Abstract
Host cell cholesterol is implicated in the entry and replication of an increasing number of intracellular microbial pathogens. Although uptake of viral particles via cholesterol-enriched caveolae is increasingly well described, the requirement of cholesterol for internalization of eukaryotic pathogens is poorly understood and is likely to be partly organism specific. We examined the role of cholesterol in active host cell invasion by the protozoan parasite Toxoplasma gondii. The parasitophorous vacuole membrane (PVM) surrounding T. gondii contains cholesterol at the time of invasion. Although cholesterol-enriched parasite apical organelles termed rhoptries discharge at the time of cell entry and contribute to PVM formation, surprisingly, rhoptry cholesterol is not necessary for this process. In contrast, host plasma membrane cholesterol is incorporated into the forming PVM during invasion, through a caveolae-independent mechanism. Unexpectedly, depleting host cell plasma membrane cholesterol blocks parasite internalization by reducing the release of rhoptry proteins that are necessary for invasion. Cholesterol back-addition into host plasma membrane reverses this inhibitory effect of depletion on parasite secretion. These data define a new mechanism by which host cholesterol specifically controls entry of an intracellular pathogen.
INTRODUCTION
A requirement for host cell cholesterol in entry and intracellular replication of microbial pathogens is a recently recognized phenomenon (Norkin, 2001; Samuel et al., 2001; Shin and Abraham, 2001a,b; Coppens et al., 2000; Charron and Sibley, 2002). Central roles for cholesterol are suggested at the attachment, penetration, and intracellular multiplication stages.
A particularly well understood example in which cholesterol plays a role is the process of pathogen uptake via caveolae. Caveolae are small, specialized invaginations of the cell surface that participate in important physiological functions such as cell surface signaling, endocytosis, and intracellular cholesterol transport (reviewed in Ikonen and Parton, 2000). Caveolae are enriched in cholesterol, glycolipids such as the glycosphingolipid GM1, glycosylphosphatidylinositol-anchored proteins, and a specific protein, caveolin. Depletion of membrane cholesterol leads to a loss of invaginated caveolae (Chang et al., 1992). Viruses that enter cells via caveolae trigger signal transduction events in the host cell via ligand binding, resulting in actin rearrangement in the host cell and pathogen uptake (Pelkmans et al., 2001; Norkin et al., 2002). The viral particle is delivered to a unique compartment, the caveosome, which does not intersect with the endocytic apparatus of the mammalian cell.
It is less clear how cholesterol in the host plasma membrane or elsewhere contributes to uptake of larger pathogens, including bacteria and protozoa. For example, host cell plasma membrane cholesterol is required for invasion of the malarial Plasmodium parasite into erythrocytes (Lauer et al., 2000); however, mature red blood cells contain neither caveolae nor any of the machinery of other uptake pathways, such as endocytosis, phagocytosis, or macropinocytosis. Nonetheless, both Plasmodium sp. and the closely related apicomplexan parasite Toxoplasma gondii, the latter of which causes infection in immunocompromised individuals (Luft et al., 1993) as well as congenital infection (Wong and Remington, 1994), are capable of actively invading specific mammalian cells (Dobrowolski and Sibley, 1996; Dobrowolski et al., 1997). These invasion processes require parasite motility, orientation toward to host cell, and sequential discharge of three secretory organelles termed micronemes, rhoptries, and dense granules (Dubremetz et al., 1993; Carruthers and Sibley, 1997).
The biogenesis, structure, and function of the T. gondii secretory organelles are increasingly well understood. Rhoptries, which contribute to the formation of the Toxoplasma and Plasmodium parasitophorous vacuole membranes (PVMs) by releasing their contents from the anterior end of the parasite during invasion (Nichols et al., 1983; Porchet-Hennere and Torpier, 1983; Carruthers and Sibley, 1997), are elongated club-shaped organelles, containing a densely packed granular material in their basal bulbous portion. T. gondii rhoptries are formed via the endocytic pathway (Hoppe et al., 2000) and are related to secretory lysosomes or exosomes (Que et al., 2002). Toxoplasma rhoptries also contain lipids, including large amounts of cholesterol and phosphatidylcholine (Foussard et al., 1991; this study). In these organelles, the cholesterol/phospholipid molar ratio (1.5/1) is too high for lipid bilayer stability, suggesting that some rhoptry cholesterol molecules may be organized in a crystalline array inside the organelle. Although it is plausible that rhoptry cholesterol is incorporated into the PVM during invasion, and that rhoptry discharge can effectively compensate for the absence of caveolae in host cells, neither supposition has been tested.
The biogenesis of the PVM surrounding Toxoplasma is only partially understood. Capacitance measurements in patch-clamped host cells indicate that at least 80% of the membrane in the nascent vacuolar membrane is host cell derived (Suss-Toby et al., 1996). This is consistent with observations showing that fluorescent tracers inserted in the host plasma membrane before infection are incorporated into the nascent PVM of Toxoplasma (Mordue et al., 1999a,b; Pacheco-Soares and de Souza, 2000) as well as into the Plasmodium PVM (Ward et al., 1993). On the other hand, multiple lines of evidence suggest that rhoptry contents contribute directly to formation of the vacuolar membrane (Aikawa et al., 1977; Nichols et al., 1983; Porchet-Hennere and Torpier, 1983; Bannister et al., 1986; Beckers et al., 1994). These apparently conflicting observations can be resolved by postulating a two-step process of invasion. Initial discharge of the rhoptry contents directly into the host cytoplasm leads to coalescence of multivesicular structures, which then fuse with the nascent vacuole that is derived primarily from the host cell plasma membrane (Hakansson et al., 2001). Whether and how cholesterol contributes to this unusual process of cell invasion by T. gondii is not clear. As just one example, although it is logical to assume that cholesterol is present in the PVM of Apicomplexa, this membrane is not readily permeabilized with the cholesterol-binding, pore-forming agent streptolysin O 24 h postinfection (Joiner, personal observation), suggesting indirectly that the PVM may be relatively poor in cholesterol. In this article, we therefore undertook a direct experimental strategy to answer the following questions, none of which have been previously approached: Is cholesterol present in the PVM at the time of invasion? Does rhoptry cholesterol, present at high concentration in the organelle, play a role at the time of invasion? Is host plasma membrane cholesterol necessary for parasite attachment to the cell surface or for parasite internalization? If host cholesterol is required, are cholesterol- and glycosphingolipid-enriched microdomains and/or caveolae, involved in Toxoplasma entry?
We have evaluated the ability of cholesterol-depleted parasites to invade normal cells, of cholesterol-depleted cells to be invaded by untreated parasites, as well as of normal parasites to invade caveolin-minus cells. Our results demonstrate that T. gondii is dependent upon host plasma membrane cholesterol to trigger organelle discharge. Neither rhoptry-derived cholesterol nor caveolae microdomains in the host cell plasma membrane, are required to complete the invasion event. These results identify a heretofore unsuspected mechanism by which cholesterol regulates microbial entry into mammalian cells.
MATERIALS AND METHODS
Reagents and Antibodies
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or Boehringer Mannheim Biochemicals (Indianapolis, IN), unless indicated otherwise. Analytical grade solvents were used for lipid analysis. Ionophore A23187 (free acid) and thapsigargin were obtained from Calbiochem (San Diego, CA). The nitrobenzoxadiazole (NBD)-cholesterol was from Molecular Probes (Eugene, OR). Radiolabeled reagents, purchased from Amersham Biosciences (Piscataway, NJ), included [5,6-3H]uracil (specific activity 45 Ci/mmol) and [1,2-3H]cholesterol (specific activity 48 Ci/mmol). Primary antibodies used in this study are rabbit polyclonal anti-GRA3 (Bermudes et al., 1994), mouse monoclonal antibodies T62H11 against GRA3 (Leriche and Dubremetz, 1991), T42F32F5 against MIC3 (Garcia-Reguet et al., 2000), T34A71C8 against ROP2,3,4 (Beckers et al., 1994), and T41E5 against SAG1 (Couvreur et al., 1988), all generously provided by Dr. J.F. Dubremetz (University of Montpellier, Montpellier, France). The mouse monoclonal antibody anti-myc was from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Lines and Culture Conditions
The cell lines used in this study are the primary human foreskin fibroblasts (HFF; ATCC CRL-1635), Chinese hamster ovary (CHO; ATCC CCL-163) cells, 3T3-Swiss albino cells (ATCC CCL-92), and the somatic 2-2 mutant cells of CHO cells kindly provided by Dr. L. Liscum (Tufts University School of Medicine, Medford, MA) (Dahl et al., 1993, 1998), which were grown as monolayer as described previously (Coppens et al., 2000). The L1210-JF cell line, wild type or transfected with the expression vector pCI-NEO-caveolin (Uittenbogaard et al., 1998) and clonally selected, was generously given by Drs. E. Smart and A. Uittenbogaard (University of Kentucky, Lexington, KY). These cell lines were grown in suspension and propagated by dilution at 37°C in an atmosphere of 5% CO2 in HEPES-buffered RPMI (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum, 2 mM l-glutamine and penicillin/streptomycin (100 U/ml per 100 μg/ml). For the caveolin-transfected L1210-JF cell line, 0.3 mg/ml geneticin was added in the culture medium.
Parasite Propagation and Purification
The RH strain tachyzoite of T. gondii was used throughout this study and was propagated in vitro by serial passages in monolayers of HFF (Roos et al., 1994). Intracellular parasites were purified by density gradient using Nycodenz and isopycnic centrifugation as detailed previously (Coppens et al., 2000). To isolate intact parasitophorous vacuoles (PVs) containing a single parasite, monolayers recently (1–2 min) infected were disrupted by scraping and passage through a 23-gauge syringe in phosphate-buffered saline (PBS) containing 137 mM NaCl, 2.7 mM KCl, 10 mM NaH2PO4, 1.8 mM KH2PO4 adjusted to pH 7.4, before their purification as described above. Parasite concentration, replication, and viability were routinely determined for all studies using standard methods (Coppens et al., 2000).
