ROP, a sharp observable stationary border consisting of a line or ridge between vascularized and nonvascularized retina becomes apparent. In all patients with proliferative ROP (n = 13), there was a demarcation line anterior to which no vessels were seen. In all infants with no ROP (n = 38), there was no ridge and no demarcation line, indicating more normal growth of the vascular front (data not shown). The mean ± SEM level of IGFBP3 at 30 to 35 weeks PMA for infants with proliferative ROP was 802 ± 66 μg/liter and for infants with no ROP was 974 ± 41 μg/liter. The Mann–Whitney U test yielded a P value of 0.03, indicating a significant difference between the two groups in mean IGFBP3 at this time point (Fig. 4).
Fig. 4.
Lower serum levels of IGFBP3 are associated with reduction in ROP in children. The mean ± SEM level of IGFBP3 at 30–35 weeks PMA for infants with proliferative ROP was 802 ± 66 μg/liter and for infants with no ROP was 974 ± 41 μg/liter. The Mann–Whitney U test yielded a P value of 0.03, indicating a significant difference between the two groups in mean IGFBP3 at this time point.
Discussion
In a prospective clinical study of premature infants, we found that increasing levels of IGFBP3 are associated with decreased retinal NV (ROP) and therefore with increased normal vascular growth. In studies using Igfbp3−/− transgenic mice and in other studies using supplemental recombinant IGFBP3 in wild-type mice, there is a dose–response of increasing pathological retinal NV to decreasing IGFBP3 levels in vivo. Increasing IGFBP3 decreases retinal vascular loss, increases vessel regrowth, and thereby decreases NV. Furthermore, the protective effect of IGFBP3 appears to be independent of IGF1 because serum IGF1 levels in transgenic and wild-type mice were the same, and adding exogenous IGFBP3 above the normal level further promoted vessel regrowth. Although IGFBP3 actions have been studied in vitro, there are few, if any, experimental studies in vivo that directly address the role of IGFBP3 in vascular development.
In a clinical study in premature infants, we also show that low levels of serum IGFBP3 between PMA weeks 30–35 (when induction of ROP occurs) (20) are correlated with increased risk of developing proliferative ROP. These data correspond to earlier findings (1) that premature infants with low levels of IGF1 in early development have less vascular growth and an increased risk of ROP. Interestingly, we observed that the IGF1/IGFBP3 ratio is similar between premature infants who develop proliferative ROP and those who do not (data not shown). Moreover, there were no significant differences in acid labile subunit levels between non-ROP and ROP children (data not shown), suggesting that the critical factors in the serum IGF1 complex (IGF1, IGFBP3, and acid labile subunit) are IGF1 and IGFBP3. This result suggests that restoring both IGF1 and IGFBP3 levels simultaneously in children at risk of developing ROP, thereby keeping the IGF1/IGFBP3 ratio intact, is important in preventing destructive retinal NV (ROP). IGFBP3 is currently available as an rhIGF1/rhIGFBP combination.
The mechanism by which IGFBP3 helps prevent vessel loss and improves repair is likely to be multifactorial. We do not know the relative contribution of systemic versus local IGFBP3. However, Igfbp3 mRNA is found in the retinal vasculature and increases substantially with hypoxia. This local source may play a significant role in controlling vascular growth. This observation is consistent with findings in other tissues (16, 17). Because IGFBP3 promotes the growth of stem cells in vitro (13), we speculate that stem cell recruitment to blood vessels may be stimulated by increasing IGFBP3 levels. Here we show that deficiency of IGFBP3 is associated with decreased number of CD34+ EPCs in the retina, suggesting that IGFBP3 might improve vessel repair by promoting the incorporation of bone marrow-derived EPCs into the retina. In a complementary work by Chang et al. (27) in this issue of PNAS, the authors show that exposure of CD34+ cells to IGFBP3 resulted in rapid differentiation into endothelial cells. This result correlates with our findings that increasing IGFBP3 protects against oxygen-induced vessel loss and promotes regrowth of vessels, thereby protecting against retinopathy.
