Abstract
We have conducted a comprehensive metabolic profiling on tomato (Lycopersicon esculentum) leaf and developing fruit tissue using a recently established gas chromatography-mass spectrometry profiling protocol alongside conventional spectrophotometric and liquid chromatographic methodologies. Applying a combination of these techniques, we were able to identify in excess of 70 small-Mr metabolites and to catalogue the metabolite composition of developing tomato fruit. In addition to comparing differences in metabolite content between source and sink tissues of the tomato plant and after the change in metabolite pool sizes through fruit development, we have assessed the influence of hexose phosphorylation through fruit development by analyzing transgenic plants constitutively overexpressing Arabidopsis hexokinase AtHXK1. Analysis of the total hexokinase activity in developing fruits revealed that both wild-type and transgenic fruits exhibit decreasing hexokinase activity with development but that the relative activity of the transgenic lines with respect to wild type increases with development. Conversely, both point-by-point and principal component analyses suggest that the metabolic phenotype of these lines becomes less distinct from wild type during development. In summary, the data presented in this paper demonstrate that the influence of hexose phosphorylation diminishes during fruit development and highlights the importance of greater temporal resolution of metabolism.
Hexokinase (E.C. 2.7.1.1) catalyzes the phosphorylation of hexoses to form hexose monophosphates. This reaction is especially important in plants because the use of free phosphates is particularly complex in higher plants (Kruger, 1997). There have been many reports on the presence of glucokinase and hexokinase enzymes in a wide variety of plant species including tomato (Lycopersicon esculentum; Martinez-Barajaz and Randall, 1998), maize (Zea mays; Doehlert, 1989; Schnarrenberger, 1990; Galina et al., 1995), potato (Solanum tuberosum; Renz and Stitt, 1993; Veramendi et al., 1999), pea (Pisum sativum; Turner et al., 1977; Turner and Copeland, 1981), avocado (Persea americana; Copeland and Tanner, 1988), castor bean (Ricinus communis; Miernyk and Dennis, 1983), soybean (Glycine max; Copeland and Morrell, 1985), tobacco (Nicotiana tabacum; Sindelar et al., 1998), spinach (Spinacia oleracea; Wiese et al., 1999), and Arabidopsis (Jang et al., 1997).
Recently, transgenic manipulations of the activity of hexokinase have been carried out in tomato, potato, and Arabidopsis (Jang et al., 1997; Dai et al., 1999; Veramendi et al., 1999, 2002). The results of these manipulations varied greatly between species. Transgenic Arabidopsis seeds that exhibited decreased or increased activities of hexokinase 1 displayed hyposensitive or hypersensitive responses to growth on high (6% [w/v]) Glc containing agar (Jang et al., 1997). The authors concluded that the hexokinase protein acts as a sensor for Glc in an analogous manner to those operating in yeast (Saccharomyces cerevisiae) and that the modulation in the abundance of this sensor led to changes in gene expression that were responsible for the phenotype observed. The overexpression of this Arabidopsis hexokinase isoform in tomato plants led to growth inhibition, reduced photosynthesis, and an accelerated leaf senescence (Dai et al., 1999). In contrast, independent genetic manipulation of both potato isoforms of hexokinase that have been cloned to date did not result in dramatic phenotypic changes with the exception that potato plants inhibited in the expression of hexokinase 1-overaccumulated transitory starch (most probably as a result of reduced Glc export form the chloroplast; Veramendi et al., 1999, 2002). Interestingly, despite the fact that a broad biochemical characterization of the potato transgenic lines revealed that neither hexokinase isoform had a large influence on morphology or metabolism in the potato on transformation into the Arabidopsis hexokinase antisense plants described above, both isoforms were able to complement the sensitivity to growth on high levels of Glc (Veramendi et al., 2002).
