Abstract
In plants, Glc-6-phosphate dehydrogenase (G6PDH) isoenzymes are present in the cytosol and in plastids. The plastidic enzymes (P1 and P2) are subject to redox regulation, but mechanisms that adjust cytosolic G6PDH activity are largely unknown. We adopted a leaf disc system for monitoring the effects of various conditions on G6PD isoform expression and enzyme activities in potato (Solanum tuberosum). Cytosolic G6PDH activity remained constant during water incubation in the dark. In continuous light or in the presence of metabolizable sugars in the dark, cytosolic G6PDH activity increased 6-fold within 24 h. Cycloheximide incubation demonstrated that enhanced cytosolic G6PDH activity depends on de novo protein synthesis. Osmotic change, phosphate sequestration, or oxidative stress did not affect cytosolic G6PDH activity. Furthermore, enzyme activity and protein contents closely followed the corresponding mRNA levels. Together with the fact that multiple SURE elements are present in the promoter region of the gene, these results suggest that cytosolic G6PDH activity is regulated by sugar availability at the transcriptional level. Plastidic G6PDH activity stayed constant during water incubation in the light and dropped to minimal levels within 6 h in the dark. Conversely, plastidic G6PDH activity of leaf discs incubated on Paraquat rose to 10-fold higher levels, which was not prevented by cycloheximide. Similar increases were found with nitrite, nitrate, or sulfate. No major changes in protein or mRNA contents of the plastidic P1 and P2 isoforms were registered. Km (Glc-6-phosphate) values of plastidic G6PDH activity differed between samples incubated on water or Paraquat, suggesting posttranslational modification of the plastidic enzyme(s). Immunoprecipitation of 32P-labeled samples with P1 isoform-specific antibodies showed that the chloroplast enzyme is subject to protein phosphorylation. Obviously, in extended dark periods, G6PDH activity in the stroma is restricted but can be stimulated in response to high demands for NADPH.
Glc-6-phosphate dehydrogenases (G6PDHs, EC 1.1.1.49) catalyze the oxidation of Glc-6-phosphate (G6P) to 6-phosphogluconolactone concomitant with reducing NADP to NADPH. The product 6-phosphogluconolactone is first converted to 6-phosphogluconate by 6-phosphogluconolactonase (EC 3.1.1.31) and then decarboxylated by 6-phosphogluconate dehydrogenase (6PGDH, EC 1.1.1.44), yielding another mole of NADPH and ribulose-5-phosphate. The first enzyme, G6PDH, controls the flux through this nonreversible limb of the oxidative pentose phosphate pathway (OPPP; Williams, 1980; Copeland and Turner, 1987). In higher plants, G6PDH isoenzymes reside in two compartments, the cytosol and plastids (Heber et al., 1967; Schnarrenberger et al., 1973). Reducing power (NADPH) generated by the OPPP sustains reductive biosyntheses (e.g. fatty acids, isoprenoids and aromatic amino acids) in the dark and nitrogen assimilation in heterotrophic tissues (Bowsher et al., 1992). In addition, OPPP intermediates are continuously withdrawn to fuel other metabolic pathways. For example, erythrose-4-phosphate, produced by the Calvin cycle in the light and by the OPPP in the dark, is a substrate of the shikimate pathway and, thus, needed as precursor of aromatic amino acids, cell wall polymers, phytoalexins, and pigments. All enzymatic steps of the shikimate pathway up to chorismate are confined to plastids (Schmid and Amrhein, 1995). Schnarrenberger et al. (1995) postulated that in plants, only the irreversible reactions of the OPPP might be present in the cytosol. This has recently been supported by the work of Eicks et al. (2002), who characterized a pentose phosphate translocator of the inner chloroplast membrane from Arabidopsis. Bioinformatic analyses of the Arabidopsis genome revealed that genes for both cytosolic and plastidic isoforms of Rib-5-phosphate isomerase and ribulose-5-phosphate-3-epimerase are present, but for transketolase and transaldolase, cytosolic isoforms are missing. Thus, the oxidative branch of the OPPP in the cytosol probably operates in close connection with the complete pathway in plastids.
Sequences coding for NADP-dependent G6PDH enzymes exist in all organisms except for Archaebacteria (Wendt et al., 1999). Plant G6PD-cDNA sequences were initially elucidated from potato (Sola- num tuberosum; cytosolic isoform, Graeve et al., 1994; plastidic P1 isoform, von Schaewen et al., 1995; plastidic P2 isoform, Wendt et al., 1999) and are now known for several higher plant species. In the past, regulation of G6PDH isoenzyme activities was examined in many different systems and under various aspects. Analysis of purified or enriched enzyme preparations revealed that G6PDH activity largely depends on reduction charge (i.e. NADPH to NADP ratio). It was shown that high concentrations of the product NADPH inhibit the chloroplast enzyme (Lendzian and Bassham, 1975) and cytosolic G6PDH (Fickenscher and Scheibe, 1986). To avoid futile cycling of carbon between the OPPP and the Calvin cycle, chloroplast G6PDH is inactivated in the light by the ferredoxin-thioredoxin system (for review, see Scheibe, 1990; Buchanan, 1991). Reductive inactivation can be mimicked in vitro by pre-incubating samples with reduced dithiothreitol (DTTred; Johnson, 1972). We used this feature in the past to discriminate in crude plant extracts between G6PDH activity derived from the two compartments (Scheibe et al., 1989; Graeve et al., 1994; Wenderoth et al., 1997; Wendt et al., 2000).
Information on in planta regulation of G6PD isoforms is limited. Several studies in the past describing conditions that stimulate G6PDH activity (pathogen attack and elicitation) did not distinguish between the different isoenzymes nor analyze possible regulatory mechanisms involved (Endo and Veech, 1969; Börner and Grisebach, 1982; Daniel et al., 1988, 1990). Steady-state transcript levels of a cytosolic and a plastidic G6PD isoform were first analyzed in potato (von Schaewen et al., 1995). In most tissues, the gene coding for cytosolic G6PDH is constitutively expressed. Compared with mature leaves, elevated mRNA levels were found in heterotrophic tissues (etiolated shoots, tubers, or roots). Analyses of G6PDH activity during leaf development revealed more or less constant levels of the cytosolic isoform. Antisense suppression, however, did not provoke a growth phenotype (K. Graeve and A. von Schaewen, unpublished data). Wendt et al. (2000) showed that a second plastidic isoform (P2) is transcribed more or less ubiquitously in potato, with highest levels in stems and roots and lowest levels in tubers. Steady-state mRNA amounts of the first characterized plastidic isoform (P1) are most prominent in green tissues and accumulate in stolons or root tips in the light but can hardly be detected in soil-grown tubers (von Schaewen et al., 1995; Wendt et al., 2000). In tobacco (Nicotiana tabacum) seedlings, transcript levels of the P1 isoform also increase during greening, but DTTred-sensitive enzyme activity does not correlate well with the mRNA amounts, suggesting either involvement of another G6PD isoform or regulation at a posttranscriptional level (C. Lange, R. Hauschild, and A. von Schaewen, unpublished data). Large variations in plastidic G6PDH activities were also reported in other studies (Schnarrenberger et al., 1995, and refs. therein), but the basis for the effects of different stimuli on G6PDH isoenzyme activities in higher plants remained obscure.
In heterotrophic tissues, plastidic G6PDH (and 6PGDH) activities provide reductive power (NADPH) for nitrogen assimilation. Several situations are known to modify metabolic fluxes through the plastid-localized OPPP: Oji et al. (1985) showed in wheat (Triticum aestivum) root plastids that electrons are transferred from G6P to nitrite (NO2-) via NADP and ferredoxin-NADP reductase (FNR). Bowsher et al. (1989, 1992) demonstrated that electrons for NO2- reduction and Glu synthesis stem from the plastidic OPPP. Thom and Neuhaus (1995) showed that carbohydrate flux through the plastidic OPPP can be stimulated by feeding NO2- or Glc to isolated plastids and by generating a requirement for reducing equivalents to sustain metabolic fluxes in isolated chloroplasts of green pepper (Capsicum annuum) fruits. However, regulatory mechanisms that control increased fluxes through the plastidic OPPP were not investigated. Wright et al. (1997) showed in barley (Hordeum vulgare) root plastids that levels of specific G6PDH activity decrease during nitrogen starvation concomitant with a slight decrease in apparent Km for NADP. This was suggested to result from expression of another G6PD isoform. Batz et al. (1998) demonstrated that genes coding for cytosolic or plastidic G6PDH enzymes are differentially transcribed upon elicitor treatment in parsley (Petroselinum crispum) suspension culture cells. Wang et al. (2000) found stimulated expression of two P2 isoform genes after switching Arabidopsis plants growing in liquid culture under constant illumination from ammonium (reduced nitrogen source) to nitrate (NO3-) feeding. Knight et al. (2001) characterized a first genomic G6PD sequence (P2 isoform) from tobacco. KNO3 treatment stimulated expression in root and leaf tissue that is assumed to be mediated through presence of several NIT2 elements identified in the promoter region. In contrast to nitrogen, no information is available about the impacts of enhanced sulfur assimilation, which is entirely located in plastids (Hell, 1997).
This work aimed at elucidating factors that result in changes of cytosolic and plastidic G6PDH activity. We adopted a leaf disc system to examine the effects of various treatments (feeding metabolites, inhibitors, etc.) on G6PD isoforms in potato. We determined maximal G6PDH activities (by differential inactivation of plastidic G6PDH with DTTred), protein abundance (with isoenzyme-specific antibodies), and transcript levels (using isoform-specific cDNA probes) and sequenced the promoter region of a genomic DNA fragment coding for the cytosolic isoform. Based on the obtained results, we suggest that discrete mechanisms contribute to the regulation of cytosolic and plastidic G6PDH isoenzyme activity in planta.
RESULTS
Rational for the Method of Choice
We chose incubation experiments to study short-term influences of water-soluble substances on G6PDH-isoenzyme activities in potato leaf tissue. Activities were determined in leaf disc extracts after different incubation times in the dark and in the light. In addition, mRNA levels of the different G6PD isoforms were analyzed by northern-blot hybridization and protein contents by immunodetection on western blots. Leaf surface served as reference to account for deviations in protein content that result from degradation of Rubisco during long-term incubations in the dark. In this way, dark- and light-incubated samples and also different experimental series can be compared.