IGF1 has been suspected to be involved in diabetic retinopathy for decades, but clinical studies of a correlation have had varied results (21). In one study, however, patients with type 1 diabetes had reduced serum levels of IGFBP3 (and reduced levels of free plus dissociable IGF1) compared to nondiabetic controls (22). Patients with proliferative retinopathy have increased vitreous levels of IGF1 and IGFBP3, as well as other binding proteins thought to be because of increased vascular leakage (23).
Patients with ROP or diabetes are susceptible to retinal vascular loss that can then precipitate hypoxia-induced proliferation of blood vessels, causing retinal detachments and blindness. Interventions that prevent vessel loss or promote efficient vessel regrowth can prevent pathologic NV, as well as many complications of diabetes that involve vascular loss, such as heart disease. These findings are likely to apply to other disease pathologies involving the regulation of vascular networks.
In summary, premature infants with lower levels of serum IGFBP3 are at greater risk for retinopathy. In the mouse model of ROP, the protective effect of IGFBP3 appears to be independent of IGF1 and is a result of increased protection against vessel loss and increased regrowth of vessels after damage. The mechanism of vascular stabilization is consistent with recruitment of vascular precursor cells. These results have significant implications for treatment of patients with diabetes and ROP, diseases associated with vascular loss and subsequent destructive NV. Improved vascularization of the retina will tend to decrease the hypoxic stimulus required for later development of pathological neovascularization, reducing the degree of ROP. This work suggests that the use of exogenous IGFBP3 in patients at risk for retinal NV would have similar protective effects.
Materials and Methods
Animals.
These studies adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research (www.arvo.org/AboutARVO/animalst.asp). Igfbp3−/−, and Igfbp3+/−, Igfbp3+/+ mice were previously described (19) and characterized by expression of Igfbp3 mRNA in tail samples with real time RT-PCR and confirmed by Southern blot analysis (data not shown). When Igfbp3-null and littermate wild-type mice were compared, there was no reduction in birth weight, litter size, or postnatal growth (19).
O2-Induced Vessel Degeneration.
To induce vessel loss, P7 mice with their nursing mother were exposed to 75% oxygen for times ranging from 18 h to 5 days (18). After O2 exposure, we anesthetized mice with Avertin (Sigma–Aldrich, St. Louis, NO) and killed them by intracardiac perfusion with 20 mg/ml of 2 × 106 molecular weight FITC-dextran in saline (24). We enucleated and fixed eyes in 4% paraformaldehyde for 2 h at 4°C. We isolated and whole-mounted retinas with glycerol-gelatin (Sigma–Aldrich) onto polylysin-coated slides with the photoreceptor side down. We examined retinas with a fluorescence microscope (Olympus, Tokyo, Japan), digitized images using a three-charge-coupled device color video camera (DX-950P; Sony, Tokyo, Japan), and processed them with Northern Eclipse software (Empix Imaging, Toronto, ON, Canada).
Analysis of the Effect of IGFBP3 on Oxygen-Induced Vessel Loss.
Igfbp3 transgenic mice and nursing mothers were exposed to 75% oxygen from P7 to P8. At P8, sibling mice were killed and tail snips were evaluated for Igfbp3 mRNA expression levels. Eyes were isolated and whole-mounted, and an area of vessel loss was evaluated in Igfbp3+/+ or Igfbp3+/− sibling mice (n = 22 eyes). Variability in gene expression was noted among individual wild-type and heterozygote mice, which allowed us to establish a dose–response.
Analysis of the Effect of Low IGFBP3 on Persistence of Vasoobliteration at P17.
Between P7 and P12, heterozygote IGFBP3 mothers with pups were exposed to 75% oxygen, removed to room air from P12 to P17, and subsequently killed. Retinas from Igfbp3−/− (n = 52 eyes) and Igfbp3+/+ mice (n = 38 eyes) were isolated and whole-mounted, and the area of vasoobliteration was evaluated.
Analysis of the Effect of Exogenous IGFBP3 on Vessel Regrowth.
