Abstract
Hypothalamic orexin/hypocretin neurons recently emerged as key orchestrators of brain states and adaptive behaviors. They are critical for normal stimulation of wakefulness and breathing: Orexin loss causes narcolepsy and compromises vital ventilatory adaptations. However, it is unclear how orexin neurons generate appropriate adjustments in their activity during changes in physiological circumstances. Extracellular levels of acid and CO2 are fundamental physicochemical signals controlling wakefulness and breathing, but their effects on the firing of orexin neurons are unknown. Here we show that the spontaneous firing rate of identified orexin neurons is profoundly affected by physiological fluctuations in ambient levels of H+ and CO2. These responses resemble those of known chemosensory neurons both qualitatively (acidification is excitatory, alkalinization is inhibitory) and quantitatively (≈100% change in firing rate per 0.1 unit change in pHe). Evoked firing of orexin cells is similarly modified by physiologically relevant changes in pHe: Acidification increases intrinsic excitability, whereas alkalinization depresses it. The effects of pHe involve acid-induced closure of leak-like K+ channels in the orexin cell membrane. These results suggest a new mechanism of how orexin/hypocretin networks generate homeostatically appropriate firing patterns.
Keywords: arousal, hypocretin, hypothalamus, pH, breathing
Even small changes in extracellular levels of protons ([H+]e) are fatal to mammalian cells and tissues. The brain rapidly counteracts such changes with finely tuned adaptations in breathing, behavioral arousal, and aversive panic responses (1–4). Breathing controls [H+]e through CO2 removal (in the body, H+ + HCO3− ↔ CO2 + H2O), while behavioral arousal ensures that any obstructions to breathing can be effectively removed. For example, respiratory acidosis produced by sleep apnea causes awakening and stimulates breathing, which normalizes [H+]e. Failure of such reflexes is thought to contribute to fatal conditions such as sudden infant death syndrome (5, 6).
To elicit appropriate changes in breathing and arousal, neurons need to translate changes in [H+]e into appropriately coordinated responses. In many neurons, [H+]e depresses electrical excitability (i.e., acidosis is inhibitory and alkalosis is excitatory) (7). However, the opposite type of cellular response is thought to be important for the vital adaptive reflexes mentioned earlier: a steep increase in firing rate by physiological acidification and a steep decrease in firing upon alkalinization (4). Such specialized chemosensory firing responses are traditionally associated with neurons of the brainstem, a key center in the control of arousal and breathing (1, 3, 4). The ionic basis of these responses is controversial and has been proposed to involve ion currents mediated by GABAA Cl− channels, Ca2+ channels, inwardly rectifying K+ channels, voltage-gated K+ channels, and leak-like K+ channels (4). However, all these channels are also expressed in many nonchemosensory neurons (8), and thus their presence alone does not signify whether a neuron is chemosensory. To draw this conclusion, it is necessary to perform direct measurements of firing rate and membrane potential at different levels of [H+]e.
Although until now most work on neuronal chemosensing has focused on the brainstem, historical evidence indicates that the hypothalamus is also vital for maintaining normal breathing and arousal (9, 10). Hypothalamic neurons responsible for these functions have only recently been identified and found to contain peptide transmitters called orexins/hypocretins (11, 12). Orexin-containing neurons (orexin neurons) are located in the lateral hypothalamus (LH), but project widely throughout the brain, where orexins act on two specific G protein-coupled receptors (13). Both arousal and breathing centers receive stimulatory inputs from orexin neurons (14–16). Orexin neurons attracted great attention when they proved to be targets in narcolepsy, where their destruction causes irresistible daytime sleepiness, disturbed night-time sleep, and cataplexy (17–19). The activity of orexin neurons is also essential for adaptive exploratory responses to food shortage (20). Furthermore, recent data show that orexin knockout severely compromises the ability to increase breathing during acidosis (16).
