Abstract
Two parkinsonian patients who experienced transient hypomanic states when the subthalamic nucleus (STN) was stimulated during postoperative adjustment of the electrical parameters for antiparkinsonian therapy agreed to have the mood disorder reproduced, in conjunction with motor, cognitive, and behavioral evaluations and concomitant functional neuroimaging. During the experiment, STN stimulation again induced a hypomanic state concomitant with activation of cortical and thalamic regions known to process limbic and associative information. This observation suggests that the STN plays a role in the control of a complex behavior that includes emotional as well as cognitive and motor components. The localization of the four contacts of the quadripolar electrode was determined precisely with an interactive brain atlas. The results showed that (i) the hypomanic state was caused only by stimulation through one contact localized in the anteromedial STN; (ii) both this contact and the contact immediately dorsal to it improved the parkinsonian motor state; (iii) the most dorsal and ventral contacts, located at the boundaries of the STN, neither induced the behavioral disorder nor improved motor performance. Detailed analysis of these data led us to consider a model in which the three functional modalities, emotional, cognitive, and motor, are not processed in a segregated manner but can be subtly combined in the small volume of the STN. This nucleus would thus serve as a nexus that integrates the motor, cognitive, and emotional components of behavior and might consequently be an effective target for the treatment of behavioral disorders that combine emotional, cognitive, and motor impairment.
Keywords: basal ganglia, emotion, deep brain stimulation, neuroimaging
In humans, motor activity consists of complex behavioral sequences that are subtly but strongly influenced by the cognitive and emotional context in which they are executed. However, how emotions interact with cognition and motor activity in the brain is still not well known (1). Experimental studies in monkeys have identified well individualized cortical areas involved in the processing of motor, cognitive and emotional information that is transmitted to separate territories in the striatum, globus pallidus, and subthalamic nucleus (2, 3), suggesting a segregated model of information processing. The existence of a limbic circuit in the basal ganglia of humans has been suggested by the results of studies in volunteers subjected to emotional stimuli (4) and in patients with psychiatric disorders and focal lesions in the basal ganglia (5), using functional MRI and positron emission tomography (PET) (6, 7). Electrical stimulation of the subthalamic nucleus (STN), used to treat patients with Parkinson's disease (8, 9), has recently proved to be a powerful and accurate tool for testing these functions and their related circuits (10–12), because the functioning of the stimulated structure can be reversibly altered in a spatially and temporally controlled manner. Manic episodes have been reported after stimulation of the STN (13, 14) or the substantia nigra (15, 16). Hypomania induced by high frequency STN stimulation has also been reported (14, 17, 18), but the structure that was stimulated was not localized with precision. In this study, we used an interactive brain atlas to precisely localize each contact of the quadripolar electrodes in the STN of two parkinsonian patients who had experienced transient hypomanic states during postoperative adjustment of the electrical parameters for therapeutic stimulation of the STN, and who agreed to undergo experimental STN stimulation to reproduce the mood disorder, in conjunction with motor, cognitive and behavioral evaluations and concomitant functional neuroimaging.
Results
Postoperative evaluation of the effects of stimulation through each of the four contacts (numbered ventrodorsally from 0 to 3) on the implanted electrodes showed that bilateral stimulation (pulse width, 60 μs; frequency, 130 Hz; voltage, 2.1–2.7 V) from contact 2 (hereafter called the “dorsal contact”) caused maximal improvement of motor symptoms in both patients [78% and 86%; Unified Parkinson's Disease Rating Scale (UPDRS) Part III]. Contacts 0 and 3 did not improve motor symptoms in patient 1 and provoked aversive effects (dysarthria, diplopia) in patient 2. In the absence of any change in medication or voltage, stimulation through contact 1, the “ventral contact,” induced a persistent abnormal expansive mood in both patients, associated with clinical features that correspond to hypomania as defined by the Diagnostic and Statistical Manual of Mental Disorders (19). Only a slight worsening of their motor condition was induced by stimulation of this ventral contact.
Because stimulation through the dorsal and ventral contacts, which are only 2 mm apart, resulted in completely different clinical pictures, we undertook to determine the exact anatomical localization of each contact, using a method previously developed in our center (Fig. 1). The placement of the electrodes within the STN was assured by intra-operative electrophysiological recordings to identify the structure. The precise anatomical localization of each of the four contacts of each electrode was determined postoperatively by MRI, as described (20, 21). The patients' MRIs were registered by a computerized method to the brain images of a three-dimensional histological atlas (21) in which the three functional territories of the human STN (sensorimotor, associative, limbic) were defined by homology with the same territories in the monkey identified by axonal tracing (22). The ventral and dorsal contacts of the electrodes in both patients were found to be localized in the associative territory of the STN (Fig. 2a). The ventral contacts, however, bordered on the limbic territory, whereas the dorsal contacts bordered on the motor territory.