Light and Electron Microscopy
Light and epifluorescence microscopy were performed on infected cells seeded on sterile coverslips in 24-well culture dishes using a Microphot FXA microscope (Nikon, Melville, NY). Images were captured on a Photometrics SenSys charge coupled-device camera, processed using Image-ProPlus software (Media Cybernetics, Silver Spring, MD), and scanned (ScanJet Iicx; Hewlett Packard, Palo Alto, CA) into Adobe Photoshop software (Adobe Systems, Mountain View, CA). For thin-section transmission electron microscopy (TEM), cells were fixed in 2.5% glutaraldehyde (Electron Microscopy Sciences) in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at room temperature. They were washed three times in 0.1 M cacodylate buffer and then postfixed for 1 h in 1% osmium tetroxide (Electron Microscopy Sciences, Gibbstown, NY) in the same buffer at room temperature. After three washes in water the samples were stained for 1 h at room temperature in 2% uranyl acetate (Electron Microscopy Sciences), and then washed again in water and dehydrated in a graded series of ethanol. The samples were then embedded in Embed-812 epoxy resin (Electron Microscopy Sciences). Ultrathin (50–60-nm) sections were cut using an Ultracut ultramicrotome (Reichart-Jung, NuBlock, Germany) and collected on formvar- and carbon-coated nickel grids, stained with 2% uranyl acetate and lead citrate before examination with a 410 electron microscope (Philips, Eindhoven, The Netherlands) under 80 kV.
Filipin Labeling and Visualization
Filipin (10 mg/ml in dimethyl sulfoxide) was stored at –20°C. For cytochemical staining of β-hydroxysterols with filipin, infected cells seeded on coverslips were fixed in 3% paraformaldehyde and then incubated with 25 μg/ml filipin for 30 min, before being viewed by UV-fluorescence microscopy as described previously (Coppens et al., 2000). For cofluorescence microscopy of parasite proteins with antibodies and filipin, infected cells on coverslips were rinsed with PBS and quenched with PBS containing 50 mM NaH4Cl for 15 min. Coverslips were rinsed with PBS, blocked for 60 min with PBS containing 10% of goat serum and 50 μg/ml filipin, and then incubated with primary antibodies at 1:100 for anti-SAG1 and 1:50 for anti-ROP2,3,4 in the blocking buffer containing filipin. After washing, coverslips were incubated with mouse fluorescein isothiocyanate (FITC)-conjugated goat secondary antibodies (Calbiochem) in the same buffer, washed and mounted onto slides with moviol. For ultrastructural localization of β-hydroxysterols with filipin, samples were first briefly fixed with glutaraldehyde for 10 min before the addition of 0.4 mg/ml filipin to the fixative solution for 60 min at room temperature. Samples are then processed for the electron microscopy observation.
Cholesterol Incorporation in Host Cell Plasma Membrane
To load cholesterol on methyl-β-cyclodextrin (MβCD), cholesterol resuspended in ethanol was dried under nitrogen and subsequently dissolved in buffer A (150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, pH 7.4) containing MβCD in a molar ratio of MβCD/cholesterol of 10:1. The resulting suspension was sonicated until clarification, incubated overnight at 37°C, and centrifuged to eliminate insoluble aggregates, as described previously (Sheets et al., 1999). Host cells were incubated with [3H]cholesterol– or NBD-cholesterol–MβCD complexes at the final concentration from 0.1 to 5 mM for 30 min at 4°C in buffer 1 before infection and PV isolation.
Cholesterol Depletion Treatments
Cellular cholesterol content was monitored by the intensity of fluorescence emission from filipin at 325–510 nm after incubation of cells with 0.1 mg/ml filipin for 60 min. Cholesterol levels in mammalian cells were reduced by incubation of cells for 60 h in 4 μM lovastatin and 250 μM mevalonate, followed by 10 mM MβCD for 45 min in serum-free medium in the continuous presence of lovastatin and mevalonate (Gatfield and Pieters, 2000). Alternatively, acute host cell cholesterol depletion was performed by treatment with 10 mM MβCD for 45 min in serum-free medium (Subtil et al., 1999). Toxoplasma cholesterol levels were only decreased by MβCD treatment in the conditions described for acute cholesterol depletion. Control treatments contained medium alone or 10 mM MβCD complexed to cholesterol. Alternatively, depletion of parasite cholesterol levels was obtained after incubation of intravacuolar parasites in the 2-2 mutant cells or in cells treated with progesterone at 10 μg/ml for 24 h, as described previously (Coppens et al., 2000). Cholesterol depletion on mammalian cells or extracellular parasites was monitored by thin layer chromatography (TLC) as reported previously (Yancey et al., 1996) and UV-fluorescence microscopy by using filipin. Cholesterol back-addition experiments were realized as reported previously (Falguieres et al., 2001).
Quantitative Analysis of Lipid Content in Parasites
Enriched rhoptry was prepared from purified parasite preparations as described previously (Leriche and Dubremetz, 1991; Que et al., 2002). Purified cholesterol and total phospholipids associated with the parasite fractions were assayed by TLC as reported by Coppens et al. (1995).
Immunofluorescence Microscopy Analysis
HFF monolayers grown on coverslips were infected with T. gondii, and coverslips were prepared for immunofluorescence as described previously (Karsten et al., 1998). Differential immunofluorescence staining was used to analyze parasite attachment or internalization in host mammalian cells. Freshly lysed-out parasites resuspended in culture medium were added to monolayers grown on coverslips to achieve a final parasite/cell ratio of 10:1. The coverslips were incubated for 60 min at 37°C in 5% CO2, and then extensively washed with cold medium at 4°C to remove unattached parasites. Cells were fixed with absolute methanol for 10 min, blocked with PBS containing 10% goat serum and 0.01 mg/ml saponin, incubated with rabbit anti-GRA3 diluted at 1:500 in the same buffer, and then with secondary FITC-conjugated goat anti-rabbit IgG. Coverslips were sequentially incubated with mouse anti-SAG1 diluted at 1:500 in the blocking buffer, and then with the secondary rhodamin-conjugated goat anti-mouse IgG before their observation by epifluorescence microscopy. The total number of parasites is estimated by their surface red staining. The discrimination between extracellular and intracellular Toxoplasma is based on the FITC pattern associated with GRA3, i.e., largely dispersed in the vacuolar space and delimitating the PVM (Bermudes et al., 1994) for intracellular parasites and strictly secluded into dense granules for extracellular parasites. Experiments were repeated three times independently, with a population of at least 300 cells counted on each coverslip. To identify microneme or rhoptry proteins discharged at the interface between Toxoplasma and host cells, infected cells were semipermeabilized as described previously (Carruthers et al., 1999) before immunolabeling with anti-MIC3 or anti-ROP2,3,4 antibodies diluted at 1:100.
Transient Caveolin Expression
3T3 cells were transfected with a mammalian expression vector pcDNA3Myc-caveolin (Liu et al., 1999) kindly provided by Dr. R. Anderson and Dr. P. Liu (University of Texas Southwestern Medical Center, Dallas, TX). Transfections were performed using the Fu-GENE transfection reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. After transfection, cells were plated on coverslips and cultured for an additional 18–24 h before being challenged with parasites. Cells were then fixed, permeabilized with Triton X-100 at 0.03%, stained with mouse anti-Myc to reveal the transfected gene product and rabbit anti-GRA3 to label the PVM before examination by fluorescence microscopy.
Protein Determination
Protein content was determined by the bicinchoninic acid assay (Smith et al., 1985), using serum albumin as a standard.
Statistical Analysis
For comparison of means, P was determined by analysis of variance against control (ANOVA 2).
RESULTS
Cholesterol Is Present in the Parasitophorous Vacuole Membrane at the Time of Invasion
We wanted to provide fine details on sterol distribution in T. gondii-infected cells. Ultrathin sections of infected cells were incubated in the presence of the polyene antibiotic filipin during fixation and then visualized by TEM. Sterol-containing membranes treated with filipin show a wavelike appearance, in cross sections, as a result of planar filipin–sterol complexes in the hydrophobic core of a bilayer. As seen on purified preparations of Toxoplasma vacuoles isolated 1–2 min postinfection, the PVM showed a regularly corrugated aspect after reaction with filipin (Figure 1, A and B). Fluorescence microscopy with filipin as a fluorescent probe for cholesterol also revealed labeling of the PVM. This labeling was maximal at the time of invasion and progressively decreased as parasite replication proceeded (Figure 1C). Although the decrease in filipin staining of the PVM over time has several potential explanations (see below), the data clearly indicate that the newly formed vacuole contains substantial levels of cholesterol. We therefore sought to determine the source of this cholesterol and the mechanism for its delivery to the PVM.
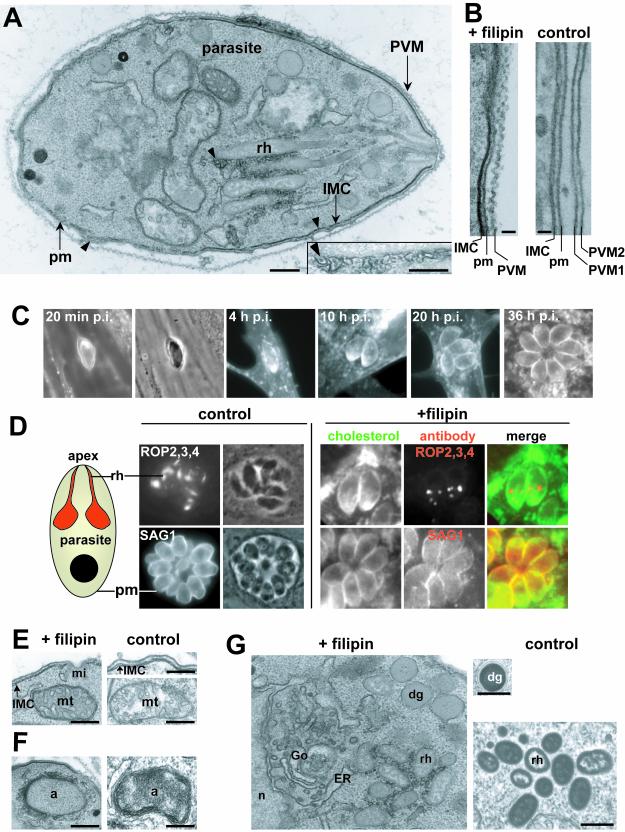
Figure 1.
