Abstract
Progression through the G1/S transition commits cells to synthesize DNA. Cyclin dependent kinase 2 (CDK2) is the major kinase that allows progression through G1/S phase and subsequent replication events. p27 is a CDK inhibitor (CKI) that binds to CDK2 to prevent premature activation of this kinase. Speedy (Spy1), a novel cell cycle regulatory protein, has been found to prematurely activate CDK2 when microinjected into Xenopus oocytes and when expressed in mammalian cells. To determine the mechanism underlying Spy1-induced proliferation in mammalian cell cycle regulation, we used human Spy1 as bait in a yeast two-hybrid screen to identify interacting proteins. One of the proteins isolated was p27; this novel interaction was confirmed both in vitro, using bacterially expressed and in vitro translated proteins, and in vivo, through the examination of endogenous and transfected proteins in mammalian cells. We demonstrate that Spy1 expression can overcome a p27-induced cell cycle arrest to allow for DNA synthesis and CDK2 histone H1 kinase activity. In addition, we utilized p27-null cells to demonstrate that the proliferative effect of Spy1 depends on the presence of endogenous p27. Our data suggest that Spy1 associates with p27 to promote cell cycle progression through the G1/S transition.
INTRODUCTION
Cell cycle progression is regulated by the activity of cyclin-dependent kinases (CDKs). CDK activity is dependent on interactions with their regulatory subunits called cyclins (Sherr, 1994; Morgan, 1995, Sherr and Roberts, 1999). At the G1/S transition of the cell cycle, cyclin D, cyclin E, and cyclin A associate with CDKs to promote cell cycle progression. Particularly important at the G1/S transition is the activation of cyclin E/CDK2, which is necessary for DNA replication. CDK activity is also dependent on phosphorylation events. To activate CDK2, the cdc25 phosphatases remove two inhibitory phosphates on Thr 14 and Tyr 15 and cyclin activating kinase (CAK) phosphorylates CDK2 at Thr 160. Phosphorylation of Thr 160 is especially important for CDK activation, because it is believed to promote additional conformational changes that may expose the catalytic cleft for substrate binding (Jeffrey et al., 1995). CDK regulation is also mediated in part by the cyclin-dependent kinase inhibitors (CKIs), which bind to cyclin/CDK complexes and inhibit CDK activity. There are two CKI families: Cip/Kip, which includes p21Cip1, p27Kip1, and p57Kip2, and INK4, which includes p16INK4a, p15INK4b, p18INK4c, and p19INK4d. p27Kip1 interaction with cyclin E/CDK2 prevents premature entry into S phase (Nakayama et al., 2001).
Normally, p27Kip1 protein levels are high during G0 and G1 phases of the cell cycle, resulting in cyclin/CDK inhibition. In addition, p27 protein expression can be stimulated in other phases of the cell cycle by contact inhibition, mitogen withdrawal, differentiation signals, or other antiproliferative cues that result in G1 arrest (Koff et al., 1993; Polyak et al., 1994; Slingerland et al., 1994; Toyoshima and Hunter, 1994; Durand et al., 1997; Baldassarre et al., 2000). p27 has been found to associate with cyclin A/CDK2, cyclin E/CDK2, and cyclin D/CDK4,6 (Slingerland and Pagano, 2000). When mitogens are present, cyclin D/CDK4 forms a trimeric complex with p27 that results in less p27 available to inhibit cyclin E/CDK2. Interestingly, one of the substrates of cyclin E/CDK2 is p27, which is phosphorylated on Thr 187 as a result of activated CDK2. Phosphorylation at Thr 187 targets p27 for degradation via the SCFSkp2 ubiquitin ligase pathway (Pagano et al., 1995; Shaeff et al., 1997; Vlach et al., 1997; Carrano et al., 1999; Montagnoli et al., 1999, Sutterluty et al., 1999; Tsvetkov et al., 1999). Decreasing protein levels of p27 results in cell cycle progression through the G1/S transition.
Human Spy1 is a novel cell cycle regulator found to bind and activate CDK2 histone H1 kinase activity (Porter et al., 2002). Spy1 mRNA is expressed during G1/S phase in a variety of human tissues and cell lines such as 293T and NTERA-2 cells (Porter et al., 2002). In these cell lines, Spy1 enhances cell proliferation by shortening G1 phase in a manner that is dependent on CDK2 kinase activity. Small interfering RNA (siRNA) directed against Spy1 demonstrates that Spy1 is an essential regulator of cell cycle progression. Taken together, these data indicate that Spy1 regulates cell cycle progression through activation of CDK2.
In this study, we sought to determine the mechanism by which Spy1 regulates cell cycle progression. Toward this end, we used a yeast two-hybrid screen using full-length human Spy1 as bait to identify other proteins that may be interacting with Spy1. One of the clones isolated was p27. We have confirmed this interaction using various in vitro and in vivo binding assays. Interestingly, CDK2 binding to Spy1 is enhanced in the presence of p27. Functionally, when Spy1 is coexpressed with p27 compared with cells expressing p27 alone, there is an increase in the number of cells in S phase and enhanced CDK2 kinase activity. This indicates that Spy1 can overcome p27-mediated growth arrest. In addition, we demonstrate that Spy1-enhanced proliferation depends on the presence of endogenous p27. Our data suggest that Spy1 enhances cell cycle progression by preventing the inhibitory actions of p27 on CDK2.
MATERIALS AND METHODS
Two-hybrid Constructs
The two-hybrid plasmids pBTM116 (constructed by P. Bartel and S. Fields), pVP16, and pLexA-lamin (Vojtek et al., 1993; Vojtek and Hollenberg, 1995) were generous gifts from S. Hollenberg and J.A. Cooper (Fred Hutchinson Cancer Research Center). LexA-Spy1 was constructed by first inserting oligos D2421/D2422 into pSP64-Spy1 (pJT013; Porter et al., 2002) via EcoRI and NsiI sites to generate a BamHI site preceding the start site of human Spy1 (pMK64). The pBTM116 vector was digested with BamHI and PstI; pMK64 was digested with BamHI and NsiI. The pBTM116 vector and Spy1 fragment were then ligated to produce LexA-Spy1 (pMK65).
Two-hybrid Screen
The two-hybrid screen was performed according to previously published protocols (Vojtek et al., 1993; Vojtek and Hollenberg, 1995). LexA-Spy1 was cotransformed with a 9.5 dpc mouse embryo cDNA library fused to pVP16 into the L40 strain of Saccharomyces cerevisiae. The transformants were selected on His–plates for 3–4 d at 30°C with the addition of 2.5 mM 3-aminotriazole (3-AT; Sigma, St. Louis, MO). An interaction was scored positive for the ability of the yeast to grow beyond bait plus empty pVP16 vector and to overcome 3-AT inhibition. A β-galactosidase filter-lift assay was also performed to confirm a positive interaction. False positives were discriminated by testing cDNA-pVP16 against LexA-lamin. The remaining cDNA-pVP16 clones were then shuttled into Escherichia coli HB101 cells, rescued, and then sequenced in the Core facility at the UCSD Cancer Center. The sequences were then entered into the NCBI BLAST program for identification.
