Abstract
Prenylation of Rab GTPases regulating vesicle traffic by Rab geranylgeranyltransferase (RabGGTase) requires a complex formed by the association of newly synthesized Rab proteins with Rab-escort-protein (REP), the choroideremia-gene-product that is mutated in disease, leading to loss of vision. After delivery to the membrane by the REP–Rab complex, subsequent recycling to the cytosol requires the REP-related guanine-nucleotide-dissociation-inhibitor (GDI). Although REP and GDI share common Rab-binding properties, GDI cannot assist in Rab prenylation and REP cannot retrieve Rab proteins from the membranes. We have now isolated REP mutant proteins that are able to partially function as both REP and GDI. These results provide molecular insight into the functional and evolutionary organization of the REP/GDI superfamily.
INTRODUCTION
Rab proteins form a large family of GTPases that are prenylated at their carboxyl terminus and play a critical role in the vesicular trafficking in eukaryotic cells (Peirera-Leal and Seabra, 2001). Mammalian Rab GTPases and their yeast Sec4p and Yptp counterparts promote the assembly and disassembly of tethering/fusion complexes that direct the interaction of membranes comprising the exocytic and endocytic pathway (Stenmark et al., 1995; Allan et al., 2000; Moyer et al., 2001; for reviews, see Pfeffer, 2001; Zerial and McBride, 2001).
Targeting of Rab proteins to the membrane requires prenylation by Rab escort protein (REP), the product of the choroideremia gene (CHM) (Seabra et al., 1992a,b; Andres et al., 1993) that when deleted in hereditary disease leads to loss of vision. In mammalian cells, two related REP proteins, REP1 (also known as CHM) and REP2 (also referred to as CHML), which share 90% identity, have been identified (Seabra et al., 1993; Cremers et al., 1994). In the budding yeast Saccharomyces cerevisiae, REP is encoded by a single essential gene MRS6/MSI4 (Fujimura et al., 1994). The functional properties of the Mrs6 protein have been recently characterized at the biochemical and molecular levels (Anant et al., 1998; Alexandrov et al., 1999; Alory and Balch, 2000; Thoma et al., 2001a). REP binds newly synthesized Rab proteins forming a cytosolic complex that presents Rab to the catalytic component, Rab geranylgeranyltransferase (RabGGTase). RabGGTase is a heterodimer composed of two tightly associated α- and β-subunits (Armstrong et al., 1993; Zhang et al., 2000). In S. cerevisiae, α- and β-subunits are encoded by the BET4 (known as MAD2) and BET2 genes, sharing 24 and 52% identity with the α- and β-subunits of the mammalian enzyme, respectively (Rossi et al., 1991; Jiang et al., 1993). Prenylation by RabGGTase involves the addition of two geranylgeranyl groups via a thioether linkage to carboxyl-terminal cysteine containing CC or CXC motifs (where X is any amino acid) (Seabra et al., 1992b; Shen and Seabra, 1996; Thoma et al., 2001b,c). After prenylation, the RabGGTase dissociates from the REP–Rab complex that delivers prenylated Rab to the membrane (for review, see Alory and Balch, 2001).
Although REP promotes delivery of newly synthesized Rab to the membrane, Rab proteins are retrieved to the cytosol for use in subsequent rounds of vesicle formation by complexing to guanine nucleotide dissociation inhibitor (GDI). GDI binds all Rab GTPases (for reviews, see Wu et al., 1996; Alory and Balch, 2001) through a common Rab-binding platform (Schalk et al., 1996; Luan et al., 1999; Luan et al., 2000). At least five mammalian GDI isoforms have been identified to date (Janoueix-Lerosey et al., 1995). In S. cerevisiae, Gdi1p, the product of the single GDI1/SEC19 gene is highly related to mammalian GDI (>50% identity with α-GDI) and is essential for cell growth (Garrett et al., 1994). GDI isoforms play a key role in the Rab GTPase cycle by retrieving prenylated Rab proteins from membranes, maintaining Rab GTPases as a soluble cytosolic complex, and delivering Rab to discrete compartments for vesicle formation.
Sequence comparison of members of the GDI and REP protein families has shown five sequence conserved regions (SCRs 1A, 1B, 2, 3A, and 3B) (Waldherr et al., 1993). The three dimensional structure of bovine α-GDI (Schalk et al., 1996) has been crucial to elucidate the functional significance of SCRs (Schalk et al., 1996; Luan et al., 2000) (for review, see Wu et al., 1996). Bovine α-GDI is constructed of two main structural units, a large multisheet domain I and a small α-helical domain II found at the base of the protein. All SCRs are located on one face of the molecule that is likely to be the face that interfaces with the lipid bilayer (Luan et al., 2000; Sakisaka et al., 2002; An et al., 2003). Residues comprising SCR1 and 3B form a compact structure at the apex of GDI that has been shown to be the Rab-binding platform in α-GDI (Schalk et al., 1996; Wu et al., 1998; Luan et al., 1999) and Mrs6p (Alory and Balch, 2000). The two other evolutionary conserved regions, SCR2 and SCR3A, contribute to the structural organization of domain II. In particular, residues found in SCR3A form the mobile effector loop (MEL), a region critical for extraction of membrane-bound Rab proteins through a geranylgeranyl switching mechanism (An et al., 2003), which in the case of Rab3A occurs through interaction with Hsp90 (Sakisaka et al., 2002). Domain II in Mrs6p has been shown to be important for the interaction of REP1 with the RabGGTase by using both molecular (Alory and Balch, 2000) and structural approaches (Pylypenko et al., 2003).
Although REP and GDI share similar functions, such as the ability to deliver Rab proteins to membranes and to inhibit the release of GDP from Rab, GDI cannot assist in the prenylation of newly synthesized Rab proteins and REP cannot retrieve Rab proteins from the membranes. Moreover, neither GDI1 nor MRS6 can suppress lethality in response to the disruption of the MRS6 or GDI1 gene, respectively (Garrett et al., 1994).
In this study, we have analyzed the conservation of function of the REP and GDI protein families. We find that yeast REP can be mutated to function as yeast GDI and can separately complement both yeast Δmrs6 and Δgdi1 null strains. Mapping of mutants revealed that structural modifications leading to gain-of-function occurred in both the Rab-binding domain (I) and in domain (II) involved in recognition of RabGGTase by REP and membrane receptors by GDI. Our results now provide general insight into the molecular and functional organization of the REP/GDI superfamily.
MATERIALS AND METHODS
Strains and Media
The S. cerevisiae strains used in these studies are listed in Table 1. Yeast strains were grown in standard yeast extract-peptone-dextrose (YPD) or synthetic medium with dextrose (SD) supplemented as needed with amino acids (Sherman et al., 1979).