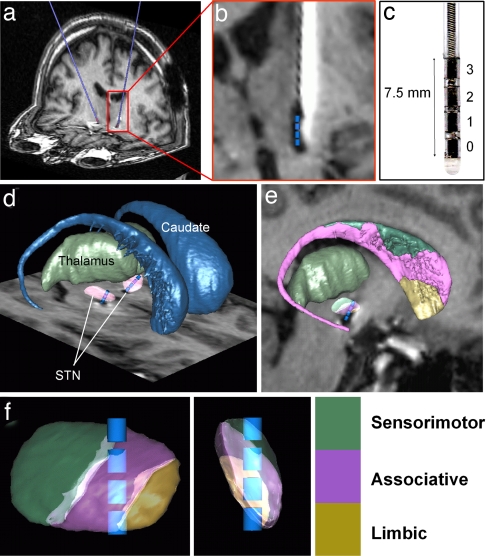
Fig. 1.
Method for localizing electrodes implanted in the brain of a patient with Parkinson's disease for stimulation of the STN. (a) Postoperative MRI of a patient showing the electrodes in blue. (b) Identification of the four contacts (blue cylinders) in the electrode MRI artifact. The MRI was reformatted along the trajectory of each electrode and a scaled template of the contacts (1.5 × 1.27 mm cylinder) was positioned at the center of the artifact (20). (c) Medtronic 3389 electrode. (d and e) The three dimensional histological atlas of the basal ganglia is fitted to the patient MRI as described in Experimental Procedures (21). (d) 3D view of caudate, thalamus, and STN after registration with the atlas. (e) Sagittal view of the fitted atlas image including sensorimotor, associative and limbic territories of caudate and STN. (f) Electrode contacts and STN territories after rotation along STN main axes.
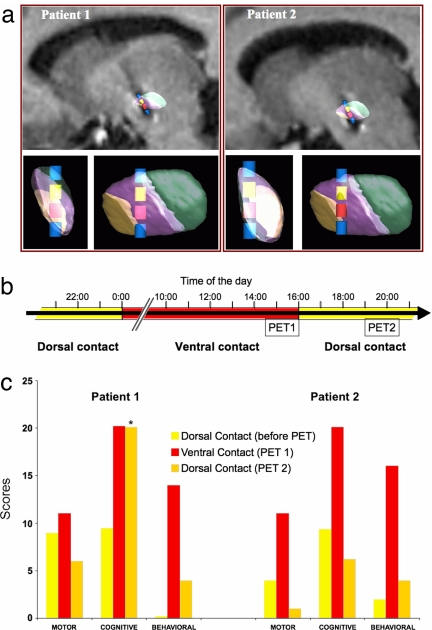
Fig. 2.
Motor, cognitive and behavioral status of two parkinsonian patients undergoing stimulation of the STN through a ventral contact that induces hypomania and a dorsal contact that ameliorates motor performance. (a) 3D views of the STN territories and contacts for patients 1 and 2 in sagittal views with MRI and after rotation along main STN axes. Therapeutic dorsal contact, yellow; ventral hypomania-inducing contact, red. (b) Schematic representation of the protocol for evaluating cerebral blood flow by PET. Yellow bars represent the normal state during dorsal contact stimulation, red bars the hypomanic state induced by ventral contact stimulation. (c) Motor, neuropsychological and behavioral scores the day before and during the PET protocol. Motor performance: UPDRS III (28) score indicating the severity of parkinsonism. Cognitive abilities: CPT-II score of attention (values >11 are indicative of problems) (24). Behavior: YMRS score (values >12 are considered pathological) (23). The baseline score for patient 1 before PET, which was zero, coincides with the base of the histograms. The CPT-II score returned to baseline values a few hours after the second series of PET acquisitions.
Because the hypomanic state induced by stimulation through the ventral contact was reversible, we proposed to determine whether correlative responses would be observed in brain regions associated with this kind of psychic disorder. The patients were asked to undergo a PET study of regional cerebral blood flow (rCBF) during stimulation of the dorsal and ventral contacts, in parallel with assessments of their motor, cognitive and behavioral status (Fig. 2b). When stimulation was switched from the dorsal (therapeutic) to the ventral contact, a hypomanic state was again observed, as during the initial evaluation of the stimulation parameters, and was quantified by using the Young Mania Rating Scale (YMRS) (23). The computerized neuropsychological assessments [CPT-II (24)] revealed attention impairment as reported in mania (25–27). Motor performance, quantified by using the Unified Parkinson Disease Rating Scale part III (UPDRS III) (28), was little affected (Fig. 2c).