When taken together, these results led us to postulate that although the basic mechanisms of sugar regulation are conserved in plants, but that the display of sugar-mediated phenotypes is dependent on the plant species, the physiological state of the plants, and other environmental factors (Veramendi et al., 2002). It seems reasonable that the difference in response observed on the manipulation of hexokinase activities between tomato and potato merely reflects the dominant pathways of Suc degradation in these species. Tomato leaves contain far higher invertase activities and correspondingly far higher Glc levels than potato leaves, and in the developing tomato fruit, Suc mobilization is dominated by invertase (with the exception of very young fruit; D'Aoust et al., 1999; Miron et al., 2002), whereas in the potato tuber, Suc mobilization is dominated by Suc synthase (with the exception of very young tubers; Appeldoorn et al., 1997; Viola et al., 2001). To test this hypothesis, we report here the detailed biochemical characterization of developing fruit of the tomato transgenic lines. To perform this study, we adapted a gas chromatography (GC)-mass spectrometry (MS) protocol that we have established for potato tubers (Roessner et al., 2000) to make it suitable for the analysis of tomato tissues and performed these analyses in parallel with HPLC and spectrophotometric assays to afford a very complete view of primary metabolism in these lines. The resultant metabolic complements were then compared with one another both on a point-by-point basis and utilizing principal component analysis (PCA). A similar approach was also carried out in leaf material from these lines. Finally, the influence of enhancing the activity of hexokinase under the developmental conditions was assessed.
RESULTS
Evaluation of the Hexokinase Activity in Various Tissues of the Transgenic Lines
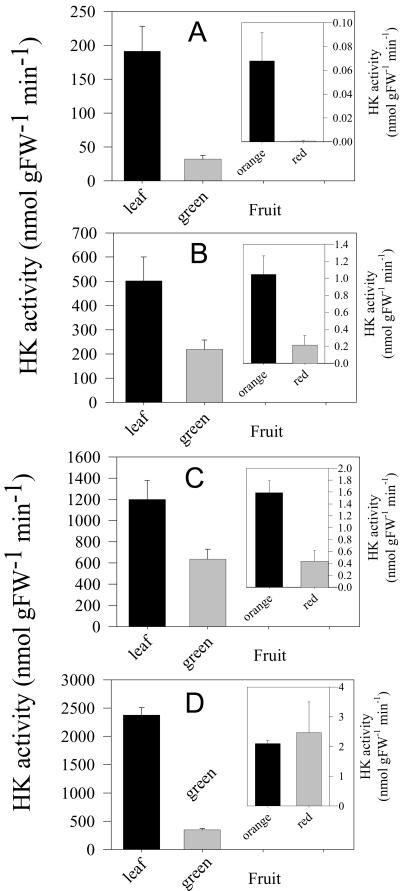
The aim of this work was to investigate the influence of the hexokinase reaction on metabolism in developing tomato plants. To this end, we decided to characterize previously generated plant material in which the Arabidopsis hexokinase AtHXK1 was expressed in tomato cv MP1 under the control of the cauliflower mosaic virus 35S promoter (Dai et al., 1999). As an initial experiment, we verified that the level of expression of the transgene in leaf tissue (data not shown) and the resultant elevation in hexokinase activity were in accordance with those presented previously (Fig. 1). After this, we grew plants to maturity and harvested fruits, 6 h into the light period at 30 (hereafter designated green), 45 (hereafter designated orange), and 60 (hereafter designated red) d after flowering. Then, we determined both the level of expression of the transgene (data not shown) and the hexokinase activity in these samples (Fig. 1). The activity of hexokinase drops massively during development in both the wild type and in the transgenics—routinely being three orders of magnitude lower in red fruit than in green. Intriguingly, the relative hexokinase activity in the transgenic lines increases throughout development with lines containing 6- to 20-fold increases in hexokinase activity in green fruit but much higher relative activity in orange and red fruits. Next, we performed a screen of a range of other enzymes of carbohydrate metabolism in both leaf and green fruit (Table I). Although the activity of enolase was unaltered in either tissue of the transgenics, most of the other enzymes displayed marked changes. Aldolase, glyceraldehyde 3-phosphate dehydrogenase, and triose phosphate isomerase were all reduced with respect to wild type in leaves from line HK4, whereas phosphoglucomutase and glyceraldehyde 3-phosphate dehydrogenase activities were reduced in leaves of line HK37. In contrast, the activities of fructokinase and phosphoglucomutase (line HK4) increase. In general, opposite changes were noticeable in the activities of green fruits than those seen in leaves, with observable increases in the activities of aldolase (in all lines), fructokinase (in all lines), phospho-Glc isomerase (in all lines), phosphofructokinase, glyceraldehyde 3-phosphate dehydrogenase (both in lines HK37 and HK38), pyruvate kinase (in all lines), and phosphoglucomutase (in all lines).