Plasmid Construction
Flag-tagged Spy1 was constructed by first adding a BglII site by QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA) downstream of the NdeI site of human Spy1 in pJT013. Oligonucleotides (D2479/D2480) containing the flag tag were synthesized and inserted between NdeI and BglII sites of pJT013. This construct was then digested with EcoRI and XhoI and inserted into the pLXSN vector at the same sites. Finally, the flag-tagged Spy1 gene was cloned into pcDNA3 via EcoRI and BamHI. Myc-tagged Spy1 was constructed as previously described (Porter et al., 2002).
The p27 clones isolated from the two-hybrid screen were subcloned from the pVP16 vector to the pCS3+MT vector to produce myc-tagged p27. First, oligonucleotides D2541/D2542 were inserted between the NcoI and BamHI sites in pCS3+MT to make the p27 clones in-frame with the myc tags (pMK92). p2723–128 and 2732–162 were digested with BamHI and EcoRI and each fragment was inserted into the modified pCS3+MT vector via BglII and EcoRI sites to produce pMK93–3 and pMK93–4, respectively. myc-p2725–51 was constructed by digesting pMK92 with BglII and EcoRI to insert oligos D2564/D2565 containing the mouse cyclin-binding region of p27 to produce pMK94. myc-p2743–128 was constructed by digesting pCS3+MT vector with NcoI and EcoRI and inserting oligos D2566/D2567 containing a unique SmaI site to produce pMK95. pMK95 and pMK93-3 were then digested with SmaI and XbaI. The vector from pMK95 was ligated with the fragment from pMK93-3 (containing the CDK-binding region of p27) to produce pMK96.
Cell Lines
p27 null immortalized MEFs (MEF–/–) from 129/sv × C57BL/6 hybrid mice were obtained from Jim Roberts and were previously described (Cheng et al., 1999). p27 null NIH3T3 (3T3–/–) cells were obtained from Andrew Koff and are previously described (Vidal et al., 2002). Both 293T and MEF–/– were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C in 10% CO2. COS-1 cells were maintained in DMEM supplemented with 10% FBS at 37°C in 5% CO2. NIH3T3 and 3T3–/– cells were cultured in DMEM supplemented with 10% calf serum at 37°C in 10% CO2.
Coimmunoprecipitation and Immunoblotting
Subconfluent 293T cells were transfected with 2.5 μg of p27 and/or Spy1 by the calcium phosphate precipitation method (Chen and Okayama, 1987) in a 10-cm dish. Additional empty pcDNA3 vector was added to produce a total of 5 μg DNA transfected into each dish. Twenty-four hours after transfection, cells were harvested and lysed in 0.5% NP-40 lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM Na3VO4, 1 mM NaF, 1 mM DTT, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin). Lysates (1 mg protein) were precleared with 40 μl Protein A-Sepharose beads and incubated with 2 μg of α-flag (Sigma), α-myc (9E10; Santa Cruz Biotechnology, Santa Cruz, CA), α-p27 (Zymed, South San Francisco, CA, or Santa Cruz) or α-CDK2 (Santa Cruz) overnight at 4°C. For the endogenous Spy1 interaction with endogenous p27, 293T cells were lysed in 0.1% NP-40 lysis buffer and 6 mg of lysate was immunoprecipitated with α-p27 sera (Zymed) or α-Spy1 sera as previously described by Porter et al. (2002). Protein A-Sepharose beads were then added for at least 2 h, and the immunoprecipitated samples were washed three times, boiled 5 min in sample buffer, and then analyzed on 10% SDS-PAGE. Half of each immunoprecipitate and 50 μg of lysate were loaded into the gels. The gels were then transferred to nitrocellulose membrane, immunoblotted with 1:2000 α-p27 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:2000 α-CDK2, 1:4000 α-myc (9E10), 1:2000 α-flag (Sigma), or 1:500 α-Spy1 followed by ECL (Amersham, San Francisco, CA). When it was necessary to reprobe with other antibodies recognizing proteins of similar molecular weight, the membranes were stripped of bound antibodies in stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.8) and incubated for 30 min at 60°C with rotation.
In Vitro Binding Assay
p27-pGEX2T was obtained from Makoto Nakanishi (Morisaki et al., 1997). GST-p27 and GST control proteins were purified using standard methods (Nakanishi et al., 1995). Briefly, a single colony of the pGEX transformant was grown in 100 ml LB/ampicillin medium for 15 h at 37°C. This culture was then diluted 1:10 into 1 liter fresh media for 2 h at 37°C followed by the addition of 1 mm IPTG for 4 h at 30°C. Cells were collected in 20 ml ice-cold PBS containing 1% NP-40, 10 mM DTT, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin and sonicated using a 5-mm-diameter probe for 30 s. Triton X-100 (10%) was added to 1% and mixed, and insoluble material was removed via centrifugation. One milliliter of 50% glutathione-agarose beads (Sigma) was added to the supernatant and mixed gently for 1 h at 4°C. Beads were then collected via centrifugation and washed three times in 50 ml of ice-cold PBS.
In vitro–translated proteins were produced using an SP6-coupled rabbit reticulocyte lysate system (Promega, Madison, WI), with the procedures performed as described by the manufacturer.
Both in vitro–translated and GST protein products were first analyzed by SDS-PAGE and then binding assays were carried out in 200 μl of binding buffer (50 mM K2HPO4, 150 mM KCl, 1 mM MgCl2, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). Binding interactions were incubated at 4°C with gently rotation for 2 h, washed three times with binding buffer, boiled 5 min in 2× sample buffer, and then analyzed by SDS-PAGE.
In Vitro Kinase Assay
293T cells were transfected as described above. Twenty-four to 36 h after transfection the cells were harvested with 0.5% NP-40 lysis buffer. Each sample was immunoprecipitated with α-CDK2 sera overnight at 4°C with rotation. Protein A-Sepharose beads were added for 2 h with rotation and then subjected to an in vitro kinase assay as previously described (Kong et al., 2000).
Immunofluorescence Microscopy
For Spy1 and p27 localization studies, COS-1 cells were plated onto glass coverslips and cultured as described above. Cells were transiently transfected with 10 μg of DNA in a 60-mm dish. Cells were then fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min and permeabilized with 0.1% Triton X-100 for 10 min. Cells were blocked with 3% BSA for 30 min and then incubated with α-p27 (1:500) antibody for 1 h, washed, and incubated for 30 min with 1:500 rhodamine-conjugated goat anti-rabbit IgG (Boehringer Mannheim, Indianapolis, IN). To detect myc-Spy1, α-myc (9E10) antibody (1:500) was added for 1 h, washed, and visualized with FITC-conjugated goat anti-mouse secondary antibody (Boehringer Mannheim). Coverslips were mounted on glass slides with 100 mM Tris, pH 8.0, 90% glycerol, 1 mg/ml phenylenediamine, and 1 μg/ml Hoechst dye 33342 to detect DNA.
