Abstract
Aggregation of the amyloid-β (Aβ) peptide in the extracellular space of the brain is critical in the pathogenesis of Alzheimer's disease. Aβ is produced by neurons and released into the brain interstitial fluid (ISF), a process regulated by synaptic activity. To determine whether behavioral stressors can regulate ISF Aβ levels, we assessed the effects of chronic and acute stress paradigms in amyloid precursor protein transgenic mice. Isolation stress over 3 months increased Aβ levels by 84%. Similarly, acute restraint stress increased Aβ levels over hours. Exogenous corticotropin-releasing factor (CRF) but not corticosterone mimicked the effects of acute restraint stress. Inhibition of endogenous CRF receptors or neuronal activity blocked the effects of acute stress on Aβ. Thus, behavioral stressors can rapidly increase ISF Aβ through neuronal activity in a CRF-dependent manner, and the results suggest a mechanism by which behavioral stress may affect Alzheimer's disease pathogenesis.
Keywords: Alzheimer's disease, synaptic activity, environmental stress, microdialysis, transgenic
Evidence indicates that the aggregation and accumulation of the amyloid-β (Aβ) peptide in the brain extracellular space is a key initiating event in the pathogenesis of Alzheimer's disease (AD) (1). A number of studies demonstrate that aggregation of Aβ is concentration-dependent (2). Increasing the amount of Aβ produced by 50% or specifically increasing the more fibrillogenic Aβ42 either by APP gene dose or mutations in amyloid precursor protein (APP), PS1, or PS2, accelerates the onset of Aβ deposition and AD (3). Conversely, decreasing Aβ by decreasing cleavage of APP or by enhancing clearance of Aβ delays the onset of Aβ deposition (4). Thus, determining factors that regulate the levels of Aβ in the brain extracellular space, where it likely changes conformation and aggregates, may provide insight into AD pathogenesis and treatment.
Aβ is produced in the brain primarily by neurons after cleavage of APP by β- and γ-secretase (1). Aβ levels in the extracellular space are then influenced by factors regulating its release from neurons as well as postsecretory events such as transport and clearance. Recent evidence (5, 6) has shown that Aβ release from neurons is regulated by neuronal and specifically synaptic activity over minutes to hours. However, whether behavioral manipulations regulate synaptic activity and interstitial fluid (ISF) Aβ levels has not been addressed.
Evidence in both humans and animals suggests that environmental stressors may increase risk for AD or AD pathology. In humans, persons without dementia who are prone to psychological distress are more likely to develop AD (7, 8). Also, plasma levels of the stress hormone, cortisol, are correlated with the rate of dementia progression in patients with AD (9). In mouse models of AD, animals subjected to isolation stress over months had decreased learning performance and accelerated Aβ deposition (10). To explore the potential mechanisms and links between behavioral stressors and Aβ, we assessed the effects of acute restraint stress and chronic isolation stress on ISF Aβ in the brain of APP transgenic mice by in vivo microdialysis. Our results suggest that acute stress can lead to increases in hippocampal ISF Aβ over hours and that these increases require neuronal activity and are corticotropin-releasing factor (CRF)-dependent.
Results
Chronic Isolation Stress Increases ISF Aβ Levels.
Chronic isolation accelerates the onset of and exacerbates Aβ deposition in the hippocampus and cortex of Tg2576 mice (10), a transgenic mouse model expressing a mutated form of human APP that causes an autosomal dominant form of early-onset AD in humans (11). Because the formation of Αβ-containing plaques within the extracellular space is concentration-dependent, we hypothesized that behavioral stressors may increase ISF Aβ levels early in life, thereby leading to Aβ aggregation. Using the same paradigm that accelerated Aβ deposition previously (10), we subjected Tg2576 mice at weaning to 3 months of isolation stress. This time point was selected because we wanted to avoid assessing animals in which plaques were already present. Isolation consisted of rearing a single mouse in a small cage (≈one-third the size of a standard mouse cage). In previous experiments with Tg2576 mice, this treatment was associated with impairments in contextual memory, decreased neurogenesis, and greater Aβ deposition (10). In contrast, control littermate Tg2576 mice were reared under standard rodent housing conditions (two to five mice per standard-size cage). Brain Aβ levels were assessed in all mice at 4 months of age, an age before Aβ deposition even in stressed mice.
