Abstract
Human HT2-19 cells with a conditional cdk1 mutation stop dividing upon cdk1 inactivation and undergo multiple rounds of endoreplication. We show herein that major cell cycle events remain synchronized in these endoreplicating cells. DNA replication alternates with gap phases and cell cycle-specific cyclin E expression is maintained. Centrosomes duplicate in synchrony with chromosome replication, giving rise to polyploid cells with multiple centrosomes. Centrosome migration, a typical prophase event, also takes place in endoreplicating cells. The timing of these events is unaffected by cdk1 inactivation compared with normally dividing cells. Nuclear lamina breakdown, in contrast, previously shown to be dependent on cdk1, does not take place in endoreplicating HT2-19 cells. Moreover, breakdown of all other major components of the nuclear lamina, like the inner nuclear membrane proteins and nuclear pore complexes, seems also to depend on cdk1. Interestingly, the APC/C ubiquitin ligase is activated in these endoreplicating cells by fzr but not by fzy. The oscillations of interphase events are thus independent of cdk1 and of mitosis but may depend on APC/Cfzr activity.
INTRODUCTION
The role of the cell cycle mechanism is to ensure accurate genome and organelle replication and their correct segregation into two daughter cells. Eukaryotic cells perform this task by undergoing a sequence of phases that must be carried out once and only once each cycle. It has been pointed out early on that such a series of events could be controlled either by an autonomous oscillator or as a sequence of interdependent events (Hartwell et al., 1974). The oscillator model was supported by the discovery that maturation promoting factor cycling activity does not depend on other cell cycle events (Newport and Kirschner, 1984). Work with yeast cell cycle mutants supported, to a great extent, the alternative model of interdependent events (Hartwell et al., 1974). The gradual insight gained into the molecular mechanisms of cell cycle control helped to largely reconcile these two conflicting views (Murray and Kirschner, 1989). The “reckless” oscillations of maturation promoting factor are mainly confined to the rapid early divisions of some embryos such as Xenopus and clam. Most other cells seem to use the more rigorous pathway of interdependent events to control their cycle. The interdependence of these events is, however, not necessarily intrinsic, but is controlled by checkpoint mechanisms. The existence of an autonomously running oscillator in cells is hard to prove because it is masked by these checkpoint mechanisms. Recently Haase and Reed, 1999 found indications for the existence of such an autonomous cell cycle oscillator in budding yeast. They arrested cells by perturbing cdk1 (p34CDC28) activity and found that cells continued to carry out various cell cycle activities on schedule.
We have used the HT2-19 human cell line that carries a conditionally targeted cdk1 gene to study this issue in mammalian cells. When cdk1 is down-regulated in these cells they stop dividing and undergo multiple rounds of endoreplication. We show herein that breakdown of the nuclear lamina and activation of APC/Cfzy, events previously shown to depend directly on cdk1 activity, indeed do not take place in these cells. Interphase events such as replication, cyclin E expression, centrosome duplication, and segregation and APC/Cfzr activation, however, do take place in these endoreplicating cells, regardless of the fact that they do not undergo mitosis.
MATERIALS AND METHODS
Tissue Culture
HT2-19 cells were grown in DMEM supplemented with 10% fetal calf serum, glutamine, pyruvate, nonessential amino acids, and penicillin/streptomycin (Beit Haemek Biological Industries, Kibbutz Beit Haemek, Israel). Cells were cultured in the presence of 2 mM isopropyl β-d-thiogalactoside (IPTG). To suppress cdk1 expression 105 cells were plated per 10-cm dish and cultured without IPTG for 7 d. Both cycling and endoreplicating cells were synchronized in S phase with 2 mM freshly prepared hydroxyurea (HU) (Sigma-Aldrich, St. Louis, MO) for 19 h. Cells were subsequently released into fresh medium with, or without, IPTG as indicated in the text and figure legends.
Antibodies
Monoclonal antibodies A17 for cdk1 (400 ng/ml), V152 for cyclin B1 (330 ng/ml), AR-38 for fzr (hybridoma supernatant), AF3 for cdc27 (hybridoma supernatant), and rabbit antibodies for cyclin A2 (serum diluted 1:300) were a gift from Drs. J. Gannon and T. Hunt (London, United Kingdom). Rabbit polyclonal antibody for Nap1 (serum diluted 1:500) was a gift from Dr. E. Nigg (Martinshied, Germany). Rabbit polyclonal antibody 860 for cdc27 (serum diluted 1:1000) was a gift from Dr. P. Hieter (Vancouver, British Columbia). Rabbit polyclonal antibodies for emerin, lamin A/C, and lamin B1 (serum diluted 1:200) were a gift from Drs. K.L. Wilson (Baltimore, MD), K. Weber (Goettingen, Germany), and E.C. Schirmer and L. Gerace (La Jolla, CA), respectively. Human autoimmune antibodies, which recognize centromeres (serum diluted 1:5000), were a gift from W. Earnshaw (Edinburgh, United Kingdom). The following antibodies were purchased: mouse monoclonal antibody (mAb) GTU88 for γ-tubulin (ascites fluid diluted 1:300; Sigma-Aldrich), mouse mAb HE12 for cyclin E (400 ng/ml; Upstate Biotechnology, Lake Placid, NY), and mouse mAb mAb414 for FG-repeat nucleoporins (nup358, nup214/CAN, nup153, nup98, and p62414) (Babco, Richmond, CA). Goat polyclonal antibodies p55cdc-N19 for fzy (400 ng/ml) and I-19 for actin (200 ng/ml) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All secondary antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Horseradish peroxidase-tagged secondary antibodies were used for Western blotting at a 1:5000 dilution. Fluorescein isothiocyanate and Cy3-tagged secondary antibodies were used for immunofluorescence at 1:200 and 1:300 dilutions, respectively.