Fine distribution of cholesterol in T. gondii and the PVM. (A and B) TEM of T. gondii tachyzoite isolated from fibroblast 1–2 min postinfection but surrounded by its PVM. Fixation performed in the presence of filipin for an ultrastructural cholesterol detection reveals a continued labeling of the PVM, rhoptries (rh), plasma membrane (pm), and inner membrane complex (IMC). Arrowheads pinpoint the filipin-labeled areas. Inset shows a magnification of the rhoptry posterior part. Bars, 0.2 μm. (B) Thin sections of parasites treated with filipin, showing the wavelike appearance of the PVM or untreated parasites as control where two PVM from adjacent parasites are visible. Bars, 50 nm. (C) Fluorescence microscopy of filipin on intravacuolar parasites showing the pattern of filipin staining of the PVM during parasite development. At 20 min postinfection, a clear filipin labeling of the PVM is visible (compare the fluorescence profile with the phase contrast image, where the vacuolar space and corresponding PVM are clearly delineated). The parasite plasma membrane was also labeled with filipin (Coppens et al., 2000). At 4, 10, 20, and 36 h postinfection, no labeling of the PVM was observed at any level of focusing. (D) Fluorescence microscopy of ROP2,3,4, SAG1, and filipin. Infected fibroblasts were staining for either rhoptries by using anti-ROP2,3,4 antibodies or plasma membrane by using anti-SAG1 antibodies, and cholesterol by using filipin. The control conditions illustrate immunolabeled parasites after Triton X-100 permeabilization. The filipin conditions represent immunolabeled parasites after filipin permeabilization, in addition to a merge representation with cholesterol staining. (E–G) TEM of intracellular organelles of T. gondii fixed in the presence of filipin or not (control). Filipin staining of IMC (E), apicoplast (F, a), rhoptries (G, rh), and dense granules (G, dg). Absence of filipin labeling of mitochondria (E, mt), micropore (E, mi), nucleus (G, n), and Golgi (G, Go) showing a smooth membrane appearance. Bars, 0.2 μm.
Cholesterol Is Highly Concentrated in Rhoptries and Is Heterogeneously Distributed between Parasite Organelles
Rhoptries are cholesterol enriched (Foussard et al., 1991) and could contribute cholesterol to the nascent PVM. Nonetheless, the distribution and organization of cholesterol in these organelles is unknown. Longitudinal electron microscopic sections of the apical secretory rhoptries show a strong membrane filipin labeling (Figure 1A), mainly at the wider posterior part of these organelles (see detail in Figure 1A, inset). No filipin labeling is visible within rhoptries. This suggests that rhoptries contain no luminal store of membrane-derived cholesterol such as found in mammalian “lamellar bodies” (reviewed in Schmitz and Muller, 1991). The association of sterols with the basal bulbous portions of rhoptries was confirmed by immunofluorescence on preparations permeabilized by filipin. In contrast, preparations permeabilized by Triton X-100 show a labeling of the whole rhoptry organelle (Figure 1D). Only organelles, or portions, with sterolenriched membranes are accessible to antibodies, because sterol–filipin complexes lead to gross membrane fragmentation and permeabilization. The immunolabeling of the basal part of rhoptries permeabilized by filipin (Figure 1D) likely corresponds to the cholesterol-enriched regions observed by electron microscopy (Figure 1A).
Filipin labeling of other membranes was less homogeneous. Although filipin colocalized with the plasma membrane marker SAG1 (Figure 1D), heterogeneity of labeling suggested the presence of specialized surface microdomains with different protein/lipid ratios (Figure 1, A and E). This is commonly observed in mammalian cells or other protozoa (Karnovsky et al., 1982; Ginsbach and Fahimi, 1987; Yoshikawa et al., 1992; Weiss Bizzoco and Reyes, 1992). Similarly, nonhomogeneous staining of the inner membrane complex (a pair of closely apposed membranes forming a discontinuous set of sheets beneath the outer plasma membrane) was observed (Figure 1E). This is consistent with previous data (Cintra and de Souza, 1985), although no staining of the cup-shape invaginated micropore was detected (Figure 1E). Filipin also labeled membranes of the apicoplast (a DNA-containing organelle capable of some lipid biosynthetic activities) (Jelenska et al., 2001) (Figure 1F) and membranes of secretory dense granules (Figure 1G). No prominent filipin alterations were seen on the membranes of mitochondrion (Figure 1E), nucleus, endoplasmic reticulum, and Golgi (Figure 1G). This is consistent with observations in mammalian cells (Korn, 1969; McGookey et al., 1983; Ginsbach and Fahimi, 1987).
Parasite Rhoptries Can Be Depleted of Cholesterol
To test the contribution of rhoptry cholesterol to PVM formation, we next explored mechanisms to deplete parasite rhoptries of cholesterol. We have previously shown that cholesterol is delivered to intracellular parasites via the endocytic pathway of the host cell (Coppens et al., 2000). At the light microscopy level, a dramatic reduction of parasite cholesterol content, including that of rhoptries, can be obtained by incubating parasites in host cells with defective mobilization of cholesterol from lysosomes. These cells include for example the Niemann-Pick type C (NPC) fibroblasts (Liscum et al., 1989) or normal fibroblasts treated with progesterone (Butler et al., 1992; Liscum, 2000). This was pursued further, because progesterone, by an unknown mechanism, has been described to be able to mobilize cholesterol from other sterol pools, such as lipid droplets or organelle membranes (McGookey and Anderson, 1983; Butler et al., 1992). Fluorescent microscopic examination of infected or uninfected fibroblasts cultured 24 h with progesterone revealed extensive filipin staining of perinuclear lysosomes, compared with normal cells (Figure 2, A and B; Blanchette-Mackie, 2000). TEM illustrated cholesterol-laden lysosomes consisting of multilamellar inclusions formed by accumulation of cholesterol-enriched lipid bilayers, clearly visible after filipin reaction (Figure 2C).
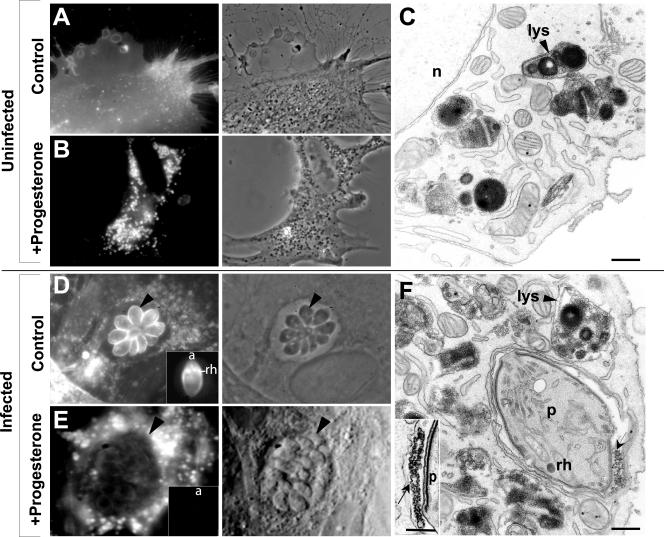
Figure 2.
Morphological detection of cholesterol in intravacuolar Toxoplasma in progesterone-treated fibroblasts. (A and B) Fluorescence microscopy of filipin on uninfected HFFs (A) and uninfected fibroblasts incubated in medium containing 10% fetal bovine serum and 10 μg/ml progesterone for 24 h and showing an extensive filipin labeling of perinuclear lysosomes (B). (C) TEM of progesterone-treated fibroblasts illustrating scalloped deformations of core lamellae and outer membranes of enlarged lysosomes (lys, arrowheads). n, nucleus. (D and E) Fluorescence microscopy of filipin on parasites grown during 24 h in control fibroblasts (D, arrowheads showing fluorescent parasites) or in progesterone-treated fibroblasts (E, arrowheads showing a weak filipin labeling of parasites). Parasites isolated from both cells are illustrated at the insets in D and E. The rhoptry (rh) labeling at the anterior region (a) of the parasites clearly visible in controls (D), is absent in parasites cultivated in the presence of progesterone (E). (F) TEM of fibroblasts infected by Toxoplasma for 6 h and then treated with progesterone for 24 h, showing a weak filipin labeling of the parasite (p) and its vacuole, except intravacuolar structures (arrows). Lysosomes, lys (arrowhead). Bars, 0.3 μm.
Parasites cultivated for 24 h in progesterone-treated cells showed a very weak filipin labeling in their membranes and apical rhoptries compared with untreated parasites (Figure 2, D and E; see insets of isolated parasites in both conditions). Ultrastructural studies of Toxoplasma in progesterone-treated cells confirmed an absence of filipin reactivity on parasites (Figures 2F and 4B). Abnormal sterol-containing structures or deposits reacting with filipin were apparent in the vacuolar space (Figures 2F, arrows; and 3B).
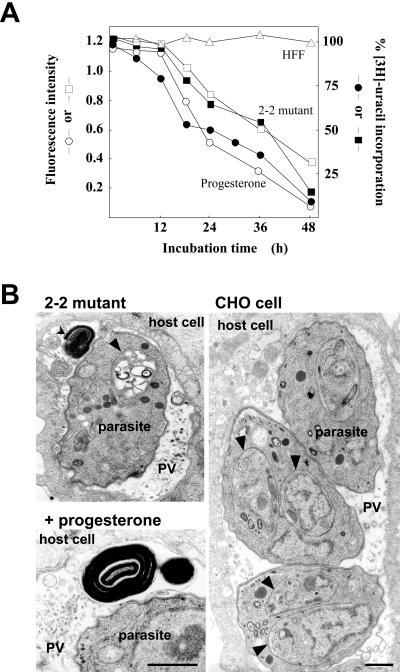
Figure 3.
Reduction of Toxoplasma cholesterol content and parasite replication in the 2-2 mutant or in progesterone-treated HFF. (A) Kinetics of fluorescence intensity associated with extracellular parasites isolated from HFF (open triangles), the 2-2 mutant cells (open squares), or progesterone-treated HFF (open circles). After purification, parasites were adjusted to a density of 107/ml, incubated for 1 h with 0.1 mg/ml filipin, washed, and analyzed by fluorometry. Kinetics of uracil incorporation was assayed on intravacuolar parasites incubated in the 2-2 mutant cells (solid squares) or progesterone-treated fibroblasts (solid circles). Data in percentage are expressed relative to control (CHO cells for the 2-2 mutant cells or untreated HFF for progesterone-treated HFF), taken as 100%. The study was done in triplicate with <15% variation. (B) TEM of intravacuolar parasites incubated in the 2-2 mutant cells, progesterone-treated HFF or CHO cells. Arrowheads pinpoint new daughters in formation within the cytoplasm of the mother, normal in appearance for parasites grown in CHO cells, whereas lethal for parasites in the 2-2 mutant cells. Arrows indicate abnormal multilamellar bodies within the PV of parasites cultivated in the 2-2 mutant cells or in progesterone-treated HFF. Bars, 0.5 μm.