These studies established that orexin neurons are essential for appropriate adjustments of brain and behavioral states to the internal and external environments (13). However, the regulation of orexin neurons is much less understood. In particular, it is unknown whether the firing of orexin neurons is sensitive to physiologically relevant changes in fundamental homeostatic signals such as [H+] and [CO2]. To document such responses and compare them to known chemosensory neurons, we performed electrophysiological recordings from orexin neurons identified by targeted expression of GFP in transgenic mice. We show that orexin cell firing is potently stimulated by CO2 and H+ through a mechanism involving acid-induced inhibition of postsynaptic leak-like K+ channels. We find that this enables orexin cell firing to encode small physiological changes in pH with high sensitivity, comparable to that of classical chemosensory neurons of the brainstem. These results identify a new mechanism by which hypothalamic orchestrators of adaptive behavior generate homeostatically appropriate patterns of activity.
Results
Firing Responses of LH Orexin Cells to Physiological Fluctuations in pHe.
Living orexin neurons are difficult to identify because they exhibit few defining anatomical features and are intermixed with many other types of cells in the LH. To overcome this problem, we used brain slices from transgenic mice that selectively express GFP in orexin neurons, allowing precise identification of living orexin cells for electrophysiological recordings (see Methods).
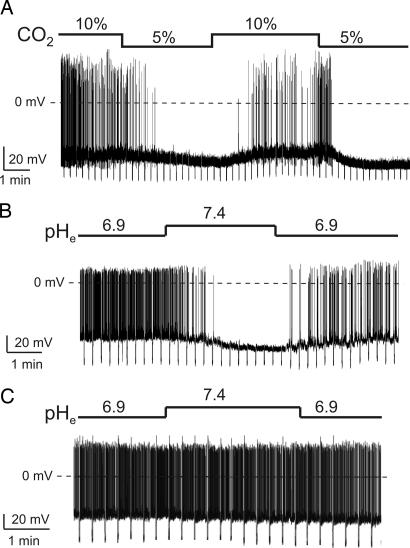
In the intact brain in vivo, the interstitial pH is generally ≈7.1 to 7.25, but can fluctuate between 6.9 and 7.4, for example, in response to hypo- and hyperventilation, respectively (21). We first mimicked the natural shifts in extracellular pH (pHe) by changing bath [CO2] (see Methods), which in the body controls [H+]e via the reaction H+ + HCO3− ↔ CO2 + H2O. Switching from 10% to 5% CO2 (which creates pHe of 7.02 and 7.25, respectively) reversibly suppressed the spontaneous firing of orexin cells by ≈4-fold, hyperpolarized their membrane potential, and increased their membrane conductance (Fig. 1A; the statistics are given in the figure legends). To confirm that this was due to changes in [H+] rather than [CO2] or [HCO3−], we also varied pHe without changing the latter two parameters, using bicarbonate-free solutions buffered with the artificial H+ buffer Hepes (see Methods). Switching pHe from 6.9 to 7.4 with Hepes-buffered solutions suppressed the spontaneous firing of orexin cells ≈10-fold, induced membrane hyperpolarization, and increased membrane conductance (Fig. 1B). Hepes-buffered solutions were used in subsequent experiments for better pH control (22).
Fig. 1.
Effects of H+ and CO2 on orexin and nonorexin cells of the LH. Current-clamp whole-cell recordings with K-gluconate intracellular solution. To monitor membrane conductance, cells were injected with periodic hyperpolarizing current pulses. The size of the resulting downward voltage deflections is inversely proportional to membrane conductance. (A) Effect of CO2-induced changes in pHe on an orexin neuron. Firing rate was 0.8 ± 0.3 Hz in 5% CO2 and 3.7 ± 0.4 Hz in 10% CO2 (n = 5; P < 0.01, two-tailed t test). (B) Effect of Hepes-buffered changes in pHe on an orexin neuron. Firing rate was 4 ± 0.6 Hz at pH 6.9 and 0.5 ± 0.2 Hz at pH 7.4 (n = 15; P < 0.001, two-tailed t test). (C) No effect of changes in pHe on firing of a nonorexin neuron.
Firing Responses of LH Nonorexin Cells to Physiological Fluctuations in pHe.
To examine the specificity of the [H+]e effects, we also performed recordings from other LH neurons. We chose cells that were near orexin neurons, but did not display GFP fluorescence and did not possess the defining electrical fingerprints of orexin neurons [electrical fingerprinting involving hyperpolarizing current injections was routinely performed as in our previous studies (23, 24)]. In contrast to orexin neurons, the firing and membrane resistance of these nonorexin cells was either unaffected by a pHe switch from 6.9 to 7.4 (see Fig. 1C) or accelerated by this alkalinizing switch (n = 3, data not shown). These data imply that the changes in orexin cell firing on physiological changes in pHe (Fig. 1 A and B) are not a general characteristic of LH neurons, but represent a more specific property of orexin cells.