Figure 1.
Total hexokinase activities of extracts from fully expanded tomato leaves and from the pericarp of tomato fruit harvested 30 (green), 45 (orange), and 60 (red) d after flowering. Values presented are mean ± se (n = 6), and those marked with an asterisk were revealed to be significantly different from the wild type. Plant genotypes are as follows: A, HK38; B, HK4; C, HK37; D, WT.
Table I.
Enzyme activity determination in leaves and green fruits of wild type and hexokinase over-expressing plants
Single leaf or fruit samples of six plants were measured. Leaves were harvested six h into the light period from fully-expanded mature leaves of six-week-old plants. Green fruit samples were taken at 30 d after flowering. Values are presented as the mean ± se. Values that were significantly different from WT are set in bold. AGPase, ADPglucose pyrophosphorylase; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; PFK, Phosphofructokinase; PGI, Phosphoglucose isomerase; PGM, Phosphoglucomutase; TPI, triose-phosphate isomerase; ϕ for PGM in leaves and TPI in fruit activities are presented in μmol min−1 g fresh wt−1.
| Enzyme | Wild-Type | HK4 | HK37 | HK38 |
|---|---|---|---|---|
| nmol min−1 g fresh wt−1 | ||||
| Leaf activity | ||||
| AGPase | 437 ± 24 | 421 ± 64 | 477 ± 51 | 434 ± 54 |
| Aldolase | 6,728 ± 252 | 4,287 ± 291 | 6,631 ± 401 | 5,689 ± 510 |
| Enolase | 623 ± 76 | 777 ± 100 | 447 ± 136 | 476 ± 109 |
| Fructokinase | 227 ± 61 | 2,029 ± 155 | 109 ± 31 | 879 ± 124 |
| PGI | 6,070 ± 282 | 6,766 ± 362 | 6,008 ± 290 | 5,491 ± 661 |
| PGMϕ | 12.6 ± 0.3 | 13.9 ± 0.4 | 10.8 ± 0.6 | 11.7 ± 0.9 |
| Pyruvate kinase | 6,021 ± 392 | 4,938 ± 325 | 5,115 ± 212 | 5,272 ± 474 |
| PFK | 345 ± 41 | 485 ± 67 | 346 ± 33 | 283 ± 26 |
| GAPdH | 8,789 ± 647 | 5,126 ± 547 | 6,421 ± 309 | 6,441 ± 832 |
| TPI | 9,560 ± 375 | 8,152 ± 476 | 9,436 ± 406 | 9,193 ± 891 |
| Fruit activity | ||||
| AGPase | 21 ± 5 | 12 ± 1 | 19 ± 1 | 21 ± 1 |
| Aldolase | 264 ± 13 | 290 ± 18 | 343 ± 16 | 501 ± 37 |
| Enolase | 200 ± 79 | 212 ± 29 | 256 ± 40 | 231 ± 3 |
| Fructokinase | 136 ± 16 | 232 ± 41 | 303 ± 32 | 398 ± 24 |
| PGI | 32 ± 7 | 51 ± 11 | 55 ± 3 | 15 ± 4 |
| PGM | 580 ± 66 | 765 ± 51 | 782 ± 46 | 969 ± 46 |
| Pyruvate kinase | 407 ± 23 | 643 ± 67 | 673 ± 74 | 1,091 ± 56 |
| PFK | 152 ± 19 | 155 ± 11 | 231 ± 14 | 237 ± 22 |
| GAPdH | 39 ± 5 | 42 ± 6 | 81 ± 22 | 110 ± 28 |
| TPIϕ | 12.4 ± 2.0 | 12.3 ± 0.2 | 11.7 ± 0.5 | 12.3 ± 0.0 |
Development of a Method for Metabolite Analysis in Tissues of Tomato Using GC-MS
Having confirmed the genetic identity of the lines and quantified the elevation in activity achieved in leaf tissues and at various developmental stages in the fruit, next wanted to biochemically phenotype material harvested in parallel to that used for the above determinations. To be able to analyze the levels of primary metabolites in various tissues of tomato, we adopted and optimized a GC-MS method established in our institute. For tomato leaves, the routine method of extraction, derivatization, measurement and chromatogram evaluation for Arabidopsis and potato leaves (Fiehn et al., 2000a; Lytovchenko et al., 2002a) was utilized. In addition to the metabolites that have previously been reported for Arabidopsis and potato, the organic acids saccharate, 3-hydroxypropanate, 5-aminopentanoate, 2-aminoadipate, and pyruvate, and the secondary metabolites tocopherol and allantoin were identified by the injection of authentic standards. Furthermore, four new peaks of putative chemical structure (dehydroxyascorbate, pyrrol-2-carboxylate, caffeate, and ribonate) were identified by comparison with commercial mass spectral libraries. When the remaining unknown peaks were compared with our collections of unknown metabolites of Arabidopsis, potato leaf and tuber, and Cucurbita maxima leaf tissue (Fiehn et al., 2000a, 2000b; Roessner et al., 2000; http://www.mpimp-golm.mpg.de), we found several that were common to all species but also many that are unique to the tomato leaf.