Table 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| SEY6210 | Matα leu2-3,112 ura3-52 his3-Δ200 trpl- Δ901lys2-801 suc2- Δ9 | Robinson et al. (1988) |
| SEY6210Δmrs6 | SEY6210; mrs6Δ::HIS3; MRS6::URA3 | Alory and Balch (2000) |
| SEY6210 Δgdi1 | SEY6210; gdi1Δ::HIS3; GD11::URA3 | Luan et al. (1999) |
| sec19-1 | NY420; MATα, ura3-52, sec19-1 | Novick et al. (1980) |
Bacterial strains (DH5α) were grown on standard media (Miller, 1972), supplemented with 100 μg/ml ampicillin for plasmid retention.
DNA Methods
Standard DNA manipulation (Maniatis et al., 1982) was used with restriction endonucleases and modification enzymes from Roche Applied Science (Indianapolis, IN), PerkinElmer Life Sciences (Boston, MA), and Promega (Madison, WI). Point mutations were introduced by using the QuikChange site-directed mutagenesis Kit (Stratagene, Cedar Creek, TX).
Bacterial and Yeast Methods
Standard yeast and Escherichia coli genetic techniques were carried out as described previously (Miller, 1972; Sherman et al., 1979). Yeast transformations were performed with the lithium acetate method (Ito et al., 1983). E. coli transformations were done as described previously (Hanahan, 1983).
Random Mutagenesis of the MRS6 and GDI1 Genes
The MRS6 gene was subjected to random polymerase chain reaction (PCR) mutagenesis by using 5′-ccggatctcgagtttttattccgttcatc-3′ and 5′-cggcatgagctcggatcctttttttacatatatatactatc-3′ as primers. To introduce incorporation errors during amplification, the PCR was performed with a reduced deoxyribonucleotide triphosphate concentration (1/5 concentration of one of the four dNTPs compared with the others). As a result, PCR fragments of 2123 base pairs in length containing random mutation were amplified. A similar approach was used to mutagenize the GDI1 gene. The GDI1 gene was subjected to random PCR mutagenesis by using 5′-ggatcccgtaatacacccatattcttgtac-3′ and 5′-gaatactagtgttcttgacatggtactgcg-3′ as primers. As a result, PCR fragments of 2459 base pairs in length containing random mutation were amplified.
Immunoblotting
Cultures were grown to exponential phase at 30°C in minimal medium. Cells were lysed (5 OD600 units/ml) in standard lysis buffer (20 mM HEPES, pH 6.2, 200 mM sorbitol, 100 mM potassium acetate, 2 mM MgCl2, 1 mM dithiothreitol [DTT]) containing glass beads and subjected to centrifugation. For immunoblotting, a rabbit anti-Mrs6p antibody and the alkaline phosphatase-linked goat anti-rabbit immunoglobin G (Pierce Chemical, Rockford, IL) were used at 1:10,000 and 1:3,000 dilution, respectively.
Fluorescence Assay for Rab–REP Interaction
Rab-REP interactions with fluorescence were performed as described previously (Alory and Balch, 2000). Briefly, recombinant His6-tagged Rab3A or Ypt1p were loaded with the fluorescent GDP analog methylanthraniloyl guanosine diphosphate (mant-GDP) (Molecular Probes) by incubating at 32°C for 45 min with mant-GDP and Rab proteins at a 100:1 M ratio in 50 mM Tris-HCl, pH 7.2, 10 mM EDTA, 1 mM DTT. The mixture was then adjusted to 20 mM MgCl2 and incubated for 15 more min at 32°C. The free mant-GDP was removed by using a MicroSpin G25 column (Amersham Biosciences, Piscataway, NJ). Dissociation was measured by using 100 nM Rab(mant-GDP) incubated with increasing amount of Mrs6p wild-type or mutants in 300 μl of fluorescence buffer (25 mM Tris-HCl, pH 7.2, 0.5 mM MgCl2, 0.6 mM EDTA, 0.3 mM GDP) using an LS50B fluorescence spectrometer (PerkinElmer Life Sciences) with a λ excitation at 360 nm and λ emission at 440 nm.
Geranylgeranylation Assay
In vitro geranylgeranylation were performed by incubating recombinant Rab proteins with [3H]geranylgeranylpyrophosphate as described previously (Alory and Balch, 2000). Briefly, a 60-μl volume reaction containing 50 mM Tris-HCl, pH 7.5, 5 mM DTT, 10 mM MgCl2, 0.5 μl of [3H]geranylgeranylpyrophosphate (20 Ci/mmol), 10 μg of Ypt1p was mixed with 250 μg of yeast crude extract. After incubation for 30 min at 30°C, 1 ml of ethanol/0.1 N HCl was added to the reaction mixture and incubated for 10 min at room temperature. The reaction mixture was applied onto GF/A paper (Whatman, Maidstone, UK) and washed twice with 2 ml of ethanol. The filters were dried and radioactivity was counted in a scintillation counter.
Membrane Extraction of Rab Proteins
Yeast membranes were treated with either recombinant wild-type or mutant Gdi1p or Mrs6p as indicated in RESULTS. In a 50-μl volume reaction, 100 μg of Golgi-enriched membranes was incubated for 2 h at 30°C in membrane extraction buffer (22 mM HEPES-KOH, pH 7.2, 20 mM Tris-HCl, 116 mM KCl, 4.3 mM MgOAc, 1 mM GDP, 2 mM DTT) containing protease inhibitors (2 mM phenylmethylsulfonyl fluoride, 5 μg/ml antipain, 1 μg/ml aprotinin, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 10 μg/ml α2-macroglobulin). Subsequently, 5 μg of recombinant proteins were added and incubated for 5 min at 30°C. The soluble and membrane bound Rab-containing fractions separated by centrifugation were analyzed by 12% SDS-PAGE, immunoblotted using polyclonal anti-Ypt1p antibody, and quantified by densitometry as described previously (Alory and Balch, 2000).
RESULTS
A Model System to Study the Functional Evolution of the REP/GDI Superfamily
GDI and REP share functional attributes, including the ability to 1) bind a diverse group of Rab proteins for delivery to the membranes, and 2) prevent GDP-dissociation from Rab GTPases. However, neither GDI nor MRS6 can complement the Δmrs6 and Δgdi1 null strains, respectively. Thus, the functions of Rab prenylation and Rab retrieval from membranes to the cytosol have diverged. To begin to elucidate the structural foundation for this divergence, we reasoned that functional domains of REP or GDI involved in prenylation or retrieval of Rab proteins may be interconvertable through mutagenesis. For this purpose, we took advantage of the fact that the budding yeast S. cerevisiae contains only a single REP (MRS6) gene and a single GDI (GDI1) gene. Each of these genes is essential for yeast cell growth (Fujimura et al., 1994; Garrett et al., 1994). Therefore, we carried out a screen to isolate Mrs6p or Gdi1p mutants, which can function as Gdi1p or Mrs6p, respectively, and tested these mutants for each of the essential functions of the REP/GDI superfamily, including support of cell growth, Rab-binding, Rab-prenylation, and membrane extraction of Rab.