During the hypomanic state, rCBF was significantly greater than normal (P < 0.00001, uncorrected; cluster size: 100 voxels) in the left thalamus, right middle and inferior temporal gyrus, right inferior parietal gyrus and right inferior frontal gyrus (Table 1, Fig. 3). In both subjects, the subcortical activation peak was located in the ventral anterior nucleus of the thalamus (THVA) as demonstrated by coregistration of the individual PET and MRI acquisitions with the atlas (Fig. 3). Deactivation was observed in left posterior middle temporal and occipital gyrus, left middle frontal gyrus, bilateral cuneus and right medial prefrontal/anterior cingulate gyrus (Table 1).
Table 1.
Significant clusters of regional activation–deactivation detected by PET analysis of cerebral blood flow
| Contrast | Anatomical structures | Brodmann areas | MNI coordinates (x y z), mm | Z value at peak |
|---|---|---|---|---|
| Activation | Left thalamus | −6 −8 6 | 6.04 | |
| Right middle/inferior temporal cortex | 20/21 | 48 −22 −30 | 5.95 | |
| Right angular/supramarginal gyrus | 40 | 54 −50 24 | 5.34 | |
| Right inferior frontal/sup. temporopolar cortex | 45/38 | 58 16 0 | 5.12 | |
| Deactivation | Left superior temporal sulcus | 19/37/21/39 | −46 −50 10 | 6.92 |
| Left middle frontal cortex | 8/9 | −36 16 44 | 6.04 | |
| Left and right cuneus | 18 | 4 −76 24 | 5.99 | |
| Right medial prefrontal/anterior cingular cortex | 11/10 | 10 44 −20 | 5.15 |
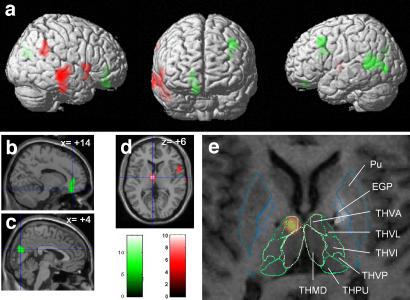
Fig. 3.
Brain regions showing activation (red) or deactivation (green) during hypomania induced by stimulation of the STN in patients with Parkinson's disease. (a) Statistical parametric contrast t-maps showing clusters of voxels in which rCBF differed significantly in hypomanic and baseline states. (b and c) Parasagittal sections superimposed on significantly deactivated clusters in deeper brain regions (b, anterior cingulate/subgenual medial prefrontal cortex; c, cuneus). (d) Activated cluster in the left thalamus on an axial section. (e) Superimposition of MRI, single-subject SPM{t} map of patient 1 on the patient-fitted histological atlas showing that the left thalamic activation was centered on the THVA. Similar data were obtained for patient 2. Pu, putamen; GPe, external globus pallidus; THVA, ventral anterior thalamic nucleus; THVL, ventral lateral thalamic nucleus; THVI, ventral intermediate thalamic nucleus; THVP, ventral posterior thalamic nucleus; THPU, pulvinar; THMD, dorsal medial thalamic nucleus.
Discussion
In this paper we report the case of two parkinsonian patients who experienced transient hypomanic states when stimulated from an electrode contact located in the anteromedial STN. Simultaneously, rCBF was modified in the same cortical areas in both patients. We will first discuss the methodological aspects of our approach and then the possible physiopathological implications of these observations.
Clinical Evaluation.
These two fortuitous observations, made during the standard postoperative phase of electrical tuning, are experimentally significant because (i) the hypomanic state could be reproducibly induced before and during the PET study in each individual and (ii) the behavioral picture and PET results were highly similar in the two patients. Non-motor side effects have already been observed in patients with STN lesions (29, 30) or in parkinsonian patients undergoing stimulation of the STN [for review see (31, 32)] or substantia nigra (15, 16) but the two cases reported here are particularly interesting because they corresponded to hypomania not to bipolar mania (13, 14). The few cases of hypomania that have been reported (17, 33) were not studied by brain imaging. In this study, we diagnosed the hypomanic state using the DSM-IV criteria, and quantified the symptoms using a standardized scale [YMRS (23)]. Although this scale is usually used to assess changes which occur over several days, the time frame is not restrictive, and the scale can be used to assess acute mood changes. It takes only 15–30 min to administer the YMRS and most items reflect symptoms that can fluctuate over hours or even minutes.
STN Identification and Electrode Localization.
Intra-operative microelectrophysiological recordings were performed to identify the STN (34, 35). The two definitive electrodes were implanted along the central trajectory in both of our patients (35). The electrophysiological recordings, during the operation, did not detect differences in the response of neurons in different parts of the STN. It was only, postoperatively, that two different behavioral responses, motor improvement and hypomania, were obtained when two different parts of the STN were stimulated. The precise localization of the two different contacts was then determined with respect to the three subdivisions of the STN as in the computerized atlas.
Reliability of Atlas/MRI-Based Localization.