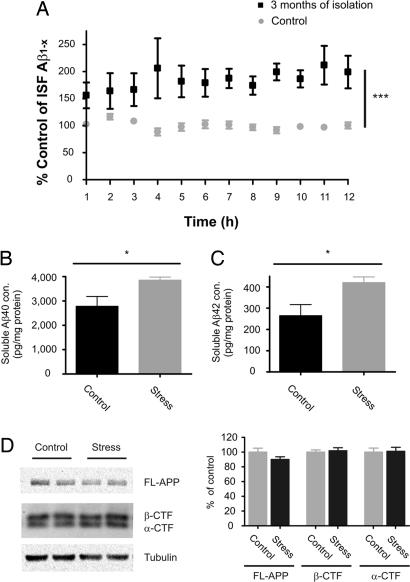
To measure specifically soluble Aβ levels in the extracellular space, we used in vivo microdialysis to measure ISF Aβ every 60 min for 12 h in freely moving mice (6, 12). ISF Aβ1–x levels were increased by 84% in Tg2576 mice exposed to 3 months of isolation stress, compared with control (Fig. 1A). This increase in ISF Aβ levels was likely a precipitating factor that resulted in accelerated Aβ deposition in Tg2576 mice subjected to 6 months of isolation stress (10).
Fig. 1.
Effects of 3 months of isolation stress on soluble Aβ levels within the ISF, tissue lysates, and APP fragments in the hippocampus. (A) Three months of isolation stress increased ISF Aβ levels to 184 ± 23% of control levels in 4-month-old Tg2576 mice (P = 0.0006; n = 10 per group). In vivo concentrations of ISF Aβ in the hippocampus were 5,309 ± 145.0 and 2,881 ± 61.0 pg/ml in mice exposed to 3 months of isolation and control condition, respectively (data not shown). To assess the levels of soluble Aβ in the hippocampus, hippocampal tissues were processed at the end of 3 months of isolation and under control conditions. As determined by ELISA, both Aβ40 (B) and Aβ42 (C) were elevated by 37.9 ± 4.4% and 57.7 ± 9.4%, respectively in a carbonate-soluble fraction of hippocampal lysates from mice after 3 months of isolation stress vs. controls (P = 0.02; n = 7–8 per group). The same tissues were also assessed for the levels of full-length APP (FL-APP), APP α-CTF and APP β-CTF (n = 7–8 per group). (D) Representative lanes from Western blots for FL-APP, α-CTF, and β-CTF. The levels of FL-APP, α-CTF, and β-CTF were not changed after 3 months of isolation stress compared with control. Each band was normalized to the amount of α-tubulin in each lane. Data represent mean ± SEM.
The levels of Aβ within hippocampal brain tissue were also assessed in control and chronically isolated Tg2576 mice. Hippocampal tissue was biochemically processed by sequential extraction in carbonate buffer then 5 M guanidine. Carbonate-soluble Aβ40 and Aβ42 levels were elevated by 38% and 59%, respectively, in 3-month isolated mice compared with controls (Fig. 1 B and C). There was not a significant change in the Aβ40/42 ratio in the isolated vs. control mice. There were also no significant differences between groups in guanidine-soluble Aβ levels, and as expected, neither the isolated nor the control mice contained Aβ deposition as assessed by immunostaining (data not shown).
To determine whether isolation stress altered APP levels or processing, the levels of full-length APP as well as the α- and β- C-terminal fragment (CTF) of APP were assessed with Western blotting. There was no difference in the levels of full-length APP protein, nor was there a difference in α- and β-CTF in mice subjected to 3 months of isolation stress compared with control mice (Fig. 1D). To examine whether isolation stress altered the protein levels of Aβ degrading enzymes and apoE, we assessed the levels of insulin-degrading enzyme (IDE) and neprilysin (NEP) in hippocampal tissue by Western blotting and apoE by ELISA. There were no differences in the levels of IDE, NEP, or apoE in mice exposed to 3 months of isolation stress compared with controls (data not shown).
Acute Restraint Stress Increases ISF Aβ Levels.