Effect of Incubation Conditions and Sugar Availability on Cytosolic G6PDH
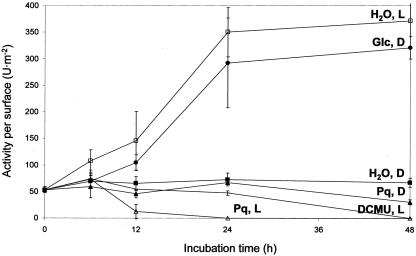
Cytosolic G6PDH activity remained constant during water incubation of leaf discs in the dark (Figs. 1, 2, 3). In the light, activities increased 5- to 7-fold compared with water controls in the dark (Figs. 1 and 3). Within 48 h, cytosolic G6PDH activity increased steadily and then remained at a constant level. Comparable stimulation was also triggered by incubation of leaf discs on 50 mm Glc in the dark (Figs. 1, 2, 3). Incubation on 50 mm mannitol or 100 mm KCl did not affect G6PDH activity, demonstrating that the observed increases are not due to osmotic or salt effects (data not shown). Presence of the electron-consuming herbicide Paraquat (5 μm methylviologen) or an inhibitor of photosynthetic electron transport (100-500 μm DCMU) in the light had no effect, indicating that oxidative stress and light as such are not responsible for the activity increases.
Figure 1.
Cytosolic G6PDH activities per surface (units per meter squared) in leaf discs incubated for 48 h on water in the dark (H2O, D), in continuous light (H2O, L), or on 50 mm Glc in the dark (Glc, D). In addition, 5 μm Paraquat in the dark (Pq, D) or in the light (Pq, L) and incubation on 100 μm 3-(3,4-dichlorophenyl)-1,1′-dimethyl urea (DCMU), which uncouples photosynthetic electron transport in the light (DCMU, L), was tested.
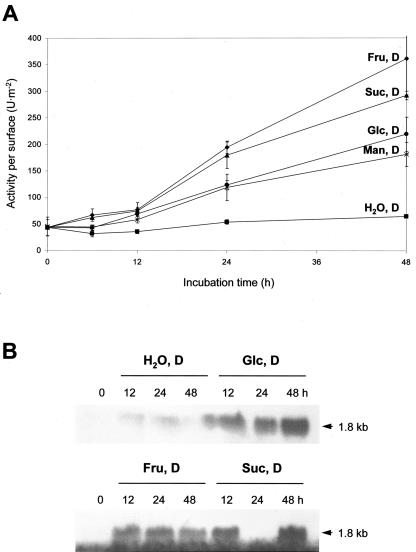
Figure 2.
A, cytosolic G6PDH activities per surface (units per meter squared) in leaf discs incubated for 48 h in the dark on either water (H2O, D), 50 mm Man (Man, D), 50 mm Glc (Glc, D), 50 mm Fru (Fru, D), or 25 mm Suc (Suc, D), respectively. B, Northern-blot analyses conducted with total RNA (15 μg each) isolated from potato leaf discs incubated on different sugars in the dark. Samples were separated in denaturing agarose gels. After northern-blot transfer, membranes were hybridized with radiolabeled cDNA fragments of the cytosolic isoform, washed under stringent conditions (three times at 68°C with 0.1× SSC and 0.1% [w/v] SDS), and exposed to x-ray film. Numbers above the lanes represent incubation time in hours. Note that an RNA sample is missing (in the lane labeled 24 h Suc, D).
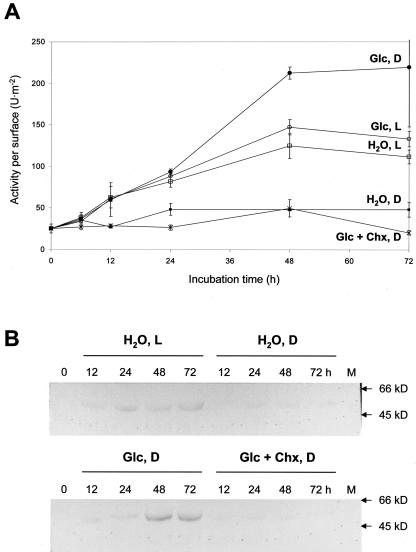
Figure 3.
A, Cytosolic G6PDH activities per surface (units per meter squared) in leaf discs incubated for 72 h on water in the light (H2O, L) or in the dark (H2O, D), on 50 mm Glc in the light (Glc, L) or in the dark (Glc, D), and in additional presence of 1 mm cycloheximide (Chx; Glc + Chx, D). Note that water incubation in the light was not always lower compared with Glc incubation in the dark (compare graphs H2O, L and Glc, D in Figs. 1 and 3). B, Immunolabeling of cytosolic G6PDH protein on western blots of cleared extracts prepared from leaf discs incubated under conditions indicated. M, Molecular mass standard; arrows indicate sizes of apparent kilodaltons. Numbers above the lanes represent incubation times in hours.
The following experiments demonstrate the effects of different sugars on cytosolic G6PDH activity. Leaf discs were incubated either on Suc, Fru, Glc, or Man, respectively. Incubation on Suc (25 mm) or Fru (50 mm) in darkness reproducibly led to higher increases of cytosolic G6PDH activity and corresponding mRNA levels compared with Glc (Fig. 2, A and B). Stimulation by Fru was always higher than by Suc. Hybridization of total RNA isolated from leaf discs incubated on water or different sugars, using cDNA fragments of the cytosolic isoform (von Schaewen et al., 1995) as a probe, revealed that mRNA levels changed similarly (Fig. 2B). Although the Glc epimer Man (50 mm) led to activity increases comparable with Glc incubations in the dark (Fig. 2A), 3-O-methyl-Glc (3-O-MG; 50 mm) had no effect, and 2-deoxy-Glc (5 mm) inhibited G6PDH activity (data not shown). Presence of inorganic phosphate (50 mm) did not affect the stimulatory effect of Glc or Man, showing that elevated cytosolic G6PDH activity is not a consequence of phosphate sequestration but depends on the presence of metabolizable sugars (data not shown).
Chx (1 mm) inhibited both the increase of cytosolic G6PDH activity in water-incubated samples in the light (not shown) and upon feeding Glc in the dark (Fig. 3A). Water incubation in the light was always equivalent to Glc incubation in the dark, although here Glc in the dark stimulates cytosolic G6PDH activity to higher levels compared with water in the light (compare with Fig. 1). Western-blot analyses using a polyclonal antiserum specific for cytosolic G6PDH revealed that elevated protein contents correspond to the observed increases in activity and mRNA levels. Because Chx prevents de novo synthesis of cytosolic G6PDH protein (Fig. 3B), elevated activity of this isoform seems to result from regulation at the transcriptional level, most likely triggered by accumulation of metabolizable sugars in the cytosol.
Other Substances Tested
Similar to Paraquat, incubation of potato leaf discs in the presence of hydrogen peroxide (H2O2; 0.05, 0.1, and 10 mm), iron (100 μm FeIIICl3), ammonium (20 mm NH4Cl), NO2- (20 mm NaNO2), NO3- (20, 40, 100, and 250 mm NaNO3), or sulfate (SO42-; 50, 100, and 250 mm KSO4) had no influence on cytosolic G6PDH activity. Incubation with phosphatase inhibitors (50 μm Endothall, 0.5 μm Okadaic acid, or 20-100 mm NaF) also had no effect.
Effect of Incubation Conditions and Stromal Reduction Charge on Plastidic G6PDH Activity
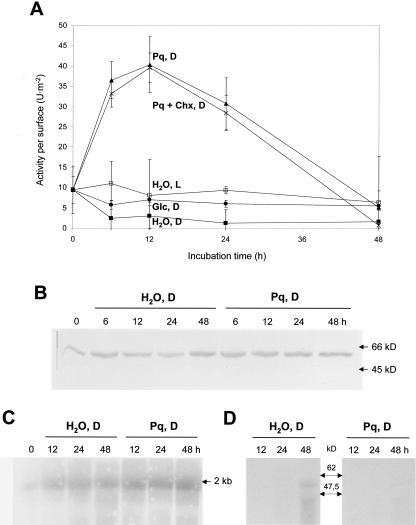
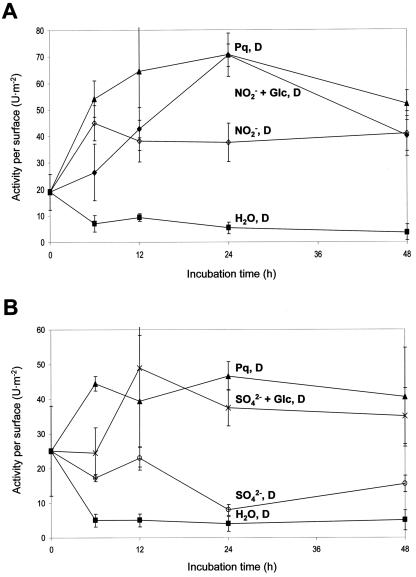
Incubation of leaf discs in the presence of Paraquat (methylviologen) in the dark has been described previously to induce oxidative stress (Bowler et al., 1991). In our experiments, incubation on 5 μm Paraquat led to remarkable increases in DTTred-sensitive (i.e. plastid-localized) G6PDH activity. Stimulation raised about 4- to 5-fold over the initial value and differed about 10-fold from the control (water incubation) determined in parallel (Fig. 4). Already after 6 h, plastidic G6PDH activity reached a maximum followed by either slightly decreased or constant G6PDH activities (compare Figs. 4A and 5, A and B). Under these conditions, no apparent damage of the leaf discs was visible. In continuous light, the same Paraquat concentration did not alter plastidic G6PDH activity during the entire incubation period, but bleaching of the tissue was visible after about 12 h.
Figure 4.