MRS6 and GDI1 genes were subjected to random PCR mutagenesis, and a gapped plasmid repair transformation was subsequently used to replace the wild-type and MRS6 genomic copies (Alory and Balch, 2000). Growth of the resultant strains was assayed after restreaking to 5-fluoroorotic acid medium to select for loss of the URA3 GDI1 or URA3 MRS6 plasmid, leaving the Mrs6p or Gdi1p mutant proteins as the only source of Gdi1p or Mrs6p, respectively. Using this scheme neither wild-type GDI1 nor MRS6 was able to complement mrs6Δ or gdi1Δ null mutation, respectively (Table 2; data not shown). Despite a number of screens, gdi1 clones could not be found that complemented the mrs6Δ null mutation. However, from two independent screens, four mrs6 clones were found to complement the gdi1Δ null mutation. The mutations were mapped and each of these clones was found to contain two to five mutations (Figure 1A and Table 2).
Table 2.
Summary of effects of single and double mutations in Mrs6p on growth of the gdilΔ null strain
| Homologous residues corresponding to:b
|
|||||
|---|---|---|---|---|---|
| Domain in REP | Yeast REP1 residue | Growth at 30°Ca | Rat REP1 residue | Bovine α-GDI residue | Yeast GDI residue |
| - | GDI wild-type | ++++ | - | - | - |
| I | K303R | + | C396 | M250 | M258 |
| I | K325R | ++ | Q422 | E273 | G283 |
| I | K303R-K325R | +++ | C396-Q422 | M250-E273 | M258-G283 |
| II | F1841 | ++ | F279 | L127 | I135 |
| II | M222V | + | F317 | M168 | M176 |
| II | F1841-M222V | +++ | F279-F317 | L127-M168 | I135-M176 |
| II | D171G | + | G266 | G114 | G122 |
| II | T177P | + | P272 | P120 | P128 |
| II | D171G-T177P | + | G266-P272 | G114/P120 | G122/P128 |
| II | R195A | - | R290 | R138 | R146 |
| - | MRS6 wild-type | - | - | - | - |
(++++) like wild-type; (+++, ++) slower than wild-type; (+) very slow; (-) dead.
Homologous residues based on the sequence alignment of members of the CHM/GDI superfamily.
Figure 1.
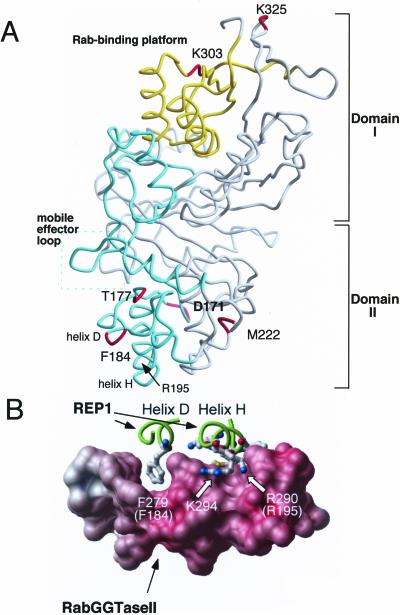
Structural location of Mrs6p residues based on sequence alignment with α-GDI. (A) Indicated residue (Mrs6p numbering) superimposed on the crystal structure of α-GDI (Schalk et al., 1996; Luan et al., 2000). α-GDI is composed of two main domains (I and II). SCRs 1B and 3B (yellow) contribute to domain I that forms the Rab-binding platform (yellow box), a common feature of the GDI/CHM superfamily (Wu et al., 1996; Alory and Balch, 2001). SCRs 2 and 3A (green) link domains I and II and contribute residues that promote prenylation by RabGGTase through the REP–Rab complex (Alory and Balch, 2000) and recycling of Rab from the membrane by GDI (Schalk et al., 1996; Wu et al., 1998; Luan et al., 1999, 2000). The mobile effector loop facilitating interaction of REP with RabGGTase and GDI with RRF is highlighted by the green box (Luan et al., 2000). This interpretation is consistent with the closely related structure of REP1 (Pylypenko et al., 2003). (B) Key role of Mrs6p residue F184 corresponding to mammalian REP1 residue F279 in mediating the interaction between REP1 and RabGGTase (figure from Pylypenko et al., 2003, with permission). Carbon backbone drawing of Helix D (containing F279 (yeast F184)) and helix H of REP1 (containing R209 (yeast R195)) are shown in green. The interaction platform of RabGGTase with REP1 is shown as a space filling model.
Single and Double Mutations in Mrs6p Complement Loss of Gdi1p Function
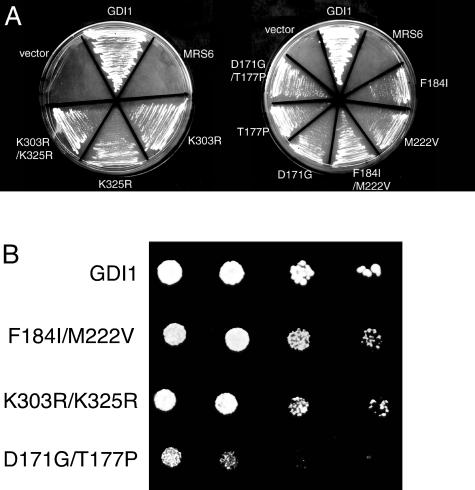
Because each of the mrs6 mutants that could complement the gdi1Δ null mutation had multiple mutations, we determined the contribution of individual amino acids residues for complementation of Gdi1p function. For this purpose, we used a plasmid shuffle-based complementation assay that allows rapid screening of site-directed point mutants for their ability to complement the gdi1Δ null mutation (Alory and Balch, 2000). Each of the mrs6 mutants was expressed from either centromeric single-copy or 2μ multi-copy vectors. Six single mrs6 mutations complemented the gdi1Δ null strain (Table 2 and Figure 2A). The ability of these mutants to complement the gdi1Δ null strain was weaker than that observed for wild-type GDI1 (Figure 2B). Although wild-type GDI1 rescued growth in one day, 3–5 d was required for the mutants. However, these mutants were able to rescue the growth defect phenotype of the gdi1Δ null strain even more efficiently when they were expressed from 2μ multi-copy vectors (our unpublished data).
Figure 2.
Ability of single and double mutants to complement growth of gdi1Δ null strain. (A) Indicated mutants were expressed in the gdi1Δ null strain using the multicopy 2 μ plasmid as described in MATERIALS AND METHODS. (B) Indicated mutants were expressed in the gdi1Δ null strain by using the multicopy 2μ plasmid and 5000, 2000, 500, and 50 cells were spotted onto a plate. The growth was assessed after a 4- to 6-d incubation at 30°C.
Mutation of residues K303 and K325 showed weak rescue of GDI function (Table 2 and Figure 2A). Based on the crystal structure of bovine α-GDI (Schalk et al., 1996; Luan et al., 2000), K303 and K325 are residues found in domain I that are associated with the SCR3B region (Figure 1A). Previous mutational analysis of this region demonstrated that it contributes to the Rab-binding platform (Schalk et al., 1996; Luan et al., 1999; Alory and Balch, 2000; Luan et al., 2000). Thus, changes in the Rab binding domain may contribute to the efficiency of interaction of REP with membrane-associated Rab.
