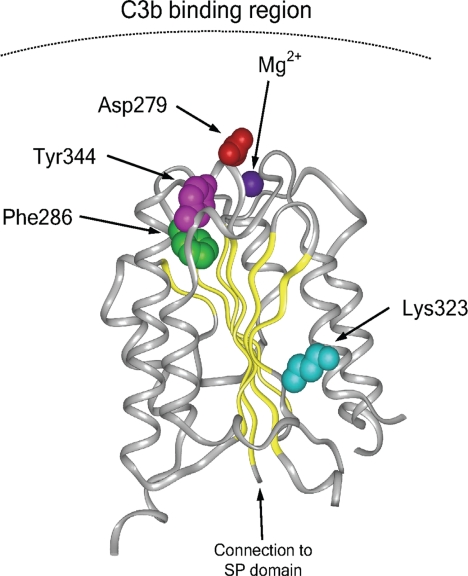
IMMUNOLOGY. For the article “Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome,” by Elena Goicoechea de Jorge, Claire L. Harris, Jorge Esparza-Gordillo, Luis Carreras, Elena Aller Arranz, Cynthia Abarrategui Garrido, Margarita López-Trascasa, Pilar Sánchez-Corral, B. Paul Morgan, and Santiago Rodríguez de Córdoba, which appeared in issue 1, January 2, 2007, of Proc Natl Acad Sci USA (104:240–245; first published December 20, 2006; 10.1073/pnas.0603420103), the authors note that in Fig. 2, the Lys323 residue of factor B was placed incorrectly; the correct location is outside of the C3b–Bb interface. In addition, residue Tyr344 was mislabeled as Tyr319. The corrected figure and its legend appear below. These errors do not affect the conclusions of the article.
Fig. 2.
Diagram of the von Willebrand type A domain of fB. Insight II (BYOSYM software package; Molecular Simulations, San Diego, CA) was used to draw structure by using PDB files 1Q0P and 1RS0_A. The positions of the residues Phe286 and Lys323 that are mutated in the HUS patients are indicated. The position of the Mg2+ ion and that of the Asp279 and Tyr344 residues are also indicated. Note the edge-to-face stacking of the Phe286 and Tyr344 residues. Numbering of residues is referred to with the initial methionine as “1” and therefore includes the sequence of the N-terminal signal peptide. Residue Asp279 was described previously as Asp254 by Hourcade et al. (32).