Plastidic (DTTred-sensitive) G6PDH activity of leaf discs incubated for 48 h. A, Leaf discs were incubated on water in the light (H2O, L) or in the dark (H2O, D), on 5 μm Paraquat in the dark (Pq, D), and in additional presence of 1 mm Chx (Pq + Chx, D). Decreases of plastidic G6PDH activity after 24 h were observed repeatedly but not in all experiments (compare Fig. 5). B, Immunodetection of plastidic G6PDH (P1 isoform) on western blots of leaf discs incubated on either water or Paraquat in the dark for the hours indicated. C, Northern-blot analysis of total RNA (20 μg per lane) isolated from leaf discs incubated on either water or Paraquat in the dark for the hours indicated. The blot was hybridized with radiolabeled cDNA fragments of the P1 isoform (von Schaewen et al., 1995; Wendt et al., 1999) and washed under stringent conditions (three times at 68°C with 0.1× SSC and 0.1% [w/v] SDS). D, Autoradiogram of immunoprecipitated P1 protein after radiolabeling of leaf discs with 500 μCi 32P in the dark incubated on either water or Paraquat. kD, Molecular mass standards. Numbers above the lanes refer to incubation time in hours.
Figure 5.
Plastidic (DTTred-sensitive) G6PDH activities in leaf discs incubated in the dark for 48 h. A, Incubations were conducted either on water (H2O, D), 5 μm Paraquat (Pq, D), 20 mm NO2- (D), or 20 mm NO2- plus 50 mm Glc (NO2- + Glc, D). B, Incubations were conducted either on water (H2O, D), 5 μm Paraquat (Pq, D), 100 mm SO42- (D), or 10 mm SO42- plus 50 mm Glc (SO42- + Glc, D).
Interestingly, stimulation of DTTred-sensitive G6PDH activity was not inhibited by Chx (Fig. 4A). Concomitantly, protein contents of the P1 isoform remained unchanged in Paraquat- or water-incubated samples (Fig. 4B). Hybridization of total RNA isolated from leaf discs incubated on water or Paraquat, using cDNA fragments of the plastidic P1 isoform (von Schaewen et al., 1995) as a probe, revealed no major changes (Fig. 4C). Protein levels of the P2 isoform (Wendt et al., 1999) remained below the detection limit (data not shown).
To assess kinetic parameters, Km and Vmax values were determined in extracts prepared from leaf discs incubated on water or Paraquat, respectively. The results are shown in Table I. The values for cytosolic G6PDH activity did not change considerably (and can be regarded as internal control). However, for the redox-sensitive plastidic enzyme(s), the Km for binding substrate (G6P) was about 10 times lower upon Paraquat incubation, with no dramatic change in Km for binding cosubstrate (NADP). The Vmax values for G6P remained more or less unchanged, whereas those for NADP doubled in Paraquat-incubated samples.
Table I.
Kinetic parameters of G6PDH isoenzymes in different subcellular compartments
Data were determined with crude extracts of leaf discs incubated for either 12 or 24 h on water (H2O) or Paraquat (Pq) in the dark (D). Km values are given in millimolars, and Vmax values are given in milliunits per milligram protein (for way of measuring isoenzyme-specific G6PDH activities, see “Materials and Methods”).
| Incubation Condition
|
|||||
|---|---|---|---|---|---|
| G6PDH Isoenzyme(s) | Kinetic Parameter | H2O, D
|
Pq, D
|
||
| 12 h | 24 h | 12 h | 24 h | ||
| Cytosolic | Vmax (G6P) | 27.9 | 29.5 | 27.6 | 18.5 |
| Km (G6P) | 0.8 | 1.0 | 0.7 | 0.8 | |
| Vmax (NADP) | 11.7 | 20.0 | 20.4 | 12.1 | |
| Km (NADP) | 5.5 | 5.2 | 9.7 | 5.3 | |
| Plastidic | Vmax (G6P) | 20.4 | 13.3 | 18.7 | 13.4 |
| Km (G6P) | 3.9 | 3.0 | 0.3 | 0.5 | |
| Vmax (NADP) | 4.3 | 7.5 | 15.0 | 9.7 | |
| Km (NADP) | 9.0 | 12.6 | 7.0 | 6.1 | |
Because stimulation of DTTred-sensitive G6PDH activity was not accompanied by increased protein contents of the plastidic enzymes, nevertheless resulting in a Km (G6P) change for binding substrate, we examined whether posttranslational modification, e.g. phosphorylation, could account for the observed effect. Dark incubation of leaf discs on either water or Paraquat was conducted in the additional presence of 500 μCi 32P-orthophosphate. Protein extracts of labeled leaf discs were immunoprecipitated with antibodies specific for the P1 isoenzyme (von Schaewen et al., 1995), separated by SDS-PAGE, and visualized by autoradiography. Figure 4D shows that the molecular mass of the largest labeled band lies in the range of 56 kD, the expected size for G6PDH monomers of the P1 isoform (Wendt et al., 2000). Labeling increased with incubation time and was more pronounced in water-incubated samples compared with those treated with Paraquat. The tested phosphatase inhibitors Endothall (50 μm), Ocadaic acid (0.5 μm), and NaF (20-100 mm), however, were unable to prevent stimulation (data not shown).
Unlike for the cytosolic enzyme, incubation of leaf discs with 50 mm Glc in the dark had no effect on DTTred-sensitive G6PDH activity (Fig. 4A). Combined incubation on Paraquat plus Glc in the dark did not influence the stimulation found with Paraquat alone. Incubation of leaf discs on Paraquat plus 500 μm DCMU in the light led to a 6-fold stimulation of DTTred-sensitive G6PDH activity, whereas Paraquat in the light alone had no effect (data not shown).
Increased DTTred-sensitive G6PDH activity was also observed for NO2- incubations in the dark (NO2-, Fig. 5A) and similar time courses also for NO3- (data not shown), either alone or in combination with Glc. High concentrations (100 mm) led to significant stimulation of DTTred-sensitive G6PDH activity, and simultaneous feeding of Glc sustained the effect. For SO42- (Fig. 5B), the extent of stimulation depended on the additional presence of sugar (acceptor carbon skeletons). Incubation on equimolar salt solution (KCl served as a control) did not result in altered plastidic G6PDH activities (data not shown).
In summary, G6PDH activity in chloroplasts of dark-held leaf tissue is rapidly inactivated at the posttranslational level, probably via phosphorylation of the existing enzyme pool. Conversely, enhanced activity of plastidic G6PDH would be triggered by dephosphorylation in situations imposing high demands for NADPH in the stroma.
DISCUSSION
Conditions Stimulating Cytosolic G6PDH Activity
During incubation of potato leaf discs, cytosolic G6PDH activity was stimulated about 5- to 7-fold above the initial value on either water in the light or upon feeding sugar in the dark. Blocking photosynthetic electron transport by DCMU in the light prevented the effect, which demonstrates that stimulation of cytosolic G6PDH activity is not due to light as such, but results from translocation of photosynthate (sugar) into the cytosol. In the leaf disc system, export of endogenously synthesized sugars via active phloem loading is unlikely because, after excision, sieve elements are rapidly clogged by callose deposition (Müller-Röber et al., 1990). Therefore, the metabolic situation of leaf discs incubated on water in the light can be considered equivalent to those incubated on sugars in the dark. Presence of mannitol or KCl did not alter cytosolic G6PDH activity and, in combination with Glc, did not affect stimulation. Thus, osmotic changes do not influence cytosolic G6PDH activity. Because non-metabolizable sugar analogs (3-O-MG and 2-desoxy-Glc) were ineffective, the stimulus can be narrowed down to elevated carbohydrate (sugar) availability in the cytosol.
Analysis of the 5′ Region of a Genomic Fragment Coding for Cytosolic G6PDH
According to the Chx and western-blot experiments, increases in cytosolic G6PDH activity require de novo protein synthesis. Northern-blot analyses showed that the mRNA levels correlate well with enhanced enzyme activities and protein amounts of cytosolic G6PDH. These results prompted us to examine the 5′ region of the corresponding gene. A clone hybridizing to the cDNA sequence of cytosolic G6PDH (Graeve et al., 1994) was isolated from a genomic potato library. Restriction analysis yielded fragments analogous to those detected on southern blots (von Schaewen et al., 1995). A large BamHI fragment (8 kb) was subcloned and mapped. Sequence analyses revealed that the fragment comprises 2 kb of the 5′-untranslated region. Several sequence motifs with high homology to elements identified in promoters of other sugar-regulated plant genes (SURE, e.g. of patatin and Suc synthase from potato, Grierson et al., 1994; Fu et al., 1995) were also found in the promoter region of the cytosolic G6PD gene (Fig. 6). This supports the results described above, pointing at transcriptional regulation of the cytosolic isoform in response to sugar availability. Other possible mechanisms, such as slower degradation of the mRNA or protein, were not addressed and, hence, cannot be excluded. However, posttranslational mechanisms do not seem to be involved in cytosolic G6PDH regulation. We propose that regulation of cytosolic G6PDH activity occurs mainly at the level of transcription and that enzyme activity is more or less proportional to the rate of transcription and translation.
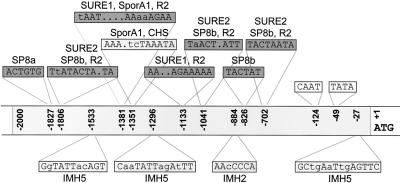
Figure 6.
Sketch of the promoter region of the gene coding for cytosolic G6PDH. Motifs similar to sugar regulatory elements in other plant genes are shown above and below the sequenced 2-kb region of the promoter (gray box) and are indicated as follows: CHS, conserved region in chalcone synthase genes of Petunia hybrida and Arabidopsis (Tsukaya et al., 1991); IMH2 and IMH5, homologous motifs in isocitrate lyase and malate synthase promoters from Cucumis sativus (Sarah et al., 1996); R2, repetitive sequence of patatin class I promoters from potato (Mignery et al., 1988); SP8a and SP8b, recognition sequences of a DNA-binding protein in sugar-regulated genes of sporamin and β-amylase from Ipomoea batatas (Ishiguro and Nakamura, 1994); SporA1, repetitive sugar regulatory region of Sporamin from I. batatas (Kim et al., 1991); and SURE1 and SURE2, sugar-responsive elements 1 and 2 identified in several genes isolated from potato (Fu et al., 1995). CAAT and TATA boxes are also indicated. Position numbers refer to the translation start (+1ATG).