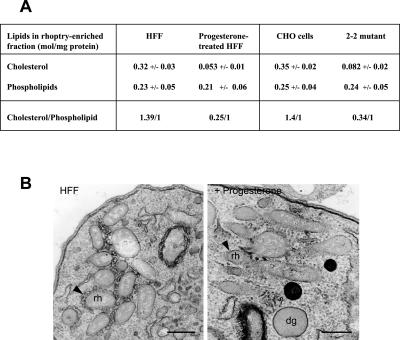
Figure 4.
Depletion in rhoptry cholesterol in Toxoplasma. (A) Phospholipid and cholesterol composition of rhoptry-enriched fractions from parasites incubated in HFF (control), progesterone-treated HFF or 2-2 mutant cells was analyzed by TLC. Data are expressed in moles of lipid per milligram of cell protein are the means ± SE of three different rhoptry preparations. (B) TEM of extracellular T. gondii fixed in the presence of filipin. Arrowheads delineate the rhoptry (rh) membrane, corrugated in parasites cultivated in untreated HFF (control), whereas smooth in parasites incubated in progesterone-treated HFF. An absence of filipin labeling on the dense granule (dg) membrane is also observed. Bars, 0.4 μm.
To quantify parasite cholesterol content, parasites were isolated from cells, labeled with filipin (Figure 2, D and E, arrows) and analyzed by fluorometry. Parasites cultivated in progesterone-treated cells or in the CHO somatic 2-2 mutant cells expressing a NPC phenotype (Dahl et al., 1992, 1993) showed a time-dependent decrease of fluorescence (Figure 3A). This curve reflects the parasite cholesterol content and parallels parasite replication as monitored by specific radioactive uracil incorporation into parasite nucleic acids (Figure 3A). Values of cholesterol content in parasites isolated from untreated fibroblasts or CHO cells are constant during the parasite development at 6–48 h postinfection, as monitored by fluorometry.
The impairment of parasite growth in progesterone-treated cells or in the 2-2 mutant cells was examined by TEM (Figure 3B). Parasites cultivated in normal cells divide by endodyogeny, which is a specialized form of reproduction packaging two newly formed progenies (see arrowheads in control CHO cell at Figure 3B) within the cytoplasm of the mother. Parasites incubated in the 2-2 mutant cells or in the presence of progesterone showed an abnormal process of parasite division. Vacuolized and misshapen progenies were observed within the mother (see arrowhead in 2-2 mutant cell at Figure 3B). This phenomenon is likely correlated to an unavailability of lysosome-sequestered cholesterol for new parasites in formation. In addition, electron-dense multilamellar structures accumulated in the vacuolar space, suggestive of lipid disorders (Figure 3B, arrows).
Quantitative analyses of lipids were performed on rhoptry-enriched fractions from parasites isolated from either progesterone-treated cells or 2-2 mutant cells. Twenty-four hours postinfection resulted in a dramatic reduction of cholesterol of 83 and 77%, respectively, compared with controls (Figure 4A). The total phospholipid content was unchanged. Hence, the cholesterol/phospholipid molar ratio was decreased four- to fivefold in these cholesterol-depleted parasites. These biochemical data have been confirmed at the ultrastructural level, showing minimal or no filipin labeling of rhoptries in treated compared with control rhoptries (Figure 4B).
Parasites Depleted of Rhoptry Cholesterol Are Not Impaired in Invasion of Host Cells
A global population of parasites depleted of rhoptry cholesterol by growth in progesterone-treated cells or 2-2 mutant cells were tested for the capacity to invade mammalian cells. For the same total number of parasites associated with at least 300 cells, the number of parasites internalized per cell was not significantly different from that for parasites isolated from normal CHO cells (Figure 5A) or HFF (our unpublished data). This suggests that rhoptry cholesterol is not required for efficient invasion of host cells.
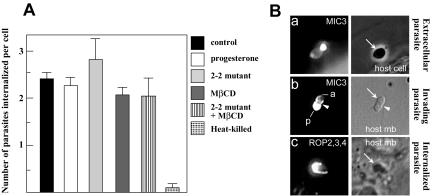
Figure 5.
Host cell invasion by cholesterol-depleted Toxoplasma. (A) Invasion assays with parasites isolated from HFF (control), progesterone-treated cells (progesterone), 2-2 mutant cells, with parasites treated for 45 min with 10 mM MβCD (MβCD), with MβCD-treated parasites isolated from 2-2 mutant cells (2–2 mutant + MβCD), with parasites incubated at 60°C for 15 min (heat killed). Intracellular parasites were quantified by immunofluorescence microscopy. Data are expressed as the mean number of intracellular parasites per cell ± SE for three different assays. (B) Immunofluorescence assay performed under conditions of semipermeabilization by using anti-MIC3 antibodies, showing micronemal secretion (a and b) by MβCD-treated parasites (representative of a population of 150 parasites). Initially secreted at the anterior pole (indicated by a), MIC3 is progressively recapped behind the moving junction (arrowheads) toward the posterior end of the parasite (indicated by p). Anti-ROP2,3,4 antibodies have been used to analyze the rhoptry secretion (c). Arrows localize the parasites.
Given the importance of this result, an alternative approach was taken to investigate the role of rhoptry cholesterol in host cell invasion. Extracellular parasites were exposed to cyclic oligosaccharides, the MβCDs. At high concentration, MβCD extracts cholesterol molecules from a membrane surface and the lipid is directly trapped in the internal hydrophobic cavity of an MβCD molecule (Yancey et al., 1996). Cholesterol depletion with MβCD at 10 mM for 45 min decreased by ∼80–90% the total cholesterol levels in Toxoplasma compared with parasites incubated with MβCD-cholesterol complexes (positive control), as determined by TLC or fluorometry. Quantitative analyses of cholesterol content on rhoptry-enriched fractions from parasites treated with MβCD revealed a reduction of cholesterol by ∼85% compared with control. As seen in Figure 5A, MβCD-treated parasites were capable of invading host cells as efficiently as control organisms. As a negative control, heat-inactivated parasites showed no invasion.
Parasite Organelles Are Discharged in Cholesterol-depleted Parasites
The above-mentioned results suggest that the correct sequence of parasite organelle discharge needed for invasion is preserved in cholesterol-depleted parasites. This was directly tested. We used an assay that exclusively detects proteins released at the host/parasite interface and inside the still open PV (Carruthers et al., 1999). MβCD-treated parasites were competent to discharge the content of their micronemes (Figure 5B, a and b) and rhoptries (Figure 5B, c). Dissection of the process of microneme and rhoptry discharge by cholesterol-depleted parasites revealed no defect compared with control parasites. Indeed, after secretion (Figure 5B, a), micronemal proteins such as the protein MIC3 were rapidly redistributed onto the apical end of the parasite at the attachment site. These proteins progressively recapped behind a “moving junction,” a region of intimate membrane apposition between the host and the parasite (Aikawa et al., 1977). After translocation to the posterior protruding extremity of the parasite (Figure 5B, b), micronemal proteins were finally released from the parasites. Subsequently, rhoptries discharged their proteins such as ROP2,3,4 shown herein (Figure 5B, c) from the anterior end of the parasite into the newly formed PV. Concomitantly, the formation of the PVM was initiated. Quantitative analysis revealed no significant difference in the number of surface microneme-positive parasites and of rhoptry-positive vacuoles after incubation with MβCD or MβCD–cholesterol complexes.
Together, our results demonstrate that parasite organelle discharge and host cell invasion is not dependent on parasite cholesterol.
Host Cell Plasma Membrane Cholesterol Is the Main Source of Cholesterol within the Vacuolar Membrane at the Time of Invasion
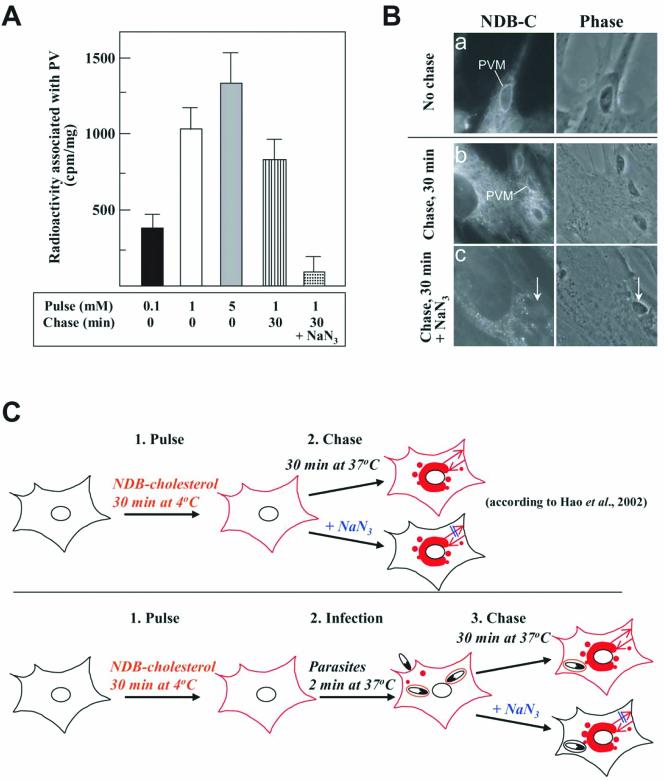
Because parasite cholesterol is not required for invasion, we therefore explored the alternative possibility that host plasma membrane cholesterol may be the main source of sterol within the nascent vacuole. Fibroblasts were pulse-labeled with different concentrations of [3H]cholesterol]-loaded MβCD for 30 min at 4°C. This allows the incorporation of radioactive cholesterol into the plasma membrane of the host cell, but blocks its delivery to intracellular compartments (Hao et al., 2002). After the pulse labeling, cells were infected with Toxoplasma at 37°C for 2 min. Toxoplasma-containing vacuoles were then isolated from host cells and counted for radioactivity. Figure 6A shows radioactivity incorporated in the PV, which is proportional with the concentrations of [3H]cholesterol–MβCD complexes to cells.
Figure 6.