Steepness of the Relationship Between pHe and Orexin Cell Firing.
The slope of the relationship between spontaneous firing rate and extracellular pH is considered of critical importance for classifying neurons as chemosensory (4). To investigate this parameter in orexin neurons, we exposed them to different levels of pHe and measured steady-state firing rates at each of these levels (Fig. 2). pHe shifts that were much smaller than the extreme changes used in Fig. 1 were also effective in changing orexin cell firing: Even switching pHe from 7.15 to 7 increased the firing rate >4-fold in some cells (e.g., Fig. 2A). The same cells were able to integrate both small and more extreme physiological changes in extracellular acidity (Fig. 2A). Within the physiological range of pHe (6.9–7.4), the firing rate was steeply dependent on pHe, decreasing from ≈4 Hz at pHe of 6.9 to near zero at pHe of 7.4 (Fig. 2B). The slope of the steep part of the firing-pHe curve was close to 10 Hz per unit change in pH (Fig. 2B), which is comparable to values observed in chemosensitive cells of the brainstem (4). We also calculated the percent change in firing rate per 0.1 unit change in pHe (see Methods), which is thought to be a better comparative measure of chemosensitivity than the absolute changes in firing rate (4). In orexin neurons, the increase in firing rate per 0.1 pH unit acidification was 108% (see Discussion).
Fig. 2.
Sensitivity of orexin cell firing to extracellular [H+] (pHe). Current-clamp whole-cell recordings with K-gluconate intracellular solution. (A) Effect of small and large pHe changes on spontaneous firing in the same cell. (B) Mean steady-state firing rate plotted against pHe. Each point represents more than three cells.
Effects of Physiological pHe Changes on Evoked Firing and Intrinsic Excitability.
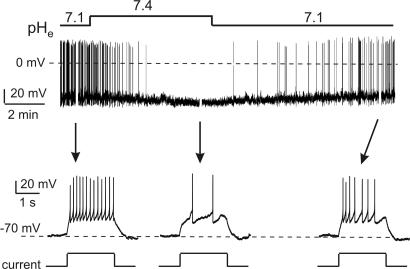
In the behaving animal, the firing of orexin neurons is likely to be modulated by an array of neurotransmitters, hormones, and nutrients that act by triggering membrane currents (25). It is therefore of interest to define how the sensitivity of orexin cell firing to current inputs (intrinsic excitability) is affected by pHe. We measured excitability as the number of action potentials induced by a fixed-amplitude current pulse (2-sec duration) from the same baseline membrane potential (−70 mV). The baseline potential was imposed by offsetting pHe-induced Vm changes by sustained current injection (26). Current amplitude was chosen that evoked 10 to 15 action potentials at pHe of 7.1 (typically this was 30 pA). After extracellular alkalinization from pH 7.1 to 7.4, there was a significant decrease in the number of evoked action potentials (Fig. 3). This decrease was reversible on return to pHe of 7.1 (Fig. 3). These data suggest that, like the spontaneous firing rate, the intrinsic excitability of orexin neurons is increased by acidification and depressed by alkalinization.
Fig. 3.
Effects of extracellular pH on evoked firing of orexin cells. Current-clamp whole-cell recordings with K-gluconate intracellular solution. The cell was set to the same baseline of −70 mV (see Methods). The time scale is expanded in the lower part to show responses to 30-pA current pulses (shown schematically below the traces) (n = 4; 12 ± 1 spikes at pHe 7.1 vs. 3.3 ± 0.6 spikes at pHe 7.4; P < 0.005, two-tailed t test).
Effects of Physiological pHe Changes on Membrane Currents.