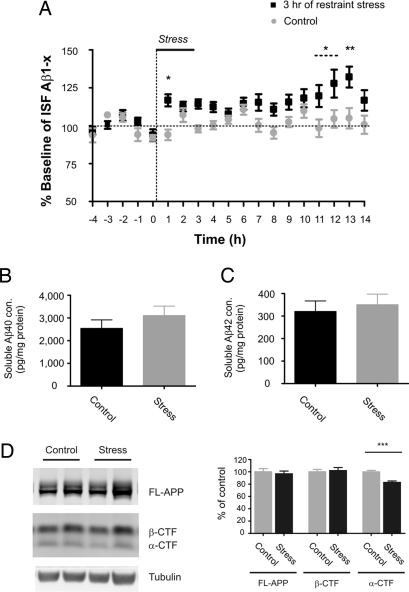
Because chronic stress elevated ISF Aβ, we then sought to determine whether an acute behavioral stressor could rapidly increase ISF Aβ levels as well. To this end, 3- to 4-month-old Tg2576 mice were subjected to 3 h of restraint stress (13). In vivo microdialysis was used to assess ISF Aβ levels before, during, and for 11 h after the end of restraint. Three hours of restraint stress increased ISF Aβ levels within 1 h of the initiation of restraint, and a peak increase of 32% was seen by 13 h (Fig. 2A). At 13 h from the beginning of restraint stress, carbonate-soluble Aβ40 and Aβ42 levels were not significantly increased within hippocampal tissue (Fig. 2 B and C). Similar to isolation stress, acute restraint stress did not alter the levels of full-length APP or β-CTF in hippocampal tissue at 13 h from the beginning of restraint (Fig. 2D). Interestingly, there was a small but significant 17% decrease in α-CTF levels in mice subjected to restraint stress (Fig. 2D). Given that the decrease in α-CTF is small compared with the 32% increase in ISF Aβ levels, if a change in α-secretase cleavage contributes to altered Aβ levels, it likely represents a small contribution to the overall effect. We also examined the levels of insulin-degrading enzyme and neprilysin protein by Western blotting and apoE by ELISA in hippocampal tissue 13 h after the beginning of acute restraint stress. Similar to chronic isolation stress, the levels were not changed in stressed mice compared with controls (data not shown).
Fig. 2.
Effects of 3 h of restraint stress on soluble Aβ levels within the ISF and tissue lysates, and APP fragments in the hippocampus. (A) Three hours of acute restraint stress increased ISF Aβ levels to 132 ± 6.9% of baseline by 13 h after the beginning of stress initiation in 3- to 4-month-old Tg2576 mice (P = 0.003; n = 10 per group). Hippocampal tissues were processed at 14 h after the beginning of 3 h of restraint stress initiation vs. the control condition. There were no significant differences in the levels of either Aβ40 (B) or Aβ42 (C) in stressed vs. control mice in the carbonate-soluble fraction of the tissue lysates as measured by ELISA (n = 8 per group). To determine whether APP processing was altered in stressed mice, the same tissues were also assessed for the protein expression levels of FL-APP, APP α-CTF, and APP β-CTF. (D) Representative lanes from Western blots for FL-APP, α-CTF, and β-CTF are shown. The levels of FL-APP and β-CTF were not different between groups. The levels of α-CTF were significantly decreased by 17.23 ± 3.404% in Tg2576 mice after 3 h of restraint stress compared with controls (P = 0.0005; n = 8 per group). Each band was normalized to the amount of α-tubulin in each lane. Data represent mean ± SEM.
Acute Corticosterone Does Not Mimic Stress-Induced Increase in ISF Aβ Levels.
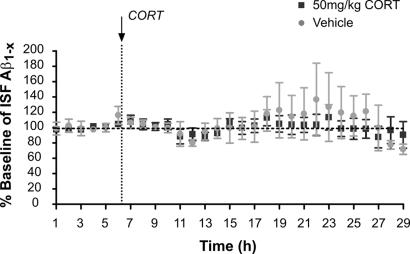
One effect of stress is to cause release of CRF from the hypothalamus into the hypophyseal portal system, where it travels to the pituitary gland to cause adrenocorticotropic hormone release, thereby inducing adrenal glucocorticoid release. Glucocorticoids act peripherally as well as within the brain in response to stressful stimuli. We asked whether systemic administration of corticosterone, the most abundantly produced endogenous glucocorticoid hormone in rodents, could mimic the effect of acute restraint stress on ISF Aβ levels. Three- to 4-month-old Tg2576 mice were treated with either vehicle or corticosterone (50 mg/kg, i.p.). Basal ISF Aβ levels were measured every hour for 6 h as well as an additional 23 h after treatment. Corticosterone did not alter ISF Aβ levels in Tg2576 compared with vehicle-treated mice (Fig. 3), suggesting that corticosterone does not mediate the acute stress-induced increase in ISF Aβ levels.