Our approach is based on the registration of the atlas with each patient's postoperative MRI. The possibility that the MR signals were distorted was excluded before MRI was chosen as the standard procedure for DBS targeting and electrode localization (35, 36). As stimulating electrodes are known to produce artifacts on MRI (37), we previously verified that the electrode itself was localized at the geometric center of the artifact (20). In addition, the correspondence between the electrophysiological identification and the atlas-based identification of the STN was demonstrated to be excellent (21). The subdivisions of the STN were delimited by atlas/MRI registration, the reliability of which was verified with respect to specific landmarks (21). Fig. 3 shows that the atlas contour of the putamen fits perfectly with the corresponding MRI hyposignal. The submillimetric resolution of the atlas was finally sufficient to show that the therapeutic and hypomania inducing contacts were both localized in the associative region of the STN but bordered respectively on the limbic and motor territories (Fig. 2). We therefore consider that electrode contacts were accurately localized, although the absence of motor improvement obtained by stimulation of contact 3 in the dorsal STN was surprising with regard to current literature (20, 37–40). This discrepancy was primarily due to the fact that this contact produced aversive effects in one patient whereas contacts 2 and 1 did not and were therefore privileged.
How the Stimulating Current Induced Hypomania.
The distribution of afferent projections from the external globus pallidus (GPe) (22, 41, 42) and the selective distribution of the calcium binding proteins parvalbumin in neurons in the motor territory of the STN and calretinin in the associative and limbic territories (43, 44) suggest that the STN is divided into separate sensorimotor, associative and limbic territories. How STN simulation induced a hypomanic state therefore depends on the distance over which the stimulating current diffuses, which in turn depends on the physical properties of both the current itself and the local nervous tissue (45–47). Given the small size of the STN (3 × 5 × 12 mm) (48), it may be asked whether the stimulating current in the patients spread over the whole of the STN and beyond, or affected different specific subregions, or was restricted to a single territory. These three possibilities are discussed below.
Four-Millimeter Diffusion Hypothesis.
A current diffusing over a distance of 4 mm would have affected about two thirds of the entire STN as well as neurons or axon bundles outside the STN (Fig. 4a). Because the contacts of stimulating electrodes were only 2 mm apart, the areas they stimulate would be expected to overlap and produce the same effect, which was not the case. Only the ventral contact induced hypomania. In addition, stimulation of the region medial to the STN was previously shown to produce aggressive behavior (49), not hypomania. The same type of argument holds for motricity. Contacts 3 and 0, localized at borders of the STN, did not improve motricity, again suggesting that the stimulating current did not diffuse over a large area, otherwise these contacts would have been as efficient as contacts 1 and 2 localized within the STN. Finally, the changes in rCBF in the cortex elicited by stimulation of the dorsal and ventral contacts would also have been similar if the current had diffused over such a large area, but we in fact observed significant differences in the limbic and para-limbic cortical networks. Stimulation of structures outside the STN does not, therefore, seem to explain the hypomanic state produced by STN stimulation in our patients.
Fig. 4.
Mechanisms by which stimulation of the ventral contact (red cylinder) but not the dorsal contact (yellow cylinder) could induce a hypomanic state. (a–c) The STN is represented along its longest axis with its three functional territories, sensorimotor, associative, and limbic, color coded as in Fig. 1. Green circles are superimposed to indicate different possible zones of diffusion of the stimulating current from the dorsal and ventral contacts: in a radius of 4 mm to structures outside the STN (a), to different STN territories within a radius of 2 mm (b), to the associative territory of the STN within a radius of 1 mm (c). (d) Schematic illustration of the convergence of projections from the cerebral cortex (CX), caudate nucleus (CD), and globus pallidus (GP) on the STN. (e) Schematic illustration, based on data from refs. 22 and 50–52, showing the gradient organization of the different territories of the STN and the disposition of the dendrites of individual neurons which extend over neighboring territories.
Two-Millimeter Diffusion Hypothesis.
If the stimulating current diffused over a smaller distance, 2 mm for example, substructures of the STN implicated in different functions might have been affected specifically by the two contacts. Indeed, the dorsal contact that bordered on the motor territory might be expected to affect motricity, whereas current from the ventral contact that bordered on the limbic territory might be expected to affect behavior (Fig. 4b). This was the effect observed, which is compatible with a model of the basal ganglia based on the parallel processing of information through sharply segregated channels (2). However, this interpretation would not explain why motor improvement was observed when the associative STN territory was stimulated through both the dorsal and ventral contacts. Furthermore, this model is not supported by the anatomical data: firstly, axon tracing studies in primates (22) and calbindin labeling in humans (50) have shown that the subdivisions of the STN do not have sharply defined boundaries, but are separated by functional gradients; secondly, STN neurons have long dendrites (51, 52) that extend to neighboring territories, suggesting a certain degree of convergence of afferent information on individual neurons (53). Furthermore, although previous studies have shown that STN stimulation at rest strongly increases rCBF in premotor and motor networks compared with the unstimulated condition (54–56), the PET images did not show any significant differences in those motor regions whether the motor territory of the STN was stimulated through the dorsal contact or when a supposedly non-motor territory was stimulated through the ventral contact. This pattern is hardly compatible with the view that moving the stimulation from the dorsal to the ventral site within the STN would correspond to a switch between motor and limbic circuits.