Immunological Procedures
Cells were fixed for IF in their culture dishes for 10 min with cold methanol (–20°C) and blocked with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Cells were incubated with antibodies at the indicated dilutions in 2% BSA in PBS for 1 h, washed twice with PBS, incubated with the fluorescently tagged secondary antibody in a dark drawer for 1 h, washed again, stained in the dark with 4,6-diamidino-2-phenylindole (DAPI) (1 μg/ml) for 5 min, mounted with 2% n-propyl gallate, and sealed with a coverslip. In experiments where two different proteins were stained simultaneously, a mix of the two primary and the two secondary antibodies was used. The cells were fixed and stained on the plastic culture dishes to avoid moving the huge endoreplicated cells to coverslips. To visualize the cells the rims of the dishes were removed after the staining and the plates were viewed upside down on an inverted microscope.
Immunobloting was performed by standard methods (Harlow and Lane, 1999) at the indicated antibody concentrations.
The APC/C was immunoprecipitated with cdc27 antibodies (AF3) coupled to protein A Affiprep beads (Bio-Rad, Hercules, CA) as described previously (Harlow and Lane, 1999). Fluorescent in situ hybridization (FISH) was performed as described with a probe at the FRA7H locus (Hellman et al., 2000).
Images of cells were captured with an Olympus IX70 microscope with 60× and 100× UplanFI oil objectives mounted with an Optronics Magnafire or a DVC1300 charge-coupled device camera. Confocal imaging was performed with a Bio-Rad 1024 system. Images were processed with ImagePro 4.5 (Media Cybernetics) and Photoshop 5 (Adobe Systems, Mountain View, CA).
Bromodeoxyuridine (BrdU) Incorporation Assay
Cells were incubated for 45 min in the presence of 30 μM BrdU and subsequently harvested into PBS. The cells were spun for 2 min in a microcentrifuge at 2700 × g, gently resuspended in 1 ml of hypotonic buffer (0.5% KCl) and incubated for 10 min at room temperature. The cells were spun again, resuspended in 1 ml of fixative (MeOH/glacial acetic acid, 3:1) and incubated for at least 0.5 h at –20°C. Cells can be stored in this fixative for weeks. The fixed cells were spun, resuspended in fixative, dripped on glass slides, and spread by blowing. The slides were air dried and dehydrated by sequential incubation in 70, 90, and 100% ethanol, 5 min in each concentration at –20°C. The cells were dried again and baked for 10 min in a 55°C oven. To denature the DNA, the slides were overlaid with 70% formamid in 2× SSC, covered with parafilm, and placed for exactly 2 min on a heated plate at 70°C. The slides were then immediately transferred to 70%, and subsequently to 90 and 100% ethanol (–20°C), 5 min each, and dried completely. The slides were blocked with 2% BSA in 4× SSC and incubated with the BrdU antibody (antibody-2 purchased from NeoMarkers, Freemont, CA, and diluted 1:200 in 4× SSC, 2% BSA, and 0.1% Tween 20), and with Cy3-conjugated goat anti-mouse (diluted 1:500). Blocking and incubation with the antibodies were carried out for 10 min each and the slides were washed three times for 5 min each with 4× SSC, 0.1% Tween 20 after incubation with the primary and the secondary antibodies. The slides were stained with DAPI, dried, mounted, and sealed.
RESULTS
Endoreplicating cdk1-deficient HT2-19 Cells Have Distinct Cell Cycle Phases
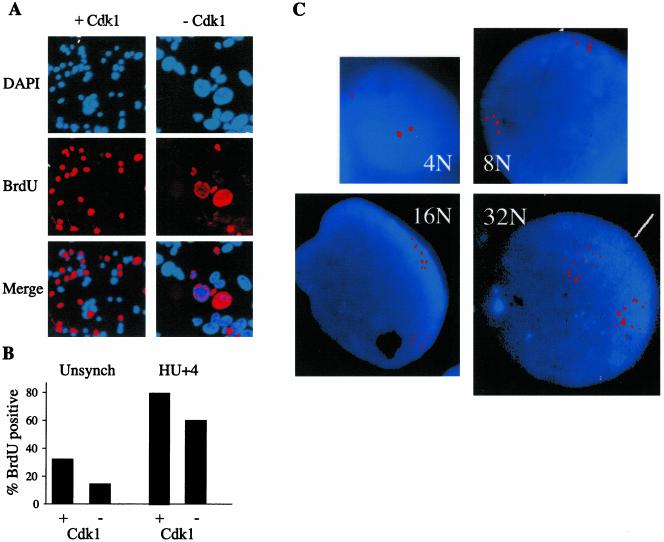
The HT2-19 cell line was generated by gene targeting of a human fibrosarcoma HT1080 cell line. HT2-19 cells carry a single conditionally active cdk1 allele expressed in the presence of IPTG. In the presence of IPTG, the level of cdk1 is about one-third of that of wild-type cells, sufficient for normal cell division. (Itzhaki et al., 1997). Deprivation of IPTG leads to a gradual decline in the levels of cdk1 mRNA, protein, and associated kinase activity. Once cdk1 levels have dropped, HT2-19 cells stop dividing and no longer enter mitosis. They do, however, endoreplicate their genome to high levels of ploidy (Itzhaki et al., 1997). Endoreplication could either be the result of continuous DNA replication or of multiple S phases separated by gap, but no M phases. The former case would point to a complete breakdown of cell cycle regulation and the latter could indicate that some, or most, of the cell cycle events still take place. To distinguish between these two scenarios, we pulsed HT2-19 cells, grown for 10 d without IPTG, with BrdU for 45 min. Cells were subsequently fixed and BrdU incorporation was analyzed by indirect immunofluorescence (see MATERIALS AND METHODS). Slides were stained with DAPI and photographed to assess the fraction of cells in S phase (Figure 1A). Figure 1A also shows that the nuclei of cells grown without IPTG are considerably larger than those that grow with IPTG. Figure 1B shows that ∼15% (n = 3287) of the cells that were grown for 10 d without IPTG, and 30% (n = 3228) of control cells, incorporated BrdU. In a second experiment cells were first arrested in S phase with 2 mM hydroxyurea (HU), released for 4 h and pulsed with BrdU for 45 min. Figure 1B shows that in this experiment ∼60% (n = 506) of the endorep-licating cells, and 80% (n = 809) of the control cells, incorporated BrdU. These results show that not all the endoreplicating cells are replicating their DNA at a given time point and imply the existence of some sort of gap phases.