SURE Elements in the Promoter Region
The promoter of the cytosolic G6PD gene harbors several conserved elements in identical or slightly modified form known to be present in promoters of genes that are regulated by carbohydrate availability (Fig. 6). Among others, SURE1 and SURE2 motifs of patatin (Mignery et al., 1988; Grierson et al., 1994) and Suc synthase (Fu et al., 1995) from potato are also present in the promoter of the gene encoding cytosolic G6PDH. According to our data transcript levels, protein abundance and enzyme activity of cytosolic G6PDH rise in metabolic sink situations. This regulation seems to be comparable with the one of patatin (Rocha-Sosa et al., 1989; Wenzler et al., 1989), ADPGlc-pyrophosphorylase (Müller-Röber et al., 1990), Suc synthase (Salanoubat and Belliard, 1989; Fu et al., 1995), nitrate reductase (Cheng et al., 1992; Vincentz et al., 1993), chalcone synthase (Tsukaya et al., 1991), and sporamine (Hattori et al., 1990; for further sink-regulated genes, see Koch, 1996). In contrast to patatin, Suc synthase, and ADP-Glc-pyrophosphorylase, cytosolic G6PDH is expressed in most organs of mature potato plants (von Schaewen et al., 1995).
Several systems involved in sugar-mediated gene regulation are currently discussed (for review, see Koch, 1996; Smeekens and Rook, 1997; Halford et al., 1999). A system analogous to hexokinase-mediated signal transduction in yeast (Saccharomyces cerevisiae) has been described by Jang and Sheen (1994) using protoplasts. Another system involving hexose transporters or membrane-bound receptor proteins was reported by Hilgarth et al. (1991) for the unicellular green alga Chlorella kessleri, and for higher plants by Godt et al. (1995) and Roitsch et al. (1995). Involvement of a Suc sensor in sugar-mediated signal transduction in Arabidopsis was reported by Rook et al. (1998). From analyses of transgenic tobacco plants expressing yeast invertase in the apoplast, Herbers et al. (1996) concluded that sugar sensing can also occur in the plant secretory system, i.e. in the endoplasmic reticulum and/or Golgi apparatus (connected to the plasma membrane and the vacuole via vesicle-mediated transport).
Due to the results of various sugar feeding studies, the response pattern of cytosolic G6PDH activity can be compared with the regulation of genes encoding enzymes of the glyoxylate pathway (described for suspension cultures of Cucumis sativus; Graham et al., 1994) and with those involved in photosynthesis (described for mesophyll protoplasts of maize [Zea mays]; Jang and Sheen, 1994), however, in the other direction: Whereas sugar incubation reduced gene expression in these systems, carbohydrate availability induces expression of the gene coding for cytosolic G6PDH in potato.
In the leaf disc system, enhanced cytosolic G6PDH activity (resulting from increased gene expression) is also induced by Man. In the past, Man was frequently used to induce phosphate sequestration (Brouquisse et al., 2001, and refs. therein). Combined incubation on Man and phosphate did not prevent stimulation of cytosolic G6PDH activity in the dark. Hence, phosphate does not influence transcription of the cytosolic G6PD gene, and its sequestration in a metabolic sink situation plays probably not the same role as during photosynthesis, where phosphate is needed as counterexchange substrate for triosephosphate export from the chloroplasts. Man is known to be a substrate for hexokinase. The resulting Man-6-phosphate is a precursor of ascorbate biosynthesis (Wheeler et al., 1998) and can be converted to G6P via Fru-6-phosphate. In contrast, the Glc analog 3-O-MG (substrate for hexokinase but not further metabolized; Cortès et al., 2003) had no effect, and 2-deoxy-Glc incubation in the dark abolished cytosolic G6PDH activity. We observed that Man and 2-desoxy-Glc cause unpredictable effects in the light (data not shown) similar to previous reports (Klein and Stitt, 1998; Brouquisse et al., 2001). Therefore, we assume that only metabolizable sugars can act as inducers of enhanced cytosolic G6PD expression.
A considerable part of photosynthate is deposited as transitory starch in the stroma and can exit the chloroplast in two ways: (a) as triosephosphate (upon phosphorolytic starch breakdown via G6P) using the triose-phosphate/3-phosphoglycerate/phosphate anti-porter (Fliege et al., 1978), or (b) as Glc (upon hydrolytic starch mobilization) mediated by a hexose facilitator in the inner chloroplast membrane (Weber et al., 2000). The dominant pathway seems to be the hydrolytic one, resulting in Glc export at night (Gleixner at al., 1998; Schleucher et al., 1998; Weber et al., 2000). During light-to-dark transitions, concentrations of the regulatory metabolite 2,6-Fru-bisphosphate increase in leaves (Servaites et al., 1989a, 1989b). Concomitantly, cytosolic and stromal levels of Fru-1,6-bisphosphate and triose-phosphates fall to zero (Gerhardt et al., 1987), indicating that in the dark, the synthesis of triose-phosphates and their conversion to hexose-phosphates in the cytosol are negligible.
Here, we demonstrate that water incubation of leaf discs in the light has the same effect as sugar incubation in the dark, and, thus, can be considered a metabolic sink situation. We did not observe an additive effect of Glc and light incubation (data not shown). Supposedly, the responsible sugar sensor in the cytosol cannot differentiate between Glc imported across the plasma membrane and Glc exported from chloroplasts. Hexose accumulation could be sensed by hexokinase (or a yet unknown downstream sensor), and further signaling leads to up-regulation of the gene encoding cytosolic G6PDH in the nucleus.
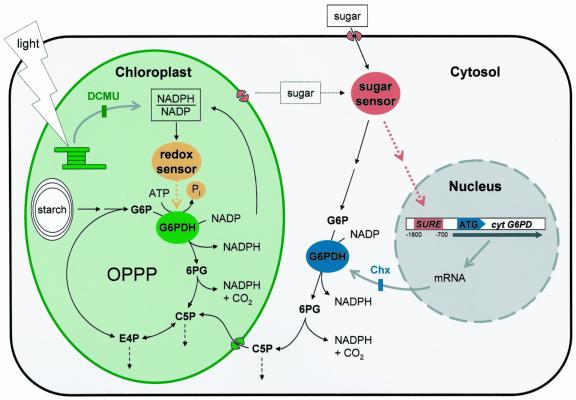
The results are summarized in a model of G6PDH regulation in potato leaf tissue (Fig. 7). Substrate availability, i.e. hexoses accumulating in the cytosol (either upon export of Glc from the chloroplasts or supplied exogenously), is perceived via stimulation of an intracellular sugar sensor—possibly by hexokinase or a yet unknown downstream sensor (Huijser et al., 2000; Xiao et al., 2000; Eastmond et al., 2002). Mediated by a signaling cascade, cognate transcription factors bind to SURE elements in the promoter region and trigger enhanced transcription of the cytosolic G6PD gene. Increased mRNA amounts result in enhanced translation and elevated activity of cytosolic G6PDH.
Figure 7.
Model of G6PDH regulation in the cytosol and in chloroplasts of potato leaf tissue. We chose the more general terms “sugar” and “sugar sensor,” although our results indicate that sugar sensing occurs at the level of hexoses (with Hexokinase as possible sensor). SURE stands for all regulatory promoter elements in the cytosolic G6PD gene involved in sugar-mediated signaling to the nucleus (compare with Fig. 6). For clarity, redox regulation of the chloroplast enzyme was omitted. ATG, Translation start; Chx, inhibitor of cytosolic protein translation; DCMU (inhibitor of photosynthetic electron transport); C5P, C5-sugar phosphates; E4P, erythrose-4-phosphate; 6PG, 6-phosphogluconate. For further explanations, see “Conclusions.”
Because incubation of leaf discs on FeIIICl3, Paraquat, H2O2, Endothall, Okadaic acid, NO2-, NO3-, or SO42- did not alter cytosolic G6PDH activity, we predict that compounds known to cause oxidative stress (FeIIICl3, Paraquat) or defense reactions (H2O2) do not influence cytosolic G6PDH activity directly but act through changes in the cellular carbohydrate state known to switch from “source” to “sink” under these conditions.
Conditions Stimulating DTTred-Sensitive (Plastidic) G6PDH Activity
Increased demand for reductant triggered either by Paraquat, NO2-, NO3-, or SO42- in the dark led to rapid posttranslational stimulation of DTTred-sensitive G6PDH activity. Thus, regulation of the plastidic enzyme(s) completely differs from the cytosolic counterpart. Conditions stimulating cytosolic G6PDH did not affect plastidic activity; conversely, sugar availability (the stimulus leading to upregulation of the cytosolic isoform) had no effect on DTTred-sensitive G6PDH activity. This is surprising and demonstrated best by two graphs that are based on the same incubation experiment (compare Fig. 1 with Fig. 4A). Glc in the dark stimulates only the cytosolic isoform, and Paraquat in the dark only stimulates plastidic G6PDH activity. Substrate for plastid-localized G6PDH in the dark is most likely provided by mobilization of transitory starch. This was shown previously by Thom and Neuhaus (1995) for isolated chloroplasts of green bell pepper fruits. Similar to potato leaf discs in the dark, green fruits represent a chloroplast-containing tissue in a heterotrophic situation.
The herbicide Paraquat, a bipyridin derivative (1,1′-dimethyl-4,4′bipyridin) is known to act as strong electron acceptor of PSI in the light and of NADPH in the dark, mediated by either FNR or ferredoxin (Brian, 1964; Farrington et al., 1973; Oji et al., 1985). Concomitant with auto-oxidation, electrons are transferred from Paraquat to oxygen with the consequence that the produced radicals have to be converted to H2O2 by superoxide dismutase. To avoid formation of the highly toxic hydroxyl radical (OH.-) in the stroma, H2O2 is quickly dissipated via the Halliwell-Asada pathway, consuming NADPH in the final glutathione reductase reaction (Foyer and Halliwell, 1976). In the light, NADPH is amply provided via photosynthetic electron flow through FNR (Shin and Arnon, 1965). In the dark, however, NADPH can only be supplied by the oxidative branch of the plastidic OPPP, i.e. through sequential action of G6PDH and 6PGDH. The importance of up-regulation of chloroplast G6PDH for sustained stromal NADPH provision in the absence of photosynthetic electron transport is emphasized by incubation on Paraquat in the light in combination with the uncoupling agent DCMU (Fig. 4A). In this condition, photosynthetic electron transport to ferredoxin is interrupted, but demand for NADPH production in the stroma prevails due to Paraquat acting as dominant electron acceptor and through the detrimental effects (reactive oxygen intermediate production) caused by the herbicide.