Fig. 3.
Systemic administration with corticosterone (CORT) did not acutely alter ISF Aβ levels. The effect of a high dose of CORT on hippocampal ISF Aβ levels in 3- to 4-month-old Tg2576 mice is shown. After the basal ISF Aβ levels were obtained for 10 h, animals received an i.p. injection of 50 mg/kg CORT. An equal volume of vehicle solution (100 μl of 15% 2-hydroxypropyl-β-cyclodextrin in water) was used for control. There was no difference in ISF Aβ levels in CORT-treated vs. vehicle-treated mice (n = 8 per group).
CRF Mediates the Acute Stress-Induced Increase in ISF Aβ Levels.
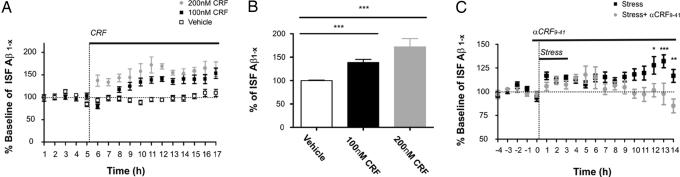
Given that corticosterone is a major hormone in the stress response, we sought to determine whether a step upstream of corticosterone release contributes to alterations in ISF Aβ levels. In response to stress, CRF peptide is synthesized and released from the hypothalamus to stimulate corticosterone release from the adrenal gland (14). CRF is also produced in many brain regions where it can bind to CRF receptors and facilitates excitatory neurotransmission (15). As a response to stress, CRF is released locally and activates CRF receptors that are expressed in a majority of CA1 and CA3 pyramidal cells in the hippocampus (16). Therefore, we examined whether CRF could alter the levels of ISF Aβ in the hippocampus by infusing CRF directly into the hippocampus through reverse microdialysis. CRF caused an immediate increase in ISF Aβ levels in a dose-dependent manner; 100 and 200 nM CRF increased ISF Aβ levels to 138.3 and 171.9% over 12 h, respectively (Fig. 4 A and B). These data suggest that CRF may mediate increases in ISF Aβ levels produced by behavioral stressors.
Fig. 4.
Effects of CRF on ISF Aβ levels. To examine the effect of CRF on hippocampal ISF Aβ levels, 100 and 200 nM CRF were administrated by reverse microdialysis in the hippocampus of 3- to 4-month-old Tg2576 mice. (A) One hundred nanomolar CRF in the microdialysis fluid resulted in an increase ISF Aβ levels at 3 h after drug infusion, whereas 200 nM CRF increased ISF Aβ levels immediately after drug infusion (n = 5 per group). (B) Both 100 and 200 nM CRF increase ISF Aβ levels in a dose-dependent manner, reaching 138.3 ± 7.027% and 171.9 ± 17.83% of baseline by 12 h, respectively (P < 0.0001 and P = 0.0001, respectively). (C) Three-hour restraint stress increased ISF Aβ levels to 132 ± 6.896% compared with baseline by 13 h after the beginning of stress initiation (P = 0.003; n = 10 for stress). Treatment with α-helical CRF9–41 (αCRF9–41), a CRF receptor antagonist, given from 30 min before restraint stress until the end of the experiment, blocked the stress-induced increase in ISF Aβ levels (P = 0.006; n = 5 for stress + αCRF9–41).