Figure 1.
The cell cycle of endoreplicating HT2-19 cells consists of defined S and gap phases. (A) HT2-19 cells, growing in the presence (+ Cdk1) or absence (–Cdk1) of IPTG were pulsed for 45 min with BrdU. Cells were subsequently fixed and stained with BrdU antibodies and Cy3-labeled secondary antibodies (red) and DAPI (blue). (B) About 3000 cells were scored to establish the proportion of the endoreplicating and normally growing cells that replicate their DNA in an unsynchronized cell population. In a second experiment, cells were synchronized with HU and released for 4 h before the BrdU pulse. The proportion of cells in S phase in both populations was established in the same way. (C) Cdk1 depleted cells were analyzed by FISH with a unique probe on chromosome 7. Cells with 4, 8, 16, and 32 dots, representing a ploidy of 4, 8, 16, and 32C respectively, are shown.
Polyploid cells that undergo successive S phases, separated by gap phases, should contain a distinct number of chromosome sets (2, 4, 8, 16, 32C, etc.). Analysis of DNA content of cdk1-deficient HT2-19 cells by flow cytometry indeed showed defined peaks of up to 64C (Itzhaki et al., 1997). We further tested the ploidy of cdk1-deficient cells by FISH analysis with a probe that identifies a single locus on chromosome 7 (Hellman et al., 2000). Figure 1C shows cells that carry 4, 8, 16, and 32 copies of this locus, always grouped into two distinct clusters, presumably representing the two homologs. FISH analysis thus confirms that endoreplicating HT2-19 cells have a defined ploidy. This result supports our hypothesis that the cell cycle of endoreplicating HT2-19 cells is composed of alternating S phases during which the entire genome is replicated once and only once, and of gap phases.
Cell Cycle-specific Pattern of Cyclin E Expression Is Maintained in Endoreplicating Cells
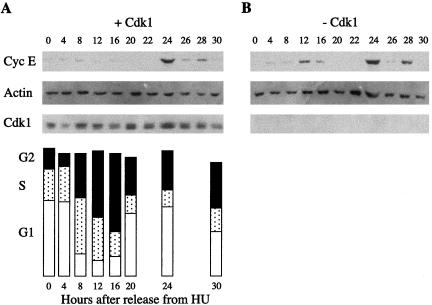
The activation of cdk2 by cyclin E is essential for the entry of cells into S phase. Cyclin E protein levels oscillate sharply during the cell cycle, peaking at the G1/S transition (Dulic etal., 1992). Endoreplicating cells cultured for 10 d in the absence of IPTG and normally cycling HT2-19 cells were analyzed by indirect immunofluorescence with cyclin E antibodies. A roughly similar proportion of the cells, 10% (n = 582) and 13% (n = 612) of cycling and endoreplicating cells, respectively, stained positive for cyclin E. We tried several synchronization protocols for endoreplicating cells to find out whether these data also represent similar cell cycle-specific oscillations. We tested both single and double blocks with several reagents but none of these protocols yielded the perfect synchronization reported for other cell lines (Spector et al., 1997). A single HU arrest proved finally to be the most useful approach and was used throughout this work. HT2-19 cells were deprived of IPTG for 7 d and arrested in S phase by treating them with HU for 19 h. Control cells grown in the presence of IPTG were arrested in a similar way. The arrested cells were released from the HU block and harvested at different time points for up to 40 h. Protein extracted from the released cells was subjected to immunoblotting with cyclin E, actin, and cdk1 antibodies. To determine cell cycle distribution patterns, the control cells were further subjected to fluorescence-activated cell sorting (FACS) analysis. FACS analysis of the endoreplicating cells was not possible because of their complex profile. Figure 2A shows that cyclin E peaked sharply 24 h after the release from the HU block. This time point coincides with late G1 as shown in the FACS analysis. Figure 2B shows that the time of the peak of cyclin E expression in endoreplicating cells was strikingly similar to the peak in cycling cells. The minor peaks of cyclin E at 12 and 28 h observed in both cell populations are probably due to incomplete synchronization of the cells. Levels of actin were used as a loading control. Cdk1 was undetectable in the cells that were grown without IPTG and relatively constant in the control cells. This experiment shows that cyclin E expression in late G1 depends neither on mitosis taking place nor on cdk1.
Figure 2.
Cell cycle-specific oscillations of cyclin E are conserved in endoreplicating cells. (A) Normally cycling HT2-19 cells were arrested in S phase with HU and released into fresh medium. Cells were harvested at the indicated times and analyzed by immunobloting with cyclin E, actin, and cdk1 antibodies, as well as by FACS. (B) Cells were deprived of IPTG for 7 d to deplete their cdk1 and were subsequently arrested with HU, released and analyzed like in A.