Possible Regulation through Phosphorylation
The stimulation of chloroplast G6PDH activity triggered by an increased stromal demand for electrons in the dark prompted us to investigate the responsible regulatory mechanism. First, the increase in DTTred-sensitive G6PDH activity induced by Paraquat in the dark was not inhibited by Chx. Simultaneous incubation on Glc, Paraquat, and Chx led to stimulation of only plastidic but not cytosolic G6PDH activity (data not shown), which proved that the chosen Chx concentration inhibited translation of nuclear-encoded mRNA without toxic effects on the tissue. Second, for the incubation period studied (72 h), we demonstrate that regulation of cytosolic and plastidic G6PDH activities operate independently. The two identified stimuli (Glc for the cytosolic isoform, increased demand for NADPH in case of the plastidic enzyme[s]) do not influence each other, despite a possible interaction between cytosolic and plastidic OPPP at the level of C5 sugar phosphates via the recently characterized pentosephosphate translocator (Eicks et al., 2002) of the inner chloroplast membrane.
In contrast to cytosolic G6PDH, stimulation of plastidic G6PDH activity did not depend on de novo protein synthesis. Independent of Chx inhibition, DTTred-sensitive G6PDH activity increased about 4-fold (when compared with the initial value) and 10-fold (when compared with water controls incubated in parallel, see Figs. 4 and 5). Protein amounts detected on western blots using isoform-specific antibodies for P1 (Fig. 4B) or P2 (data not shown) did not change and, hence, cannot be responsible for the rapid stimulation of DTTred-sensitive G6PDH activity.
The differences in apparent Glc6P of DTTred-sensitive G6PDH activity indicated that Paraquat incubation probably influences the plastidic enzyme(s) by covalent modification. Km and Vmax were determined in crude extracts and, therefore, are difficult to compare with values previously reported for enriched or partially purified enzyme preparations. Kinetic comparisons must also take into account that the values published by Scheibe et al. (1989) probably represent the harvest situation (mixed state), whereas the data of Wenderoth et al. (1997), determined with recombinant P1 variants expressed in Escherichia coli, most likely reflect the stimulated state. In all previous studies, G6PDH activity in plastids was calculated as difference measured in the oxidized (active) and the reduced (inactive) state, but here we compare for the first time, to our knowledge, plastidic G6PDH activity of the active but unstimulated state (water incubation in the dark) to the maximally stimulated state (Paraquat, NO3-, or SO42- incubations in the dark, compare with Figs. 4 and 5).
The obtained results led to the conclusion that posttranslational modification of plastidic G6PDH is responsible for the rapid activity changes. An effect of redox state can be excluded because for both stimulated and unstimulated enzyme forms, G6PDH activity was determined as difference between samples pre-incubated with either buffer or DTTred. Incubation in the presence of 32P-labeled orthophosphate revealed that at least the P1 enzyme is subject to protein phosphorylation. Incorporation of label increased with time and was much less pronounced in Paraquat-incubated samples. In the dark, 32P-orthophosphate fed to plant cells must first enter the mitochondria for incorporation into ATP and is only then imported by plastids via counterexchange with ADP (Heldt, 1976; Neuhaus et al., 1997). The reason for the 32P labeling of the P1 protein lagging behind the activity changes, therefore, is most likely due to an excess of unlabeled ATP in the stroma at the beginning of the incubation experiments. Independent of knowing the exact kinetics, 32P labeling of the P1 protein correlates with decreased (and less labeling with enhanced) plastidic G6PDH activity (Fig. 4D). The smear below the major band on the autoradiogram could reflect rapid turnover of the phosphorylated enzyme.
For plastid-encoded genes, redox modification and phosphorylation are known to play important regulatory roles (e.g. transcription factor, Tiller and Link, 1993; Baginsky et al., 1997; endonuclease responsible for 3′-processing of plastidic RNA, Liere and Link, 1997). In C4 and CAM plants, pyruvate-pyrophosphate dikinase and phosphoenolpyruvate carboxylase are regulated by phosphorylation and are less active in the phosphorylated state (Wang and Chollet, 1993; Ashton et al., 1984). In C3 plants, chloroplast G6PDH would be the first stromal enzyme involved in primary metabolism whose activity is also regulated by phosphorylation. Until now, only one nuclear-encoded plastid-destined protein, a protein-disulfide isomerase of the green alga Chlamydomonas reinhardtii (Danon and Mayfield, 1994; Kim and Mayfield, 1997), has been shown to be regulated by both redox modification and phosphorylation.
Tests intended to prevent Paraquat-mediated stimulation of plastidic G6PDH activity using the phosphatase inhibitors Endothall or Okadaic acid were unsuccessful (data not shown), possibly due to low uptake into intact leaf tissue (discs floating upside down on the solutions) where the cuticle forms a potential barrier for these substances. Another reason could be that to act, the inhibitors have to reach the chloroplast stroma, whereas they only need to cross the plasma membrane to contact potential cytosolic targets, as described in the work of Sheen (1993) and Ehness et al. (1997). Also, different concentrations of the unspecific phosphatase inhibitor NaF (fluoride is a phosphate analogon) were without effect. On the other hand, the phosphatase involved in G6PDH regulation might be no target for these substances, which matches the recently published results of Lukaszewski et al. (2001). These authors found that in isolated pea (Pisum sativum) root plastids, three major proteins are phosphorylated. Interestingly, enhanced fluxes through the OPPP abolished phosphorylation of a 58-kD protein, and the phosphatase involved did not fall into any class known from mammalian systems. In view of the data presented here, this phosphoprotein could represent G6PDH. The P2 enzyme is the prominent G6PDH in plastids of heterotrophic tissues and migrates slightly more slowly than P1 (Wendt et al., 2000).
Large variations of G6PDH activity in chloroplasts were reported earlier (see Schnarrenberger et al., 1995, and refs. therein) and are also evident from our study (compare the different initial levels in Figs. 4 and 5). After 6 h of water incubation in the dark, DTTred-sensitive G6PDH activity dropped to minimal values (5-10 units m-2 in crude extracts) supposedly corresponding to the phosphorylated state. Because incubation on Paraquat quickly resulted in peak activities, we assume that intermediate initial G6PDH values represent mixtures of phosphorylated and dephosphorylated enzyme molecules, reflecting different levels of stimulation at the beginning of the experiments. Previous reports on obscure (Schnarrenberger et al., 1995), largely varying, or badly reproducible plastidic G6PDH activities can now be explained by differing ratios of unstimulated (phosphorylated) versus stimulated (dephosphorylated) enzyme forms, probably due to local environmental influences.
It appears that chloroplast G6PDH is affected by the stromal redox state at three levels: (a) NADPH is known to act as a competitive inhibitor of G6PDH (Lendzian and Bassham, 1975). In this case, the enzyme itself functions as kind of a sensor (“fine control”). (b) Reversible activation by the ferredoxinthioredoxin system during light/dark transitions (Scheibe and Anderson, 1981) is also closely linked to the prevailing NADPH to NADP ratio in the stroma (“coarse control”). (c) In addition, our results suggest that the oxidized (dark-activated) enzyme can be switched off posttranslationally (most likely by phosphorylation) in extended dark periods, probably to restrict reductant flow into biosynthetic pathways until carbohydrate reserves are replenished. In life-threatening situations imposing elevated demand for stromal NADPH (e.g. oxidative stress and NO2- reduction), however, the enzyme can be stimulated up to 10-fold (most likely by dephosphorylation), a process that could involve redox signaling (Fig. 7). The possible sensor might be equivalent to the one regulating protein-disulfide isomerase in C. reinhardtii (Danon and Mayfield, 1994; Kim and Mayfield, 1997). Target proteins may be inactivated by a redox-sensitive kinase similar to NPH1 (Huala et al., 1997), and reactivation would require interaction with a phosphatase. Redox-sensitive kinases and/or phosphatases have been described to regulate components of PSII, the D1 protein and light-harvesting complex II (Bennet, 1991; Elich et al., 1993, 1997; Silverstein et al., 1993; Durnford and Falkowski, 1998).
It remains to be shown whether there is hierarchy between redox regulation and phosphorylation in vivo. Phosphorylation could act either directly by influencing the catalytic properties of the enzyme or indirectly by interfering with redox regulation. We currently favor the idea that regulation by phosphorylation developed as a means to restrict plastidic G6PDH activity in the oxidized (dark-activated) state. In any case, the two posttranslational mechanisms ensure a quick and tight adaptation of plastidic G6PDH activity to alterations in stromal redox state simply by changing the kinetic properties of the enzyme.
Other Physiological Stimuli
The observation that plastidic G6PDH activity is stimulated by the demand for stromal NADPH prompted us to test other potential stimuli. The importance of plastidic electron transport for NO2- assimilation was reported earlier (Paneque et al., 1964; Oji et al., 1985). Close interaction between nitrogen assimilation and the OPPP was shown for different systems (Bowsher et al., 1989, 1992, 1993; Aubert et al., 1994; Ritchie et al., 1994; Jin et al., 1998). In contrast, there was no experimental evidence for a link between SO42- reduction and the OPPP. Because the complete sulfur reduction pathway is located in plastids, effects comparable with those elicited by nitrogen were also expected for sulfur assimilation. De Kok and Kuiper (1986) demonstrated that de novo reduction of SO42- occurs when spinach (Spinacia oleracea) leaf discs are incubated in the dark. Neuenschwander et al. (1991) showed that in the dark, SO42- assimilation in Lemna minor is limited by O-acetyl-Ser (carbon acceptor) and not by reducing equivalents. The source of the reducing equivalents, however, was not analyzed. Hell (1997) proposed that electrons for sulfur assimilation in the dark are most likely provided by the OPPP via FNR and ferredoxin-like proteins.