Between P7 and P12, C57BL/6 mice were exposed to 75% oxygen. After removal from oxygen at P12, mice were given three daily i.p. injections (P12-P14) with 60 μg of IGFBP3 (n = 16 eyes) or vehicle (n = 14 eyes). The mice were killed at P15, retinas were isolated and whole-mounted, and an area of vasoobliteration was evaluated for the effect of IGFBP3 on regrowth of vessels.
Analysis of the Effect of Low IGFBP3 on NV at P17.
Between P7 and P12, heterozygote Igfbp3 transgenic mothers with pups were exposed to 75% oxygen, removed to room air from P12 to P17, and subsequently killed. Retinas from Igfbp3−/+ and Igfbp3+/+ mice (n = 9 mice, 18 eyes) were isolated and whole-mounted, and an area of NV was evaluated and recorded as the mean of two retinas. Igfbp3 mRNA levels were determined from tail snips as described.
Quantitative Analysis of Gene Expression (Quantitative Real-Time RT-PCR).
PCR primers targeting Igfbp3 and an unchanging control gene (cyclophilin) RNA were designed by using Primer Express software (Applied BioSystems, Foster City, CA). We used three methods to analyze primer and probe sequences for specificity of gene detection. First, only primer and probe sequences that specifically detect the sequence of choice, as determined by means of the NCBI Blast module, were used. Second, amplicons generated during the PCR were analyzed by using the first derivative primer melting curve software supplied by Applied BioSystems. This analysis determines the presence of amplicons on the basis of their specific melting point temperatures. Third, amplicons generated during the PCR were gel-purified and sequenced (Children's Hospital Core Sequencing Facility, Boston, MA) to confirm the selection of the desired sequence. Quantitative analysis of gene expression was generated by using an ABI Prism 7700 Sequence Detection System (TaqMan; Applied Biosystems, Foster City, CA) and the SYBR Green master mix kit (QIAGEN, Valencia, CA). C57BL/6 mice were analyzed at P8, P10, P12, P15, P17, P26, and P33 with and without exposure to 75% oxygen by real-time PCR for levels of Igfbp3 mRNA.
Laser Capture Microdissection.
Eyes embedded in OCT compound were sectioned at 8 μm in a cryostat, mounted on uncoated glass slides, and immediately stored at −80°C. Slides containing frozen sections were immediately fixed in 70% ethanol for 30 sec and stained with H&E (Meyers), followed by four dehydration steps each in 70%, 95%, and 100% ethanol and a final 10-min dehydration step in xylene. Once they were air dried, the sections were microdissected for vessels and retinal neuron layers with a PixCell II LCM system (Arcturus Engineering, Mountain View, CA). Each population was estimated to be >95% homogenous as determined by microscopic visualization of the captured cells. Material from each cell layer from more than four mice was combined, RNA-isolated, and converted to cDNA as described. Specific cDNA was quantified by using quantitative real-time PCR.
Quantification of EPCs in the Retina.
Igfbp3−/− and wild-type control litters were exposed to 75% oxygen from P7 to P12 and then moved to room air. Animals were killed on P15. Retinas from Igfbp3−/− (n = 8 eyes) and Igfbp3+/+ mice (n = 7 eyes) were isolated and fixed in 4% paraformaldehyde for 1 h, permeabilized in PBS with 1% Triton X-100 overnight, followed by Griffonia simplicifolia isolectin I (endothelial cell-specific) (Invitrogen, Eugene, OR) and CD34-FITC antibody (Miltenyi Biotec, Auburn, CA) staining.
Analysis of Serum IGF1 and IGFBP3: Infants.
Weekly blood samples (0.5 ml) for each infant were stored at −20°C to −80°C and assayed at the same time. Serum IGFBP3 levels were measured by using a specific radioimmunoassay for IGFBP3 (Mediagnost GmbH, Tübingen, Germany) (25). For the IGFBP3 assay, the intraassay coefficients of variation (CVs) were 7.1%, 7.3%, and 7.9% at concentrations of 1,800, 3,790, and 5,776 μg/liter, respectively, and the interassay CVs were 13.4%, 10.5%, and 14.1%.
Mice.