Residues D171, T177, F184, and M222 are found in SCR2. SCR2 is located in the lower domain II of the protein (Figure 1A). SCR2 in Mrs6p has been shown previously to play an important role in the interaction with the RabGGTase (Alory and Balch, 2000). Mutation of these residues in Mrs6p results in an apparent gain of GDI function, the strongest phenotype being observed with the F184 to isoleucine mutant (Table 2 and Figure 2A). Importantly, F184 in yeast Mrs6p corresponds to the highly conserved F279 residue found in helix E of mammalian REP1 (Pylypenko et al., 2003) (Figure 1B). This residue is critical for binding of REP1 to RabGGTase through the interaction platform observed in the 2.7 Å structure of the REP1–RabGGTase complex (Pylypenko et al., 2003) (Figure 1B). Furthermore, mutation of F279 to Ala results in loss of binding of REP1 to RabGGTase in vitro (Pylypenko et al., 2003). Our results and the fact that F279 (yeast F184) is highly conserved among members of the REP gene family, but not the GDI family (Pylypenko et al., 2003), emphasizes the potential important role of this residue in domain II in contributing to the specific function of REP and GDI.
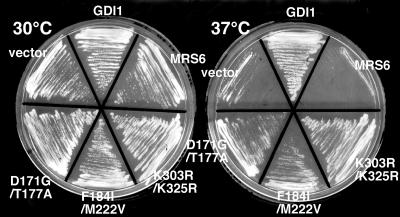
We also generated double mutants to test whether multiple mutations in Mrs6p associated with the Rab-binding platform or the RabGGTase/rab/recycling factor (RRF)-interacting regions would contribute to enhanced growth. All double mutant combinations complemented the gdi1Δ null mutation, when expressed either from centromeric single-copy or multicopy vectors. In particular, two double mutant combinations (K303R/K325R in domain I and F184I/M222V in domain II) were able to support growth of the gdi1Δ null strain much better than individual mutations (Table 2 and Figure 2A). We also examined whether they could complement the growth defect of the sec19-1 yeast strain expressing temperature-sensitive (ts) Gdi1p/Sec19p (Garrett et al., 1994). Each of the mrs6 mutant genes was transformed into the sec19-1 strain, and transformants were selected and maintained at 30°C. Transformants were then restreaked onto two separate plates, one was maintained at 30°C, whereas the second was shifted to 37°C for 4 d. The growth of each of the strains was assessed (Figure 3). Consistent with the previous results, all of the mrs6 mutants, when expressed either from centromeric single-copy or multicopy vectors, listed in Table 2 suppressed the ts growth defect of the sec19-1 strain in a comparable manner to that observed in the gdi1Δ null strain (Figure 2). Thus, the ability to suppress defective function is not restricted to the null allele.
Figure 3.
Complementation of growth of the sec19-1 strain by single and double mutants. Sec19-1 strain was transformed with a 2μ vector containing the indicated single and double mrs6 point mutants or wild-type GDI1 and MRS6. Transformants were maintained at 30°C (left) or incubated at 37°C (right) for 4 d.
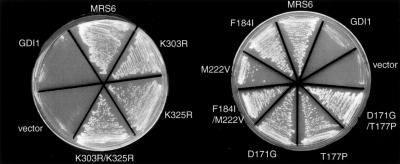
Mrs6p Mutants Function as REP
To test whether the mrs6 mutants found to complement the gdi1Δ null and sec19-1 strains were still able to function as REP, we tested their ability to complement the mrs6Δ null strain. Each of the mutant genes was transformed into the mrs6Δ null strain containing a MRS6::URA3 plasmid, and transformants were selected after restreaking onto 5-fluoroorotic acid–containing plates. All of these mrs6 mutants, expressed from either centromeric single-copy or 2μ multicopy vectors, were able to efficiently complement the mrs6Δ null strain in a manner comparable to that of the wild-type MRS6 (Figure 4). Therefore, mutations conferring apparent gain of GDI function did not detectably compromise REP function in vivo. This result is consistent with the fact that only a very low level of the prenylated Rab pool seems to support efficient growth of yeast (Alory and Balch, 2000). In contrast to the ability of the mutants to independently support growth of the mrs6Δ null or gdi1Δ null strains, we have been unable to generate a mrs6Δgdi1Δ double mutant that is complemented by any of the Mrs6p mutants, a result consistent with our inability to recover an Mrs6p function by mutation of Gdi1p.
Figure 4.
Ability of single mutants to complement growth of mrs6Δ null strain. Indicated mutants were expressed in the mrs6Δ null strain using the multicopy 2μ plasmid as described in MATERIALS AND METHODS.
Mrs6p Mutants Show Similar Distributions to Their Wild-Type Counterparts
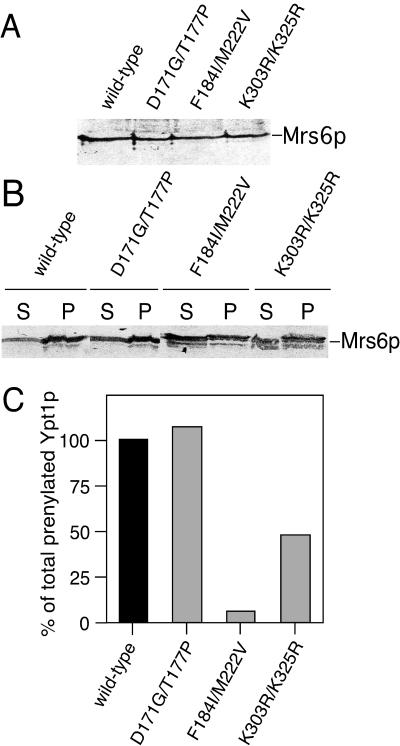
To determine the molecular basis for complementation of Gdi1p function by mutant forms of Mrs6p, we first examined whether Mrs6p mutants had altered expression levels or distributions that may influence their ability to mediate the function of Gdi1p. Total cell extracts were prepared and the distribution of Mrs6p mutants in the cytosol or membranes was detected by a polyclonal anti-Mrs6p antibody by using immunoblotting. All Mrs6 mutants were expressed at comparable levels (Figure 5A). Furthermore, the ratio between the membrane-bound pool and the cytosolic pool of the Mrs6p mutants was identical to that of wild-type Mrs6p (Figure 5B). Therefore, the observed ability of the Mrs6p mutants to support growth of the gdi1Δ null strain is not a consequence of altered expression levels and distribution in the cell.
Figure 5.
Analysis of in vitro prenylation activity of Mrs6p double mutants. (A and B) Total Mrs6p in cell lysates (A) or membrane and cytosolic fractions (B) of mrs6Δ null strains expressing either wild-type or mutant Mrs6p was detected using immunoblotting with a polyclonal anti-Mrs6p antibody. (C) Double mutants that partially complemented growth of the gdi1Δ null strain were expressed in the mrs6Δ null strain. Extracts were prepared and used for the in vitro prenylation assay in the presence or absence of recombinant Ypt1p as described in MATERIALS AND METHODS.