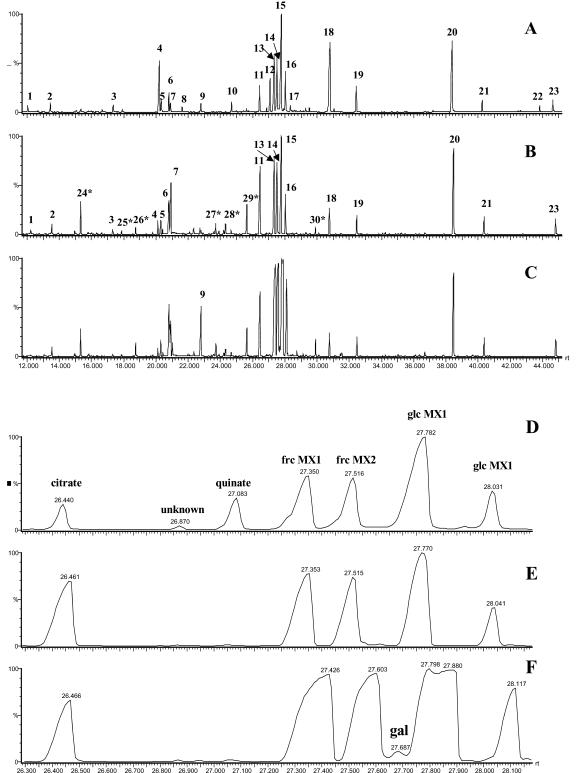
The analysis of tomato fruits represented a more difficult task because they contain high amounts of hexose sugars and citrate (Knee and Finger, 1992; Eshed and Zamir, 1994; MacDougall et al., 1995; Chen et al., 2001). This can be easily visualized in the representative chromatograms presented in Figure 2, with the pattern of peak elution between 25 and 28 min being dramatically different in leaf (Fig. 2, A and D), green fruit (Fig. 2, B and E), and red fruit (Fig. 2, C and F). Therefore, it was necessary to measure two different amounts of extract to evaluate highly abundant compounds without the problems associated with overloaded peaks and to ensure that lower abundance compounds were above the detection limits of the method. The extraction and derivatization procedure for fruits was adopted from that we have used extensively for potato tuber (see Roessner et al., 2000, 2001a, 2001b). In the chromatograms of tomato fruits, a similar set of metabolites can be detected as were found in tomato leaves; however, there are some exceptions. With GABA, His, Pro, pyrrol-2-carboxylate, galactitiol/sorbitol, glycerol, maltitol, 3-phosphoglyceric acid (3PGA), and allantoin, the unknown metabolites LE011, LE012, LE018, and LE030 were only present above the level of detection in fruits, whereas with homo-Cys, caffeate, gluconate, and ribonate, the unknown metabolites LE002, LE006, LE015, LE032, LE035, LE036, and LE038 were only detectable in leaf extracts.
Figure 2.