For analysis of 4-bromodeoxyuridine (BrdU) incorporation, NIH3T3 cells were plated onto glass coverslips and cultured as described above. Cells were transiently transfected with a total of 10.1 μg of DNA (0.1 μg GFP, 5 μg p27, 5 μg flag-Spy1). pcDNA3 was added to single transfections to bring the final DNA concentration to 10.1 μg. Eighteen hours after transfection, the medium was replaced and incubated at 10% CO2, BrdU (100 μL of 6 mg/ml; Sigma) was added to the cells for 2 h. The cells were then fixed as described above. To visualize BrdU incorporation, 1:50 α-BrdU mouse sera (Becton Dickinson, Palo Alto, CA) was added for 1 h to cells, washed, and incubated with 1:50 α-rhodamine–conjugated anti-mouse (Boehringer-Mannheim). One hundred GFP-positive cells were then counted from each coverslip and scored for BrdU incorporation. Three independent experiments were performed with similar results, and the SE of the mean is presented.
Cell Proliferation Assay
To determine the effect of Spy1 on cells lacking endogenous p27, 1 × 105 MEF–/– cells were seeded onto 10-cm dishes and cultured as described above. Twenty-four hours after seeding, cells were transiently transfected in triplicate with a total of 10 μg of DNA, and pcDNA3 was added to single transfections to make up the difference. Eighteen hours after transfection, the medium was replaced and incubated at 10% CO2 for 24 h. The cells were then trypsinized and live cells were counted via trypan blue exclusion. Four counts were made for each triplicate transfection. Four independent experiments were performed with similar results, and the SE of the mean is presented.
CDK2-Spy1 Binding Assay
NIH3T3 cells or equivalent cells null for p27 (3T3–/–) were seeded on 150-mm plates at 6 × 105 cells/plate, and 24 h after seeding cells were harvested and lysed as described above. Lysates (containing 3 mg of protein) were incubated with 6 μg of α-p27 (Santa Cruz), α-CDK2 (Santa Cruz), or α-Spy1 sera as previously described by Porter et al. (2002) overnight at 4°C. Protein A-Sepharose beads were then added for 2 h, and the immunoprecipitated samples were washed three times, boiled 5 min in sample buffer, and then analyzed by 10% SDS-PAGE as described above.
RESULTS
Two-Hybrid Interaction between p27 and Spy1
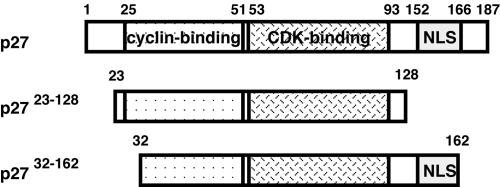
We performed a yeast two-hybrid screen using LexA-Spy1 as bait against a 9.5 dpc mouse embryo library, which resulted in 103 positives. DNA sequencing of the mouse cDNA inserts revealed that several of these proteins had been pulled out at least twice. After subjecting the clones to a β-galactosidase filter-lift assay and a false-positive test by interaction against LexA-lamin, 33 clones were identified as positive interactors. One of the clones identified was the CDK inhibitor, p27 (Table 1). As shown in Figure 1, two clones were isolated from the two-hybrid screen. One of the clones included aa 23–128 of mouse p27, and the second clone included aa 32–162 of mouse p27. Both of the mouse p27 clones isolated from the two-hybrid screen encompassed the cyclin-binding and CDK-binding regions of p27. p2732–162 also included a large portion of the nuclear localization signal (NLS) of p27.
Table 1.
Summary of yeast two-hybrid interactions
| LexA Fusion | pVP16 | Mouse p27 (aa 23-128) | Mouse p27 (aa 32-162) |
|---|---|---|---|
| LexA-Spy1 | - | + | + |
| LexA-lamin | - | - | - |
Figure 1.
Schematic representation of p27. Full-length p27 contains a cyclin-binding region (aa 25–51), a CDK-binding region (aa 53–93), and a nuclear localization signal (NLS; aa 152–166). The two clones isolated from the yeast two-hybrid screen encompassed aa 23–128 of p27 (middle) and aa 32–162 of p27 (bottom).
The CDK Binding Region of p27 Interacts with Spy1 In Vivo
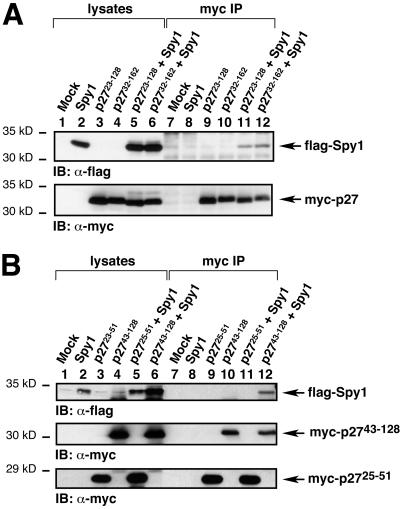
We constructed myc-tagged p27 derivatives (p2723–128 and p2732–162) to investigate whether these portions of p27 could interact with Spy1 in mammalian cells. 293T cells were transfected with mock, flag-tagged Spy1, myc-p2723–128, myc-p2732–162, and flag-Spy1 plus myc-p2723–128 or myc-p2732–162. Lysates were then immunoprecipitated with the myc antisera, separated by 10% SDS-PAGE, and transferred to nitrocellulose membrane. The membrane was then probed with α-flag sera to detect Spy1. In Figure 2A (lanes 11 and 12), we show that Spy1 can interact with both myc-p2723–128 and myc-p2732–162. The bottom panel of Figure 2A indicates approximately equal levels of myc-p27.
Figure 2.
Spy1 interacts in vivo with the mouse p27 clones isolated from the two-hybrid screen. (A) 293T cells were transiently transfected with the indicated constructs, lysed, immunoprecipitated with α-myc sera, analyzed by 10% SDS-PAGE, and transferred to nitrocellulose membrane. The membrane was then immunoblotted with α-flag sera to detect flag-tagged Spy1 (top panel). As shown in lanes 11 and 12 of the top panel, flag-Spy1 coimmunoprecipitates with myc-p2723–128 and myc-p2732–162, respectively. The membrane was then stripped and reprobed with α-myc sera to demonstrate the presence of the myc-tagged p27 constructs (bottom panel). (B) 293T cells were transfected with the indicated constructs, lysed, immunoprecipitated with α-myc sera, and analyzed as above. Myc-p27 25–51, which is the cyclin-binding region of p27, did not appear to bind to flag-Spy1 (lane 11). In contrast, myc-p27 43–128, which encompasses the CDK-binding region of p27, interacted with flag-Spy1 (lane 12).