To examine further whether endogenous CRF is responsible for modulating ISF Aβ in mice subjected to 3 h of acute restraint stress, 3-month-old Tg2576 mice were pretreated with either vehicle or αCRF9–41, an antagonist of CRF receptors (17), by reverse microdialysis. αCRF9–41 was continuously infused from 30 min before the onset of 3 h of restraint stress until the end of the experiment. αCRF9–41 prevented the stress-induced increase in ISF Aβ levels (Fig. 4C), suggesting that endogenous CRF likely mediates the increase in ISF Aβ levels caused by restraint stress. Infusion with αCRF9–41 in the hippocampus, in the absence of stress, had no significant effect on ISF Aβ levels (data not shown). Increases in ISF Aβ levels mediated by endogenous CRF could be the result of increased endogenous CRF, enhanced sensitivity of CRF receptors, or both. CRF levels were measured by ELISA in hippocampal ISF assessed by microdialysis in 3-month-old Tg2576 mice subjected to acute restraint stress and chronic isolation stress. After obtaining microdialysis samples for 10 h, 3 h of restraint stress was given to mice, and samples were collected every 3 h up to 12 h from the end of restraint. CRF levels were significantly higher in the 3-h period immediately after 3 h of acute restraint stress compared with controls (stressed mice, 173.0 ± 24% vs. control mice, 100.0 ± 15%; expressed as mean percent control ± SEM; P = 0.02; n = 5 per each group). This stress-induced increase in CRF suggests that increases in endogenous CRF may play a role in the acute CRF-mediated increase in ISF Aβ levels. We also assessed CRF levels in the mice exposed to chronic isolation vs. control conditions. There was no difference in CRF levels in the mice exposed to 3 months of isolation stress vs. controls (stressed mice, 104.8 ± 12% vs. control mice, 100.0 ± 19%; expressed as mean percent control ± SEM; n = 5 per each group, P = 0.83). The absence of a change in CRF in chronic stress suggests that the mechanisms by which acute vs. chronic stress leads to increased ISF Aβ are likely to differ.
Neuronal/Synaptic Activity Is Involved in Stress-Induced Increases in ISF Aβ Levels.
Within the hippocampus, CRF potentiates excitatory neurotransmission (15). Intracellular electrophysiological recordings from rat hippocampal pyramidal neurons determined that exogenously applied CRF increases the firing of CA1 pyramidal neurons in response to excitatory input (18). Endogenous CRF during stress also enhances hippocampal synaptic plasticity (19). Our group has demonstrated previously that neuronal and synaptic activity regulates ISF Aβ release from neurons (6). Taken together, these studies suggest that the effect of stress on ISF Aβ levels through the actions of CRF and its receptors may result from an increase in excitatory synaptic transmission.
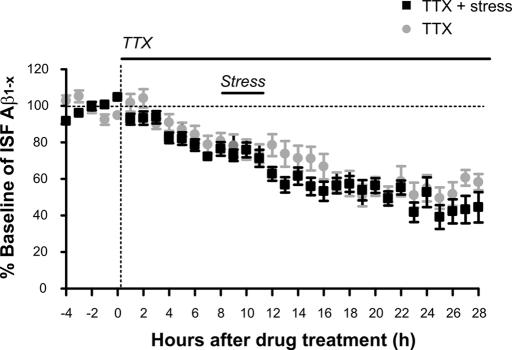
To address this issue, we decreased neuronal activity by infusing tetrodotoxin (TTX) directly into the hippocampus through reverse microdialysis. Consistent with our previous observations (6), TTX treatment decreased ISF Aβ levels in Tg2576 mice by ≈60% over 16 h compared with baseline (Fig. 5). ISF Aβ levels remained low for an additional 12 h in the presence of TTX. TTX almost completely blocks neuronal activity in the hippocampus by 6 h of treatment as assessed by extracellular field potential recordings (6). Therefore, after 8 h of TTX administration, mice were subjected to 3 h of restraint stress. In the presence of TTX, restraint stress did not result in an increase in ISF Aβ (Fig. 5). That TTX blocked the restraint stress-induced increase in ISF Aβ levels suggests that neuronal activity is required for the acute stress-induced increase in ISF Aβ levels. These data are also consistent with findings that neuronal activity is linked to Aβ release (5, 6) and suggest that modulation of ISF Aβ levels through environmental and physiological alterations may result from neuronal activity mediated by specific neuromodulators such as CRF.
Fig. 5.
Neuronal/synaptic activity is involved in the stress-induced increase in ISF Aβ levels. Infusion with 5 μM TTX in the hippocampus by reverse microdialysis immediately decreased ISF Aβ levels, reaching 58.5% of baseline by 17 h from drug treatment in 3- to 4-month-old Tg2576 mice. Three hours of restraint stress was given to mice at 8 h after TTX treatment, which resulted in no significant change in ISF Aβ levels compared with controls treated with TTX alone controls (n = 5 per group).