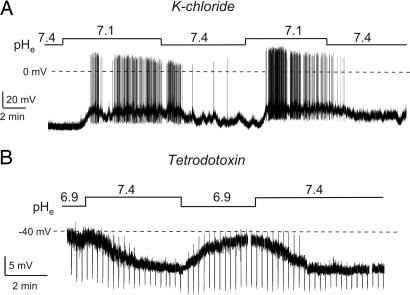
To address the mechanism underlying H+-sensing by orexin neurons, we combined intracellular ion substitutions with measurements of the equilibrium potential of the H+-modulated conductance. In Figs. 1–3, we used a low [Cl−] intracellular solution (K-gluconate; see Methods), which meant that, at typical membrane potentials of orexin cells, only K+ and Cl− conductances were hyperpolarizing, and all other ion channels were depolarizing (27). Therefore, the hyperpolarizing conductance activated by rises in pHe (i.e., a fall in [H+]e) (Fig. 1) must have been mediated by K+ or Cl− channels. We next switched to a high [Cl−] intracellular solution (K-chloride), which sets ECl to 0 mV, thereby making K+ channels the only ion channels that can hyperpolarize resting potentials of orexin cells (27). Neither this manipulation (Fig. 4A) nor blocking action potential-dependent synaptic communication with tetrodotoxin (Fig. 4B) affected the membrane potential and membrane conductance responses to H+, implying that postsynaptic K+, and not Cl−, channels are responsible. In direct confirmation of this conclusion, the He-+ modulated current had a mean reversal potential of −93 ± 4 mV (n = 14; mean ± SEM), close to that of K+ (theoretical EK = −97 mV). This current was activated by physiological alkalinization and inhibited by acidification (Fig. 5A).
Fig. 4.
Effects of intracellular ion substitutions and action potential blockade on H+ responses of orexin cells. Current-clamp whole-cell recordings with K-chloride intracellular solution. (A) Effects of pHe changes on firing and membrane potential. (B) Effects of pHe changes in the presence of tetrodotoxin (0.7 μM). The amplitude of downward deflections is inversely proportional to membrane conductance (see Fig. 1).
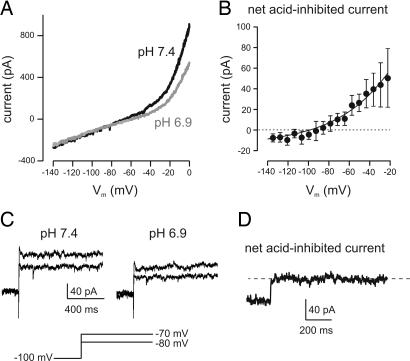
Fig. 5.
Ionic basis of H+ responses of orexin cells. Voltage-clamp whole-cell recordings with K-chloride intracellular solution and bath tetrodotoxin (0.7 μM). (A) Effect of pHe changes on membrane current-voltage relationship of the cell shown in Fig. 4B. The current-voltage relationship was obtained using slow voltage ramps (see Methods). (B) Current-voltage relationship of the net current (n = 4; data are means ± SEM) inhibited by acid (i.e., current in pH 7.4 minus current in pH 6.9). The line through the points is a fit of the Goldman–Hodgkin–Katz equation to the data (see Methods). (C) Effects of pHe on membrane current obtained with voltage-clamp steps (shown schematically below the traces). (D) Time course of activation and inactivation of the net acid-inhibited current (pH 7.4–6.9) obtained by a step to −60 mV (from −100 mV). The trace is representative of results obtained in five cells.
Several ion channels implicated in chemosensing have recently been found in orexin neurons (27, 29). Among these are “leak” K+ channels (K2P channels) (27). We next directly examined the effects of pHe on membrane currents using whole-cell voltage-clamp recordings (Fig. 5) to test whether acid-inhibited leak K+ channels are involved in firing and membrane potential responses of orexin cells to extracellular H+. We found strong support for this idea: The net H+-inhibited current in orexin neurons exhibited (i) current-voltage rectification well described by the Goldman–Hodgkin–Katz current equation (Fig. 5 A and B), and (ii) leak-like rapid activation and minimal time-dependent decay in response to voltage steps (Fig. 5 C and D). Because these two properties, together with inhibition by H+, are not features of any known ion channels apart from acid-sensitive K2P channels (28), our findings are strong evidence that they mediate the K+ currents involved in H+ sensing in orexin neurons.