Mrs6p Mutants Have Altered Properties in Rab Prenylation
Because one of the unique functions of Mrs6p is to deliver Rab to RabGGTase for prenylation, one possibility was that some of these mutants may have partially lost prenylation activity, because they need to interact with RRF to gain Gdi1p retrieval function (Luan et al., 2000). Therefore, we analyzed the ability of select Mrs6p double mutants to support prenylation by using an in vitro assay that measures the incorporation of [3H]geranylgeranyl/pyrophosphate into recombinant Rab proteins (Alory and Balch, 2000). Wild-type or double mutants that complement the growth of the gdi1Δ null strain were expressed from multi-copy vectors in the mrs6Δ null strain. Cell homogenates were prepared and assayed in the presence or absence of recombinant Ypt1p. The domain I double mutant K303R/K325R had a 50% decrease in prenylation activity when compared with a similar level of wild-type Mrs6p activity in cell homogenates (Figure 5C). Because residues K303 and K325 are located in the Rab-binding region (Figure 1A), a decrease in the in vitro prenylation activity is likely a result of a reduction of the binding of these mutants to Rab.
In domain II, the double mutant D171G/T177P had no effect on the in vitro prenylation activity of Mrs6p (Figure 5C), a result consistent with the observation that this double mutant and the corresponding single mutants more weakly complemented the growth of the gdi1Δ null strain (Figure 2 and Table 2). This is also consistent with the fact that these residues lie outside the REP1–RabGGTase interaction platform (Pylypenko et al., 2003) and thus are likely to have indirect effects on the structural organization of domain II. In contrast, the domain II double F184I/M222V mutant had a striking effect on the ability of Mrs6p to prenylate Ypt1 in vitro with nearly complete loss of activity (Figure 5C). Given the importance of domain II in recognition of RabGGTase (Alory and Balch, 2000; Pylypenko et al., 2003), these results raise the possibility that GDI function can be gained by the reduction in interaction with RabGGTase.
To explore the above-mentioned possibility, we have previously shown that residue R195 in domain II of Mrs6p (Figure 1A) is critical for Rab prenylation because activity is lost when it is mutated to alanine (Alory and Balch, 2000). The role of R195 is now apparent in the REP1-RabGGTase crystal structure where the corresponding conserved, homologous residue in mammalian REP1 (R290) is also an important residue in the REP1–RabGGTase interaction platform (Pylypenko et al., 2003) (Figure 1B). When we analyzed the effect of the R195A mutant in Mrs6p on its ability to complement the growth defect of the gdi1Δ null strain, it was unable to rescue the growth (Table 2). These results suggest that reduced interaction of REP with RabGGTase through domain II is not sufficient for mrs6 mutants to gain GDI function. It is more likely that F184 is a key residue that distinguishes the function of domain II in REP from domain II in GDI. The M222V substitution in the F184I/M222V double mutant that enhances GDI-like activity may contribute indirectly by promoting subtle changes in domain II structural organization to permit this region to function more efficiently in Rab extraction.
Domain I and II Mrs6p Mutants That Function as Both GDI and REP Show Differences in Rab Binding
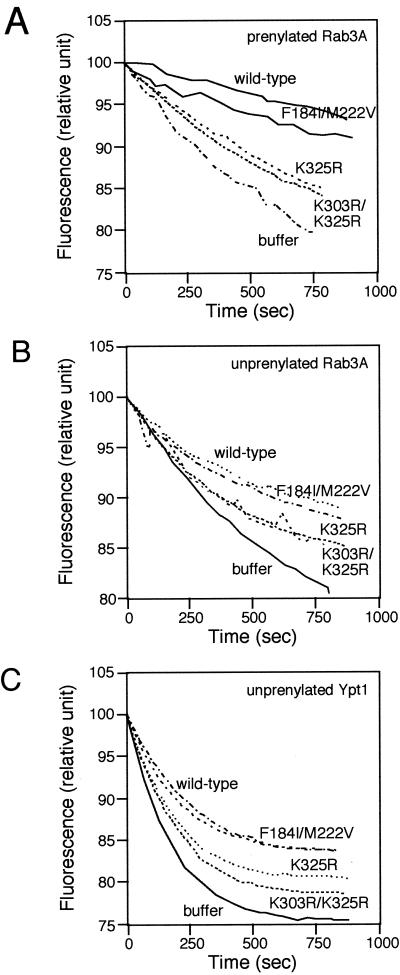
To assess the strength of interaction of Mrs6p mutants with Rab, we used an in vitro Rab-binding assay (Alory and Balch, 2000). This assay is based on the ability of Mrs6p to prevent the intrinsic dissociation of GDP from Rab3A by using methylanthraniloylguanosine diphosphate (mantGDP or mGDP) as a fluorescence probe. Addition of 2.5 μM recombinant Mrs6p to prenylated Rab3A led to a fivefold decrease in the loss of intrinsic fluorescence through the formation of a Rab3A–Mrs6p–mGDP complex (Figure 6A). As expected, the Mrs6p mutant that carried the double mutation F184I/M222V in domain II prevented the dissociation of mGDP from prenylated Rab3A in a manner comparable to wild-type Mrs6p. Thus, the F184I/M222V double mutant still binds Rab efficiently. In contrast, mutations at positions K325R and K303R/K325R interfered with the ability of Mrs6p to prevent mGDP dissociation (Figure 6A). This result demonstrates that the loss of in vitro prenylation observed for these mutants is a consequence of a change in Rab-binding properties (Figure 5C).
Figure 6.
Fluorescence assays to measure the strength of interactions between Rab proteins and wild-type or mutant Mrs6p. (A and B) Effect of mutant Mrs6p on the intrinsic dissociation of mant-GDP from prenylated (A) or nonprenylated (B) Rab3A as described in MATERIALS AND METHODS. (C) Effect of mutant Mrs6p on the intrinsic dissociation of mantGDP from nonprenylated Ypt1p.
Because one function of GDI in vivo is to extract and recycle Rab proteins from the membrane, one prediction would be that Mrs6 mutant proteins that complement the growth of the gdi1Δ null strain can either bind prenylated Rab proteins with higher efficiency than the wild-type Mrs6p, or bind prenylated Rabs preferentially compared with unprenylated Rab. To test the latter, unprenylated Rab3A, was expressed and purified from E. coli, and incubated with wild-type or mutant Mrs6p. As expected, the domain II F184I/M222V mutant bound unprenylated Rab and prevented the loss of fluorescence in the mant-nucleotide assay, a result similar to its effect on prenylated Rab3A (Figure 6, A and B). Moreover, domain I mutants K325R and K303R/K325R were defective in binding both unprenylated and prenylated Rab (Figure 6, A and B). Similar results were observed for the interaction of Mrs6p with the yeast Rab protein Ypt1 (Figure 6C). Thus, the mutations do not seem to specifically contribute to the recognition of Rab through the carboxyl-terminal prenyl groups, a result consistent with our recent observation that in the crystal structure of the GDI-geranylgeranyl lipid complex, the prenyl group binding pocket is located below the Rab-binding domain (An et al., 2003).