Discussion
Sporadic, late-onset AD accounts for the majority of cases of AD; however, unlike the familial forms, the etiology remains largely unknown. The only genetic risk factor that influences late-onset AD that has been confirmed in multiple studies is the APOE genotype (3). Environmental factors such as head trauma (20) and education (21) also appear to influence disease risk. There are likely other environmental factors that determine risk for AD. Recent evidence from both humans and animal models has suggested that stress can increase the risk for developing AD (7–10). Whether stress plays a role in disease progression by direct effects on a specific molecule such as Aβ or by indirect effects on other downstream targets is unknown. Our work demonstrates that two forms of stress directly increase ISF Aβ. The effect on ISF Aβ is greatest when mice are subjected to several months of stress; however, a significant effect of stress can be detected in as little as 1 h. Additionally, CRF and neuronal activity appear to play key mechanistic roles linking an acute behavioral stressor and ISF Aβ levels. Results from many studies suggest that the concentration of Aβ is linked to the onset of Aβ deposition and toxicity. We hypothesize that the concentration in the brain ISF pool is directly linked to this process. ISF Aβ constitutes a small overall pool of Aβ in the brain, and further evidence is required to understand whether the concentration in this pool is directly linked with the onset of Aβ aggregation and its effects.
CRF is a 41-aa peptide that is synthesized within the hypothalamus and stimulates the release of adrenocorticotropic hormone from the anterior pituitary (22). In addition to the hypothalamus, CRF and its receptors are expressed in a variety of other locations in the CNS where it acts as a neuropeptide to modulate neuronal activity and signaling (23, 24). It has been shown that behavioral stressors acutely release CRF from nerve terminals in the limbic system (15), where it can propagate and integrate stress-related behaviors (25). Both exogenous and endogenous CRF can increase neuronal activity and excitability as well as influence synaptic plasticity in the hippocampus both in vitro and in vivo (15, 19). Our observation that CRF increases ISF Aβ levels, coupled with the facts that CRF increases neuronal activity and neuronal activity results in Aβ release from neurons, suggests that CRF modulates ISF Aβ through effects on neuronal activity. This observation is supported by the finding that TTX blocked the ability of acute stress to increase ISF Aβ.
CRF effects are mediated by CRF receptors 1 and 2, although CRF1 in particular, appears to modulate stress-mediated effects of CRF in the hippocampus (26, 27). CRF receptors are G protein-coupled, and their stimulation results in activation of adenylate cyclase and protein kinase A (28, 29). It is possible that these signaling pathways link acute stress to increases in neuronal activity and Aβ levels. Another possibility is that CRF binding to its receptors has an effect on CRF receptor-mediated endocytosis and Aβ production that is not G protein-dependent. It has recently been shown that activation of the β2-adrenergic receptor can increase Aβ levels, and this effect requires receptor endocytosis, as is associated with γ-secretase tracking to later endosomes and lysosomes (30). If stress and CRF are involved in regulating ISF Aβ and contributing to whether Aβ aggregates, the involvement of stress and CRF would likely be relevant to the onset of Aβ deposition as well as its progression. Once AD pathology is more significant with tauopathy and cell loss, a variety of secondary changes could take place. In fact, in patients with AD, it has been shown that CRF-like immunoreactivity is decreased and CRF receptor binding is increased (31). Whether and how CRF is responsible for the changes that result from chronic stress will need to be defined in future studies.
A recent study suggests that additional mechanisms may regulate the effects of glucocorticoids on brain Aβ. We found that acute systemic treatment with the endogenous steroid corticosterone had no acute effect on ISF Aβ levels; however, treatment of triple transgenic APP/PS1/MAPT mice with dexamethasone increased brain Aβ levels as well as β-site APP-cleaving enzyme (BACE) and the β-CTF of APP as assessed 7 days after treatment (32). Dexamethasone is a potent, synthetic, and selective glucocortocoid receptor ligand, like corticosterone, that has profound effects on the HPA axis in vivo. However, given that dexamethasone does not readily cross the blood–brain barrier (BBB), it seems likely that its primary site of action is either within the periphery or within brain regions such as parts of the hypothalamus that lack a BBB (33). Although it was found that CRF modulates ISF Aβ levels in an acute-stress paradigm, we have not addressed the mechanism of increased ISF Aβ in chronic stress, which is likely to involve additional pathways. It is possible that altered physical activity in mice subjected to isolation stress in some way resulted in long-term changes in ISF Aβ independent of effects of CRF. Although we found that total locomotor activity in animals subjected to 3 months of isolation stress vs. controls was not different at the end of 3 months (data not shown), the lack of change in locomotor activity does not rule out the possibility that changes in activity over several months are related to increases in ISF Aβ. The finding that CRF levels are increased after acute restraint but not in mice subjected to chronic isolation suggests that acute vs. chronic stress may affect ISF Aβ by different mechanisms. Although a single stressful event may affect ISF Aβ levels through CRF and synaptic activity, it may be that multiple stressful events or prolonged stress sets off a cascade of events that influence Aβ metabolism. It will be important in future studies to assess the detailed interplay among CRF, corticosteroids, and stress on ISF Aβ levels over time to determine whether and how they influence the relationship between synaptic activity and Aβ, Aβ clearance, APP processing, and Aβ aggregation.