Centrosome Duplication Is Synchronized with Replication in Endoreplication Cycles
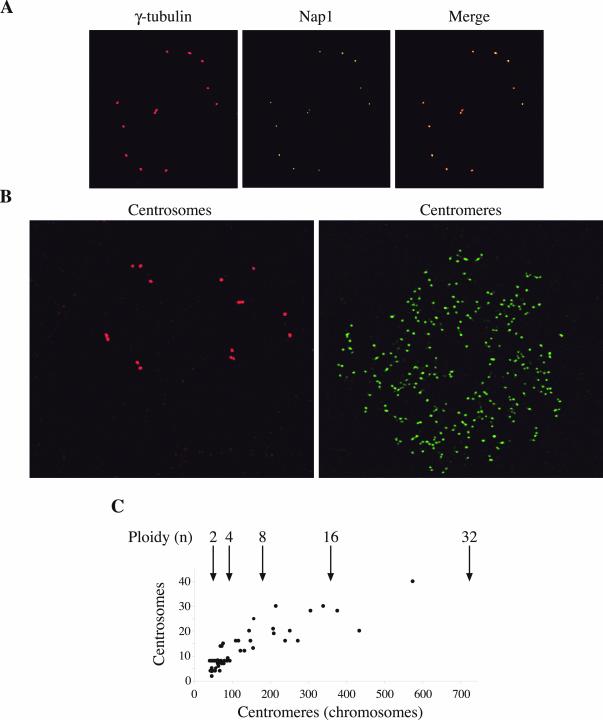
Centrosome duplication in late G1 is, like DNA replication, an event that takes place only once each cycle. This duplication was shown to be dependent on the activity of cyclin E-cdk2 (Lacey et al., 1999; Matsumoto et al., 1999) or cyclin A2-cdk2 (Meraldi et al., 1999). We studied the centrosomes in reduplicating HT2-19 cells to find out whether their number increases with the ploidy of the cell. Immunofluorescence analysis of polyploid HT2-19 cells with a γ-tubulin antibody showed that they contained multiple centrosomes. This observation was verified with an antibody against the centrosomal protein Nup1, which colocalizes with γ-tubulin (Figure 3A).
Figure 3.
Centrosomes in endoreplicating cells are duplicated in synchrony with DNA replication. (A) The centrosomes of endoreplicating cells were stained with mouse monoclonal γ-tubulin and rabbit polyclonal NAP1 antibodies. (B) Endoreplicating cells were stained in parallel for centrosomes (γ-tubulin) and for centromeres. (C) A plot of centrosomes versus centromeres of endoreplicating cells. Arrows indicate the expected number of centromeres for the respective ploidy.
To establish the relationship between the ploidy of the cell and the number of centrosomes, we required a method to assess the ploidy of single cells. For this purpose, we used a human autoimmune antibody that recognizes chromosomal centromeres. HT2-19 cells were grown for 6 or 12 days without IPTG and then fixed and stained by dual labeling with centromere and γ-tubulin antibodies (Figure 3B). Cells were photographed and both centromeres and centrosomes were counted. In some cells, >300 centromeres (16C) could safely be counted. The big and flat nuclei of the polyploid HT2-19 cells enabled a precise enough count of centromeres to estimate the ploidy of the cells. Close centromeres could not always be told apart and some centromeres were out of the focal plane. These technical limitations could explain the deviation from precise numbers (184, 368, etc.). We also used confocal microscopy but this, too, did not yield precise numbers. The results of the FISH analysis shown in Figure 1C show, however, that cells retained accurate chromosome doublings at least up to 16, and probably up to 32C. It is therefore most likely that deviations from the expected centromere numbers reflect experimental limitations rather than chromosomal instability.
Double labeling of centromeres and centrosomes showed a close correlation between the ploidy of the cells and the number of centrosomes. Cells with 16C, which had as much as 30 centrosomes, were observed. The deviation of the centrosome number from the expected powers of two is also probably mainly due to experimental limitations. Many of the centrosomes were clustered and it was impossible to establish their precise number. Other centrosomes might have been out of the focal plane. In spite of these limitations, our data strongly suggest that centrosome duplication remains synchronized in these cells and happens once every endoreplication cycle (Figure 3C).
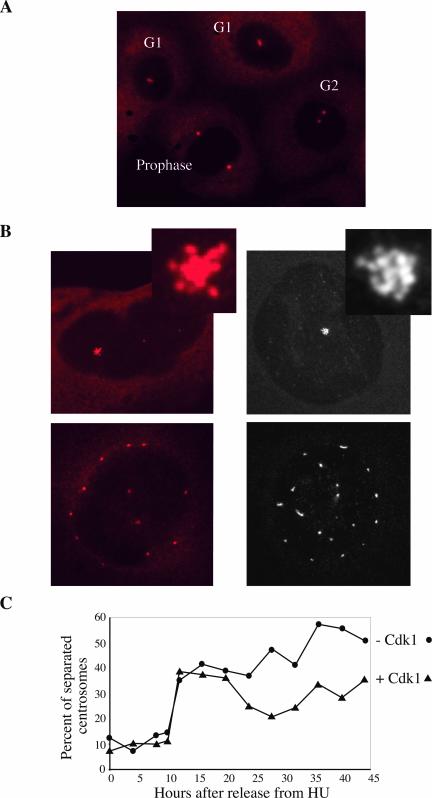
Centrosomes Migrate in Endoreplicating Cells
Centrosomes normally duplicate in late G1 or early S but remain close together until they reach G2. In G2, centrosomes separate and in prophase they migrate to opposite poles of the nucleus to form the mitotic spindle (Figure 4A). The multiple centrosomes observed in polyploid cells were either all in a single cluster or scattered all over the cell. The latter were often evenly spaced around the periphery of the nucleus like the two centrosomes in a prophase cell (Figure 4B). This phenotype suggested that centrosomes in polyploid cells migrate in prophase as in normally dividing cells. To test this hypothesis, we arrested cells with HU and released them for up to 44 h. Cells from each time point were fixed and stained with γ-tubulin antibodies. In HU-arrested cells, almost all centrosomes were clustered and remained so for ∼10 h. At the transition between 10 and 12 h, a very high proportion of the cells separated their centrosomes. Strikingly this separation happened exactly at the same time in endoreplicating cells cultured for 7 d without IPTG and in control cells (Figure 4C). The fraction of control cells with separated centrosomes significantly declined after 20 h as would be expected from cells entering G1. A similar decline was not observed in endoreplicating cells, which do not divide.