To further delineate the interaction of Spy1 with p27, separate myc-tagged clones of p27 were constructed comprised of either the cyclin-binding region or the CDK-binding regions of p27. Each clone was transfected with or without flag-Spy1 and immunoprecipitated with α-myc sera. Myc-p2725–51, which is the cyclin-binding region of mouse p27, did not interact with flag-Spy1 (Figure 2B, lane 11). In contrast, myc-p2743-128, which encompasses the CDK-binding region of p27, interacted with flag-Spy1 (Figure 2B, top panel, lane 12). The data suggest that Spy1 binds to the CDK-binding region, but not the cyclin-binding region, of p27.
p27 Interacts with Spy1 In Vitro
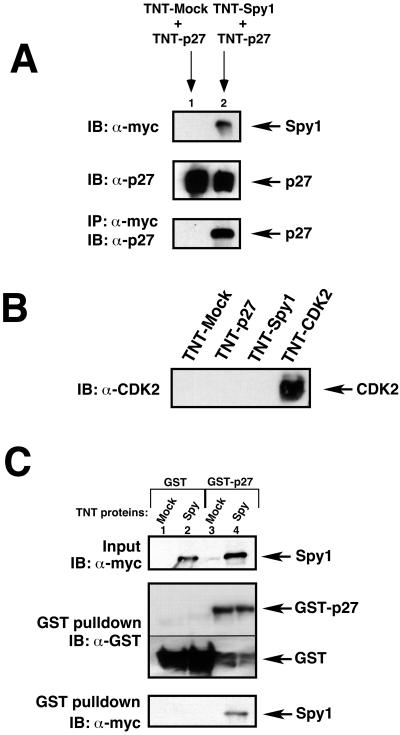
To confirm an in vitro interaction between p27 and Spy1, we first in vitro translated p27 (TNT-p27), myc-tagged Spy1 (TNT-Spy1), and pCS3+MT empty vector (TNT-mock) using a SP6-coupled rabbit reticulocyte lysate system. After a 2-h incubation of TNT-p27 with either TNT-Spy1 or TNT-mock (Figure 3A), TNT-p27 selectively immunoprecipitated with TNT-Spy1 but not with the TNT-mock control (Figure 3A, bottom panel). As an additional control, we immunoblotted the in vitro–translated proteins with CDK2 antisera to determine if there were trace levels of CDK2 protein in the reticulocyte lysate. As seen in Figure 3B, there is no CDK2 protein that can be detected within the limit of Western blot analysis.
Figure 3.
Human p27 interacts with human Spy1 in vitro. (A) In vitro–translated p27 (TNT-p27) was incubated for 2 h with either in vitro–translated pCS3-MT empty vector (TNT-mock) or in vitro–translated myc-tagged Spy1 (TNT-Spy1). The top panel demonstrates the amount of input TNT-Spy1 protein (lane 2), the middle panel demonstrates the input TNT-p27 protein (lanes 1 and 2). Both samples were immunoprecipitated with α-myc sera, and proteins were separated by 10% SDS-PAGE and immunoblotted with p27 sera (bottom panel). (B) In vitro–translated pCS3-MT (TNT-mock), p27 (TNT-p27), myc-Spy1 (TNT-Spy1), or CDK2 (TNT-CDK2) were separated by 10% SDS-PAGE and immunoblotted with α-CDK2 sera as a control. (C) Purified GST-p27 or GST-control protein conjugated to GST beads were incubated in binding buffer with either in vitro–translated (TNT) mock or Spy1 protein (top panel), both of which were previously immunodepleted for any CDK2 protein as an additional control. A GST pull-down assay was performed. Proteins were separated by 12.5% SDS-PAGE and immunoblotted with α-myc sera to detect immunoprecipitation of GST proteins with myc-tagged proteins (bottom panel). α-GST immunoblot is presented to demonstrate the GST proteins present in the GST pull-down experiment. Different exposures of the same immunoblot are presented in the middle panel because of the high amount of protein in the GST-control lanes.
Next, we determined whether TNT-Spy1 would also bind to bacterially produced GST-p27. We purified both GST-p27 fusion proteins and GST control proteins using standard methods and conjugated the bacterial proteins to GST beads. To rigorously ensure that CDK2 protein, possibly existing at undetectable levels in the reticulocyte lysate, was not playing a role in the p27-Spy1 interaction, we also immunodepleted both TNT-Spy1 and TNT-mock products with CDK2 antisera before binding. Figure 3C demonstrates that after a 2-h incubation with GST control (middle panel, lanes 1 and 2) or GST-p27 (middle panel, lanes 3 and 4), TNT-Spy1 selectively bound to GST-p27 and not to the GST control (bottom panel, lane 4 compared with lane 2).
Full-length p27 Interacts with Spy1
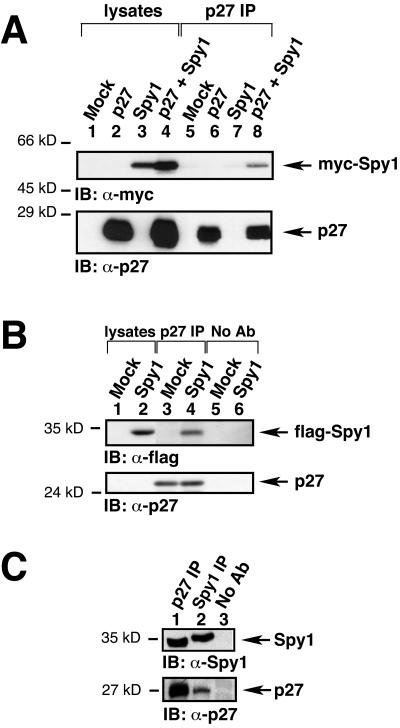
We next confirmed the interaction between full-length human p27 and Spy1 by coimmunoprecipitation in mammalian cells. 293T cells were transfected with mock, full-length p27 (untagged), myc-tagged Spy1, or both p27 and myc-Spy1. Precleared cell lysates were then subjected to immunoprecipitation by α-p27 sera, analyzed by 10% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with α-p27 or α-myc sera. The top panel of Figure 4A shows that myc-Spy1 can coimmunoprecipitate with p27 (lane 8). The bottom panel of Figure 4A confirms approximately equal levels of transfected p27. The data also indicate that endogenous p27 levels are low as expected in these cycling cells, requiring the transfection of exogenous p27 to visualize the protein. Thus, full-length p27 can interact with Spy1.