Recent in vitro (5) and in vivo (6) studies demonstrate that neuronal activity, specifically synaptic activity and synaptic vesicle release, is linked with the release of Aβ from neurons. This work suggests that physiologic levels of neuronal activity also rapidly modulate ISF Aβ levels. In humans, the brain areas that are most vulnerable to Aβ deposition are also areas with the highest metabolic activity and likely synaptic activity (34). These areas overlap with brain regions that make up what is termed the “default network” (35), regions that have the highest activity when a person is not carrying out a specific mental task. It has been estimated that the majority of the brain's energy consumption supports synaptic activity (35). However, the additional energy burden associated with the momentary demands of a specific mental task may be as little as 0.5–1.0% of the brain's total energy budget (35). The possibility exists that environmental manipulations, such as behavioral stressors, may affect synaptic activity in brain regions over longer periods of time (e.g., hours to days) and may have marked effects in the physiological regulation of extracellular brain Aβ levels and potentially long-term risk for AD. Recent observations with APP transgenic mice exposed to different environments over time may be relevant to this issue. It has been shown that exposure of APP transgenic mice to differing environmental conditions and different levels of physical, cognitive, and social activity over months results in increased or decreased Aβ deposition depending on the conditions (36–38). Determining how environmental manipulations affect synaptic activity and ISF Aβ levels may be important in understanding the vulnerability of specific brain regions to AD-like changes.
In sum, our findings demonstrate that acute and chronic behavioral stressors increase ISF Aβ levels. The acute effects of restraint stress are mediated through effects of CRF and require neuronal activity. The relationship among stress, CRF, and ISF Aβ levels suggests that CRF may play a role in AD pathogenesis and that CRF and CRF signaling pathways are therapeutic targets to modulate processes that affect Aβ metabolism.
Materials and Methods
Animals.
All experimental procedures involving animals were performed in accordance with guidelines established by the Animal Studies Committee at Washington University. We bred Tg2576+/− hemizygous male mice (a generous gift from Dr. K. Ashe, University of Minnesota) to C57BL6/SJL female mice (Taconic Farms, Germantown, NY). The Tg2576+/− littermates of both sexes were used equally for the experimental groups. Animals were screened for the Tg2576 transgene by PCR using DNA obtained from postweaning toe biopsies. Animals were raised, and all experiments were performed in 12-h dark/12-h light-controlled room. The animal had access to food and water ad libitum.
Isolation and Restraint Stress.
To induce chronic isolation stress, Tg2576 mice were housed individually in cages one-third the size of a standard mouse cage from weaning until 4 months of age (10, 39). The control animals were group-housed (n = 2–5 per standard-sized cage). All mice received food and water ad libitum. For restraint stress, mice at 3–4 months of age were subjected to 3 h of restraint in a 50-ml polypropylene tube (4 × 5 × 4 cm) similar to a method described previously (40). The stress was initiated at the beginning of the dark period during microdialysis. Mice subjected to restraint were raised under standard group-housing conditions until stress was given. The control animals were subjected to only microdialysis without additional stress.
In Vivo Microdialysis.
In vivo microdialysis to assess brain ISF Aβ1–x in the hippocampus of awake, freely moving Tg2576 mice was performed as described previously (6, 12). This technique samples soluble molecules within the extracellular fluid that are smaller than 38 kDa, the molecular mass cutoff of the microdialysis probe membrane. Basal levels of ISF Aβ were defined as the mean concentration of Aβ from hours 5–10 after probe insertion. In all data from microdialysis experiments, time 1 indicated 1 h after the beginning of the dark period unless specifically noted. After each experiment, animals were killed.
Aβ, ApoE, and CRF Quantification.