Figure 4.
Centrosome segregation timing is conserved in endoreplicating cells. (A) Normally growing cells stained with a γ-tubulin antibody with clustered (G1), separated (G2), and fully segregated (prophase) centrosomes. (B) Endoreplicating cells stained with γ-tubulin antibodies were imaged either by wide field (left) or by confocal microscopy (right). The confocal image is a projection of the series of z-stacks spanning the entire depth of the cell to show centrosomes at all focal planes. The centrosomes in cells were either clustered (top) or segregated (bottom). (C) Cells cultured without IPTG for 7 d (–Cdk1) and control cells (+ Cdk1) were arrested with HU and released into fresh medium. Cells were fixed at the indicated times and stained with a γ-tubulin antibody. Two hundred cells of each time point were photographed to score the proportion of cells with separated centrosomes.
Oscillation of APC/C Activity in Endoreplicating Cells
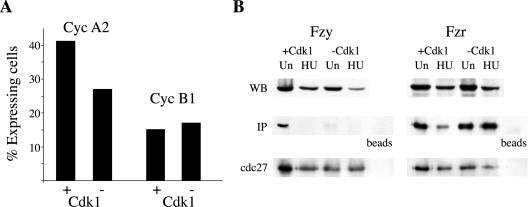
Mitotic A and B type cyclin levels oscillate during the cell cycle. They accumulate during S and G2 and are degraded by the APC/C pathway of ubiquitin-mediated proteolysis in mitosis. Both cyclins are regulated at the transcriptional level (Henglein et al., 1994; Cogswell et al., 1995); however, their oscillations are largely due to proteolysis (Glotzer et al., 1991). Cycling and endoreplicating cells were stained with antibodies against cyclin A2 and cyclin B1. The percentage of cells that stained positive for the nuclear cyclin A2 was 41% (n = 554) and 27% (n = 507) in cycling and endoreplicating cells, respectively. The percentage of cyclin B1-positive cells was 15% (n = 549) and 17% (n = 456) in cycling and endoreplicating cells, respectively (Figure 5A). These data suggested that periodic APC/C-mediated degradation might be maintained in endoreplicating cells.
Figure 5.
The APC/C is activated by fzr in endoreplicating cells. (A) Endoreplicating and control cells were stained with antibodies against cyclin A2 or B1. About 500 cells of each population were photographed and the proportion of positive cells was counted. (B) The APC/C was immunoprecipitated with cdc27 antibodies from control (+ Cdk1) or endoreplicating (–Cdk1) cells deprived of IPTG for 10 d. Cells were either unsynchronized or arrested with HU for 19 h as indicated. Coimmunoprecipitation of fzy and fzr was analyzed by immunoblotting. Total cell extracts were blotted in parallel with fzy and fzr antibodies.
The APC/C can be activated by either of two WD repeat proteins: fizzy (fzy/cdc20) or fizzy related (fzr/cdh1/hct1). The initial activation of the APC/C by fzy normally takes place in mitosis and requires the phosphorylation of several APC/C subunits by cdk1 (Shteinberg et al., 1999; Kramer et al., 2000; Rudner and Murray, 2000). After cell division, the APC/C is activated by fzr for the duration of G1 (Zur and Brandeis, 2002). Cdc27 antibodies coupled to protein Affiprep beads were used to immunoprecipitate APC/C complexes from unsynchronized or HU-arrested, cycling (+ cdk1) or endoreplicating (–cdk1) cells. The immunoprecipitated proteins were used, in parallel with whole cell extracts, for immunoblotting with fzy and fzr antibodies. Figure 5B shows that fzy binds to the APC/C only in cycling cells that express cdk1. Binding does not take place in cells arrested with HU, in agreement with previous reports that this binding is restricted to mitosis (Kramer et al., 1998; Listovsky et al., 2000). Fzy binding does not take place in cells lacking cdk1, conforming with reports that fzy can only bind to APC/C that has been phosphorylated by cdk1 (Shteinberg et al., 1999; Kramer et al., 2000; Rudner and Murray, 2000). Figure 5B further shows that fzr binds the APC/C both in cycling and in endoreplicating cells. Fzr binds the APC/C during G1 and is released when cells enter S phase (Zur and Brandeis, 2001). In cycling (+ cdk1) cells arrested with HU the level of fzr binding was indeed markedly reduced. We did not observe a similar reduction in endoreplicating (–cdk1) presumably due to a high proportion of cells that stopped dividing and are arrested in a G1 or G0-like phase of the cell cycle. Figure 1B shows that ∼40% of the cells released from a HU block did not resume replication. The majority of these cells are likely to be arrested in G1 or G0-like phase and contain an active APC/Cfzr. Due to this incomplete level of synchronization there is no direct way to show that fzr is binding the APC/C only during part of the cell cycle. The fact that both mitotic cyclins and APC/Cfzr are detected in endoreplicating cells implies, however, that the APC/C is periodically activated because mitotic cyclins do not accumulate in cells with an active APC/C.