Figure 4.
p27 binds to Spy1 in vivo. (A) 293T cells were transiently transfected with the indicated constructs, lysed, immunoprecipitated with α-p27 sera, analyzed by 10% SDS-PAGE, and transferred to nitrocellulose membrane. The membrane was then immunoblotted with α-myc sera to detect myc-tagged Spy1 (top panel). As shown in lane 8 of the top panel, p27 can coimmunoprecipitate with myc-tagged Spy1. The bottom panel demonstrates the approximately equal expression of exogenous p27. (B) 293T cells were transfected with mock or flag-Spy1 constructs, starved, lysed, and immunoprecipitated with α-p27 sera. Immunoblotting with α-flag sera shows that flag-Spy1 interacts with endogenous p27 (lane 4). (C) 293T cells were starved, lysed, and immunoprecipitated with α-p27 or α-Spy1 sera. Immunoblotting demonstrates that endogenous Spy1 interacts with endogenous p27 (lanes 1 and 2). A sample with no primary antibody was used as a negative control (lane 3).
We examined 293T cells transfected with or without flag-tagged Spy1 followed by serum starvation overnight with 0.2% FBS to induce p27 expression. The top panel of Figure 4B shows that flag-Spy1 coimmunoprecipitates with endogenous p27 (lane 4). Immunoblotting with α-p27 sera shows the presence of endogenous p27 in the α-p27 immunoprecipitated samples (Figure 4B, bottom panel). Protein A-Sepharose beads alone were used as a negative control (lanes 5 and 6). We also examined the interaction of endogenous Spy1 and endogenous p27. Lysates from starved cells were then immunoprecipitated with α-p27 sera followed by immunoblotting with α-Spy1 sera. Figure 4C demonstrates the presence of endogenous Spy1 in the p27 immunoprecipitate (top panel, lane 1). Similarly, endogenous p27 coimmunoprecipitated with endogenous Spy1 (bottom panel, lane 2). Once again Protein A-Sepharose was immunoprecipitated alone as a negative control (top and bottom panel, lane 3). Taken together, endogenous Spy1 and endogenous p27 can interact in human cells.
Spy1 and p27 Colocalize in the Nucleus
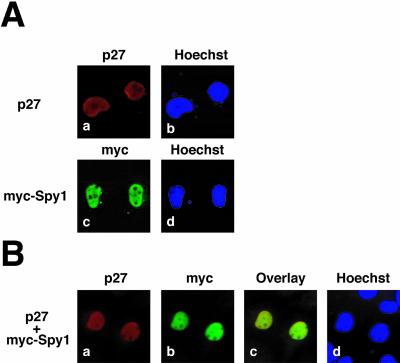
p27 must be imported into the nucleus to inhibit CDK2 activity (Reynisdóttir and Massagué, 1997; Slingerland and Pagano, 2000). This inhibition of CDK2 appears to be a dichotomy because cyclin E/CDK2 phosphorylation of p27 on Thr 187 is a trigger for degradation of p27 (Morisaki et al., 1997). It has been hypothesized that there may be alternate triggers for p27 degradation that will relieve the inhibition of CDK2 to a certain threshold, allowing for phosphorylation of p27. This would begin a feedback loop resulting in the eventual degradation of p27 and resumption of the cell cycle (Rodier et al., 2001). One of these pathways requires cytoplasmic relocalization aided by Jab1 (Tomoda et al., 1999). Because Spy1 has also been shown to be primarily nuclear in localization, although Spy1 can undergo relocalization upon DNA damage (Porter et al., 2002; unpublished results), we wanted to determine the effect, if any, that coexpression of Spy1 had on p27 localization. Immunofluorescence studies were performed on both single- and double-transfected COS-1 cells. As shown in Figure 5Aa, p27 and myc-Spy1 (Figure 5Ac) are each localized in the nucleus. Cotransfection of p27 and myc-Spy1 results in the nuclear localization of both proteins (Figure 5Bc). The data indicate that neither p27 nor myc-Spy1 alter their nuclear localization upon coexpression.
Figure 5.
p27 and Spy1 colocalize in the nucleus. COS-1 cells were transiently transfected with p27, myc-Spy1, or p27 plus myc-Spy1. (A) p27 localizes to the nucleus as shown in panel a. Similarly, myc-Spy1 localizes in the nucleus as shown in panel c. (B) Cells transfected with both p27 and myc-Spy1 demonstrate that both proteins localize to the nucleus (panel c). Hoechst dye was used to stain the nucleus.
p27, CDK2, and Spy1 Form a Complex
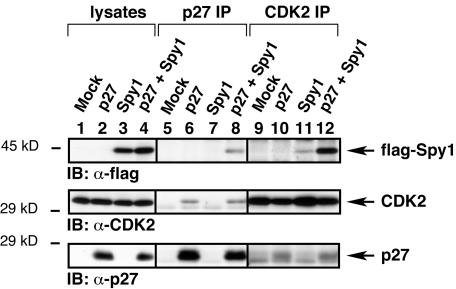
Studies in our laboratory have shown that CDK2 and Spy1 form a complex (Lenormand et al., 1999, Porter et al., 2002). To determine whether CDK2 was part of the p27/Spy1 complex, we transfected 293T cells with mock, p27, flag-Spy1, or p27 plus flag-Spy1. The lysates were immunoprecipitated with α-p27 sera and immunoblotted with α-flag, α-CDK2, or α-p27 sera. In Figure 6, lane 8, we demonstrate that Spy1, CDK2, and p27 can form a complex. Interestingly, immunoprecipitation with α-CDK2 antibody resulted in a significant difference in Spy1/CDK2 binding. In Figure 6, lane 11, we demonstrate that CDK2 and Spy1 can interact when p27 levels are low. When exogenous p27 was transfected into the cells, there is an increase in the amount of Spy1 in the complex (Figure 6, lane 12). These data suggest that the presence of p27 increases the amount of Spy1 association to the p27/CDK2 complex.
Figure 6.
p27 interacts with Spy1 and CDK2. 293T cells were transfected with the indicated constructs. The lysates were immunoprecipitated with α-p27 or α–CDK2 sera and immunoblotted for Spy1, CDK2, and p27. Lane 8 demonstrates that flag-Spy1 (top panel), endogenous CDK2 (middle panel), and p27 (bottom panel) can coimmunoprecipitate. The top panel of lane 11 shows that Spy1 interacts with CDK2 and that this interaction is increased when p27 is overexpressed as demonstrated in lane 12.