Microdialysis samples and hippocampal tissue lysates were analyzed for Aβ by using a denaturing, sandwich ELISA specific for human Aβ1–x, Aβ1–40, or Aβ1–42 as described previously (12). Free CRF levels from microdialysis samples were analyzed by using a sandwich ELISA kit (COSMO BIO Co., Tokyo, Japan). ApoE levels were assessed by ELISA in tissue lysates as described previously (41).
Western Blotting.
Hippocampal tissues were harvested at the end of 3 months of isolation stress and control conditions or at 14 h after the beginning of 3 h of restraint stress initiation and control conditions. Western blotting was performed as described previously (12).
Drug Treatment.
TTX was purchased from Sigma–Aldrich (St. Louis, MO) and dissolved in water at 3.13 mM as a stock solution. TTX was diluted in artificial cerebrospinal fluid (aCSF), prepared as described (12), to a final concentration of 5 μM immediately before the experiments and delivered into the hippocampus by reverse microdialysis. Corticosterone was purchased from Sigma-Aldrich and dissolved in 15% of 2-hydroxypropyl-β-cyclodextrin (HPB) at 15 mg/ml. Fifty mg of corticosterone per kg of body weight or 15% HPB alone as a vehicle in a 100-μl total volume was injected i.p. into mice. Human/rat CRF peptide (h/r CRF) and αCRF9–41 peptide were purchased from Bachem (King of Prussia, PA). For h/r CRF, 400 ng/μl stock solution was prepared in 10 mM acetic acid and diluted in aCSF to final concentrations of 100 and 200 nM. For αCRF9–41, 3 μg/μl stock solution was prepared in 10 mM acetic acid and diluted in aCSF to final concentration of 860 nM. Both h/r CRF and αCRF9–41 were diluted in aCSF immediately before the experiments and administered directly into the hippocampus by reverse microdialysis.
Statistical Analysis.
Data in the figures represent mean ± SEM. All statistical analysis was performed by using Prism version 4.02 for Windows (GraphPad, San Diego, CA). Statistical analysis was performed by using a nonparametric Mann–Whitney t test and was accepted as significant if P ≤ 0.05. Comparisons between two groups were performed by using two-way ANOVA with a Bonferroni post test.
Acknowledgments
This work was supported by National Institutes of Health Grants AG025824 (to J.G.C. and D.M.H.) DA 07261 (to J.R.C.), and AG13956 (to D.M.H.), a Zenith Award from the Alzheimer's Association (to D.M.H.), the Cure Alzheimer's Fund (D.M.H.), and Eli Lilly (D.M.H.).
Abbreviations
- Aβ
amyloid-β
- αCRF9–41
antagonist of CRF receptors
- aCSF
artificial cerebrospinal fluid
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- CRF
corticotropin-releasing factor
- CTF
C-terminal fragment
- h/r CRF
human/rat CRF
- ISF
interstitial fluid
- TTX
tetrodotoxin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Selkoe DJ. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 2.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 3.Tanzi RE, Bertram L. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Golde TE. J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 5.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 6.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 9.Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Youkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 12.Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, et al. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RB, Zhou J, Shi M, Redmann S, Mynatt RL, Ryan DH. Physiol Behav. 2001;73:599–608. doi: 10.1016/s0031-9384(01)00508-x. [DOI] [PubMed] [Google Scholar]
- 14.Watts AG. Front Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Baram TZ, Hatalski CG. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunson KL, Schultz L, Baram TZ. Brain Res Dev Brain Res. 1998;111:119–128. doi: 10.1016/s0165-3806(98)00130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 19.Blank T, Nijholt I, Eckart K, Spiess J. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, et al. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 22.Vale W, Spiess J, Rivier C, Rivier J. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 23.Chang CP, Pearse RV, 2nd, O'Connell S, Rosenfeld MG. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 24.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. Ciba Found Symp. 1993;172:277–295. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- 26.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Brain Res. 1986;381:49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- 29.Haug T, Storm JF. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- 30.Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang Z, Song M, Xiong J, Bai Y, Pei G. Nat Med. 2006;12:1390–1396. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- 31.De Souza EB, Whitehouse PJ, Kuhar MJ, Price DL, Vale WW. Nature. 1986;319:593–595. doi: 10.1038/319593a0. [DOI] [PubMed] [Google Scholar]
- 32.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raichle ME, Mintun MA. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 36.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adlard PA, Perreau VM, Pop V, Cotman CW. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S. Psychoneuroendocrinology. 2003;28:540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Mol Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]