One-Millimeter Diffusion Hypothesis.
If the stimulating current diffused even less, on the order of 1 mm, which would be expected for a 2.5 V current (45), the dorsal and ventral contacts would then both affect the same associative territory of the STN (Fig. 4c) and thus have the same clinical effect. Because this similarity was not observed, a model in which the stimulation of two locations in the same territory could have different consequences should be considered. We propose a model in which the STN would serve as a nexus that subtly integrates the motor, cognitive and emotional components of behavior (Fig. 4 d and e) and could combine exaggerated motor activity, cognitive fluidity and emotionality in the hypomanic state. Four observations support this hypothesis. (i) The STN, the smallest structure in the basal ganglia circuitry (0.16 cm3), receives convergent information from much larger structures, the external globus pallidus (GPe, 0.8 cm3) and the striatum (10 cm3), which itself receives motor, associative and limbic information from the entire cerebral cortex (500 cm3) (48) (Fig. 4d). (ii) Motor, associative and limbic territories of the STN are separated by functional gradients, not by sharp boundaries (22). (iii) The motor, associative and limbic input to the STN from the GPe (22) overlaps with input from the motor cortices (57), which can explain the motor improvement obtained in the associative territory. (iv) The output of STN neurons to its different targets is also unsegregated (58). These observations suggest that the STN does not preserve a clear-cut segregation between different functional modalities. Conversely, it could combine limbic, associative and motor information into an output message which would integrate the emotional, cognitive and motor components of behavior. It is our hypothesis that limbic, associative and motor information is distributed in a medio-lateral gradient in the STN of all individuals, but that the precise anatomical limits of each component are progressive and might probably vary among individuals. Consequently, only slight variations in the placement of the stimulating electrode could induce different effects in different patients. This model would also explain why stimulation from a contact in the middle of the associative STN would have effects on both motor and limbic functions. In our study, the behavioral effects were indeed correlated with changes in rCBF in widely distributed associative regions of the cortex along with clusters in the limbic-related anterior cingulate cortex.
Cortical Regions Involved in the Hypomanic State.
Deactivation of the anterior cingulate gyrus in the right hemisphere and activation of the THVA, which receives associativo-limbic information from the substantia nigra and the ventral pallidum (42) and projects onto the dorsolateral frontal cortex (2, 3, 59), have already been implicated in mood or neuropsychological dysfunction (60–63). However, it should be noted that the manic episodes produced by stimulation of the substantia nigra (not the STN) were associated with activation (not deactivation) of the left (not right) dorsal anterior cingulate gyrus (16). This difference suggests that the substantia nigra and STN both influence behavior, but in different, perhaps opposing ways, in line with the opposite roles they play in the direct and indirect circuits of the basal ganglia (64). The other changes observed were less clearly related to hypomania, but the cortical regions involved are known to process limbic (emotional) or associative (cognitive) information. Increased activity in the right inferior frontal gyrus and the posterior middle temporal gyrus has been described in emotion appraisal (65). Changes in the right parietal and medial occipital areas might be related to the role of these structures in attention (66). Those in the left posterior superior temporal gyrus might underlie altered social cognition (67, 68) during the hypomanic state.
Concluding Remarks.
This study has shown that stimulation of a particular site in the anteromedial STN induced a hypomanic state in two parkinsonian patients. Paradoxically, STN stimulation has also been reported to improve behavioral disorders in parkinsonian patients (18, 69–71). This contradiction indicates that processing of non-motor information in the STN cannot be simply explained in terms of global activation or inhibition, as in the classical model of the basal ganglia circuit (64). In contrast to the substantia nigra, in which stimulation induces acute and violent maniac or depressive symptoms (15, 16, 49), STN stimulation gives rise to more subtle hypomanic changes (14, 17). Our study suggests that the three functional modalities (sensorimotor, cognitive and emotional) can be combined in the very small volume of the STN which thus would serve as a nexus for the integration of motor, cognitive and emotional components of behavior. This model differs from that of Haber et al. (72, 73) in which integration is based on the reciprocal striato-nigro-striatal and cortico-thalamo-cortical circuits and from that of Temel and colleagues (11, 74) in which the STN is a regulator of associative and limbic circuits, but not an integrator. Techniques that provide more precise information about the neuronal architecture of the STN in individual patients are urgently needed. The detailed three-dimensional atlas used in this study has greatly advanced our ability to perform such analyses, but rapidly developing techniques such as diffusion tensor imaging or high resolution MRI will help us go even further in unraveling the intricate neural relationships that integrate the motor, cognitive and emotional components of behavior at all levels of the basal ganglia. Finally, given the major role that the STN plays in behavioral integration, it might represent an effective target for the treatment of basal ganglia diseases which combine, to different degrees, motor, cognitive and emotional impairment. Further studies that test the effects of stimulation in different parts of the STN with smaller electrodes, a larger number of contacts and varied voltage that would change the area stimulated are indispensable if we want to understand how motor and non-motor information is processed in the STN.