Mrs6 Mutant Proteins Show Rab-recycling Activity
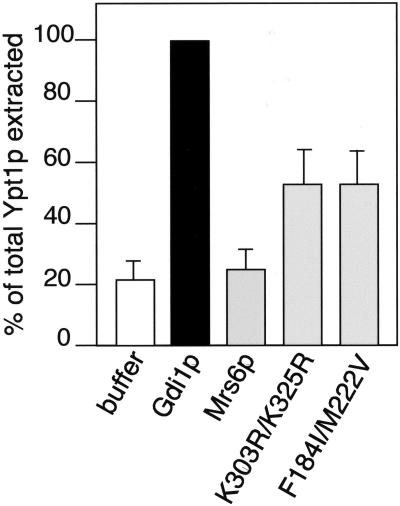
Because the unique function of GDI compared with REP is to extract Rab proteins from the membranes, we assessed the ability of Mrs6p mutants to retrieve Rab proteins in vitro. For this purpose, membranes were prepared from wild-type strains, and incubated with equivalent concentrations of either recombinant wild-type Gdi1p, recombinant Mrs6p, or recombinant single and double Mrs6p mutants. As shown in Figure 7, incubation of yeast membranes with wild-type Gdi1p extracted 62% of the total Ypt1p pool relative the buffer control (10%), a value similar to the extraction efficiency of mammalian GDI for different Rab proteins (Soldati et al., 1993; Peter et al., 1994; Ullrich et al., 1994; Schalk et al., 1996). In contrast, recombinant wild-type Mrs6p had only a marginal effect on the recovery of Ypt1p (12%) relative to the buffer control. However, both K303R/K325R (with reduced Rab binding) and F184I/M222V (which binds Rab proteins with similar efficiency to wild-type Mrs6p, but is deficient in prenylation activity) mutants had increased in efficiency, and we were able to extract 29% of total Ypt1p or 50% of the value of Gdi1p. These results suggest that the gain of function observed in the Mrs6p F184I/M222V double mutant reflects new structural properties of domain II that lead to an increased interaction with Rab during recycling from membranes.
Figure 7.
Effect of mutant Mrs6p on the extraction of Ypt1p from yeast membranes. Yeast membranes were incubated with either wild-type Mrs6p and Gdi1p, or the indicated Mrs6p mutants. After centrifugation, Ypt1p remaining on the membranes was detected by immunoblotting with a polyclonal anti-Ypt1p antibody. The amount of Ypt1p released into the supernatant as the complex is reported as a percent of the total Ypt1p in the yeast membrane preparation. Results typical of three independent experiments with SD of the mean are indicated.
DISCUSSION
Our genetic and biochemical analyses provide new insight into the molecular and structural origins of the REP/GDI superfamily. Using random mutagenesis, we could readily detect Mrs6p mutants that gained partial GDI function in response to amino acid substitutions in either domain I or II. These results suggest a common molecular organization to GDI and REP function. We suggest that an ancestral REP was the evolutionary precursor of GDI. Changes in both the Rab-binding platform and the RabGGTase-binding region in domain II promoted evolutionarily divergence of REP to GDI to accommodate more specialized aspects of Rab recycling in vesicular membrane traffic.
Domain I: The Rab-binding Platform, a Universal Functional Feature of the CHM/GDI Superfamily
Several Mrs6p mutations conferring gain of function of Gdi1p activity localized to the Rab-binding platform, which has molecular (Alory and Balch, 2000) and structural homology between GDI and REP (Pylypenko et al., 2003). Substitutions in two residues (K303R/K325R) found in the Rab-binding platform allow the Mrs6 protein to function not only as a REP but also weakly as a GDI. Given that these residues are not the invariant residues found in SCR3B (285GExxQGFxRxxAxxG299) (where x is any residue) participating in general Rab binding by both REP and GDI (Schalk et al., 1996; Wu et al., 1998; Luan et al., 1999; Alory and Balch, 2000; Luan et al., 2000), these results suggest that additional structural features of the Rab-binding platform play an important role in its specialized function in Rab recycling. This is unlikely to involve the prenyl group because we have recently shown they are bound in a shallow hydrophobic groove beneath the Rab-binding domain (An et al., 2003). A second possibility is that the mutant residues might affect the strength of interaction with different Rab proteins, possibly by interacting with additional membrane-associated effectors facilitating normal Rab recycling (Sakisaka et al., 2002).
Domain II: An Evolutionarily Divergent Feature of the REP/GDI Superfamily Function
Domain II has a more divergent function than domain I (Schalk et al., 1996; Luan et al., 2000; Sakisaka et al., 2002). Domain II promotes interaction of GDI with RRF (Luan et al., 1999; Luan et al., 2000; Sakisaka et al., 2002) and the interaction of REP with the RabGGTase (Alory and Balch, 2000). Because we were able to isolate domain II mutants that were able to function as both GDI and REP, we conclude that this domain functions as a regulatory “adaptor” region. One possibility is that domain II has become evolutionarily specialized for recognizing proteins that mediate different steps in Rab function, In particular, an important residue identified in our studies by using random mutagenesis was F184, which when mutated to isoleucine, confers upon Mrs6p the ability to partially function as Gdi1p in yeast. Strikingly, F184 is a highly conserved residue found in mammalian REP1 (F279) that is crucial for interaction with RabGGTase (Pylypenko et al., 2003). Mutation of this residue results in the loss of binding to recombinant RabGGTase in vitro (Pylypenko et al., 2003). Consistent with the structural data, we observed a dramatic loss of prenylation activity in vivo and in vitro. Although F184 is highly conserved among REP family members, it is absence in GDI family members (Pylypenko et al., 2003).
Model for the Evolution of the REP/GDI Superfamily
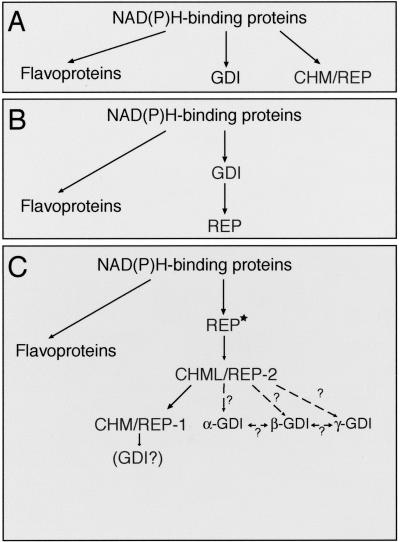
The fact that members of both protein families share a common function (Rab binding), a relatively high homology, a similar structural organization (as suggested by the molecular analysis of the distribution and function of SCRs), and now, the ability of REP to functionally substitute for GDI, leads us to the conclusion that these proteins were derived from a common origin. Three possible evolutionary models can be envisaged (Figure 8). Previous studies indicated that the central structural fold of GDI is identical to that of flavoprotein oxidases such as p-hydroxybenozate hydroxylase and cholesterol oxidase (Schalk et al., 1996). In the first model, CHM/REP, GDI and flavoprotein oxidases may have evolved independently from an ancestral NAD(P)H-binding protein (Figure 8A). However, random mutagenesis experiments performed on MRS6 and GDI1 gene showed that Mrs6p mutants were able to complement Gdi1p function. Moreover, despite the striking homology of GDI to p-hydroxybenozate hydroxylase and cholesterol oxidase, there is no sequence homology. Thus, model A is unlikely.