Serum IGF1 levels were measured on Igfbp3−/− (n = 8) and Igfbp3−/+ (n = 11) and Igfbp3+/+ (n = 10) sibling mice by using an IGFBP-blocked radioimmunoassay with a large excess of IGFI for determination of IGF-1 (Mediagnost GmbH) (25). The intraassay CVs for the IGF1 assay were 11.1%, 7.2%, and 7.4% at concentrations of 36, 204, and 545 μg/liter, respectively, and the interassay CVs were 13.5%, 8.8%, and 9.9%. This assay can be used as heterologous assay for mouse by using rat IGF1 in the standard curve (26).
Study Participants.
Infants born at gestational age <32 weeks were recruited at the Queen Silvia Children's Hospital in Göteborg, Sweden, and at Uppsala University Hospital, Sweden (190 eligible, 79 enrolled between December 1999 and April 2002). Inability to complete postnatal followup until PMA 40 weeks or discharge to home and conspicuous congenital anomaly were exclusion criteria. The group included 19 twins, 8 pairs and 3 whose siblings died. All infants were hospitalized in a neonatal intensive care unit. Enteral feeding with increasing amounts of breast milk was introduced early (2 to 48 h after birth). Until full enteral feeding was achieved, supplementary parenteral nutrition with glucose, amino acids, and fat was given. Breast milk fortified with 0.8 g of protein per 100 ml was given to infants <1,500 g from ≈10 days PMA until the infant weighed 2,000 g. The Ethics Committees of the Medical Faculties at Göteborg and Uppsala Universities gave approval (#Ö594–00), and informed consent was obtained from parents.
ROP Examinations.
Dilated retinal examinations with indirect ophthalmoscopy were performed weekly or biweekly from the age of 5 to 6 weeks after birth until the retina was fully vascularized or the condition was considered stable. Children with plus disease and/or stage 3 ROP had more frequent examinations. ROP changes were classified according to the International Classification of ROP.
Acknowledgments
We thank Argiris Efstratiadis (Columbia University) for Igfbp3−/− mice, Insmed Inc. for the kind gift of IGFBP3, and Jan-Rung Mo for technical help. This work was supported by the V. Kann Rasmussen Foundation, a Research to Prevent Blindness Lew Wasserman Merit Award (to L.E.H.S.), National Eye Institute Grants EY008670 and EY14811 (to L.E.H.S.), Children's Hospital Boston Mental Retardation and Developmental Disabilities Research Center Grant P01 HD18655 (to L.E.H.S.), a Fulbright Commission for a Fulbright Visiting Research Scholarship (to C.L.), the Swedish Research Council (C.L.), National Eye Institute Fellowships T32EY017145 and IF32EY017789-01A1 (to K.M.C.), and a Juvenile Diabetes Research Foundation International Postdoctoral Fellowship (to J.C.).
Abbreviations
- CV
coefficient of variation
- EPC
endothelial progenitor cell
- NV
neovascularization
- OIR
oxygen-induced retinopathy
- Pn
postnatal day
- PMA
postmenstrual age
- ROP
retinopathy of prematurity.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Hellstrom A, Engstrom E, Hard AL, Albertsson-Wikland K, Carlsson B, Niklasson A, Lofqvist C, Svensson E, Holm S, Ewald U, et al. Pediatrics. 2003;112:1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 2.Mohan S, Baylink DJ. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 3.Firth SM, Baxter RC. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 4.De Mellow JS, Baxter RC. Biochem Biophys Res Commun. 1988;156:199–204. doi: 10.1016/s0006-291x(88)80824-6. [DOI] [PubMed] [Google Scholar]
- 5.Conover CA. Endocrinology. 1992;130:3191–3319. doi: 10.1210/endo.130.6.1375895. [DOI] [PubMed] [Google Scholar]