We observed stimulation of plastidic G6PDH by NO2-, NO3-, and also by SO42- plus Glc that were comparable with those seen with Paraquat. This shows that physiological electron acceptors can replace Paraquat in stimulating plastidic G6PDH activity in the dark. A major function of the OPPP in darkened leaves seems to be sustained provision of reducing equivalents for biosyntheses like nitrogen and sulfur assimilation. To our knowledge, this is the first direct experimental evidence for an interaction of the plastidic OPPP with SO42- reduction and proves that stimulation by Paraquat is not a result of unspecific tissue damage. The concentrations of NO3- or SO42- used in this study were fairly high but were chosen to provoke a clear response and to yield plastidic G6PDH activities comparable with those observed with Paraquat. Because equimolar KCl concentrations did not stimulate plastidic G6PDH activity, salt effects can be excluded. The leaf disc system seems to be rather insensitive to SO42- or NO3- feeding, which probably reflects absence of high-affinity uptake mechanisms in leaf tissue. The effect of NO2- in lower concentrations (20 mm) may be due to its high membrane permeability and/or toxicity and the resulting need for rapid reduction. It is important to note that feeding cytosolic electron acceptors (FeIIICl3 and NO3-) did not lead to increased cytosolic G6PDH activity, showing that this enzyme is not directly regulated by the demand for reductant.
Our experiments clearly demonstrate interaction of carbohydrate metabolism with nitrogen and sulfur assimilation. Addition of Glc (50 mm) to NO2-, NO3-, or SO42- incubations resulted in higher plastidic G6PDH stimulation than with NO3-, NO2-, or SO42- alone because sugars provide the carbon backbone for ammonium and sulfide acceptors (Glu and O-acetyl-Ser, respectively). The finding that NO2-, NO3-, and SO42- exert the same effect as Paraquat in the dark demonstrates that up-regulation is not stimulus specific but a result of NADPH shortage in the stroma, which somehow influences the catalytic state of the plastidic enzyme(s). Thus, posttranslational regulation of chloroplast G6PDH can be summarized as follows: Redox modification via dithiol/disulfide interchange of two regulatory Cys in the cosubstrate binding domain represents a coarse “off/on” switch during light/dark transitions, and the phosphorylation state probably determines the extent of catalytic activity of the dark-activated (oxidized) enzyme.
CONCLUSIONS
The results suggest that regulation of G6PDH enzymes in the cytosol and in chloroplasts are governed by distinct mechanisms. Figure 7 shows a hypothetical model based on the elucidated regulation principles. High sugar levels in the cytosol trigger elevated transcription of the cytosolic G6PD gene via sugar-mediated signaling to the nucleus. Increased mRNA expression results in higher enzyme levels and G6PDH activity. C5 sugar phosphates formed in the cytosol can be used for nucleotide synthesis or are transported into plastids to replenish continuous withdrawals of R5P and E4P (by plastid-localized nucleotide synthesis and the shikimate pathway, respectively), which is especially important in metabolic sink situations (i.e. in darkness and in heterotrophic tissues). In contrast, G6PDH activity in plastids can be stimulated by low NADPH to NADP ratios (most likely via dephosphorylation of the existing enzyme pool) and is restricted in prolonged dark periods (probably via phosphorylation). This ensures quick adaptation of the OPPP to short-term NADPH shortage in the stroma and could help to poise the important but labile balance of stromal reduction charge in the night, when alternative mechanisms like the malate valve (Fickenscher and Scheibe, 1983) are inactive.
MATERIALS AND METHODS
Harvest and Incubation of Potato (Solanum tuberosum L. cv Désirée) Leaf Discs
Potato seed tubers stored at 16°C for approximately 6 months were planted in a soil:compost:sand mixture (3:3:1 [v/v]) and grown in climate chambers under controlled conditions (10 h of light, 200 μmol m-1 s-1 at 25°C; 14 h of dark at 20°C). When the plants were about 4 weeks old, discs were cut from both sides of the midrib using a cork borer (i.d. = 7 mm) of leaves numbered 3 to 5 (counted from the top, the first leaf measuring about 1 cm in length) always at the beginning of the light period. Up to 30 leaf discs were floated upside-down (to minimize anaerobic effects) on different solutions. Water served as a control. The following substances were tested alone or in combination: 50 mm Glc, Fru, Man, 3-O-MG, or mannitol, respectively; 25 mm Suc; 100 mm KCl; 50 mm KH2PO4; 5 mm 2-deoxy-Glc; 1 mm Chx; 0.1 mm DCMU; 5 μm Paraquat (methylviologen); KNO3 (20, 40, 100, and 250 mm); 20 mm NaNO2 (to avoid toxic effects); K2SO4 (50, 100, and 250 mm); KCl (100 and 250 mm); 50 μm Endothall; 0.5 μm Ocadaic acid; and NaF (20, 40, and 100 mm). All incubations were carried out on 20-mL volume in disposable petri dishes at room temperature for up to 72 h in the dark or in continuous light. After 6, 12, 24, 48, and 72 h, incubated leaf discs were removed from the solutions with forceps, briefly dried on filter paper, snap frozen, and stored in liquid nitrogen. To determine initial values of G6PDH activity (0 h of incubation), samples were directly harvested from the plants and frozen as above.
Enzyme Assays
To reduce variations in the measurements due to the start material (discs cut from different regions of the leaf), we sampled three times two to three leaf discs and extracted them separately. Enzyme activities were determined twice from each of the three extracts. Thus, means ± sds are based on six single values. The leaf disc extraction buffer consisted of 100 mm Trismaleate (pH 8), 5 mm β-mercaptoethanol, 0.1 mm NADP, 1 mm Pefabloc SC (Serva, Heidelberg), and 1:100 (v/v) volume “protease-inhibitor mix for use with plant extracts” (Sigma, Deisenhofen, Germany). Frozen material was crushed in microcentrifuge tubes under liquid nitrogen and suspended in ice-cold extraction buffer (1 μL mg-1 tissue). To each sample, 0.1 mg mg-1 tissue insoluble polyvinylpolypyrrolidone (Sigma) was added. After thawing, samples were thoroughly mixed and kept on ice until centrifugation for 5 min at 4°C and maximum speed. To differentiate between cytosolic and plastidic (DTTred-sensitive) isoenzymes in the supernatant (cleared extract), G6PDH activity was determined immediately under nitrogen atmosphere using Parafilm-sealed glass cuvettes in a dual-wavelength spectrophotometer at 334 nm (with 410-nm reference; Sigma-Eppendorf, Hamburg, Germany). The standard test mixture was also set up under nitrogen atmosphere and consisted of 2 mm G6P and 0.2 mm NADP in 100 mm Trismaleate buffer (pH 8) essentially as described by Graeve et al. (1994). To determine Km and Vmax values, the concentration of one substrate was held constant, and the other one was varied. Before measurements, up to 30 μL of crude extract was used for 10 min of pre-incubation at RT (40-μL total volume), one sample in the presence (i.e. 10 μL of 250 mm DTTred in 300 mm Tris [pH 8], final concentration = 62.5 mm DTTred) and another one in the absence (10 μL of buffer) of reductant. Contribution of cytosolic and plastidic isoenzymes is based on differential calculation of G6PDH activity measured in the absence (total) and presence of DTTred (cytosolic activity): Total minus cytosolic is equal to plastidic G6PDH activity. Protein amounts were determined with the dye-binding assay (Bradford, 1976) and bovine serum albumin as standard protein.
Northern- and Western-Blot Analyses
Total RNA was isolated with the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Each sample consisted of six to eight leaf discs. Isolated RNA (15 μg each) was separated in denaturing agarose gels and blotted on nylon membranes (Hybond N+, Amersham Pharmacia Biotech, Freiburg, Germany). Blots were hybridized with radiolabeled cDNA fragments, washed, and exposed with x-ray film as described in von Schaewen et al. (1995). For western-blot analyses, 20-μL aliquots of three protein samples (extracted as mentioned above) were pooled, separated by SDS-PAGE, blotted on nitro-cellulose membranes (PROTRAN BA85, Schleicher & Schuell, Dassel, Germany), and subjected to immunodetection of G6PDH isoenzymes as described previously (von Schaewen et al., 1995; Wendt et al., 2000).
Immunoprecipitation of 32P-Labeled Proteins
To label phosphoproteins, leaf discs were incubated in the additional presence of 500 μCi of H332PO4 (specific activity 9,000 Ci mmol-1; NEN, Meckenheim, Germany) diluted in 100 μm KH2PO4 (5-mL total volume). Extraction of radiolabeled leaf discs was in 1 mL of Tris-maleate buffer (also used for enzyme measurements and western blots; Graeve et al., 1994). Immunoprecipitation under denaturing conditions was performed essentially as described by Anderson and Blobel (1983) using 5 μL of polyclonal rabbit antiserum raised against the recombinant P1 protein (von Schaewen et al., 1995). Complexes bound to protein-A Sepharose (Sigma) were washed extensively and then boiled for 5 min in SDS-loading buffer. Released polypeptides were separated in 12% (w/v) SDS gels. After SDS-PAGE, the gel was stained with Coomassie Brilliant Blue, dried, and exposed with Hyperfilm β-max autoradiography film (Amersham, Braunschweig, Germany) for up to 5 d.
Isolation of Genomic Clones
A genomic potato DNA library cloned in λEMBL3 (kindly provided by the group of Uwe Sonnewald, IPK Gatersleben, Germany) was screened using the entire radiolabeled NotI-cDNA fragment of the cytosolic G6PD isoform as a probe (Graeve et al., 1994). Hybridization and wash conditions were as described for tobacco (Nicotiana tabacum) and Arabidopsis cDNA libraries (Wendt et al., 1999). Phage DNA of identified clones was isolated with the Lambda System kit (Qiagen) using liquid cultures or plate lysates as start material.