Experimental Procedures
Atlas-Based Contact Localization.
The STN was first identified in each patient by microelectrode recordings, which is the standard method for intra-operative STN identification (34, 35). Unicellular STN recordings were obtained along 4.5 mm of the trajectory of three microelectrodes. The definitive stimulating electrode was implanted along the central trajectory in both hemispheres of the two patients. It was localized postoperatively with respect to the subdivision of the STN by fitting the images of a three-dimensional atlas developed from histological and MRI data (21, 75) to the MRIs of the patients. A similar procedure was used to analyze rCBF data.
PET Protocol.
Both patients gave written informed consent to have their mood disorder reproduced during a 24 h protocol with PET (Fig. 2). Six dynamic rCBF images were acquired, with patients in the resting state, 14–16 h after stimulation through the ventral contact inducing hypomania began. Stimulation was then switched to the dorsal contact and, after an interval of 3 h, a second series of six sequential acquisitions were performed at rest. During PET acquisitions, the subjects were instructed to lie still with their eyes open and ears unplugged; noise and interaction with the subjects were kept to a minimum and the room was dimly lit. For rCBF image acquisition, tracer was administered through a cannula inserted in a vein in the left arm. PET measurements were performed with a tomograph (EXACT-HR+; CTI-Siemens, Knoxville, TN) that allowed the 3-dimensional acquisition of 63 transaxial slices. Spatial resolution was 4.5 mm and 4.1 mm in the transaxial and axial directions, respectively. The rCBF images were acquired at 10 min intervals, for 80 seconds after injection of 8 mCi (1 Ci = 37 GBq) of H2O15. PET data were analyzed by statistical parametric mapping (SPM2, Wellcome Department of Imaging Neuroscience, 2003; www.fil.ion.ucl.ac.uk/spm/software/spm2/) by Matlab 6.5 software (Mathworks) on a Linux (Red Hat 9) workstation.
Detailed reports of clinical cases, clinical and neuropsychological evaluations, and PET data analysis can be found in supporting information (SI) Materials and Methods.
Supplementary Material
Acknowledgments
We thank Max Westby and Jean-Baptiste Pochon for their thoughtful comments on the manuscript. This work was supported by the Action Concertée Incitative “Plasticité Neuronale et Adaptation Fonctionnelle” (ACI 2001-6503H).
Abbreviations
- STN
subthalamic nucleus
- PET
positron-emission tomography
- UPDRS
Unified Parkinson's Disease Rating Scale
- rCBF
regional cerebral blood flow
- THVA
ventral anterior nucleus of the thalamus
- YMRS
Young Mania Rating Scale.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610849104/DC1.
References
- 1.Dalgleish T. Nat Rev Neurosci. 2004;5:583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, DeLong MR, Strick PL. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 3.Parent A, Hazrati LN. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 4.Bartels A, Zeki S. NeuroReport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia KP, Marsden CD. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 6.Baxter LR, Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Arch Gen Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- 7.Rauch SL, Whalen PJ, Curran T, Shin LM, Coffey BJ, Savage CR, McInerney SC, Baer L, Jenike MA. Adv Neurol. 2001;85:207–224. [PubMed] [Google Scholar]
- 8.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 9.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, et al. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 10.Mayberg HS, Lozano AM. Neurology. 2002;59:1298–1299. doi: 10.1212/wnl.59.9.1298. [DOI] [PubMed] [Google Scholar]
- 11.Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Hershey T, Mink JW. Neurology. 2006;66:1142–1143. doi: 10.1212/01.wnl.0000216425.34178.dd. [DOI] [PubMed] [Google Scholar]
- 13.Romito LM, Raja M, Daniele A, Contarino MF, Bentivoglio AR, Barbier A, Scerrati M, Albanese A. Mov Disord. 2002;17:1371–1374. doi: 10.1002/mds.10265. [DOI] [PubMed] [Google Scholar]
- 14.Herzog J, Reiff J, Krack P, Witt K, Schrader B, Muller D, Deuschl G. Mov Disord. 2003;18:1382–1384. doi: 10.1002/mds.10530. [DOI] [PubMed] [Google Scholar]