GC-MS total ion chromatogram of different tissues of wild-type tomato cv MP1. A to C, Complete chromatogram, 12.0 to 50.0 min. D to F, Illustration of sample complexity and analyte range by a representative expansion of chromatograms (A-C) for the region 26.3 to 28.2 min. A and D, Tomato source leaf; B and E, green fruit (30 d after flowering); C and F, Red fruit (60 d after flowering). Peak identification: 1, unknown substance; 2, dodecane (time reference); 3, Ser; 4, malate per-trimethylsilylated (TMS); 5, pentadecane (time reference); 6, Asp TMS; 7, γ-aminobutyrate (GABA) TMS; 8, threonate TMS; 9, Glu TMS; 10, ribitol TMS (quantification standard); 11, citrate TMS; 12, quinate TMS; 13, Fru MEOX1 TMS; 14, Fru MEOX2 TMS; 15, Glc MEOX1 TMS; 16, Glc MEOX2 TMS co-eluting with nonadecane (time reference); 17, gluconate TMS; 18, inositol TMS; 19, docosane (time reference); 20, Suc TMS; 21, octacosane (time reference); 22, chlorogenate TMS; 23, dotriacontane; 24, phosphate TMS; 25, Thr TMS; 26, β-Ala TMS; 27, Asn TMS; 28, unknown substance; 29, Gln TMS; and 30, unknown substance. Derivatives are TMS unless otherwise indicated. Major peaks in the expanded region are identified directly on the chromatogram. An asterisk indicates novel metabolites detected within tomato fruit extracts compared with tomato leaf extracts.
Absolute Quantification of Metabolites and Validation of the GC-MS Method
To extend the characterization of tomato leaf and green and red fruit tissue, we quantified 22 amino acids, seven sugars, and six organic acids by establishment of calibration curves as described by Roessner et al. (2000). Between the two injection amounts, we were able to observe a linear relationship covering the normal concentration range observed in plant tissues (data not shown). These metabolites were then quantified in the various tomato samples (Table II); the resultant absolute levels were found to be in the same range as previously reported by other groups using enzymatically linked photometric assays or HPLC analysis (Martinez et al., 1994; Boggio et al., 2000; Chen et al., 2001). The major exception to this statement was that the magnitude of some of the changes we measured here was somewhat different to that reported by Boggio et al. (2000). Although some of these differences are minor and can be explained by the differences in growth conditions and in cultivars studied, the absolute values we obtained for Asp and Glu were at least an order of magnitude higher than those previously reported. Repeated measurement of these amino acids using an HPLC-based protocol (Regierer et al., 2002), however, gave very similar values to those observed after GC-MS determinations (data not shown). Interestingly, comparisons of the relative levels of amino acids and organic acids reveal that, with the exceptions of Trp, Tyr, and fumarate, these are far more abundant in fruit than leaf tissue. Levels of sugars and sugar alcohols were generally in close agreement between the tissues; however, maltose, Man, and trehalose were appreciably higher in red than green fruit.
Table II.
Quantitative determination of metabolite concentrations in leaves and fruits of tomato