Nuclear Lamina Breakdown Does Not Occur in Endoreplication cdk1-deficient HT2-19 Cells
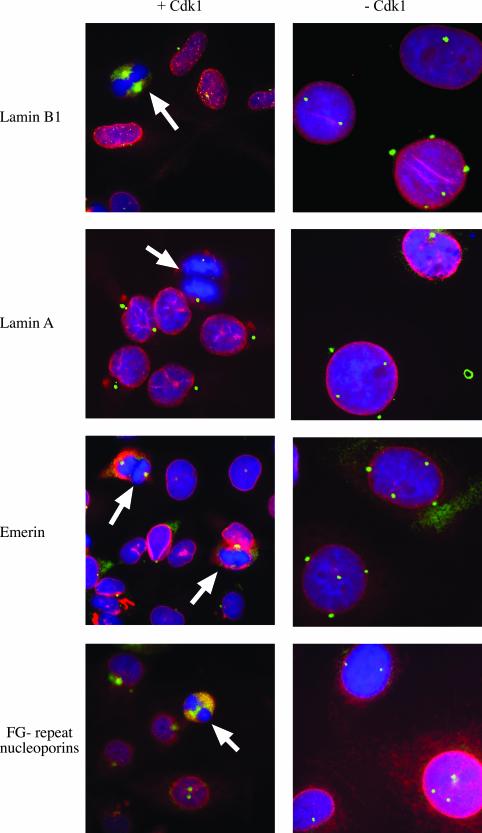
The nuclear lamina disassembles during mitosis. In mammals, this process occurs in prophase shortly after the centrosomes migrate to both poles of the nucleus (Cohen et al., 2001). Nuclear lamina disassembly depends on the phosphorylation of lamins by cdk1 (Heald and McKeon, 1990; Ward and Kirschner, 1990; Dessev et al., 1991). To follow the fate of the nuclear lamina, nuclear membranes, and nuclear pore complexes, we stained HT2-19 endoreplicating cells by indirect immunofluorescence with antibodies against A and B type lamins, emerin, and nucleoporins (Figure 6, red). The fate of the centrosomes was followed with γ-tubulin and Nap1 antibodies (Figure 6, green). To verify our interpretation of the stages of mitosis, we also labeled cells with DAPI (Figure 6, blue). Dividing HT2-19 cdk1-positive cells were used as controls. In agreement with previous data (Figures 1 and 3), the number of centrosomes and the nuclear volume increased in HT2-19 cdk1-deficient endoreplicating cells (Figure 6, right). In contrast, cdk1-positive cells retained their normal volume and had one or two centrosomes, depending on the stage of the cell cycle (Figure 6, left). In cdk-positive cells, lamin B1 (top), lamin A/C (second panel), emerin (third panel), and FG-repeat nucleoporins (bottom) stained the nuclear periphery during interphase, whereas the nuclear envelope disassembled during mitosis (white arrows), as expected. In cdk1-negative cells, all nuclear envelope markers remained in the nuclear periphery in cells with fully segregated centrosomes. As expected, lamins also labeled the nucleoplasm (Moir et al., 2000). These results demonstrate that all nuclear envelope components require cdk1 activity and proper mitosis for their disassembly. They also show that nuclear envelope disassembly is regulated differently than centrosome migration.
Figure 6.
The nuclear lamina does not disassemble in endoreplicating cdk1 deficient cells. Control cells expressing cdk1 (left) or endoreplicating cells that do not express cdk1 (right) were fixed and stained with antibodies for nuclear envelope components (red), with antibodies for centrosomes (green), and with DAPI. Centrosomes were stained with monoclonal γ-tubulin antibodies in the top three panels together with rabbit polyclonal antibodies for lamin A/C, lamin B1, and emerin. Centrosomes in the bottom panel were stained with rabbit polyclonal Nap1 antibodies together with mouse monoclonal antibodies for FG-repeat nucleoporins. White arrows indicate mitotic cells.
DISCUSSION
The eukaryotic cell cycle normally consists of alternating phases of DNA replication and cell division. These two phases are further separated by gap phases of variable length. The expression of cdk1 in HT2-19 cells can be inactivated and cells subsequently enter multiple rounds of endoreplication without dividing. We have used these cells to establish the dependence of various cell cycle events on mitosis in general and on cdk1 in particular. We found that all the interphase events we tested remained synchronized and presumably continued to oscillate in the absence of mitotic division and cdk1.
Endoreplication is a widespread phenomenon in a large number of organisms from all major groups of eukaryotes. Most endoreplication events are part of the developmental program of specialized tissues. Endoreplication has been extensively studied in many organisms as described in recent reviews (Grafi, 1998; Edgar and Orr-Weaver, 2001). The HT2-19 cell line has several advantages for the study of the regulation and characterization of endoreplication. First, the switch from mitotic to endoreplication cycles can be readily achieved in an inducible manner. Second, this switch is based on the manipulation of a single gene in cells that normally perform mitotic cycles. This is in contrast to cells in which endoreplication is part of the developmental program that leads to changes in expression of multiple genes.
The role of cdk1 in ensuring alternating S and M phases has been demonstrated in fission (Broek et al., 1991; Hayles et al., 1994; Wuarin et al., 2002) as well as in budding (Haase and Reed, 1999) yeast. In yeast, there is a single cdk that regulates both mitosis and replication. Budding yeast cdk1 is activated by different cyclins during different phases of the cell cycle. Metazoan cells express several different cdks, and each is required for a different phase of the cell cycle (Morgan, 1997). Cdk4 and 6 are active in G1, cdk2 in G1/S and G2, and cdk1 in mitosis. Cdk1 binds both A and B type cyclins. Cdk1-cyclin B1 is activated only at prophase upon its import into the nucleus and is inactivated shortly afterward in anaphase. Cyclin B1 has thus a very short window of activity but it is nevertheless an essential gene in mice (Brandeis et al., 1998). It is likely that the failure of mitosis in HT2-19 is largely due to the lack of cdk1-cyclin B1 activity. Cyclin A2 activates both cdk1 and cdk2. Cdk2-cyclin A2 plays multiple important roles from early S phase to the end of G2 (Furuno et al., 1999). It is inactivated by the degradation of the cyclin subunit by the APC/C. There is nothing known about the role of cdk1-cyclin A2 in mammalian cells and it remains to be seen whether any part of the phenotype of HT2-19 is due to the lack of cdk1-cyclin A2 activity. The G2 events studied herein seem, however, to take place on time so that the lack of cdk1-cyclin A2 activity does not seem to affect them.