Discussion
Extracellular pH is a fundamental signal regulating breathing and behavioral arousal. Although the activity of hypothalamic orexin neurons is essential for normal adaptive increases in arousal and respiration (20, 16), it was unknown whether orexin cell firing is sensitive to physiological changes in pHe. Here we show that the spontaneous firing rate of identified orexin neurons is profoundly affected by physiological fluctuations in acid and CO2 levels (Figs. 1 and 2). Crucially, these firing responses resemble those displayed by classical chemosensory neurons both qualitatively (acidification is excitatory, alkalinization is inhibitory) and quantitatively (≈100% change in firing rate per 0.1 unit change in pHe). Evoked firing of orexin cells is also modulated by physiologically relevant pHe: Acidification increases intrinsic excitability, whereas alkalinization depresses it (Fig. 3). The effects of pHe are caused by changes in postsynaptic conductance, but Cl− channels are not essential for these responses (Fig. 4). Instead, our data imply that acid-inhibited leak-like K+ channels in the orexin cell membrane are involved (Fig. 5). These results provide an insight into how behaviorally defined hypothalamic networks generate homeostatically appropriate patterns of activity.
Orexin Neurons as Potential Polymodal Chemosensors.
After the recent realization of the behavioral importance of orexin neurons (13), much work became focused on stimuli controlling their activity. This work revealed that the firing of orexin cells is regulated by diverse hormones and neurotransmitters (20, 30). Furthermore, orexin neurons were found to act as specialized electrical sensors of glucose, translating rises in ambient levels of the sugar into reductions in their firing rate (20, 31). Our new data show that orexin cell firing is also specialized to encode physiological levels of acid and CO2. This ability to sense endocrine, neural, metabolic, and physicochemical signals is reminiscent of the classical polymodal chemosensory organs, such as the carotid body (32, 33). Combined with their ability to orchestrate diverse brain states (see below), this places orexin cells in a pivotal position to guard body homeostasis through appropriate behavioral corrections. The relative role of each type of input to orexin cells during different circumstances and stages of development is an important subject for future investigation.
In the field of chemosensing, our study supports an emerging concept that different chemosensory systems operate at different levels in the brain to link pH with breathing and arousal. In particular, there appear to be chemosensors tightly linked to the control of breathing, e.g., neurons of the retrotrapezoid nucleus of the brainstem (34) and, in parallel, systems that link pH to both breathing and arousal, such as serotonin and noradrenaline neurons of the brainstem (3, 4) and orexin neurons (this study). We estimated the chemosensitivity of orexin cell firing to be ≈100% rate increase per 0.1 pH unit. In the brainstem, this parameter varies from ≈100% for regions with the highest degree of chemosensitivity, such as the medullary raphe, to ≈15% for regions with the lowest chemosensitivity, such as the locus coeruleus (4). In terms of sensitivity of firing to pHe, orexin cells thus appear to be among the most sensitive known chemosensors.
Implications for Brain States and Adaptive Behaviors.
We propose that the acid/CO2-induced changes in orexin cell firing, reported here, represent a previously overlooked physiological pathway contributing to fundamental reflexes such as increased wakefulness and breathing during sleep apnea. This sensing pathway could provide a possible explanation for the recent observation that orexin knockout reduces the breathing responses to CO2 by ≤50% (16). Orexin cells project to respiratory centers in the brainstem and spinal cord neurons where injections of orexin stimulate breathing (15), although the underlying mechanisms remain to be established in detail. With regard to behavioral arousal and alertness, it is well established that the activity of orexin neurons stimulates wakefulness by providing excitatory inputs to most classical arousal centers as well as directly to the cortex (13, 35, 36). In addition, orexins may also generate anxiety and stresslike states (37, 38, 39) probably due to the reciprocal excitatory interactions with neurons containing corticotropin-releasing factor (40, 41). This pathway is of particular interest in the context of this study because high CO2 levels are well known to induce anxiety responses, which are thought to be mediated in part by CO2/H+-sensitive neurons of the locus coeruleus (4). However, as noted above, the chemosensitivity of locus coeruleus neurons appears to be ≈6-fold lower than that of orexin neurons. The orexin system may thus ensure a more graded transition to panic states during rising CO2 levels, possibly in cooperation with the serotonin system of the brainstem (3). Finally, it is noteworthy that orexin neurons have many additional central projections that exert critical influences on appetite and reward/addiction (11, 42, 43). Our findings may thus represent a new cellular link between acid-base balance and these behavioral drives.
Methods
Preparation of Brain Slices from Transgenic Mice.