Single leaf or fruit samples of six plants were measured. Leaves were harvested six h into the light period from fully-expanded mature leaves of six-week-old plants. Green and red fruit samples were taken at 30 and 60 d after flowering, respectively. Values are presented as the mean ± se. n.d. indicates compounds that were not detected. GABA, gamma-aminobutyric acid.
| Metabolite | Leaf | Green Fruit | Red Fruit |
|---|---|---|---|
| μmol/g fresh wt−1 | |||
| Ala | 0.12 ± 0.00 | 1.85 ± 0.71 | 1.30 ± 0.84 |
| Asn | 0.02 ± 0.01 | 5.02 ± 1.29 | 3.86 ± 0.46 |
| Asp | 0.15 ± 0.03 | 82.83 ± 24.95 | 201.3 ± 22.1 |
| Cys | 0.002 ± 0.00 | 0.03 ± 0.01 | 0.07 ± 0.01 |
| GABA | 0.01 ± 0.00 | 50.83 ± 9.77 | 16.99 ± 2.47 |
| Glu | 2.32 ± 0.86 | 40.07 ± 9.13 | 282.1 ± 87.4 |
| Gln | 0.25 ± 0.14 | 62.45 ± 9.89 | 43.25 ± 6.36 |
| Gly | 0.12 ± 0.01 | 1.57 ± 0.24 | 0.38 ± 0.05 |
| His | n.d. | 1.27 ± 0.16 | 2.31 ± 0.26 |
| Ile | 0.07 ± 0.02 | 2.52 ± 0.50 | 0.79 ± 0.12 |
| Leu | 0.15 ± 0.02 | 2.51 ± 0.37 | 1.68 ± 0.24 |
| Lys | 0.01 ± 0.00 | 0.17 ± 0.03 | 0.56 ± 0.12 |
| Met | 0.08 ± 0.00 | 0.25 ± 0.07 | 0.48 ± 0.18 |
| Phe | 0.12 ± 0.01 | 2.70 ± 1.23 | 0.55 ± 0.13 |
| Pro | 0.12 ± 0.00 | 0.30 ± 0.07 | 0.68 ± 0.31 |
| Ser | 0.05 ± 0.01 | 0.54 ± 0.04 | 0.60 ± 0.10 |
| Thr | 0.00 ± 0.00 | 0.22 ± 0.03 | 0.13 ± 0.03 |
| Trp | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.03 |
| Tyr | 0.07 ± 0.01 | 0.15 ± 0.04 | 0.37 ± 0.11 |
| Val | 0.17 ± 0.03 | 4.27 ± 0.73 | 0.73 ± 0.12 |
| Gal | 0.03 ± 0.03 | 0.75 ± 0.10 | 5.19 ± 0.73 |
| Inositol | 32.05 ± 6.70 | 34.22 ± 4.09 | 33.33 ± 4.78 |
| Maltitol | 1.93 ± 0.42 | n.d. | n.d. |
| Maltose | 0.42 ± 0.17 | 0.29 ± 0.08 | 1.11 ± 0.19 |
| Mannitol | 0.22 ± 0.02 | 0.28 ± 0.02 | 0.28 ± 0.02 |
| Man | 0.22 ± 0.07 | 1.97 ± 0.17 | 5.16 ± 0.36 |
| Trehalose | n.d. | 0.10 ± 0.01 | 0.44 ± 0.09 |
| Fumarate | 19.48 ± 4.39 | 0.73 ± 0.11 | 0.03 ± 0.00 |
| Glycerate | 0.56 ± 0.13 | 0.18 ± 0.02 | 0.14 ± 0.01 |
| Malate | 1.66 ± 0.26 | 3.17 ± 0.34 | 0.67 ± 0.30 |
| Oxalate | n.d. | 0.59 ± 0.07 | n.d. |
| Succinate | 0.01 ± 0.00 | 0.21 ± 0.03 | 0.14 ± 0.01 |
Before utilizing the tomato method for the comparison of diverse genotypes, we carried out one final examination of its robustness. We performed recombination experiments, whereby extracts of potato and tomato were subjected to GC-MS investigations, both in isolation then as a stochiometric mixture, to validate that the peak identification for tomato samples was the same as that for potato tuber. This experiment is of particular importance given that different extract compositions can cause so-called “matrix effects” that can result in shifts in relative elution times (for example, see Wagner et al., 2003). Using this strategy, we evaluated whether the relative values determined in the simple tomato extracts could be quantifiably retrieved in the mixed extract. Results of this experiment are presented in Table III. Approximately 80% of the 60 chemicals that we evaluated in this manner were recovered at between 85% and 120% of the level at which they were added, and no compound was recovered at less than 68% or more than 130% of its initial level.
Table III.
Quantitative recovery of tomato metabolites following recombination with potato tube tissue extraction, derivatization, and analysis on gas chromatography—mass spectrometry protocol
Six replications were performed as described in “Materials and Methods.” Values are presented as the mean ± se. GABA, gamma-aminobutyric acid.