Spy1 Can Overcome a p27-induced G1 Arrest
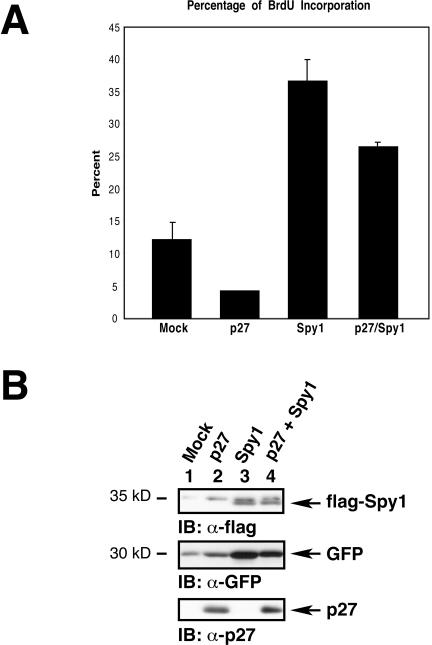
We next wanted to examine the biological effect of the interaction between p27 and Spy1. Because Spy1 has been found to enhance cell proliferation, we wanted to determine whether Spy1 could affect a G1 arrest induced by p27 overexpression. NIH3T3 cells were transfected with mock, p27, flag-Spy1, or p27 plus flag-Spy1. A GFP expression vector was used as a reporter for transfection efficiency. The cells were then treated with BrdU, a thymidine analog, for 2 h to assay for DNA synthesis. The cells were fixed, and GFP-positive cells were examined by immunofluorescence. As shown in Figure 7A, ∼12% of mock transfected cells were in S phase, and p27-transfected cells showed only ∼4% of cells in S phase. In contrast, flag-Spy1 transfected cells showed ∼36% of cells in S phase. These controls show that p27 prevents DNA synthesis and Spy1 increases the number of cells in S phase. When both Spy1 and p27 were cotransfected, ∼27% of cells are in S phase, indicating that Spy1 is able to overcome a p27-induced G1 arrest. As a control, Figure 7B demonstrates the approximately equal expression levels of exogenous flag-Spy1, GFP, and p27. Taken together, the data indicate that Spy1 enhances DNA synthesis despite p27 overexpression.
Figure 7.
Spy1 can overcome a p27-induced G1 arrest. (A) NIH3T3 wild-type cells were transfected with the indicated constructs and treated with BrdU for 2 h. The cells were then fixed and examined by immunofluorescence for BrdU incorporation by examination of GFP-positive cells. The graphed data indicate that there is an increase in DNA synthesis when p27 and Spy1 are coexpressed in comparison to p27 alone. (B) The lysates from the BrdU experiment were analyzed by 10% SDS-PAGE; immunoblotting with α-flag, α-GFP, and α-p27 sera show approximately equivalent levels of protein expression.
Expression of Spy1 Releases p27-inhibition of CDK2 Histone H1 Kinase Activity
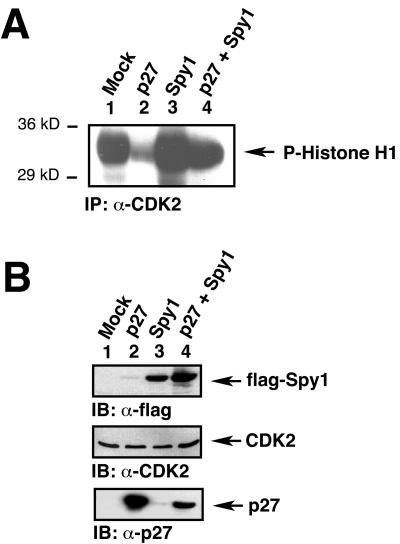
Previous studies from our laboratory have shown that overexpression of Spy1 can enhance CDK2 histone H1 kinase activity. We wanted to determine the effect on CDK2 histone H1 kinase activity when both p27 and Spy1 were expressed. 293T cells were transfected with mock, p27, flag-Spy1, or both p27 and flag-Spy1. The lysates were then immunoprecipitated with CDK2 antisera and subjected to an in vitro histone H1 kinase assay. Figure 8A demonstrates the basal levels of CDK2 histone H1 kinase activity in mock-transfected cells (lane 1). As expected, CDK2 activity is significantly inhibited when p27 is overexpressed (lane 2). Cells expressing Spy1 have a moderate increase in histone H1 kinase activity over mock, as previously demonstrated (Porter et al., 2002; lane 3). Interestingly, when both p27 and Spy1 are overexpressed, histone H1 kinase activity is significantly increased compared with p27 alone (lane 4). The data indicate that Spy1 can enhance CDK2 histone H1 kinase activity in the presence of overexpressed p27. Figure 8B shows the expression of transfected flag-Spy1 and p27 (top and bottom panels, respectively), as well as endogenous CDK2 in the lysates (middle panel).
Figure 8.
Spy1 enhances histone H1 kinase activity in the presence of p27. (A) 293T cells were transfected with the indicated constructs. The lysates were immunoprecipitated with α-CDK2 sera and subjected to an in vitro kinase assay. Lane 2 shows that p27-transfected cells have decreased kinase activity, but Spy1-transfected cells exhibit kinase activity higher than mock-transfected cells (lane 3). When both Spy1 and p27 are cotransfected, CDK2 histone H1 kinase activity is increased as shown in lane 4 compared with p27 alone. (B) The lysates were analyzed by 10% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with flag-Spy1, CDK2, and p27 antisera to demonstrate equivalent protein expression.
Spy1 Enhanced Proliferation Requires Endogenous p27
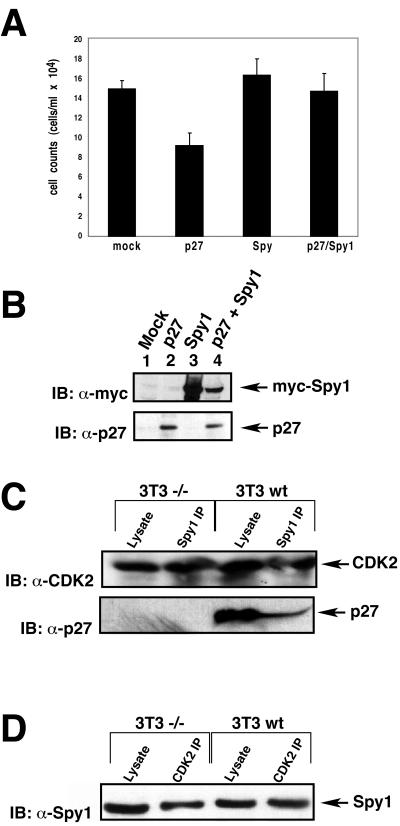
To address whether Spy1 requires p27 to induce cells to proliferate, we transiently transfected MEF cells lacking endogenous p27 (MEF–/–) with mock, p27, myc-Spy1, or p27 plus myc-Spy1 vectors. At the 48-h time point, we found that Spy1 expression alone did not stimulate an increase in proliferation of MEF–/– cells over mock, demonstrating that endogenous p27 is an essential component in Spy1-enhanced proliferation (Figure 9A, mock vs. Spy1). When p27 is expressed in these cells, there is a significant decrease in cell number, demonstrating that exogenous p27 is functional in these cells (Figure 9A, p27). Furthermore, in the presence of exogenous p27, Spy1 is capable of overriding the antiproliferative effect of p27 (Figure 9A, p27 vs. p27+Spy1). This is one representative experiment of four, all demonstrating that p27 is an essential component of the Spy1-proliferative pathway. Figure 9B is a Western blot of lysates from each of the relevant samples, demonstrating that both proteins are being expressed as indicated. However, it is interesting that endogenous Spy1 is still capable of binding to CDK2 in cells lacking endogenous p27 (Figure 9, C and D). Here, we studied the interaction between endogenous Spy1 and endogenous CDK2 in both wild-type NIH3T3 cells (3T3wt) and NIH3T3 cells null for p27 (3T3–/–). As demonstrated in Figure 9C, an immunoblot for α-CDK2 detects CDK2 in cell lysates as well as in Spy1 immunoprecipitations for both cell types (top panel). The bottom panel of Figure 9C demonstrates that p27 protein is only present in the 3T3wt cells. Similarly, a Spy1 immunoblot detects Spy1 protein in cell lysates as well as in an immunoprecipitation using CDK2 antisera (Figure 9D). Hence, Spy1 binds to CDK2 in both p27 null and wild-type 3T3 cells.