- 6.Chen JC, Shao ZM, Sheikh MS, Hussain A, LeRoith D, Roberts CT, Jr, Fontana JA. J Cell Physiol. 1994;158:69–78. doi: 10.1002/jcp.1041580110. [DOI] [PubMed] [Google Scholar]
- 7.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 8.Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng C, Lee AV, Yee D. Clin Cancer Res. 1997;3:103–109. [PubMed] [Google Scholar]
- 9.Rocha RL, Hilsenbeck SG, Jackson JG, Lee AV, Figueroa JA, Yee D. J Natl Cancer Inst. 1996;88:601–606. doi: 10.1093/jnci/88.9.601. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Levesque MA, Khosravi MJ, Papanastasiou-Diamandi A, Clark GM, Diamandis EP. Int J Cancer. 1998;79:624–628. doi: 10.1002/(sici)1097-0215(19981218)79:6<624::aid-ijc12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Levesque MA, Khosravi MJ, Papanastasiou-Diamandi A, Clark GM, Diamandis EP. Br J Cancer. 1996;74:1242–1247. doi: 10.1038/bjc.1996.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granata R, Trovato L, Garbarino G, Taliano M, Ponti R, Sala G, Ghidoni R, Ghigo E. Faseb J. 2004;18:1456–1458. doi: 10.1096/fj.04-1618fje. [DOI] [PubMed] [Google Scholar]
- 13.Liu LQ, Sposato M, Liu HY, Vaudrain T, Shi MJ, Rider K, Stevens Z, Visser J, Deng HK, Kraus M. Oncol Res. 2003;13:359–371. doi: 10.3727/096504003108748375. [DOI] [PubMed] [Google Scholar]
- 14.Spagnoli A, Hwa V, Horton WA, Lunstrum GP, Roberts CT, Jr, Chiarelli F, Torello M, Rosenfeld RG. J Biol Chem. 2001;276:5533–5540. doi: 10.1074/jbc.M005088200. [DOI] [PubMed] [Google Scholar]
- 15.Franklin SL, Ferry RJ, Jr, Cohen P. J Clin Endocrinol Metab. 2003;88:900–907. doi: 10.1210/jc.2002-020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser HM, Lunn SF, Kim H, Duncan WC, Rodger FE, Illingworth PJ, Erickson GF. J Clin Endocrinol Metab. 2000;85:1672–1677. doi: 10.1210/jcem.85.4.6497. [DOI] [PubMed] [Google Scholar]
- 17.Schmid MC, Bisoffi M, Wetterwald A, Gautschi E, Thalmann GN, Mitola S, Bussolino F, Cecchini MG. Int J Cancer. 2003;103:577–586. doi: 10.1002/ijc.10874. [DOI] [PubMed] [Google Scholar]
- 18.Smith LE, Wesolowski E, McLellan A, Kostyk S. K., D'Amato R, Sullivan R, D'Amore PA. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 19.Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. Mol Endocrinol. 2006;20:2173–2186. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- 20.Lofqvist C, Engstrom E, Sigurdsson J, Hard AL, Niklasson A, Ewald U, Holmstrom G, Smith LE, Hellstrom A. Pediatrics. 2006;117:1930–1938. doi: 10.1542/peds.2005-1926. [DOI] [PubMed] [Google Scholar]
- 21.Frystyk J. Horm Metab Res. 2005;37(Suppl 1):44–48. doi: 10.1055/s-2005-861362. [DOI] [PubMed] [Google Scholar]
- 22.Frystyk J, Bek T, Flyvbjerg A, Skjaerbaek C, Orskov H. Diabet Med. 2003;20:269–276. doi: 10.1046/j.1464-5491.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Schwickerath R, Pfeiffer A, Blum WF, Freyberger H, Klein M, Losche C, Rollmann R, Schatz H. J Clin Invest. 1993;92:2620–2625. doi: 10.1172/JCI116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Amato R, Wesolowski E, Smith LE. Microvasc Res. 1993;46:135–142. doi: 10.1006/mvre.1993.1042. [DOI] [PubMed] [Google Scholar]
- 25.Blum WF, Breier BH. Growth Reg. 1994;4:11–19. [PubMed] [Google Scholar]
- 26.Upton Z, Webb H, Tomas FM, Ballard FJ, Francis GL. J Endocrinol. 1996;149:379–387. doi: 10.1677/joe.0.1490379. [DOI] [PubMed] [Google Scholar]
- 27.Chang K-H, Chan-Ling E, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Calzi SL, et al. Proc Natl Acad Sci USA. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]