Cholesterol distribution from the host plasma membrane to nascent Toxoplasma-containing vacuoles. (A) HFF were pulse labeled with 0.1–5 mM [3H]cholesterol-loaded MβCD for 30 min at 4°C in buffer A, washed to remove free complexes, and incubated in buffer A with 108 parasites/ml for 2 min at 37°C. After washing to discard extracellular parasites, HFFs were lysed, and the PV was isolated for radioactivity determination by liquid scintillation counting. In another set of experiments, labeled cells were chased for 30 min at 37°C in buffer A containing 5 mM of unlabeled cholesterol-loaded MβCD to promote exchange of radioactive cholesterol for cholesterol at the plasma membrane, and with energy poisons (15 μM NaN3 and 15 μM 2-deoxyglucose) to induce the sequestration of cholesterol in cell interior. Chase medium in control experiments is buffer A containing 5 mM unlabeled cholesterol-loaded MβCD. After the chase, cells were incubated with 108 parasites/ml for 2 min at 37°C in buffer A containing 0.2% glucose. After washing, HFFs were lysed before PV isolation. Data, expressed in counts per minute per milligram of cell protein, are the means ± SE for three different assays. Differences between values of control and energy-depleted cells chased for 30 min are statistically significant (p < 0.005). (B) HFFs on coverslips were labeled with 1 mM NDB-cholesterol–loaded MβCD for 30 min at 4°C, washed to remove free complexes, and incubated with 108 parasites/ml for 2 min at 37°C. After washing, cells were observed by fluorescence microscope to visualize NDB-cholesterol on the PVM. For chase experiments, labeled cells were treated as described in A before infection with parasites (arrows) for 2 min at 37°C and microscopy observation. (C) Schematic representations of the pulse-chase experiments and fluorescence pattern (in red) associated with cellular compartments or the PVM.
A 30 min-chase after the infection resulted in an association of radioactivity with the isolated Toxoplasma-containing vacuoles (Figure 6A). The next step was to determine whether cholesterol within the newly formed vacuole was primarily derived from the plasma membrane of the cell, and/or was also delivered to the vacuole from an intracellular source. As previously shown for mammalian cells, by warming cells incubated with [3H]cholesterol-loaded MβCD for 30 min-chase at 37°C (Hao et al., 2002), cholesterol is delivered from the plasma membrane to juxtaperinuclear organelles (including Golgi and endocytic recycling compartments). From this location, it recycles back to the cell surface. This sterol return to the plasma membrane follows a conventional, tubulovesicular membrane-recycling pathway, which is energy-dependent (Figure 6C, scheme). We exploited these features on Toxoplasma-infected cells as follows: after a 30-min pulse with [3H]cholesterol]-loaded MβCD at 4°C, fibroblasts were chased during 30 min at 37°C in the presence of energy poisons (to trap labeled cholesterol in an intracellular location) and unlabeled cholesterol-loaded MβCD (to supply unlabeled cholesterol at the plasma membrane). After washing to remove the energy poisons, labeled cells were infected with Toxoplasma at 37°C for 2 min, and the Toxoplasma-containing vacuoles were then isolated from host cells and counted for radioactivity. PV isolated from the energy-depleted cells contained no radioactive cholesterol (Figure 6A) compared with nonenergy-depleted cells. Quantitative immunofluorescence assays revealed the same number of PV formed in energy-depleted cells and control cells. This indicates that the source of cholesterol in the nascent PVM is mainly host plasma membrane cholesterol and that intracellular pools of cholesterol are not mobilized for PVM formation.
To visualize the incorporation of host plasma membrane cholesterol into the PVM at the time of invasion, NBD-cholesterol loaded on MβCD was used to label the host plasma membrane. After a 30-min pulse with high concentration of fluorescent cholesterol-loaded MβCD (to increase the sensitivity of the labeling) at 4°C (to avoid an intracellular diffusion of the probe), cells were infected with parasites for 2 min at 37°C and observed by fluorescence microscopy. Cholesterol was concentrated at the PVM (Figure 6B, a), which correlates with our biochemical data (Figure 6A). The intensity of PV staining with NBD-cholesterol decreased over time. This may result from dilution of the label, PVM extension or possibly transfer to the parasite or host cell (our unpublished data). After a 30-min chase under conditions of energy depletion, the newly formed PVM was unlabeled (Figure 6B, c), due to the retention of labeled cholesterol inside cell compartments. This result must be directly compared with control cells pulse chased in the same conditions but without energy poisons (Figure 6B, b). Results of fluorescence cholesterol pattern are summarized at Figure 6C.
Host Plasma Membrane Cholesterol Is Necessary for Parasite Invasion
We next analyzed whether host plasma membrane cholesterol is necessary for Toxoplasma internalization. Cellular cholesterol levels were reduced by pharmacological inhibition of cholesterol biosynthesis by using lovastatin, an inhibitor of the 3-hydroxy-3-methylglutaryl CoA reductase. This was followed by extraction of residual cholesterol from the plasma membrane by MβCD. These conditions led to a ∼95% specific decrease in total cholesterol levels as determined by TLC, compared with cells incubated with MβCD complexed to cholesterol. Because cholesterol is involved in the regulation of fluidity, cholesterol depletion treatment may cause a general malfunctioning of the plasma membrane. The internalization of 3-μm latex beads was therefore followed under conditions of cholesterol depletion. The counting of fluorescent beads internalized per CHO cells revealed no difference compared with nondepleted cells (our unpublished data). Indeed, this protocol of cholesterol extraction seems to have no deleterious effect on host phagocytosis, membrane ruffling, cell locomotion or protein synthesis, as demonstrated previously (Gatfield and Pieters, 2000).
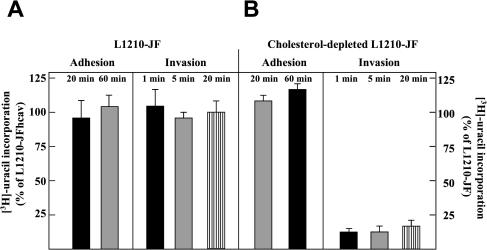
Cholesterol-depleted fibroblasts were infected with Toxoplasma and the degree of invasion was analyzed after 1 or 5 min of parasite contact with host cells. Plasma membrane cholesterol was depleted either by incubation with lovastatin/mevalonate plus MβCD (Figure 7A) or under acute treatment with MβCD alone (our unpublished data). Under both conditions, there was a drastic reduction of ∼90% of the uracil incorporation values in both cholesterol-depleted HFF and cholesterol-depleted CHO cells (our unpublished data), compared with untreated cells. Invasion assays performed during a 60-min contact of depleted cells and parasites did not increase the values of uracil incorporation (our unpublished data).
Figure 7.
Effect of host cholesterol depletion on Toxoplasma attachment and internalization. (A) Uracil incorporation assayed on control HFFs or cholesterol-depleted HFFs after incubation for 60 h in 4 μM lovastatin/250 μM mevalonate followed by treatment for 45 min 10 mM MβCD in the presence of lovastatin/mevalonate, before a 1- or 5-min infection with Toxoplasma. Data expressed in counts per minute per milligram of cell protein are the means ± SE of four different assays. Differences between values of control and depleted cells are statistically significant (p < 0.005). (B) Cell binding versus cell invasion assays. Parasites were exposed to control and cholesterol-depleted cells for 60 min, washed, and quantified after double immunofluorescence microscopy by using anti-SAG1 antibodies to label the parasite plasma membrane (in red) and anti-GRA3 antibodies to label the PV (fully internalized parasites) or the dense granules within the parasites (extracellular parasites). Data are expressed as the mean number of parasites associated per cell ± SE from three different assays. Differences between values of intracellular parasites in control versus depleted cells are statistically significant (p < 0.005).
We then discriminated between a role for cholesterol in parasite binding versus a role in internalization. The staining of the dense granule GRA3 protein was used to identify extracellular parasites (labeling concentrated in parasite dense granules) and intracellular parasites (labeling dispersed in the PV). For a same total number of parasites associated with at least 300 cells, parasite binding was not altered by cholesterol depletion, but parasite invasion was markedly impaired (Figure 7B).
Parasite Organelle Discharge Is Impaired When Parasites Bind to Cholesterol-depleted Cells
Micronemal secretion is a prerequisite for Toxoplasma attachment to mammalian cells apical orientation. Rhoptry discharge is involved in PVM formation. Because parasite binding but no internalization occurred under conditions of host cell cholesterol depletion, we wanted to explore the secretory capability of Toxoplasma after parasite binding to cholesterol-depleted cells. Surprisingly, quantitative analysis of micronemal secretion revealed that the percentage of parasites able to secrete MIC3 at the host/parasite interface (surface MIC3-positive parasites) was reduced fourfold after parasite exposure to cholesterol-depleted cells (Table 1). Although we cannot assert that parasite attachment and orientation to host cell cholesterol depletion are normal, this result may suggest the participation of parasite binding ligands other than micronemal proteins. This partial shut-off in organelle secretion was reversible. Parasites exposed to cholesterol-depleted cells and then mechanically dissociated from host cells before control with normal fibroblasts regained their ability to secrete their microneme contents and invade normally (our unpublished data).
Table 1.
Quantitative analysis of microneme or rhoptry proteins discharged at the interface between Toxoplasma and cholesterol-depleted cells
| Control | Cholesterol-depleted cells | Cholesterol-repleted cells | |
|---|---|---|---|
| MIC3-positive parasites | 78 ± 16 | 20 ± 3a | 67 ± 11 |
| 82 ± 12 (+ A23187) | 24 ± 11a | ||
| 84 ± 15 (+ thapsigargin) | 17 ± 9a | ||
| ROP2,3,4-positive vacuoles | 87 ± 10 | 4 ± 3b | 79 ± 15 |
After acute cholesterol depletion of the host cells, parasites were exposed to the depleted cells for 2 min or 7 min to follow their release of micronemal or rhoptry proteins, respectively. Infected monolayers were semipermeabilized before immunolabeling with anti-MIC3 or anti-ROP2,3,4 antibodies as described in MATERIAL AND METHODS. The discharge of microneme was also analyzed in the presence of 125 nM A23187 or 1 μM thapsigargin added to the monolayers for 30 sec before infection. Cholesterol add-back experiments were obtained by incubation of cholesterol-depleted cells with 0.3 mM cholesterol-saturated MβCD for 30 min before infection. Values for microneme-positive parasites and rhoptry-positive vacuoles are means (± SE) expressed in percent of three or four separate immunofluorescence assays. Differences between values of parasite secretion in cholesterol-depleted cells versus control cells are statistically significant.
p < 0.01.
p < 0.001.
Microneme release is calcium dependent and is stimulated using the calcium ionophore A23187 or thapsigargin, an inhibitor of the endoplasmic reticulum Ca2+-ATPase (Carruthers and Sibley, 1999). However, treatments of parasites with A23187 or thapsigargin did not result in stimulation of microneme secretion in condition of cholesterol depletion (Table 1) or in invasion (our unpublished data).