Mice were made transgenic for enhanced GFP (eGFP) under the control of prepro-orexin promoter as described in our previous study (27). This procedure results in very specific targeting of eGFP only to orexin neurons (27). Procedures involving animals were carried out in accordance with the Animals Scientific Procedures Act of 1986 (U.K.). Mice were maintained on a 12-h light:dark cycle (lights on at 0800) and had free access to food and water. Animals (14–20 days postnatal) were killed during their subjective night (the light phase), and coronal slices (250–300 μm thick) containing the lateral hypothalamus were prepared as previously described (27).
Extracellular and Intracellular Solutions.
For experiments involving changes in [CO2] (Fig. 1A), we used bicarbonate-buffered bath solution, which contained 125 mM NaCl, 2.5 mM KCl, 2 mM MgCl2, 1.2 mM NaH2PO4, and 26 mM NaHCO3. Bubbling this solution with 5% CO2 (95% N2) or 10% CO2 (90% N2) gave pHe values of 7.25 and 7.02, respectively (at 25°C). In the rest of the paper, we changed pHe using Hepes-buffered bath solutions based on Hopwood and Trapp (22). These Hepes-buffered solutions were bubbled with 100% O2 and contained 118 mM NaCl, 3 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 25 mM Hepes, and 1 mM glucose (pH adjusted with NaOH) (22). We also carried out experiments where ACSF was buffered with both bicarbonate and Hepes (27), and changes in its pH were created by addition of NaOH or HCl. The effects of pHe on firing and currents of orexin cells using this solution were similar to those shown in Figs. 1–3 (n = 15, data not shown). Intracellular (pipette) solutions were based on either K-gluconate or K-chloride. K-gluconate intracellular solution (used in Figs. 1–3) contained 120 mM K-gluconate, 10 mM KCl, 0.1 mM EGTA, 10 mM Hepes, 4 mM K2ATP, 1 mM Na2ATP, and 2 mM MgCl2 (pH = 7.3 with KOH). K-chloride solution (used in Figs. 4 and 5) contained 130 mM KCl, 0.1 mM EGTA, 10 mM Hepes, 5 mM K2ATP, 1 mM NaCl, and 2 mM MgCl2 (pH 7.3) with KOH. Tetrodotoxin was obtained from Tocris Cookson Ltd. (Avonmouth, U.K.). All other chemicals were from Sigma–Aldrich (Poole, Dorset, U.K.).
Electrophysiology and Data Analysis.
For patch-clamp recordings, living orexin-eGFP neurons were visualized in brain slices using an Olympus BX50WI upright microscope equipped with infrared gradient contrast optics, a mercury lamp, and filters for visualizing eGFP-containing cells (Olympus, Tokyo, Japan) (27). Whole-cell voltage- and current-clamp recordings from orexin-eGFP neurons were performed using an EPC-9 amplifier (HEKA, Lambrecht, Germany) at 25°C as previously described (27). Control experiments showed that physiological alkalinization of orexin cells (going from pHe 6.9–7.4) is hyperpolarizing and inhibitory, whereas acidification (going from pHe 7.4–6.9) is depolarizing and excitatory at both 25°C (n > 10 cells) and 35°C (n = 4 cells). Patch pipettes were pulled from borosilicate glass and had tip resistances of 2–3 MΩ when filled with the K-chloride pipette solution. Series resistances were in the range of 5–8 MΩ and were not compensated. Data were sampled and filtered using Pulse/Pulsefit software (HEKA) and analyzed with Pulsefit and Origin (Microcal, Northampton, MA) software.
To monitor membrane resistance together with changes in membrane potential (Figs. 1 and 4B), cells were injected every 10–20 sec with a fixed-amplitude (10–40 pA, 1-sec duration) square pulse of hyperpolarizing current (24). The amplitude of the resulting downward deflections in membrane potential is proportional to membrane resistance (Ohm's law: resistance = voltage divided by current), i.e., inversely proportional to membrane conductance (conductance = current divided by voltage = 1 over resistance).