Cloning and Sequencing Procedures
DNA fragments of isolated phage clones were ligated to compatible restriction sites of plasmid vector pBluescript SK (Stratagene, Heidelberg) using standard procedures (Sambrook et al., 1989) and introduced into RbCl competent (Hanahan, 1983) Escherichia coli XL1-Blue cells (Stratagene). Sequence analysis of positive clones was conducted as described by Wendt et al. (1999). Further analyses and assembly of nucleotide sequences employed the GCG software package (Devereux et al., 1984).
Acknowledgments
The authors thank Monika Nietschke for excellent technical assistance and the gardeners of the Plant Physiology Department in Osnabrück for continuous provision of healthy plants. They gratefully acknowledge the group of Uwe Sonnewald (IPK Gatersleben, Germany) for providing the genomic potato library and the helpful initial advice of Andrea Polle (Universität Göttingen, Germany) on setting up a leaf disc incubation system for potato.
This work was supported by the Deutsche Forschungsgemeinschaft (Scha 541/3).
References
- Anderson DJ, Blobel G (1983) Immunoprecipitation of proteins from cell-free translations. Methods Enzymol 96: 111-120 [DOI] [PubMed] [Google Scholar]
- Ashton AR, Burnell JN, Hatch MD (1984) Regulation of C4 photosynthesis: inactivation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and activation by phosphorolysis. Arch Biochem Biophys 230: 492-503 [DOI] [PubMed] [Google Scholar]
- Aubert S, Gout E, Bligny R, Douce R (1994) Multiple effects of glycerol on plant cell metabolism. J Biol Chem 269: 21420-21427 [PubMed] [Google Scholar]
- Baginsky S, Tiller K, Link G (1997) Transcription factor phosphorylation by a protein kinase associated with chloroplast RNA polymerase from mustard (Sinapis alba). Plant Mol Biol 34: 181-189 [DOI] [PubMed] [Google Scholar]
- Batz O, Logemann E, Reinold S, Hahlbrock K (1998) Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. Biol Chem 379: 1127-1135 [DOI] [PubMed] [Google Scholar]
- Bennet J (1991) Protein phosphorylation in green plant chloroplasts. Annu Rev Plant Physiol Plant Mol Biol 42: 281-311 [Google Scholar]
- Börner H, Grisebach H (1982) Enzyme induction in soybean infected by Phytophtora megasperma f. sp. glycinea. Arch Biochem Biophys 217: 65-71 [DOI] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, van Montagu M, Inze D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10: 1723-1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher CG, Boulton EL, Rose J, Nayagam S, Emes MJ (1992) Reductant for glutamate synthesis is generated by the oxidative pentose phosphate pathway in non-photosynthetic root plastids. Plant J 2: 893-896 [Google Scholar]
- Bowsher CG, Hucklesby DP, Emes MJ (1989) Nitrite reduction and carbohydrate metabolism in plastids purified from roots of Pisum sativum L. Planta 177: 359-366 [DOI] [PubMed] [Google Scholar]
- Bowsher CG, Hucklesby DP, Emes MJ (1993) Induction of ferredoxin-NADP+ oxidoreductase and ferredoxin synthesis in pea root plastids during nitrate assimilation. Plant J 3: 463-467 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of dye-binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Brian RC (1964) The classification of herbicides and types of toxicity. In LJ Audus, ed, The Physiology and Biochemistry of Herbicides. Academic Press, London, pp 1-33
- Brouquisse R, Evrard A, Rolin D, Raymond P, Roby P (2001) Regulation of protein degradation and protease expression by mannose in maize root tips: Pi sequestration by mannose may hinder the study of its signalling properties. Plant Physiol 125: 1485-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB (1991) Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Arch Biochem Biophys 288: 1-9 [DOI] [PubMed] [Google Scholar]
- Cheng CL, Acedo GN, Christinsin M, Conkling MA (1992) Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA 89: 1861-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland L, Turner JF (1987) The regulation of glycolysis and the pentosephosphate pathway. In A Marcus, ed, The Biochemistry of Plants, Vol 11. Academic Press, New York, pp 107-125 [Google Scholar]
- Cortès S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisse RM (2003) In plants, 3-O-methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol 131: 824-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S, Hinderer W, Barz W (1988) Elicitor induced changes of enzyme activities related to isoflavone and pterocarpan accumulation in chickpea (Cicer arietinum L.) cell suspension cultures. Z Naturforsch 43c: 536-544 [Google Scholar]
- Daniel S, Tiemann K, Wittkampf U, Bless W, Hinderer W, Barz W (1990) Elicitor-induced metabolic changes in cell cultures of chickpea (Cicer arietinum L.) cultivars resistant and susceptible to Ascochyta rabiei: I. Investigations of enzyme activities involved in isoflavone and pterocarpan phytoalexin biosynthesis. Planta 182: 270-278 [DOI] [PubMed] [Google Scholar]
- Danon A, Mayfield SP (1994) Light-regulated translation of chloroplast messenger RNAs through redox potential. Science 266: 1717-1719 [DOI] [PubMed] [Google Scholar]
- de Kok LJ, Kuiper PJC (1986) Effect of short-term-incubation with sulfate, chloride and selenate on the glutathione content of spinach leaf discs. Physiol Plant 68: 477-482 [Google Scholar]
- Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnford DG, Falkowski PG (1998) Chloroplast redox regulation of nuclear gene transcription during photoacclimation. Photosynth Res 53: 229-241 [Google Scholar]
- Eastmond PJ, van Dijken AJH, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JDG, Smeekens SC, Graham IA (2002) Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 29: 225-235 [DOI] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt D, Roitsch T (1997) Glucose and stress independently regulate source and sink metabolism via signal transduction pathways involving protein phosphorylation. Plant Cell 9: 1825-1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicks M, Maurino V, Fluegge U-I, Fischer K (2002) The plastidic pentosephosphate translocator represents a link between the cytosolic and the plastidic pentose-phosphate pathways in plants. Plant Physiol 128: 512-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK (1993) Dephosphorylation of photosystem II core proteins is light-regulated in vivo. EMBO J 12: 4857-4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK (1997) Evidence for light-regulated and light-independent protein dephosphorylation in chloroplasts. FEBS Lett 411: 236-238 [DOI] [PubMed] [Google Scholar]
- Endo BY, Veech JA (1969) The histochemical localization of oxidoreductive enzymes of soybeans infected with the root knot nematode Meloidogyne incognita acrita. Phytopathology 59: 418-425 [PMC free article] [PubMed] [Google Scholar]
- Farrington JA, Ebert M, Land EJ, Fletcher K (1973) Bipyridylium salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta 314: 372-381 [DOI] [PubMed] [Google Scholar]
- Fickenscher K, Scheibe R (1983) Purification and properties of NADP-dependent malate dehydrogenase from pea leaves. Biochim Biophys Acta 749: 249-254 [Google Scholar]
- Fickenscher K, Scheibe R (1986) Purification and properties of the cytoplasmic glucose-6-phosphate dehydrogenase from pea leaves. Arch Biochem Biophys 247: 393-402 [DOI] [PubMed] [Google Scholar]
- Fliege R, Flügge U-I, Werdan K, Heldt HW (1978) Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta 505: 232-247 [DOI] [PubMed] [Google Scholar]
- Foyer C, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 133: 21-25 [DOI] [PubMed] [Google Scholar]
- Fu H, Kim SY, Park WD (1995) High-level tuber expression and sucrose inducibility of a potato Sus4 sucrose synthase gene require 5′ and 3′ flanking sequences and the leader intron. Plant Cell 7: 1387-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW (1987) Subcellular metabolite levels in spinach leaves. Plant Physiol 83: 399-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner G, Scrimgeour C, Schmidt H-L, Viola R (1998) Stable isotope distribution in the major metabolites of source and sink organs of Solanum tuberosum L.: a powerful tool in the study of metabolite partitioning in intact plants. Planta 207: 241-245 [Google Scholar]
- Godt DE, Riegel A, Roitsch T (1995) Regulation of sucrose synthase expression in Chenopodium rubrum: characterization of sugar induced expression in photoautotrophic suspension cultures and sink tissue specific expression in plants. J Plant Physiol 146: 231-238 [Google Scholar]
- Graeve K, von Schaewen A, Scheibe R (1994) Purification, characterization and cDNA sequence of glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L.). Plant J 5: 353-361 [DOI] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver C (1994) Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell 6: 761-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson C, Du J-S, de Torres Zabala M, Beggs K, Smith C, Holdsworth M, Bevan M (1994) Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J 5: 816-826 [DOI] [PubMed] [Google Scholar]
- Halford NG, Purcell PC, Hardie DG (1999) Is hexokinase really a sugar sensor in plants? Trends Plant Sci 4: 117-120 [DOI] [PubMed] [Google Scholar]
- Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166: 557-580 [DOI] [PubMed] [Google Scholar]
- Hattori T, Nakagawa S, Nakamura K (1990) High level expression of tuberous root storage protein genes of sweet potato in stems of plantlets grown in vitro on sucrose medium. Plant Mol Biol 14: 595-604 [DOI] [PubMed] [Google Scholar]
- Heber U, Hudson MA, Hallier UW (1967) Lokalisation von Enzymen des Reduktiven und des Oxidativen Pentosephosphatzyklus in den Chloroplasten und Permeabilität der Chloroplastenmembranen gegenüber Metaboliten. Z Naturforsch 22b: 1200-1215 [PubMed] [Google Scholar]
- Heldt HW (1976) Transfer of substrates across the chloroplast envelope. Horiz Biochem Biophys 2: 199-299 [PubMed] [Google Scholar]
- Hell R (1997) Molecular physiology of plant sulfur metabolism. Planta 202: 138-148 [DOI] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Métraux J-P, Sonnewald U (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8: 793-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgarth C, Sauer N, Tanner W (1991) Glucose increases the expression of the ATP/ADP translocator and the glyceraldehyde-3-phosphate dehydrogenase genes in Chlorella. J Biol Chem 266: 24044-24047 [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR (1997) Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278: 2120-2123 [DOI] [PubMed] [Google Scholar]