Figure 8.
Possible pathways for the structural and functional evolution of the REP/GDI superfamily. Star indicates an ancient ancestor.
Models B and C (Figure 8, B and C) explore potential ways in which GDI and REP could be evolutionarily related. In model B, GDI is the precursor of REP. Curiously, despite a mutant screen more extensive than that used to identify Mrs6p mutants that could substitute for Gdi1p, we were unable to recover Gdi1p mutants that functioned as Mrs6p. Although further screens may uncover such mutants, we currently favor the interpretation that Gdi1p has now significantly diverged from REP to promote specialized interactions with novel protein complexes on the membrane facilitating Rab delivery and retrieval (Soldati et al., 1994; Luan et al., 2000). In contrast, the ability to recover Mrs6p mutants that function as Gdi1p suggests that the basic structural organization of domains I and II in REP remains sufficiently flexible to accommodate evolutionarily change. Moreover, because REP mediates the first step in Rab function, assisting in the attachment of geranylgeranyl groups to newly synthesized Rab proteins that are essential for membrane association, without geranylgeranyl modification of Rab there would be, in principle, no need for GDI.
Given the above-mentioned information, we favor the model C (Figure 8C) in which REP is the evolutionarily precursor. In this model, perhaps through a gene duplication event, a REP isoform specialized to become GDI through loss of RabGGTase interaction and an increased capacity to execute Rab extraction in the context of Rab-associated effectors through MEL. Interestingly, our results demonstrate that it is not Rab binding alone that represents that evolutionary hallmark of GDI specialization, rather the contribution of modulators of Rab function such as RRF (Luan et al., 2000; Sakisaka et al., 2002), or potentially GDF (Soldati et al., 1994), that interface with REP and GDI through domain II and MEL. Thus, alterations in MEL and domain II function may be a late event reflecting evolution of tethering/fusion complexes and the need to localize GDI to membranes to mediate efficient Rab retrieval.
Although yeast and Drosophila contain only one GDI and one REP, two REP homologs are expressed in mammalian cells. It is interesting to note that general Rab function is not disrupted in the CHM/REP1 deficiency choroideremia where only a restricted subset of Rab proteins fail to be prenylated (e.g., Rab27) (Seabra et al., 1995; Detter et al., 2000). We favor the possibility that REP1 is an evolutionarily specialization of REP2, although the reason for this specialization of function is not readily apparent at this time. In contrast to REP, five isoforms of GDI are expressed in mammalian cells (Janoueix-Lerosey et al., 1995). β-GDI is currently considered the housekeeping isoform because it is ubiquitously distributed, compared with, for instance, α-GDI, which is largely restricted to the brain. Based on sequence comparison, all GDI isoforms show similar levels of identity (16–18%) and homology (27–29%) to both CHM/REP1 or CHML/REP2 proteins, respectively. Thus, it is difficult to distinguish the order of evolution of GDI from REP isoforms. However, given that most Rabs, with the exception of Rab27, are not affected in choroideremia disease, we suggest that the β- and α-isoforms may have evolved as a further specialization of the more general REP2 protein. Therefore, REP2/CHML is likely the direct descendant from an ancestral REP precursor that diverged from the p-hydroxybenzoate hydroxylase/cholesterol oxidase structural fold (Schalk et al., 1996) (Figure 8C). Whether one of the other uncharacterized GDI isoforms is a specialized GDI that has evolved from REP1 for recycling of Rab27 remains an intriguing possibility.
Acknowledgments
This work was supported by a National Eye Institutes grant EY11606 and GM-33301 (to W.E.B.). This is manuscript 15048-CB from The Scripps Research Institute.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0227. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0227.
References
- Alexandrov, K., Simon, I., Yurchenko, V., Iakovenko, A., Rostkova, E., Scheidig, A.J., and Goody, R.S. (1999). Characterization of the ternary complex between Rab7, REP-1 and Rab geranylgeranyl transferase. Eur. J. Biochem. 265, 160–170. [DOI] [PubMed] [Google Scholar]
- Allan, B.B., Moyer, B.D., and Balch, W.E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448. [DOI] [PubMed] [Google Scholar]
- Alory, C., and Balch, W.E. (2000). Molecular basis for Rab prenylation. J. Cell Biol. 150, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alory, C., and Balch, W.E. (2001). Organization of the Rab-GDI/REP superfamily: functional basis for choroideremia disease. Traffic 2, 532–539. [DOI] [PubMed] [Google Scholar]
- An, Y., Shao, Y., Alory, C., Matteson, J., Sakisaka, T., Chen, W., Gibbs, R.A., Wilson, I.A., and Balch, W.E. (2003) GDI-Rab GTPase recycling. Structure 11, 347–357. [DOI] [PubMed] [Google Scholar]
- Anant, J.S., Desnoyers, L., Machius, M., Demeler, B., Hansen, J.C., Westover, K.D., Deisenhofer, J., and Seabra, M.C. (1998). Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry 37, 12559–12568. [DOI] [PubMed] [Google Scholar]
- Andres, D.A., Seabra, M.C., Brown, M.S., Armstrong, S.A., Smeland, M.C., Cremers, F.P.M., and Goldstein, J.L. (1993). cDNA cloning of component A of Rab geranylgeranyltransferase and demonstration of its role as a rab escort protein. Cell 73, 1091–1099. [DOI] [PubMed] [Google Scholar]
- Armstrong, S.A., Seabra, M., Südhof, T.C., Goldstein, J.L., and Brown, M.S. (1993). cDNA cloning and expression of the alpha and beta subunits of rat geranylgeranyl transferase. J. Biol. Chem. 268, 12221–12229. [PubMed] [Google Scholar]
- Cremers, F.P., Armstrong, S.A., Seabra, M.C., Brown, M.S., and Goldstein, J.L. (1994). REP-2, a Rab escort protein encoded by the choroideremia-like gene. J. Biol. Chem. 269, 2111–2117. [PubMed] [Google Scholar]
- Detter, J.C., et al. (2000). Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc. Natl. Acad. Sci. USA 97, 4144–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura, K., Tanaka, K., Nakano, A., and Toh-e, A. (1994) The Saccharomyces cerevisiae MSI4 gene encodes the yeast counterpart of component A of Rab geranylgeranyltransferase. J. Biol. Chem. 269, 9205–9212. [PubMed] [Google Scholar]