We next wanted to monitor the rhoptry protein secretion from parasites attached to cholesterol-depleted cells. Quantitative results showed that rhoptry proteins secreted by parasites in contact with cholesterol-depleted plasma membrane (ROP2,3,4-positive vacuoles) was negligible. This confirms the coordination between micronemal and rhoptry protein secretion as observed previously (Carruthers et al., 1999). The specificity of the cholesterol reaction was confirmed by the demonstration that cholesterol back-addition to extracted cells restored parasite protein secretion. Under these conditions, the percentage of microneme-positive parasites and rhoptry-positive vacuoles was close to normal (Table 1). These results indicate that host cell cholesterol depletion specifically reduces the discharge of organelles needed for cell invasion.
Host Cell Caveolae Microdomains Are Not Necessary for Triggering Toxoplasma Entry
The requirement of host plasma membrane cholesterol for parasite organelle discharge and cell invasion suggested that host cell caveolae might be involved. To explore this possibility, cells were pretreated for 20 min with cholera toxin B before infection. This subunit binds glycosphingolipid GM1 clustered within the cell surface caveolae microdomains and triggers caveolae-mediated internalization (Montesano et al., 1982). Over a range of concentrations of cholera toxin B known to abolish the caveolae-mediated internalization of various pathogens (20–80 μg/ml) (Shin et al., 2000), no impairment of Toxoplasma invasion was observed (our unpublished data).
To directly investigate a role of host caveolae in parasite invasion, the L1210-JF lymphocyte cell line deficient in caveolin and lacking caveolae (Uittenbogaard et al., 1998) was challenged with parasites. Toxoplasma invasion in these deficient cells was comparable to that observed in L1210-JF cells transfected with the caveolin gene and expressing surface caveolae (Uittenbogaard et al., 1998) (Figure 8). Extraction of cholesterol from the caveolin-transfected L1210-JF cells abolished parasite invasion, confirming the essential role of cholesterol plasma membrane in parasite invasion.
Figure 8.
Influence of host caveolae in Toxoplasma adhesion and invasion. (A and B) Uracil incorporation assayed on intravacuolar parasites in L1210-JF cells, cholesterol-depleted L1210-JF cells or L1210-JF cells transfected with caveolin. Cell adhesion was analyzed after 20 or 60 min of parasite contact at 4°C, whereas cell invasion was monitored after 1, 5, or 20 min of parasite contact at 37°C. After parasite-cell contact, cells were washed to remove extracellular parasites and incubated for 24 h before uracil incorporation assay. Data in percentage are expressed relative to controls taken as 100% ± SE from three separate experiments done in duplicate. Controls are L1210-JF cells transfected with caveolin for experiments with L1210-JF cells (A) and L1210-JF cells for experiments with cholesterol-depleted L1210-JF cells (B). Differences between values of parasite invasion in L1210-JF cells versus cholesterol-depleted L1210-JF cells are statistically significant (p < 0.005).
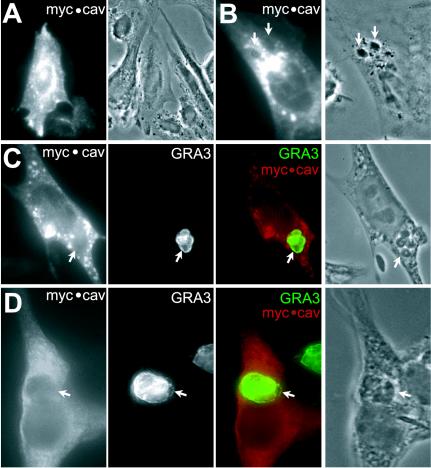
It has been reported that the mature PVM does not stain with antibodies against mammalian caveolin (Mordue et al., 1999). This suggests that the mature PVM is devoid of caveolin at steady state. However, during the development of intravacuolar parasites, the PVM is extensively modified, with rapid loss of selected host cell proteins initially incorporated into the vacuolar membrane (Mordue et al., 1999). We therefore asked whether caveolin was present in the PVM at the time of invasion, potentially reflecting a role for caveolae in the invasion process. We infected fibroblasts that had been transiently transfected with a myc-epitope-tagged version of caveolin 1. Transgenic caveolin localized primarily to the plasma membrane and Golgi of the host cells (Figure 9A). Shortly after parasite invasion in these transfected cells, the PVM was unstained (Figure 9B). Overexpressed caveolin was never observed associated with the PVM, even after infection for 24 h (Figure 9C) or 48 h (Figure 9D). Together, these results clearly establish that Toxoplasma neither interacts with caveolae at the time of invasion nor with caveolae-derived vesicles during intravacuolar development.
Figure 9.
Immunolocalization of transfected human caveolin in Toxoplasma-infected HFFs. (A) HFF were transfected with human caveolin-1 associated with the myc tag (myc · cav). Twenty-four hours posttransfection, cells were stained by fluorescence microscopy with anti-myc antibodies, followed by rhodamin-conjugated goat anti-mouse IgG. (B) Twenty-four hours posttransfection, HFFs were incubated for 20 min in the presence of parasites (arrows) and showed a nonlabeled zone corresponding to location. of the PV C-D: 24 h posttransfection, HFFs were incubated for an additional 24 h (C) or 48 h (D) in the presence of parasites (arrows). Cells were then processed for double immunolabeling with anti-Myc (red staining) and anti-GRA3 antibodies as PVM markers (green staining). The merged pictures show an absence of costaining between host caveolin and the PVM.
DISCUSSION
This report establishes that cholesterol is present in the PVM at the time of Toxoplasma invasion into mammalian cells. Rhoptry-derived cholesterol does not contribution to PVM formation but host plasma membrane cholesterol is mostly incorporated into this membrane in formation. Depletion of host plasma membrane cholesterol reversibly impairs parasite internalization by reducing the release of micronemal and rhoptry proteins from parasites. Our experiments examining the origin of cholesterol in the PVM were restricted to very short intervals of 1–2 min after parasite invasion. Toxoplasma cannot synthesize cholesterol de novo and depends upon acquisition of low-density lipoprotein (LDL)-derived cholesterol from the host cell (Coppens et al., 2000). Kinetic studies show that intracellular parasites have access to exogenous cholesterol after a lag time of 10 min after incubation with LDL. Under the short-term conditions of our assay, delivery of cholesterol from the host cell interior and lysosomes and delivered to the PVM is largely minimized, as seen in Figure 6A.
We depleted parasites of cholesterol by blocking the accessibility of exogenously derived lipoprotein cholesterol to the Toxoplasma-containing vacuole. Lipoprotein-derived cholesterol passes through the host cell lysosomes for cholesterol ester hydrolysis en route to intracellular compartments (Brown and Goldstein, 1986; Liscum and Dahl, 1992), including the Toxoplasma-containing vacuole. Trafficking of LDL-derived lysosomal cholesterol to regulatory sites of cholesterol homeostasis is mediated by NPC1 and NPC2 (reviewed in Blanchette-Mackie, 2000), which reside in vesicles shuttling the cholesterol away from lysosomes. The NPC mutation blocks the relocation of lysosomal LDL cholesterol to regulatory sites of cholesterol homeostasis (reviewed in Blanchette-Mackie, 2000) as well as to intravacuolar Toxoplasma. In cells incubated with progesterone, NPC1 is localized to cholesterol-laden lysosomes. Progesterone may inhibit NPC1 shuttling, which results in a blockade of LDL-cholesterol movement (Liscum, 2000). Cholesterol accumulates in the core of lysosomes of both NPC fibroblasts and normal human fibroblasts treated with progesterone. The consequence of this lipidosis of cholesterol in lysosomes may result in an impairment of cholesterol accessibility for the intracellular parasites. Indeed, a progressive decrease in their overall content in cholesterol, including rhoptries, is observed. Such parasites depleted of rhoptry cholesterol are still able to invade cells and secrete the protein content of rhoptries, refuting an essential involvement of rhoptry cholesterol for PVM formation. Although it remains possible that the residual cholesterol content in rhoptries after depletion (16–26%) participates in forming the nascent PVM, its contribution is minor compared with the bulk of host plasma membrane cholesterol involved in PVM generation.
The functional significance of cholesterol inside rhoptries remains enigmatic. Though our data suggest that rhoptry-derived lipids do not play a substantial role in PVM formation, they may be discharged nonetheless into the PVM throughout the parasite intracellular life cycle. This process could endow the PVM with properties favorable to survival of the parasite by giving it a lipid composition different from that of the host cell plasma membrane. However, the absence of prominent filipin staining in the mature PVM leads to the assumption that this membrane, if accessible to filipin, is poor in cholesterol, arguing against cholesterol secretion from rhoptries. Because cholesterol has a significant influence on the fluidity and permeability of membranes (Chen et al., 1978; Presti et al., 1985), the mature PVM may have unusual properties of deformability that could accommodate the increasing mass of parasites.
The standing pool of rhoptry cholesterol is likely derived primarily from the parasite plasma membrane. Indeed, the reduction of rhoptry cholesterol content obtained after MβCD treatment may be a consequence of the cholesterol egress from the parasite. It has been previously observed in mammalian cells that cholesterol arriving at the plasma membrane from intracellular compartments can be removed by MβCD, which is consistent with a loss of organelle-associated cholesterol as revealed by filipin (Subtil et al., 1999). In addition, MβCD can also prevent internalization of plasma membrane cholesterol to organelles. Similarly to these observations on mammalian cells, our experiments suggest the existence of a brisk circulation of cholesterol between the parasite plasma membrane and rhoptries.
A recent study speculates that rhoptries may be an early prototype of a lysosome-related organelle (Dell'Angelica et al., 2000; Que et al., 2002). Rhoptries do not seem to be lamellar bodies, long known, for example, to accumulate and secrete lipids in epithelial cells or to store lipid debris in macrophages (Schmitz and Muller, 1991). Nevertheless, the high cholesterol-to-protein ratio associated with rhoptries as well as the internal vesicle content is more reminiscent of mammalian multivesicular bodies in the endocytic system (Bishop and Woodman, 2000). Cholesterol is indispensable for multivesicular body reorganization, which operates to sort and transport molecules to specific cell compartments, including the trans-Golgi network (Miwako et al., 1991). Because protein–lipid interactions are key components that comprise physicochemical bases for membrane transport systems, cholesterol inside rhoptries may have a central importance in intracellular traffic of molecules between parasite organelles.