- Huijser C, Korstee A, Pego J, Wisman E, Smeekens S (2000) The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J 23: 577-585 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Nakamura K (1994) Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognises SP8 sequences in the 5′ upstream region of genes coding for sporamin and β-amylase from sweet potato. Mol Gen Genet 244: 563-571 [DOI] [PubMed] [Google Scholar]
- Jang J-C, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6: 1665-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Huppe HC, Turpin DH (1998) In vitro reconstitution of electron transport from glucose-6-phosphate and NADPH to nitrite. Plant Physiol 117: 303-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HS (1972) Dithiothreitol: An inhibitor of glucose-6-phosphate dehydrogenase activity in leaf extracts and isolated chloroplasts. Planta 106: 273-277 [DOI] [PubMed] [Google Scholar]
- Kim J, Mayfield SP (1997) Protein disulfide isomerase as a regulator of chloroplast translational activation. Science 278: 1954-1957 [DOI] [PubMed] [Google Scholar]
- Kim S-R, Costa MA, An G (1991) Sugar response element enhances wound response of potato proteinase inhibitor II promoter in transgenic tobacco. Plant Mol Biol 17: 973-983 [DOI] [PubMed] [Google Scholar]
- Klein D, Stitt M (1998) Effects of 2-deoxyglucose on the expression of rbcS and the metabolism of Chenopodium rubrum cell-suspension cultures. Planta 205: 223-234 [Google Scholar]
- Knight JS, Emes MJ, Debnam P (2001) Isolation and characterisation of a full-length genomic clone encoding a plastidic glucose-6-phosphate dehydrogenase from Nicotiana tabacum. Planta 212: 499-507 [DOI] [PubMed] [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509-540 [DOI] [PubMed] [Google Scholar]
- Lendzian K, Bassham JA (1975) Regulation of glucose-6-phosphate dehydrogenase in spinach chloroplasts by ribulose 1,5-diphosphate and NADPH/NADP+ ratios. Biochem Biophys Acta 396: 260-275 [DOI] [PubMed] [Google Scholar]
- Liere K, Link G (1997) Chloroplast endoribonuclease p54 involved in RNA 3′-end processing is regulated by phosphorylation and redox state. Nucleic Acids Res 25: 2403-2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewski KM, Bowsher CG, Savory PJ, Emes MJ (2001) Protein phosphorylation in pea root plastids. Plant Cell Physiol 42: 642-649 [DOI] [PubMed] [Google Scholar]
- Mignery GA, Pikaard CS, Park WD (1988) Molecular characterization of the patatin multigene family of potato. Gene 62: 27-44 [DOI] [PubMed] [Google Scholar]
- Müller-Röber BT, Koβmann J, Hannah LC, Willmitzer L, Sonnewald U (1990) One of two different ADP-G glucose-pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet 224: 136-146 [DOI] [PubMed] [Google Scholar]
- Neuenschwander U, Suter M, Brunold C (1991) Regulation of sulfate assimilation by light and O-acetyl-l-serine in Lemna minor L. Plant Physiol 97: 253-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus HE, Thom E, Möhlmann T, Steup M, Kampfenkel K (1997) Characterization of a novel eukaryotic ATP/ADP translocator located in the plastid envelope of Arabidopsis thaliana L. Plant J 11: 73-82 [DOI] [PubMed] [Google Scholar]
- Oji Y, Watanabe M, Wakiuchi N, Okamoto S (1985) Nitrite reduction in barley root plastids: Dependence on NADPH coupled with glucose-6-phosphate and 6-phosphogluconate dehydrogenases, and possible involvement of an electron carrier and a diaphorase. Planta 165: 85-90 [DOI] [PubMed] [Google Scholar]
- Paneque A, Ramirez JM, Del Campo FF, Losada M (1964) Light and dark reduction of nitrite in a reconstituted enzymic system. J Biol Chem 239: 1737-1741 [PubMed] [Google Scholar]
- Ritchie SW, Redinbaugh MG, Shiraishi N, Vrba JM, Campbell WH (1994) Identification of a maize root transcript expressed in the primary response to nitrate: characterization of a cDNA with homology to ferredoxin-NADP+-oxidoreductase. Plant Mol Biol 26: 679-690 [DOI] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8: 23-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Bittner M, Godt DE (1995) Induction of apoplastic invertase of Chenopodium rubrum by d-Glucose and a glucose analog and tissue-specific expression suggest a role in sink-source-regulation. Plant Physiol 108: 285-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook F, Gerrits N, Kortstee A, van Kampen M, Borrias M, Weisbeek P, Smeekens S (1998) Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J 15: 253-263 [DOI] [PubMed] [Google Scholar]
- Salanoubat M, Belliard G (1989) The steady state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis, and sucrose concentration. Gene 84: 181-185 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sarah CJ, Graham IA, Reynolds SJ, Leaver CJ, Smith SM (1996) Distinct cis-acting elements direct the germination and sugar response of the cucumber malate synthase gene. Mol Gen Genet 250: 153-161 [DOI] [PubMed] [Google Scholar]
- Scheibe R (1990) Light/dark modulation: regulation of chloroplast metabolism in a new light. Bot Acta 103: 327-334 [Google Scholar]
- Scheibe R, Anderson LE (1981) Dark modulation of NADP-dependent malate dehydrogenase and glucose-6-phosphate dehydrogenase in the chloroplast. Biochem Biophys Acta 636: 58-64 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Geissler A, Fickenscher K (1989) Chloroplast glucose-6-phosphate dehydrogenase: Km shift upon light modulation and reduction. Arch Biochem Biophys 274: 290-297 [DOI] [PubMed] [Google Scholar]
- Schleucher J, Vanderveer PJ, Sharkey TD (1998) Export of carbon from chloroplasts at night. Plant Physiol 118: 1439-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J, Amrhein N (1995) Molecular organization of the shikimate pathway in higher plants. Phytochemistry 39: 737-749 [Google Scholar]
- Schnarrenberger C, Flechner A, Martin W (1995) Enzymatic evidence for a complete oxidative pentose phosphate pathway in chloroplasts and an incomplete pathway in the cytosol of spinach leaves. Plant Physiol 108: 609-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C, Oeser A, Tolbert NE (1973) Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys 154: 438-448 [DOI] [PubMed] [Google Scholar]
- Servaites JC, Fondy BR, Li B, Geiger DR (1989b) Sources of carbon for export from spinach leaves throughout the day. Plant Physiol 90: 1168-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites JC, Geiger DR, Tucci MA, Fondy BR (1989a) Leaf carbon metabolism and metabolite levels during a period of sinusoidal light. Plant Physiol 89: 403-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1993) Protein phosphatase activity is required for light-inducible gene expression in maize: phosphatase. EMBO J 12: 3497-3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Arnon DI (1965) Enzymic mechanisms of pyridine nucleotide reduction in chloroplasts. J Biol Chem 240: 1405-1411 [PubMed] [Google Scholar]
- Silverstein T, Cheng L, Allen JF (1993) Chloroplast thylakoid protein phosphatase reactions are redox-independent and kinetically heterogeneous. FEBS Lett 334: 101-105 [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F (1997) Sugar sensing and sugar-mediated signal-transduction in plants. Plant Physiol 115: 7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom E, Neuhaus HE (1995) Oxidation of imported or endogenous carbohydrates by isolated chloroplasts from green pepper fruits. Plant Physiol 109: 1421-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller K, Link G (1993) Phosphorylation and dephosphorylation affect functional characteristics of chloroplast and etioplast transcription systems from mustard (Sinapis alba L.). EMBO J 12: 1745-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H, Ohshima T, Naito S, Chino M, Komeda Y (1991) Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol 97: 1414-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz M, Moureaux T, Leydecker M-T, Vaucheret H, Caboche M (1993) Regulation of nitrate and nitrite reductase expression in Nicotiana plum-baginifolia leaves by nitrogen and carbon metabolites. Plant J 3: 315-324 [DOI] [PubMed] [Google Scholar]
- von Schaewen A, Langenkämper G, Graeve K, Wenderoth I, Scheibe R (1995) Molecular characterization of the plastidic glucose-6-phosphate dehydrogenase from potato in comparison to its cytosolic counterpart. Plant Physiol 109: 1327-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Chollet R (1993) In vitro phosphorylation of purified tobacco-leaf phosphoenol-pyruvate carboxylase. FEBS Lett 328: 215-218 [DOI] [PubMed] [Google Scholar]
- Wang R, Guegler K, LaBrie ST, Crawford NM (2000) Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 12: 1491-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge U-I (2000) Identification, purification, and molecular cloning of a putative plastidic glucose transporter. Plant Cell 12: 787-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth I, Scheibe R, von Schaewen A (1997) Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J Biol Chem 272: 26985-26990 [DOI] [PubMed] [Google Scholar]
- Wendt UK, Hauschild R, Lange C, Pietersma M, Wenderoth I, von Schaewen A (1999) Evidence for functional convergence of redox regulation in G6PDH isoforms of cyanobacteria and higher plants. Plant Mol Biol 40: 487-494 [DOI] [PubMed] [Google Scholar]
- Wendt UK, Wenderoth I, Tegeler A, von Schaewen A (2000) Molecular characterization of a novel glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L.) Plant J 23: 723-733 [DOI] [PubMed] [Google Scholar]
- Wenzler HC, Mignery GA, Fisher LM, Park WD (1989) Analysis of a chimeric class I-patatin GUS gene in transgenic potato plants: high level expression in tubers and sucrose inducible expression in cultured leaf and stem explants. Plant Mol Biol 12: 41-50 [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393: 365-369 [DOI] [PubMed] [Google Scholar]
- Williams JF (1980) A critical examination of the evidence for the reactions of the pentose pathway in animal tissues. Trends Biochem Sci 5: 315-320 [Google Scholar]
- Wright DP, Huppe HC, Turpin DH (1997) In vivo and in vitro studies of glucose-6-phosphate dehydrogenase from barley root plastids in relation to reductant supply for NO2- assimilation. Plant Physiol 114: 1413-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Sheen J, Jang J-C (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44: 451-461 [DOI] [PubMed] [Google Scholar]