In Drosophila, cdk1 inactivation also gives rise to endoreplication (Hayashi, 1996). Preventing mitosis by cdk1 inactivation does not interfere with the normal developmental replication schedule of the cells. Moreover, as in HT2–19 cells, Drosophila cells with inactive cdk1 grow in size and duplicate their centrosomes (Weigmann et al., 1997).
We suggest that endoreplicating HT2-19 cells pass from G2 into G1, skipping mitosis. Events typical of late G2 or prophase, like centrosome migration, and of G1, like mitotic cyclin degradation and cyclin E expression, take place on schedule. An interesting question for future studies would be how late into G2/prophase do these cells proceed. The complete migration of centrosomes shown in Figure 4B suggests that cells get very close to mitosis. On the other hand, endoreplicating HT2-19 cells do not condense their chromosomes (Itzhaki et al., 1997) or break down their nuclear lamina (Figure 6). Endoreplicating megakaryocytes, in contrast, do progress into mitosis, condense their chromosomes, and break down their nuclear lamina. They even undergo anaphase but skip cytokinesis (Nagata et al., 1997). Nuclear lamina breakdown is supposed to depend directly on cdk1 activity (Heald and McKeon, 1990; Dessev et al., 1991). Oscillation in the level of cyclins E and A have also been observed in endoreplicating rodent trophoblast cells (MacAuley et al., 1998).
It has recently been suggested that two different oscillators regulate the eukaryotic cell cycle. One oscillator is based on the APC/Cfzy, which is activated in mitosis. APC/Cfzy turns the mitotic cyclins off and enables the separation of sister chromatids by degradation of securin and Xkid. The second oscillator is based on the APC/Cfzr, which is activated when cells exit mitosis, and seems to be required for G1 (Morgan and Roberts, 2002; Wasch and Cross, 2002). APC/Cfzy and APC/Cfzr are regulated by cdk1 in opposite ways: APC/Cfzy requires cdk1 for its activation (Shteinberg et al., 1999; Kramer et al., 2000; Rudner and Murray, 2000), whereas APC/Cfzr is inhibited by it (Zachariae et al., 1998; Listovsky et al., 2000). The activation of APC/Cfzr thus normally depends on cyclin B1 degradation by APC/Cfzy (Zur and Brandeis, 2002). APC/Cfzy and APC/Cfzr can, however, also work independently. During the rapid cell cycles of early Xenopus and Drosophila embryos, only the APC/Cfzy is active (Sigrist and Lehner, 1997; Lorca et al., 1998). These cell cycles consist of rapidly alternating mitosis and S phases without gap phases. It is much less common for the APC/Cfzr to work independently. It has, however, recently been shown in budding yeast that the APC/Cfzr is capable of oscillating on its own on a suitable mutant background (Wasch and Cross, 2002). We show herein that also in mammalian cells the APC/Cfzr can be activated in the absence of APC/Cfzy. The prerequisite for independent APC/Cfzr activity in mammalian cells is the absence of cdk1 as is the case in HT2-19 cells. Cdk1 inactivation thus uncouples these two oscillators without affecting the oscillations of interphase events. Interestingly, even modest constant overexpression of fzr in mammalian cells abolishes mitosis and leads to repeated DNA replication (Sorensen et al., 2000). There is also evidence from other organisms in support of a role for the APC/Cfzr in the regulation of endoreplication cycles (Edgar and Orr-Weaver, 2001).
Acknowledgments
We are grateful to Drs. J. Gannon, T. Hunt, E. Nigg, W. Earnshaw, P. Hieter, K. Wilson, E.C. Schirmer, L. Gerace, and K. Weber for generous gifts of antibodies. This work was funded by grants from the Israel-US Binational science fund (9800123), the German Israel Foundation (658/2000), the Israeli Sciences foundation (124/98), and the Association for International Cancer Research.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–12–0850. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0850.
References
- Brandeis, M., Rosewell, I., Carrington, M., Crompton, T., Jacobs, M.A., Kirk, J., Gannon, J., and Hunt, T. (1998). Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl. Acad. Sci. USA 95, 4344–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek, D., Bartlett, R., Crawford, K., and Nurse, P. (1991). Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature 349, 388–393. [DOI] [PubMed] [Google Scholar]
- Cogswell, J.P., Godlevski, M.M., Bonham, M., Bisi, J., and Babiss, L. (1995). Upstream stimulatory factor regulates expression of the cell cycle-dependent cyclin B1 gene promoter. Mol. Cell. Biol. 15, 2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M., Lee, K.K., Wilson, K.L., and Gruenbaum, Y. (2001). Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem. Sci. 26, 41–47. [DOI] [PubMed] [Google Scholar]
- Dessev, G., Iovcheva-Dessev, C., Bischoff, J.R., Beach, D., and Goldman, R. (1991). A complex containing p34cdc2 and cyclin B phosphorylates the nuclear lamin and disassembles nuclei of clam oocytes in vitro. J. Cell Biol. 112, 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic, V., Lees, E., and SI, R. (1992). Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257, 1958–1961. [DOI] [PubMed] [Google Scholar]
- Edgar, B.A., and Orr-Weaver, T.L. (2001). Endoreplication cell cycles: more for less. Cell 105, 297–306. [DOI] [PubMed] [Google Scholar]
- Furuno, N., den Elzen, N., and Pines, J. (1999). Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 147, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer, M., Murray, A.W., and Kirschner, M.W. (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138. [DOI] [PubMed] [Google Scholar]
- Grafi, G. (1998). Cell cycle regulation of DNA replication: the endoreduplication perspective. Exp. Cell Res. 244, 372–378. [DOI] [PubMed] [Google Scholar]