Figure 9.
Spy1-enhanced proliferation is dependent on endogenous p27. (A) MEF–/– cells were transfected with the indicated constructs, medium was changed 24 h posttransfection, and cells were counted via trypan blue exclusion 48 h posttransfection. The graphed data indicate that exogenous p27 slows proliferation in the MEF–/– cells and that Spy1 can overcome this inhibition of proliferation. Spy1 does not enhance proliferation over mock control in cells lacking endogenous p27. The data are one representative experiment of four. Error bars indicate the SEM of three separate transfections. (B) Lysates were analyzed by 10% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with α-myc-Spy1, and α-p27 sera to demonstrate protein expression. (C) Endogenous Spy1 was immunoprecipitated from either NIH3T3 cells null for p27 (3T3–/–) or NIH3T3 wt (3T3wt). Both lysate and immunoprecipitated samples were separated by 10% SDS-PAGE and immunoblotted with α-CDK2 sera (top panel) or α-p27 sera (bottom panel). (D) Endogenous CDK2 was immunoprecipitated from both 3T3wt and 3T3–/– cell types. Lysate samples and the immunoprecipitated samples were analyzed by 10% SDS-PAGE, and immunoblotting was carried out with α-Spy1 sera.
DISCUSSION
We have identified a novel interaction between p27 and Spy1 using a yeast two-hybrid screen. This interaction was confirmed both in vitro and in vivo using full-length p27 and Spy1 and occurs in the absence of CDK2. The binding of Spy1 to p27 requires the same domain of p27 that mediates direct interaction between p27 and CDK2 (Slingerland et al., 2000).
This leads to the question: does the interaction of Spy1 with CDK2 occur independently of p27, or rather, is this an indirect interaction mediated by p27? The answer may not be straightforward.
In support of a p27-independent interaction, we have shown here that Spy1 binds CDK2 in cells devoid of endogenous p27. This observation is consistent with prior observations that Xenopus homologues of Spy1, X-Spy1, and p33ringo, both bind directly to CDK2 (Lenormand et al., 1999; Karaiskou et al., 2001). In these systems Spy1 activation is involved in triggering meiotic maturation, demonstrating that Spy1 may have other cell cycle effects that are not dependent on p27. Previously, we also demonstrated that human Spy1 can exist in a complex with CDK2 (Porter et al., 2002), although the possible involvement of p27 in this interaction was not investigated.
On the other hand, a p27-dependent interaction between Spy1 and Cdk2 is supported by the fact that p27, but not CDK2, was detected as a positive interacting protein in a two-hybrid screen using Spy1 as the bait. Additionally, we show an increased association between Spy1 and CDK2 when p27 is exogenously expressed, demonstrating that p27 enhances the interaction between Spy1 and CDK2.
It is known that p27 interacts with cyclin/CDK2 complexes via both its CDK and cyclin-binding regions. Conceivably, when Spy1 binds to p27 it may do so by competing with CDK2 for the CDK-binding region of p27. In this case, however, CDK2 may still remain bound to the p27/Spy1 complex indirectly through its interaction with cyclin. This provides one possible explanation for the presence of a p27/Spy1/CDK2 complex. Further investigation is needed into the functional significance of an interaction between Spy1-and CDK2, which can be either p27-dependent or -independent.
An in vitro kinase assay was carried out to determine the effect of coexpressing p27 and Spy1. Here, we found that there was an increase in CDK2 histone H1 kinase activity in the presence of Spy1 compared with p27 alone. In agreement with these results, we demonstrate that DNA synthesis is also increased in Spy1/p27 coexpressed cells. The data indicate that expression of Spy1 can overcome CDK2 kinase inhibition by p27. CDK2 kinase activity is required for Spy1-proliferative effects in mammalian cells (Porter et al., 2002). Additionally, this report demonstrates that Spy1-induced proliferation requires endogenous p27. How might Spy1 be enhancing CDK2 activity in a p27-dependant manner? One possibility to investigate is whether Spy1 affects p27 degradation. Total amounts of p27 are not drastically reduced when Spy1 is coexpressed with p27, demonstrating that Spy1 is not sufficient to target p27 for degradation, but this observation does not rule out that Spy1 may play a role in initiating pathways involved in p27 degradation. Exploration of this possibility is ongoing.
In summary, we have identified a novel interaction between p27 and Spy1 by using a yeast two-hybrid screen. The interaction between p27 and Spy1 was confirmed both in vitro and in vivo in mammalian cells. Coimmunoprecipitation studies demonstrated that Spy1 can bind to the CDK-binding region, but not to the cyclin-binding region, of p27. Additionally, CDK2 remains a part of this complex, indicating how these three proteins may interact. We demonstrate that the interaction between Spy1 and p27 increases DNA synthesis, indicating that Spy1 is capable of overriding p27 inhibition of CDK2 kinase activity. Furthermore, Spy1 is not capable of enhancing proliferation in cells lacking endogenous p27, demonstrating that p27 is an essential component of the Spy1-induced cell proliferation pathway. Taken together, these data suggest that Spy1 regulates cell cycle progression by directly interacting with p27 to relieve cell cycle arrest.
Acknowledgments
We thank S. Hollenberg and J. A. Cooper for the kind gifts of pBTM116, pVP16, and LexA-lamin plasmids; P. Bartel and S. Fields for construction of the pBTM116 vector; and Jim Roberts and Andrew Koff for p27 null cell lines. We also thank S.M. Mac, R.W. Dellinger, S. Mobin, and M.C. Mendoza for technical assistance and S.M. Mac, E.A. Barnes, R. Gastwirt, and A.N. Meyer for advice and critical reading of the manuscript. M.K. was supported by the NIH/NIDCR Grant R01 DE12581. L.A.P. gratefully acknowledges support from the UCSD Cancer Center Pete Lopiccola Fellowship in Cancer Research and from the Leukemia Research Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–12–0820. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0820.