- 15.Kulisevsky J, Berthier ML, Gironell A, Pascual-Sedano B, Molet J, Pares P. Neurology. 2002;59:1421–1424. doi: 10.1212/wnl.59.9.1421. [DOI] [PubMed] [Google Scholar]
- 16.Ulla M, Thobois S, Lemaire JJ, Schmitt A, Derost P, Broussolle E, Llorca PM, Durif F. J Neurol Neurosurg Psychiatry. 2006;77:1363–1366. doi: 10.1136/jnnp.2006.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandat TS, Hurwitz T, Honey CR. Acta Neurochir. 2006;148:895–897. doi: 10.1007/s00701-006-0795-4. [DOI] [PubMed] [Google Scholar]
- 18.Houeto JL, Mallet L, Mesnage V, Tezenas du Montcel S, Behar C, Gargiulo M, Torny F, Pelissolo A, Welter ML, Agid Y. Arch Neurol. 2006;63:1090–1095. doi: 10.1001/archneur.63.8.1090. [DOI] [PubMed] [Google Scholar]
- 19.APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4th Ed. Arlington, VA: American Psychiatric Publishing, Inc; 2000. Text Revision. [Google Scholar]
- 20.Yelnik J, Damier P, Demeret S, Gervais D, Bardinet E, Bejjani BP, Francois C, Houeto JL, Arnule I, Dormont D, et al. J Neurosurg. 2003;99:89–99. doi: 10.3171/jns.2003.99.1.0089. [DOI] [PubMed] [Google Scholar]
- 21.Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tande D, Karachi C, Ayache N, Cornu P, Agid Y. NeuroImage. 2007;34:618–638. doi: 10.1016/j.neuroimage.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Karachi C, Yelnik J, Tande D, Tremblay L, Hirsch EC, Francois C. Mov Disord. 2005;20:172–180. doi: 10.1002/mds.20302. [DOI] [PubMed] [Google Scholar]
- 23.Young RC, Biggs JT, Ziegler VE, Meyer DA. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 24.Conners CK. Conners' Continuous Performance Test II. Toronto: Multi-Health Systems Inc; 2000. Version 5 for Windows. [Google Scholar]
- 25.Tavares JV, Drevets WC, Sahakian BJ. Psychol Med. 2003;33:959–967. doi: 10.1017/s0033291703008432. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain SR, Sahakian BJ. Curr Psychiatry Rep. 2004;6:451–458. doi: 10.1007/s11920-004-0010-3. [DOI] [PubMed] [Google Scholar]
- 27.Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Jr, Hawkins JM. Am J Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- 28.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Marsden CD. Mov Disord. 1994;9:493–507. doi: 10.1002/mds.870090502. [DOI] [PubMed] [Google Scholar]
- 30.Trillet M, Vighetto A, Croisile B, Charles N, Aimard G. Rev Neurol (Paris) 1995;151:416–419. [PubMed] [Google Scholar]
- 31.Takeshita S, Kurisu K, Trop L, Arita K, Akimitsu T, Verhoeff NP. Neurosurg Rev. 2005;28:179–186. doi: 10.1007/s10143-005-0387-4. [DOI] [PubMed] [Google Scholar]
- 32.Voon V, Kubu C, Krack P, Houeto JL, Troster AI. Mov Disord. 2006;21:S305–S327. doi: 10.1002/mds.20963. [DOI] [PubMed] [Google Scholar]
- 33.Herzog J, Volkmann J, Krack P, Kopper F, Potter M, Lorenz D, Steinbach M, Klebe S, Hamel W, Schrader B, et al. Mov Disord. 2003;18:1332–1337. doi: 10.1002/mds.10518. [DOI] [PubMed] [Google Scholar]
- 34.Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Ann Neurol. 1998;44:622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 35.Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, Bonnet AM, Marsault C, Agid Y, Philippon J, et al. J Neurosurg. 2000;92:615–625. doi: 10.3171/jns.2000.92.4.0615. [DOI] [PubMed] [Google Scholar]
- 36.Dormont D, Ricciardi KG, Tande D, Parain K, Menuel C, Galanaud D, Navarro S, Cornu P, Agid Y, Yelnik J. Am J Neuroradiol. 2004;25:1516–1523. [PMC free article] [PubMed] [Google Scholar]
- 37.Saint-Cyr JA, Hoque T, Pereira LC, Dostrovsky JO, Hutchison WD, Mikulis DJ, Abosch A, Sime E, Lang AE, Lozano AM. J Neurosurg. 2002;97:1152–1166. doi: 10.3171/jns.2002.97.5.1152. [DOI] [PubMed] [Google Scholar]
- 38.Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B, Weinert D, Pfister G, Muller D, Mehdorn HM, et al. Mov Disord. 2004;19:1050–1054. doi: 10.1002/mds.20056. [DOI] [PubMed] [Google Scholar]
- 39.Lanotte MM, Rizzone M, Bergamasco B, Faccani G, Melcarne A, Lopiano L. J Neurol Neurosurg Psychiatry. 2002;72:53–58. doi: 10.1136/jnnp.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zonenshayn M, Sterio D, Kelly PJ, Rezai AR, Beric A. Surgical Neurology. 2004;62:216–225. doi: 10.1016/j.surneu.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 41.Sato F, Lavallee P, Levesque M, Parent A. J Comp Neurol. 2000;417:17–31. [PubMed] [Google Scholar]