- Haase, S.B., and Reed, S.I. (1999). Evidence that a free-running oscillator drives G1 events in the budding yeast cell cycle. Nature 401, 394–397. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1999). Using Antibodies, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Hartwell, L.H., Culotti, J., Pringle, J.R., and Reid, B.J. (1974). Genetic control of the cell division cycle in yeast. Science 183, 46–51. [DOI] [PubMed] [Google Scholar]
- Hayashi, S. (1996). A Cdc2 dependent checkpoint maintains diploidy in Drosophila. Development 122, 1051–1058. [DOI] [PubMed] [Google Scholar]
- Hayles, J., Fisher, D., Woollard, A., and Nurse, P. (1994). Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell 78, 813–822. [DOI] [PubMed] [Google Scholar]
- Heald, R., and McKeon, F. (1990). Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61, 579–589. [DOI] [PubMed] [Google Scholar]
- Hellman, A., Rahat, A., Scherer, S.W., Darvasi, A., Tsui, L.C., and Kerem, B. (2000). Replication delay along FRA7H, a common fragile site on human chromosome 7, leads to chromosomal instability. Mol. Cell. Biol. 20, 4420–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henglein, B., Chenivesse, X., Wang, J., Eick, D., and Brechot, C. (1994). Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc. Natl. Acad. Sci. USA 91, 5490–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki, J., Gilbert, C., and Porter, A. (1997). Construction by gene targeting in human cells of a “conditional'CDC2 mutant that rereplicates its DNA. Nat. Genet. 15, 258–265. [DOI] [PubMed] [Google Scholar]
- Kramer, E.R., Gieffers, C., Holzl, G., Hengstschlager, M., and Peters, J.M. (1998). Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr. Biol. 8, 1207–1210. [DOI] [PubMed] [Google Scholar]
- Kramer, E.R., Scheuringer, N., Podtelejnikov, A.V., Mann, M., and Peters, J.M. (2000). Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey, K.R., Jackson, P.K., and Stearns, T. (1999). Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA 96, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listovsky, T., Zor, A., Laronne, A., and Brandeis, M. (2000). Cdk1 is essential for mammalian cyclosome/APC regulation. Exp. Cell Res. 255, 184–191. [DOI] [PubMed] [Google Scholar]
- Lorca, T., Castro, A., Martinez, A., Vigneron, S., Morin, N., Sigrist, S., Lehner, C., Doree, M., and Labbe, J. (1998). Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 17, 3565–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAuley, A., Cross, J.C., and Werb, Z. (1998). Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol. Biol. Cell 9, 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, Y., Hayashi, K., and Nishida, E. (1999). Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 9, 429–432. [DOI] [PubMed] [Google Scholar]
- Meraldi, P., Lukas, J., Fry, A.M., Bartek, J., and Nigg, E.A. (1999). Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat. Cell Biol. 1, 88–93. [DOI] [PubMed] [Google Scholar]
- Moir, R.D., Yoon, M., Khuon, S., and Goldman, R.D. (2000). Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D.O. (1997). Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell. Dev. Biol. 13, 261–291. [DOI] [PubMed] [Google Scholar]
- Morgan, D.O., and Roberts, J.M. (2002). Oscillation sensation. Nature 418, 495–496. [DOI] [PubMed] [Google Scholar]
- Murray, A.W., and Kirschner, M.W. (1989). Dominoes and clocks: the union of two views of the cell cycle. Science 246, 614–621. [DOI] [PubMed] [Google Scholar]
- Nagata, Y., Muro, Y., and Todokoro, K. (1997). Thrombopoietin-induced polyploidization of bone marrow megakaryocytes is due to a unique regulatory mechanism in late mitosis. J. Cell Biol. 139, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport, J.W., and Kirschner, M.W. (1984). Regulation of the cell cycle during early Xenopus development. Cell 37, 731–742. [DOI] [PubMed] [Google Scholar]
- Rudner, A.D., and Murray, A.W. (2000). Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149, 1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteinberg, M., Protopopov, Y., Ganoth, D., Listovsky, T., Brandeis, M., and Hershko, A. (1999). Phosphorylation of the cyclosome is required for its stimulation by Fizzy/Cdc20. Biochem. Biophys. Res. Commun. 260, 193–198. [DOI] [PubMed] [Google Scholar]
- Sigrist, S.J., and Lehner, C.F. (1997). Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90, 671–681. [DOI] [PubMed] [Google Scholar]
- Sorensen, C.S., Lukas, C., Kramer, E.R., Peters, J.M., Bartek, J., and Lukas, J. (2000). Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell. Biol. 20, 7613–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, D.L., Goldman, R.D., and Leinwand, L.A. (1997). Cells: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Ward, G.E., and Kirschner, M.W. (1990). Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell 61, 561–577. [DOI] [PubMed] [Google Scholar]
- Wasch, R., and Cross, F.R. (2002). APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418, 556–562. [DOI] [PubMed] [Google Scholar]
- Weigmann, K., Cohen, S.M., and Lehner, C.F. (1997). Cell cycle progression, growth and patterning in imaginal discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development 124, 3555–3563. [DOI] [PubMed] [Google Scholar]
- Wuarin, J., Buck, V., Nurse, P., and Millar, J. (2002). Stable association of mitotic cyclin B/Cdc2 to replication origins prevents endoreduplication. Cell 111, 419–431. [DOI] [PubMed] [Google Scholar]
- Zachariae, W., Schwab, M., Nasmyth, K., and Seufert, W. (1998). Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282, 1721–1724. [DOI] [PubMed] [Google Scholar]
- Zur, A., and Brandeis, M. (2001). Securin degradation is mediated by fzy and by fzr and is required for complete chromatid separation but not for cytokinesis. EMBO J. 20, 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur, A., and Brandeis, M. (2002). Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes. EMBO J. 21, 4500–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]