During invasion, the moving junction forming between the host plasma membrane and the parasite selectively controls internalization of host cell plasma membrane components into the parasite-containing vacuole. Previous studies reported that endogenous (e.g., glycosphingolipid GM1) and exogenous (e.g., fluorescent lipophilic dye DiIC16) membrane lipids as well as proteins devoid of cytoplasmic domains (e.g., glycosylphosphatidylinositol-anchored proteins) readily diffuse past this junction and gain entry to the nascent PVM (Mordue et al., 1999). Our experiments document the capacity of cholesterol to behave analogously, and further support the hypothesis that the bulk of the PVM, including its major lipid constituent, is derived from invagination of the host cell surface. The presence of labeled cholesterol in the PV necessarily comes from host plasma membrane since no lipid traffic from cell compartments to the preformed PVM has been observed.
As described above, caveolae and caveolae-like membrane domains are critical to a variety of pathogen-associated events. Several microbial pathogens, including viruses, intracellular bacteria, and prions have the capacity to coopt caveolae microdomains to trigger their entry into cells and avoid lysosome destruction. Indeed, caveolae-mediated entry into eukaryotic cells does not feed into the lysosomal pathway and does not result in the degradation of the content of caveolae vesicles (Norkin, 2001; Shin and Abraham, 2001a,b), clearly of benefit to these pathogens. As a requirement for caveolae entry, the respective receptor implicated in pathogen entry is a constituent of caveolae or must move to caveolae after ligation. Pneumocystis carinii has a unique interaction with the caveolar membrane system of its host cell. After alveolar cell contact, this parasite can activate the formation of caveolae vesicles in host cells. This pathogen-induced response is believed to contribute to the pneumocysts' ability to extract nutrients from the host's alveolar cells (Settnes and Nielsen, 1991).
So far, no caveolae-mediated entry has been reported for any protozoan parasites. Toxoplasma can modulate the composition of its PV by preferentially enriching the vacuole membrane with host glycosylphosphatidylinositol-anchored proteins and glycolipids (Mordue et al., 1999). Intriguingly, Plasmodium falciparum can induce the formation of a PV in mature human erythrocytes, which is also enriched in glycosylphosphatidylinositol-anchored proteins and glycolipids typically found in host cell rafts (Lauer et al., 2000). A role of such raft components in the Plasmodium vacuole membrane against organelle fusion is elusive because erythrocytes are devoid of endocytic and digestive compartments.
Cholesterol acts as the main “rigidifier” in natural membranes (Bretscher and Munro, 1993). Host cholesterol inserted in the PVM at the time of the Toxoplasma vacuole formation probably plays an important structural role. The MβCD-mediated removal of cholesterol from the plasma membrane has an influence on membrane fluidity and can impair the curvature necessary for the internalization of large pathogens. Glycerophospholipids and glycoproteins are also released from plasma membranes after treatment with MβCD as an indirect consequence of its disorganization (Ilangumaran and Hoessli, 1998). Activity of some receptors is altered, in correlation with the modulatory role of cholesterol on the function of receptors (Gimpl et al., 1997). This information implies that Toxoplasma invasion may rely on host membrane architecture and rigidity, or on the presence of a host-binding site that can trigger parasite internalization and that is extracted from plasma membrane after MβCD treatment. Although a variety of parasite-binding ligands and potential host cell receptors (Furtado et al., 1992a,b; Monteiro et al., 1998; Carruthers et al., 2000; Jacquet et al., 2001) mediating T. gondii attachment have been identified, the physiological triggers for microneme and rhoptry secretion are still unknown. Our results clearly suggest that interaction of T. gondii with cholesterol-depleted cells results in the parasite cell surface association but not the triggering of microneme and rhoptry secretion. Exposure of parasites to reconstituted liposomes with various amounts of cholesterol should elucidate the potential role of host plasma membrane cholesterol in triggering parasite organelle secretion.
Acknowledgments
We thank Vern Carruthers, Barbara Kazmierczak, and Craig Roy for critical comments of the manuscript and the members of K.A. Joiner laboratory for helpful discussions during the course of this work. We acknowledge the individuals who generously provided antibodies, plasmids and mutant cell lines used in this study (see MATERIALS AND METHODS). We thank Marc Pypaert (Yale Center for Cell and Molecular Imaging) for excellent assistance and scientific comments for electron microscopy. The work was supported by a grant from the National Institute of Health (AI488443) to K.A.J. and an American Heart Association (Scientist Development Grant 0230079N) to I.C.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–12–0830. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0830.
Abbreviations used: CHO, Chinese hamster ovary; HFF, human foreskin fibroblast; LDL, low-density lipoproteins; MβCD, methyl-β-cyclodextrin; NBD, nitrobenzoxadiazole; NPC, Niemann-Pick type C; PV, parasitophorous vacuole; PVM, parasitophorous vacuole membrane; TEM, transmission electron microscopy.
References
- Aikawa, M., Komata, Y., Asai, T., and Midorikawa, O. (1977). Transmission and scanning electron microscopy of host cell entry by Toxoplasma gondii. Am. J. Pathol. 87, 285–296. [PMC free article] [PubMed] [Google Scholar]
- Bannister, L.H., Mitchell, G.H., Butcher, G.A., and Dennis, E.D. (1986). Lamellar membranes associated with rhoptries in erythrocytic merozoites of Plasmodium knowlesi: a clue to the mechanism of invasion. Parasitology 92, 291–303. [DOI] [PubMed] [Google Scholar]
- Beckers, C.J., Dubremetz, J.F., Mercereau-Puijalon, O., and Joiner, K.A. (1994). The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 127, 947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudes, D., Dubremetz, J.F., Achbarou, A., and Joiner, K.A. (1994). Cloning of a cDNA encoding the dense granule protein GRA3 from Toxoplasma gondii. Mol. Biochem. Parasitol. 68, 247–257. [DOI] [PubMed] [Google Scholar]
- Bishop, N., and Woodman, P. (2000). ATPase-defective mammalian VPS4 localizes to aberrant endosomes and impairs cholesterol trafficking. Mol. Biol. Cell 11, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette-Mackie, E.J. (2000). Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim. Biophys. Acta 1486, 171–183. [DOI] [PubMed] [Google Scholar]
- Bretscher, M.S., and Munro, S. (1993). Cholesterol and the Golgi apparatus. Science 261, 1280–1281. [DOI] [PubMed] [Google Scholar]
- Brown, M.S., and Goldstein, J.L. (1986). A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47. [DOI] [PubMed] [Google Scholar]
- Butler, J.D., et al. (1992). Progesterone blocks cholesterol translocation from lysosomes. J. Biol. Chem. 267, 23797–23805. [PubMed] [Google Scholar]
- Carruthers, V.B., Giddings, O.K., and Sibley, L.D. (1999). Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell. Microbiol. 1, 225–235. [DOI] [PubMed] [Google Scholar]
- Carruthers, V.B., Hakansson, S., Giddings, O.K., and Sibley, L.D. (2000). Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect. Immun. 68, 4005–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers, V.B., and Sibley, L.D. (1997). Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73, 114–123. [PubMed] [Google Scholar]
- Carruthers, V.B., and Sibley, L.D. (1999). Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31, 421–428. [DOI] [PubMed] [Google Scholar]
- Chang, W.J., Rothberg, K.G., Kamen, B.A., and Anderson, R.G. (1992). Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J. Cell Biol. 118, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron, A.J., and Sibley, L.D. (2002). Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115, 3049–3059. [DOI] [PubMed] [Google Scholar]
- Chen, H.W., Heiniger, H.J., and Kandutsch, A.A. (1978). Alteration of 86Rb+ influx and efflux following depletion of membrane sterol in L-cells. J. Biol. Chem. 253, 3180–3185. [PubMed] [Google Scholar]
- Cintra, W.M., and de Souza, W. (1985). Distribution of intramembranous particles and filipin-sterol complexes in the cell membranes of Toxoplasma gondii. Eur. J. Cell Biol. 37, 63–69. [PubMed] [Google Scholar]
- Coppens, I., Levade, T., and Courtoy, P.J. (1995). Host plasma low density lipoprotein particles as an essential source of lipids for the bloodstream forms of Trypanosoma brucei. J. Biol. Chem. 270, 5736–5741. [DOI] [PubMed] [Google Scholar]
- Coppens, I., Sinai, A.P., and Joiner, K.A. (2000). Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur, G., Sadak, A., Fortier, B., and Dubremetz, J.F. (1988). Surface antigens of Toxoplasma gondii. Parasitology 97, 1–10. [DOI] [PubMed] [Google Scholar]
- Dahl, N.K., Gutheil, W.G., and Liscum, L. (1993). Abnormal regulation of low density lipoprotein-sensitive events in a cholesterol transport mutant. J. Biol. Chem. 268, 16979–16986. [PubMed] [Google Scholar]
- Dahl, N.K., Reed, K.L., Daunais, M.A., Faust, J.R., and Liscum, L. (1992). Isolation and characterization of Chinese hamster ovary cells defective in the intracellular metabolism of LDL-derived cholesterol. J. Biol. Chem. 267, 4889–4896. [PubMed] [Google Scholar]
- Dell'Angelica, E.C., Mullins, C., Caplan, S., and Bonifacino, J.S. (2000). Lysosome-related organelles. FASEB J. 14, 1265–1278. [DOI] [PubMed] [Google Scholar]
- Dobrowolski, J.M., Carruthers, V.B., and Sibley, L.D. (1997). Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26, 163–173. [DOI] [PubMed] [Google Scholar]
- Dobrowolski, J.M., and Sibley, L.D. (1996). Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84, 933–939. [DOI] [PubMed] [Google Scholar]
- Dubremetz, J.F., Achbarou, A., Bermudes, D., and Joiner, K.A. (1993). Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res. 79, 402–408. [DOI] [PubMed] [Google Scholar]
- Falguieres, T., Mallard, F., Baron, C., Hanau, D., Lingwood, C., Goud, B., Salamero, J., and Johannes, L. (2001). Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12, 2453–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussard, F., Leriche, M.A., and Dubremetz, J.F. (1991). Characterization of the lipid content of Toxoplasma gondii rhoptries. Parasitology 102, 367–370. [DOI] [PubMed] [Google Scholar]