- 42.Haber SN, Lynd-Balta E, Mitchell SJ. J Comp Neurol. 1993;329:111–128. doi: 10.1002/cne.903290108. [DOI] [PubMed] [Google Scholar]
- 43.Augood SJ, Waldvogel HJ, MÅnkle MC, Faull RLM, Emson PC. Neuroscience. 1999;88:521–534. doi: 10.1016/s0306-4522(98)00226-7. [DOI] [PubMed] [Google Scholar]
- 44.Levesque JC, Parent A. Mov Disord. 2005;20:574–584. doi: 10.1002/mds.20374. [DOI] [PubMed] [Google Scholar]
- 45.Butson CR, Cooper SE, Henderson JM, McIntyre CC. NeuroImage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Clin Neurophysiol. 2004;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. J Neurophysiol. 2006;96:1569–1580. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- 48.Yelnik J. Mov Disord. 2002;17(Suppl 3):S15–S21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]
- 49.Bejjani BP, Houeto JL, Hariz M, Yelnik J, Mesnage V, Bonnet AM, Pidoux B, Dormont D, Cornu P, Agid Y. Neurology. 2002;59:1425–1427. doi: 10.1212/01.wnl.0000031428.31861.23. [DOI] [PubMed] [Google Scholar]
- 50.Karachi C, Francois C, Parain K, Bardinet E, Tande D, Hirsch E, Yelnik J. J Comp Neurol. 2002;450:122–134. doi: 10.1002/cne.10312. [DOI] [PubMed] [Google Scholar]
- 51.Yelnik J, Percheron G. Neuroscience. 1979;4:1717–1743. doi: 10.1016/0306-4522(79)90030-7. [DOI] [PubMed] [Google Scholar]
- 52.Pearson JC, Norris JR, Phelps CH. J Comp Neurol. 1985;238:323–339. doi: 10.1002/cne.902380307. [DOI] [PubMed] [Google Scholar]
- 53.Bevan MD, Clarke NP, Bolam JP. J Neurosci. 1997;17:308–324. doi: 10.1523/JNEUROSCI.17-01-00308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F. Arch Neurol. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- 55.Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- 56.Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, Gaura V, Rascol O, Samson Y, Agid Y. Arch Neurol. 2004;61:1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- 57.Nambu A, Takada M, Inase M, Tokuno H. J Neurosci. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato F, Parent M, Levesque M, Parent A. J Comp Neurol. 2000;424:142–152. doi: 10.1002/1096-9861(20000814)424:1<142::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 59.Francois C, Tande D, Yelnik J, Hirsch EC. J Comp Neurol. 2002;447:249–260. doi: 10.1002/cne.10227. [DOI] [PubMed] [Google Scholar]
- 60.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 61.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, et al. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 62.Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, et al. Biol Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 63.Strakowski SM, Delbello MP, Adler CM. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 64.Albin RL, Young AB, Penney JB. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 65.Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. NeuroImage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Behrmann M, Geng JJ, Shomstein S. Curr Opin Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Adolphs R. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 68.Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, Pessoa L. J Neurosci. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallet L, Mesnage V, Houeto JL, Pelissolo A, Yelnik J, Behar C, Gargiulo M, Welter ML, Bonnet AM, Pillon B, et al. Lancet. 2002;360:1302–1304. doi: 10.1016/S0140-6736(02)11339-0. [DOI] [PubMed] [Google Scholar]
- 70.Fontaine D, Mattei V, Borg M, von Langsdorff D, Magnie MN, Chanalet S, Robert P, Paquis P. J Neurosurg. 2004;100:1084–1086. doi: 10.3171/jns.2004.100.6.1084. [DOI] [PubMed] [Google Scholar]
- 71.Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H, Pelissolo A, Moro E, Lhommee E, Lang AE, et al. Mov Disord. 2006;21:1941–1946. doi: 10.1002/mds.21098. [DOI] [PubMed] [Google Scholar]
- 72.Haber SN. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Haber SN, Kim KS, Mailly P, Calzavara R. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan SK, Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. J Chem Neuroanat. 2006;31:155–161. doi: 10.1016/j.jchemneu.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Yelnik J, Bardinet E, Dormont D, François C, Tandé D, Parain C, Malandain G, Ayache N, Hirsch E, Agid Y. NeuroImage. 2003;99:S48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.