- Garrett, M.D., Zahner, J.E., Cheney, C.M., and Novick, P.J. (1994). GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 13, 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. J Mol. Biol. 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Ito, H., Fukuda, Y., Murata, K., and Kimura, A. (1983). Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., Rossi, G., and Ferro-Novick, S. (1993). Bet2p and Mad2p are components of a prenyltransferase that adds geranylgeranyl onto Ypt1p and Sec4p. Nature 366, 84–86. [DOI] [PubMed] [Google Scholar]
- Janoueix-Lerosey, I., Jollivet, F., Camonis, J., Marche, P.N., and Goud, B. (1995). Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. J. Biol. Chem. 270, 14801–14808. [DOI] [PubMed] [Google Scholar]
- Luan, P., Balch, W.E., Emr, S.D., and Burd, C.G. (1999). Molecular dissection of guanine nucleotide dissociation inhibitor function in vivo. Rab-independent binding to membranes and role of Rab recycling factors. J. Biol. Chem. 274, 14806–14817. [DOI] [PubMed] [Google Scholar]
- Luan, P., Heine, A., Moyer, B.D., Greasely, S.E., Kuhn, P., Balch, W.E., and Wilson, I.A. (2000). A new functional domain of guanine nucleotide dissociation inhibitor (α-GDI) involved in Rab recycling. Traffic 1, 270–281. [DOI] [PubMed] [Google Scholar]
- Maniatis, T., Fritsch, E.F., and Sambrook, J. (1982). Molecular Cloning, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Miller (1972). Experiments in Molecular Genetics, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Moyer, B.D., Allan, B.B., and Balch, W.E. (2001). Rab1 Interaction with a GM130 Effector Complex Regulates COPII Vesicle cis-Golgi Tethering. Traffic 2, 268–276. [DOI] [PubMed] [Google Scholar]
- Peirera-Leal, J.B., and Seabra, M. (2001). Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889–901. [DOI] [PubMed] [Google Scholar]
- Peter, F., Nuoffer, C., Pind, S.N., and Balch, W.E. (1994). Guanine nucleotide dissociation inhibitor (GDI) is essential for rab1 function in budding from the ER and transport through the Golgi stack. J. Cell Biol. 126, 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S.R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487–491. [DOI] [PubMed] [Google Scholar]
- Pylypenko, O., et al. (2003). Structure of Rab escrot protein-1 in complex with Rab geranylgeranyl transferase. Mol. Cell 11, 483–494. [DOI] [PubMed] [Google Scholar]
- Rossi, G., Yu, J.A., Newman, A.P., and Ferro-Novick, S. (1991). Dependence of Ypt1 and Sec4 membrane attachment on Bet2. Nature 351, 158–161. [DOI] [PubMed] [Google Scholar]
- Sakisaka, T., Meerlo, T., Matteson, J., Plutner, H., and Balch, W.E. (2002). Rab-αGDI activity is regulated by a Hsp90 chaperone complex. EMBO J. 21, 6125–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk, I., Zeng, K., Wu, S.-K., Stura, E., Matteson, J., Huang, M., Tandon, A., Wilson, I., and Balch, W.E. (1996). Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature 381, 42–48. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., Brown, M.S., and Goldstein, J.L. (1993). Retinal degeneration in choroideremia: deficiency of Rab geranylgeranyl transferase. Science 259, 377–381. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., Brown, M.S., Slaughter, C.A., Sudhof, T.C., and Goldstein, J.L. (1992a). Purification of component A of Rab geranylgeranyltransferase: possible identity with the choroideremia gene product. Cell 70, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., Goldstein, J.L., Sudhof, T.C., and Brown, M.S. (1992b). Rab geranylgeranyltransferase, a multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-x-Cys or Cys-Cys. J. Biol. Chem. 267, 14497–14503. [PubMed] [Google Scholar]
- Seabra, M.C., Ho, Y.K., and Anant, J.S. (1995). Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J. Biol. Chem. 270, 24420–24427. [DOI] [PubMed] [Google Scholar]
- Shen, F., and Seabra, M.C. (1996). Mechanism of di-geranylgeranylation of Rab proteins. Formation of a complex between monogeranylgeranyl-Rab and Rab escort protein. J. Biol. Chem. 271, 3692–3698. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Fink, G.R., and Lawrence, L.W. (1979). Methods in Yeast Genetics, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Soldati, T., Riederer, M.A., and Pfeffer, S.R. (1993). Rab GDI: a solubilizing and recycling factor for rab9 protein. Mol. Biol. Cell 4, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati, T., Shapiro, A.D., Svejstrup, A.B., and Pfeffer, S.R. (1994). Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature 369, 76–78. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Vitale, G., Ullrich, O., and Zerial, M. (1995). Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 83, 423–432. [DOI] [PubMed] [Google Scholar]
- Thoma, N.H., Iakovenko, A., Goody, R.S., and Alexandrov, K. (2001a). Phosphoisoprenoids modulate association of Rab geranylgeranyltransferase with REP-1. J. Biol. Chem. 276, 48637–48643. [DOI] [PubMed] [Google Scholar]
- Thoma, N.H., Iakovenko, A., Kalinin, A., Waldmann, H., Goody, R.S., and Alexandrov, K. (2001b). Allosteric regulation of substrate binding and product release in geranylgeranyltransferase type II. Biochemistry 40, 268–274. [DOI] [PubMed] [Google Scholar]
- Thoma, N.H., Niculae, A., Goody, R.S., and Alexandrov, K. (2001c). Double Prenylation by RabGGTase can proceed without dissociation of the mono-prenylated intermediate. J. Biol. Chem. 276, 48631–48636. [DOI] [PubMed] [Google Scholar]
- Ullrich, O., Stenmark, H., Alexandrov, K., Huber, L.A., Kaibuchi, K., Sasaki, T., Takai, Y., and Zerial, M. (1993). Rab GDP dissociation inhibitor as a general regulator for the membrane association of Rab proteins. J. Biol. Chem. 268, 18143–18150. [PubMed] [Google Scholar]
- Waldherr, M., Ragnini, A., Schweyer, R.J., and Boguski, M.S. (1993). MRS6-yeast homologue of the choroideraemia gene. Nat. Genet. 3, 193–194. [DOI] [PubMed] [Google Scholar]
- Wu, S.K., Luan, P., Matteson, J., Zeng, K., Nishimura, N., and Balch, W.E. (1998). Molecular role for the Rab binding platform of guanine nucleotide dissociation inhibitor in endoplasmic reticulum to Golgi transport. J. Biol. Chem. 273, 26931–26938. [DOI] [PubMed] [Google Scholar]
- Wu, S.K., Zeng, K., Wilson, I.A., and Balch, W.E. (1996). Structural insights into the function of the Rab GDI superfamily. Trends Biochem. Sci. 21, 472–476. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Mol. Cell. Biol. 2, 107–119. [DOI] [PubMed] [Google Scholar]
- Zhang, H., Seabra, M.C., and Deisenhofer, J. (2000). Crystal structure of Rab geranylgeranyltransferase at 2.0 A resolution. Structure Fold. Des. 8, 241–251. [DOI] [PubMed] [Google Scholar]