- Furtado, G.C., Cao, Y., and Joiner, K.A. (1992a). Laminin on Toxoplasma gondii mediates parasite binding to the beta 1 integrin receptor alpha 6 beta 1 on human foreskin fibroblasts and Chinese hamster ovary cells. Infect. Immun. 60, 4925–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado, G.C., Slowik, M., Kleinman, H.K., and Joiner, K.A. (1992b). Laminin enhances binding of Toxoplasma gondii tachyzoites to J774 murine macrophage cells. Infect. Immun. 60, 2337–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reguet, N., Lebrun, M., Fourmaux, M.N., Mercereau-Puijalon, O., Mann, T., Beckers, C.J., Samyn, B., Van Beeumen, J., Bout, D., and Dubremetz, J.F. (2000). The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell. Microbiol. 2, 353–364. [DOI] [PubMed] [Google Scholar]
- Gatfield, J., and Pieters, J. (2000). Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288, 1647–1650. [DOI] [PubMed] [Google Scholar]
- Gimpl, G., Burger, K., and Fahrenholz, F. (1997). Cholesterol as modulator of receptor function. Biochemistry 36, 10959–10974. [DOI] [PubMed] [Google Scholar]
- Ginsbach, C., and Fahimi, H.D. (1987). Labeling of cholesterol with filipin in cellular membranes of parenchymatous organs. Standardization of incubation conditions. Histochem. 86, 241–248. [DOI] [PubMed] [Google Scholar]
- Hakansson, S., Charron, A.J., and Sibley, L.D. (2001). Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20, 3132–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, M., Lin, S.X., Karylowski, O.J., Wustner, D., McGraw, T.E., and Maxfield, F.R. (2002). Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J. Biol. Chem. 277, 609–617. [DOI] [PubMed] [Google Scholar]
- Hoppe, H.C., Ngo, H.M., Yang, M., and Joiner, K.A. (2000). Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nat. Cell Biol. 2, 449–456. [DOI] [PubMed] [Google Scholar]
- Ikonen, E., and Parton, R.G. (2000). Caveolins and cellular cholesterol balance. Traffic 1, 212–217. [DOI] [PubMed] [Google Scholar]
- Ilangumaran, S., and Hoessli, D.C. (1998). Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet, A., Coulon, L., De Neve, J., Daminet, V., Haumont, M., Garcia, L., Bollen, A., Jurado, M., and Biemans, R. (2001). The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol. Biochem. Parasitol. 116, 35–44. [DOI] [PubMed] [Google Scholar]
- Jelenska, J., Crawford, M.J., Harb, O.S., Zuther, E., Haselkorn, R., Roos, D.S., and Gornicki, P. (2001). Subcellular localization of acetylCoA carboxylase in the apicomplexan parasite Toxoplasma gondii. Proc. Natl Acad. Sci. USA 98, 2723–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky, M.J., Kleinfeld, A.M., Hoover, R.L., Dawidowicz, E.A., McIntyre, D.E., Salzman, E.A., and Klausner, R.D. (1982). Lipid domains in membranes. Ann. NY Acad. Sci. 401, 61–75. [DOI] [PubMed] [Google Scholar]
- Karsten, V., Qi, H., Beckers, C.J., Reddy, A., Dubremetz, J.F., Webster, P., and Joiner, K.A. (1998). The protozoan parasite Toxoplasma gondii targets proteins to dense granules and the vacuolar space using both conserved and unusual mechanisms. J. Cell Biol. 141, 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn, E.D. (1969). Current concepts of membrane structure and function. Fed. Proc. 28, 6–11. [PubMed] [Google Scholar]
- Lauer, S., VanWye, J., Harrison, T., McManus, H., Samuel, B.U., Hiller, N.L., Mohandas, N., and Haldar, K. (2000). Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19, 3556–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leriche, M.A., and Dubremetz, J.F. (1991). Characterization of the protein contents of rhoptries and dense granules of Toxoplasma gondii tachyzoites by subcellular fractionation and monoclonal antibodies. Mol. Biochem. Parasitol. 45, 249–259. [DOI] [PubMed] [Google Scholar]
- Liscum, L. (2000). Niemann-Pick type C mutations cause lipid traffic jam. Traffic 1, 218–25. [DOI] [PubMed] [Google Scholar]
- Liscum, L., and Dahl, N.K. (1992). Intracellular cholesterol transport. J. Lipid Res. 33, 1239–1254. [PubMed] [Google Scholar]
- Liscum, L., Ruggiero, R.M., and Faust, J.R. (1989). The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J. Cell Biol. 108, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Li, W.P., Machleidt, T., and Anderson, R.G. (1999). Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat. Cell Biol. 1, 369–375. [DOI] [PubMed] [Google Scholar]
- Luft, B. J., et al. (1993). Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 32914, 995–1000. [DOI] [PubMed] [Google Scholar]
- McGookey, D.J., and Anderson, R.G. (1983). Morphological characterization of the cholesteryl ester cycle in cultured mouse macrophage foam cells. J. Cell Biol. 97, 1156–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGookey, D.J., Fagerberg, K., and Anderson, R.G. (1983). Filipincholesterol complexes form in uncoated vesicle membrane derived from coated vesicles during receptor-mediated endocytosis of low density lipoprotein. J. Cell Biol. 96, 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwako, I., Yamamoto, A., Kitamura, T., Nagayama, K., and Ohashi, M. (1991). Cholesterol requirement for cation-independent mannose 6-phosphate receptor exit from multivesicular late endosomes to the Golgi. J. Cell Sci. 114, 1765–1776. [DOI] [PubMed] [Google Scholar]
- Monteiro, V.G., Soares, C.P., and de Souza, W. (1998). Host cell surface sialic acid residues are involved on the process of penetration of Toxoplasma gondii into mammalian cells. FEMS Microbiol. Lett. 164, 323–327. [DOI] [PubMed] [Google Scholar]
- Montesano, R., Roth, J., Robert, A., and Orci, L. (1982). Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature 296, 651–653. [DOI] [PubMed] [Google Scholar]
- Mordue, D.G., Desai, N., Dustin, M., and Sibley, L.D. (1999a). Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190, 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue, D.G., Hakansson, S., Niesman, I., and Sibley, L.D. (1999b). Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 92, 87–99. [DOI] [PubMed] [Google Scholar]
- Nichols, B.A., Chiappino, M.L., and O'Connor, G.R. (1983). Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J. Ultrastruct. Res. 83, 85–98. [DOI] [PubMed] [Google Scholar]
- Norkin, L.C. (2001). Caveolae in the uptake and targeting of infectious agents and secreted toxins. Adv. Drug. Deliv. Rev. 49, 301–315. [DOI] [PubMed] [Google Scholar]
- Norkin, L.C., Anderson, H.A., Wolfrom, S.A., and Oppenheim, A. (2002). Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 76, 5156–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Soares, C., and de Souza, W. (2000). Labeled probes inserted in the macrophage membrane are transferred to the parasite surface and internalized during cell invasion by Toxoplasma gondii. Parasitol. Res. 86, 11–17. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001). Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473–483. [DOI] [PubMed] [Google Scholar]
- Porchet-Hennere, E., and Torpier, G. (1983). Relations entre Toxoplasma et sa cellule-hote. Protistologica 19, 357–370. [Google Scholar]
- Presti, F.T., Pace, R.J., and Chan, S.I. (1985). Cholesterol-phospholipid interaction in membranes. 2. Stoichiometry and molecular packing of cholesterol-rich domains. Biochemistry 21, 3831–3835. [DOI] [PubMed] [Google Scholar]
- Que, X., Ngo, H., Lawton, J., Gray, M., Liu, Q., Engel, J., Brinen, L., Ghosh, P., Joiner, K.A., and Reed, S.L. (2002). The cathepsin B of Toxoplasma gondii, toxopain-1, is critical for parasite invasion and rhoptry protein processing. J. Biol. Chem. 277, 25791–25797. [DOI] [PubMed] [Google Scholar]
- Roos, D.S., Donald, R.G.K., Morissette, N.S., and Moulton, A.L.C. (1994). Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45, 27–63. [DOI] [PubMed] [Google Scholar]
- Samuel, B.U., Mohandas, N., Harrison, T., McManus, H., Rosse, W., Reid, M., and Haldar, K. (2001). The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J. Biol. Chem. 276, 29319–29329. [DOI] [PubMed] [Google Scholar]
- Schmitz, G., and Muller, G. (1991). Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32, 1539–1570. [PubMed] [Google Scholar]
- Settnes, O.P., and Nielsen, M.J. (1991). Host-parasite relationship in Pneumocystis carinii infection: activation of the plasmalemmal vesicular system in type I alveolar epithelial cells. J. Protozool. 38, 174S–176S. [PubMed] [Google Scholar]
- Sheets, E.D., Holowka, D., and Baird, B. (1999). Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J. Cell Biol. 145, 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J.S., and Abraham, S.N. (2001a). Caveolae as portals of entry for microbes. Microbes Infect. 3, 755–761. [DOI] [PubMed] [Google Scholar]
- Shin, J.S., and Abraham, S.N. (2001b). Co-option of endocytic functions of cellular caveolae by pathogens. Immunology 102, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J.S., Gao, Z., and Abraham, S.N. (2000). Involvement of cellular caveolae in bacterial entry into mast cells. Science 289, 785–788. [DOI] [PubMed] [Google Scholar]
- Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, N.M., Olson, B.J., and Klenk, D.C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Subtil, A., Gaidarov, I., Kobylarz, K., Lampson, M.A., Keen, J.H., and McGraw, T.E. (1999). Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96, 6775–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss-Toby, E., Zimmerberg, J., and Ward, G.E. (1996). Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl Acad. Sci. USA 9316, 8413–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uittenbogaard, A., Ying, Y., and Smart, E, J. (1998). Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J. Biol. Chem. 273, 6525–6532. [DOI] [PubMed] [Google Scholar]
- Ward, G.E., Miller, L.H., and Dvorak, J.A. (1993). The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci. 106, 237–248. [DOI] [PubMed] [Google Scholar]
- Weiss Bizzoco, R.L., and Reyes, V. (1992). Filipin labeling in Chlamydomonas gametes exposes site-specific lipid specializations. Cell. Mol. Biol. 38, 337–342. [PubMed] [Google Scholar]
- Wong, S.Y., and Remington, J.S. (1994). Toxoplasmosis in pregnancy. Clin. Infect. Dis. 18, 853–861. [DOI] [PubMed] [Google Scholar]
- Yancey, P.G., Rodrigueza, W.V., Kilsdonk, E.P., Stoudt, G.W., Johnson, W.J., Phillips, M.C., and Rothblat, G.H. (1996). Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271, 16026–16034. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, H., Furuki, J., Takahashi, Y., Morioka, H., and Yoshida, Y. (1992). Distribution of filipin-sterol complexes in the bloodstream form of Trypanosoma brucei gambiense. J. Electron Microsc. 41, 364–368. [PubMed] [Google Scholar]