Current-voltage relationships (Fig. 5 A and B) were obtained by performing voltage-clamp ramps from 0 mV to −140 mV at a rate of 0.1 mV/msec ramp, which is sufficiently slow to allow leak-like K+ currents to reach steady-state at each potential (27, 44). In Fig. 5B, the net current-voltage relationship was fitted with the Goldman–Hodgkin–Katz current equation (8) in the following form:
Here I is current, V is membrane potential, z is the charge of a potassium ion (+1), [K+]i is the pipette K+ concentration, [K+]o is the ACSF K+ concentration, T is temperature (298 K, corresponding to 25°C), and R and F have their usual meanings (8). PK (a constant reflecting the K+ permeability of the membrane) was the only free parameter during fits. The value of PK used to obtain the fit shown in Fig. 5B was 0.00686. Values are presented as means ± SEM.
To calculate chemosensitivity as percent firing change per 0.1 unit change in pHe, we adapted the method described in Putnam et al. (4) and used the following equation:
Here FR is firing rate, FRA is FR in acidic solution, FRALK is FR in alkaline solution, and ΔpH is the change in pH. The value of % change in FR given in Results (108%) was calculated using FRA and FRALK of 3.7 Hz and 1 Hz, respectively, which were taken from data in Fig. 2B at pHe of 7 and 7.25, respectively (ΔpH = 0.25).
Acknowledgments
This work was supported by the Royal Society (D.B.), the Biotechnology and Biological Sciences Research Council (studentship for R.H.W.), and the Karen Elise Jensen Foundation of Denmark (L.F. and L.T.J.).
Abbreviation
- LH
lateral hypothalamus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Nattie E. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 2.Phillipson E, Bowes G. Handbook of Physiology. The Respiratory System. Bethesda: American Physiological Society; 1986. pp. 649–689. [Google Scholar]
- 3.Richerson GB. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 4.Putnam RW, Filosa JA, Ritucci NA. Am J Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 5.Hunt CE. In: Respiratory Control Disorders in Infants and Children. Beckerman RC, Brouillette RT, Hunt CE, editors. Baltimore: Williams & Wilkins; 1992. pp. 190–211. [Google Scholar]
- 6.Richerson GB. Neuroscientist. 1997;3:3–7. [Google Scholar]
- 7.Chesler M. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 9.Redgate ES, Gellhorn E. Am J Physiol. 1958;193:189–194. doi: 10.1152/ajplegacy.1958.193.1.189. [DOI] [PubMed] [Google Scholar]
- 10.Saper CB, Chou TC, Scammell TE. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 12.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, II, et al. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai T. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 14.Sutcliffe JG, de Lecea L. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 15.Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. J Appl Physiol. 2007;102:247–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 18.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 19.Siegel JM, Boehmer LN. Nat Clin Pract Neurol. 2006;2:548–556. doi: 10.1038/ncpneuro0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, et al. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 21.Kintner DB, Anderson MK, Fitzpatrick JH, Jr, Sailor KA, Gilboe DD. Neurochem Res. 2000;25:1385–1396. doi: 10.1023/a:1007664700661. [DOI] [PubMed] [Google Scholar]
- 22.Hopwood SE, Trapp S. J Physiol. 2005;568:145–154. doi: 10.1113/jphysiol.2005.093070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdakov D, Alexopoulos H, Vincent A, Ashcroft FM. Eur J Neurosci. 2004;20:3281–3285. doi: 10.1111/j.1460-9568.2004.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdakov D, Gerasimenko O, Verkhratsky A. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burdakov D. Neuroscientist. 2004;10:286–291. doi: 10.1177/1073858404263597. [DOI] [PubMed] [Google Scholar]
- 26.Rosenkranz JA, Johnston D. J Neurosci. 2006;26:3229–3244. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Bayliss DA, Sirois JE, Talley EM. Mol Interv. 2003;3:205–319. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, van den Pol AN. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Gao XB, Sakurai T, van den Pol AN. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 31.Burdakov D. Pflugers Arch. 2007;454:19–27. doi: 10.1007/s00424-006-0189-8. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Barneo J. Curr Opin Neurobiol. 2003;13:493–499. doi: 10.1016/s0959-4388(03)00093-x. [DOI] [PubMed] [Google Scholar]
- 33.Kumar P, Bin-Jaliah I. Respir Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 35.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambe EK, Aghajanian GK. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 37.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Am J Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto F, Yamada S, Ueta Y. Regul Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris GC, Aston-Jones G. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]