References
- Baldassarre, G. et al. (2000). Retinoic acid induces neuronal differentiation of embryonal carcinoma cells by reducing proteasome-dependent proteolysis of the cyclin-dependent inhibitor p27. Cell Growth Differ. 11, 517–526. [PubMed] [Google Scholar]
- Carrano, A.C., Eytan, E., Hershko, A., and Pagano, M. (1999). SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 4, 193–199. [DOI] [PubMed] [Google Scholar]
- Chen, C., and Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, M., Olivier, P., Diehl, J.A., Matthew, R., Roussel, M.F., Roberts, J.M., and Sherr, C.J. (1999). The p21Cip1 and p27Kip1 CDK `inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18, 1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand, B., Gao, F.B., and Raff, M. (1997). Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 16, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey, P.D., Russo, A.A., Polyak, K., Gibbs, E., Hurwitz, J. Massagué, and Pavletich, N.P. (1995). Crystal structure of a cyclin A-CDK2 complex at 2.3 Å: mechanism of CDK activation by cyclins. Nature 375, 159–161. [DOI] [PubMed] [Google Scholar]
- Karaiskou, A., Perez, L.H., Ferby, I., Ozon, R., Jessus, C., and Nebreda, A. (2001). Differential regulation of Cdc2 and Cdk2 by RINGO and cyclins. J. Biol. Chem. 276, 36028–36034. [DOI] [PubMed] [Google Scholar]
- Koff, A., Ohtsuki, M., Polyak, K., Roberts, J.M., and Massague, J. (1993). Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science 260, 536–539. [DOI] [PubMed] [Google Scholar]
- Kong, M., Barnes, E.A., Ollendorff, V., and Donoghue, D.J. (2000). Cyclin F regulates the nuclear localization of cyclin B1 through a cyclin-cyclin interaction. EMBO J. 19, 1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand, J.-L., Dellinger, R.W., Knudsen, K.E., Subramani, S., and Donoghue, D.J. (1999). Speedy: a novel cell cycle regulator of the G2/M transition. EMBO J. 18, 1869–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnoli, A., Fiore, F., Eytan, E., Carrano, A.C., Draetta, G.F., Hershko, A., and Pagano, M. (1999). Ubiquitination of p27 is regulated by Cdk dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D.O. (1995). Principles of CDK regulation. Nature 374, 131–134. [DOI] [PubMed] [Google Scholar]
- Morisaki, H., Fujimoto, A., Ando, A., Nagata, Y., Ikeda, K., and Nakanishi, M. (1997). Cell cycle-dependent phosphorylation of p27 cyclin dependent kinase (Cdk) inhibitor by cyclin E/Cdk2. Biochem. Biophys. Res. Commun. 240, 386–390. [DOI] [PubMed] [Google Scholar]
- Nakanishi, M., Robetorye, R.S., Adami, G.R., Pereira-Smith, O.M., and Smith, J.R. (1995). Identification of the active region of the DNA synthesis inhibitory gene 21Sdi1/CIP1/WAF1. EMBO J. 14, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, K-I., Hatakeyama, S., and Nakayama, K. (2001). Regulation of the cell cycle at the G1/S transition by proteolysis of cyclin E and p27Kip1. Biochem. Biophys. Res. Commun. 282, 853–860. [DOI] [PubMed] [Google Scholar]
- Pagano, M., Tam, S.W., Theodoras, A.M., Beer-Romero, P., Del Sal, G., Chau, V., Yew, P.R., Draetta, G.F., and Rolfe, M. (1995). Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269, 682–685. [DOI] [PubMed] [Google Scholar]
- Polyak, K. Lee, M.H., Erdjument-Bromage, H., Koff, A., Roberts, J.M., Tempst, P., and Massagué, J. (1994). Cloning of p27kip1: a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59–66. [DOI] [PubMed] [Google Scholar]
- Porter, L.A., Dellinger, R.W., Tynan, J.A., Barnes, E.A., Kong, M., Lenormand, J.-L., and Donoghue, D.J. (2002). Human speedy: a novel G1/S cell cycle regulator. J. Cell Biol. 157, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdóttir, I., and Massagué, J. (1997). The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11, 492–503. [DOI] [PubMed] [Google Scholar]
- Rodier, G., Montagnoli, A., Di Marcotullio, L., Coulombe, P., Draetta, G.F., Pagano, M., and Meloche, S. (2001). p27 cytoplasmic localization is regulated by phophorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20, 6672–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaeff, R.J., Groudine, M., Gordon, M., Roberts, J.M., and Clurman, B.E. (1997). Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11, 1464–1478. [DOI] [PubMed] [Google Scholar]
- Sherr, C.J. (1994). G1 phase progression: cycling or cue. Cell 79, 551–555. [DOI] [PubMed] [Google Scholar]
- Sherr, C.J., and Roberts, J.M. (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Slingerland, J., and Pagano, M. (2000). Regulation of the Cdk Inhibitor p27 and its deregulation in cancer. J. Cell. Phys. 183, 10–17. [DOI] [PubMed] [Google Scholar]
- Slingerland, J., Hengst, L., Pan, C., Alexander, D., Stampfer, M.R., and Reed, S.I. (1994). A novel inhibitor of cyclin-CDK activity detected in transforming growth factor beta-arrested epithelial cells. Mol. Cell. Biol. 14, 3683–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterluty, H., Chatelain, E., Marti, A., Wirbelauer. C., Senften, M., Muller, U., and Krek, W. (1999). p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 4, 207–214. [DOI] [PubMed] [Google Scholar]
- Tomoda, K., Kubota, Y., and Kato, J.-Y. (1999). Degradation of the cyclin-dependent kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398, 160–165. [DOI] [PubMed] [Google Scholar]
- Toyoshima, H., and Hunter, T. (1994). p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell 78, 67–74. [DOI] [PubMed] [Google Scholar]
- Tsvetkov, L.M., Yeh, K.-H., Lee, S.-J., Hong, S., and Zhang, H. (1999). p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr 187 in p27. Curr. Biol. 9, 661–664. [DOI] [PubMed] [Google Scholar]
- Vidal, A., Millard, S., Miller, J.P., and Koff, A. (2002). Rho activity can alter the translation of p27 mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. J. Biol. Chem. 277, 16433–16440. [DOI] [PubMed] [Google Scholar]
- Vlach, J., Hennecke, S., and Amatic, B. (1997). Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 16, 5334–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek, A.B., Hollenberg, S.M., and Cooper, J.A. (1993). Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74, 205–214. [DOI] [PubMed] [Google Scholar]
- Vojtek, A.B., and Hollenberg, S.M. (1995). Ras-Raf interaction: two-hybrid analysis. Methods Enzymol. 255, 331–342. [DOI] [PubMed] [Google Scholar]