| Metabolite | Recovery in |
|---|---|
| % | |
| Ala | 117.82 ± 10.95 |
| Asn | 91.07 ± 9.09 |
| Asp | 88.71 ± 3.55 |
| β-Ala | 115.08 ± 6.68 |
| GABA | 108.46 ± 12.65 |
| Gln | 87.94 ± 2.70 |
| Glu | 107.69 ± 13.32 |
| Gly | 98.50 ± 2.09 |
| Ile | 117.38 ± 8.33 |
| Leu | 115.57 ± 8.40 |
| Lys | 143.12 ± 11.22 |
| Met | 132.96 ± 5.81 |
| Orn | 78.07 ± 6.77 |
| t-4-HO-Pro | 94.71 ± 1.88 |
| 5-Oxo-Pro | 80.40 ± 9.65 |
| Phe | 95.97 ± 1.22 |
| Ser | 109.55 ± 6.45 |
| Thre | 128.56 ± 7.79 |
| Trp | 107.95 ± 6.07 |
| Tyr | 121.17 ± 25.15 |
| Val | 106.80 ± 8.66 |
| Dopamine | 79.55 ± 2.52 |
| FA 16:0 | 90.38 ± 7.87 |
| FA 18:0 | 83.07 ± 8.14 |
| Hydroxylamine | 104.01 ± 24.49 |
| Putrescine | 110.65 ± 6.30 |
| Spermidine | 76.65 ± 7.40 |
| Thymine | 90.32 ± 8.73 |
| Tyramine | 70.10 ± 1.68 |
| Ara | 112.26 ± 12.87 |
| Fru | 86.74 ± 3.00 |
| Fru-6-P | 93.21 ± 1.43 |
| Fuc | 119.64 ± 29.03 |
| Glc-6-P | 98.72 ± 2.05 |
| Glycerol | 108.24 ± 24.51 |
| Glycerol-1-P | 105.67 ± 8.54 |
| Maltose | 117.37 ± 4.53 |
| Man | 88.94 ± 14.43 |
| Mannitol | 90.16 ± 2.06 |
| Inositol | 68.93 ± 6.82 |
| Rha | 94.00 ± 5.59 |
| Rib | 125.21 ± 29.57 |
| Suc | 80.44 ± 5.18 |
| Xyl | 98.01 ± 2.70 |
| α-kt-Gulonate | 74.04 ± 8.26 |
| Citramalate | 90.18 ± 4.60 |
| Citrate | 85.43 ± 2.96 |
| Fumarate | 96.14 ± 3.63 |
| Galactonate | 92.26 ± 3.07 |
| Galacturonate | 103.23 ± 5.19 |
| Gluconate | 116.22 ± 3.73 |
| Glycerate | 82.93 ± 5.36 |
| L-Ascorbate | 100.12 ± 10.47 |
| Malate | 91.85 ± 1.88 |
| Quinate | 93.64 ± 2.01 |
| Phosphate | 85.83 ± 4.61 |
| Shikimate | 102.95 ± 3.03 |
| Succinate | 88.53 ± 7.05 |
| Threonate | 110.92 ± 3.88 |
Comparison of Relative Metabolite Levels in Transgenic and Wild-Type Tomato Fruits in Fruit Harvested at Different Developmental Stages
Next, we evaluated the levels of starch, hexose phosphates, nucleotides, and the metabolites covered in the GC-MS protocol described above in ethanol, trichloroacetate, and methanol extracts of the homogenized pericarp tissue used for the enzyme determinations presented in Figure 1. The full data set comprising over 70 metabolites is presented in Table IV; the data set contains 73 metabolites of defined chemical structure, including starch, sugars, sugar alcohols, amino acids, organic acids, phosphorylated intermediates, and nucleotides. The majority of the compounds measured were found to alter both through development and across the genotypes. Furthermore, the relative changes observed between green and red fruit in the wild type were largely in accordance with the absolute data presented for the more limited data set of Table I. Although several of the changes in metabolite pools such as the large increases in hexose sugars and the transitory nature of starch accumulation have been documented in previous studies (Yelle et al., 1988; Demnitz-King et al., 1997; Schaffer and Petreikov, 1997), many of the data presented here are completely novel. In the wild-type data, a progressive increase in the majority of amino acids with developmental time is clearly observable. However, the levels of β-Ala, GABA, Gly, Ile, and Val progressively decreased over this period. With the exception of citrate and fumarate (which change contrapuntally to one another), there is no clear relationship between organic acids of the Krebs cycle with time. In contrast, the levels of several other organic compounds show clearer trends with the levels of ascorbate decreasing over time, whereas those of a derivative of ascorbate increase, and there are also large increases in the levels of citramalate, quinate, and succinate and a considerable decrease in the threonate pool size over time. Intriguingly, there is also a considerable decrease in the levels of 3PGA after the green to red color transition alongside a 50% reduction in the level of UDPGlc and minor reductions in the levels of other uridinylates and ADP. When the metabolite contents of green fruits from the transgenic lines are compared with their corresponding wild-type controls, it is clear that they are metabolically very distinct. Glc, Fru, and Suc (in the case of line HK4 only) are decreased in the transgenic lines, whereas fructose 6-phosphate (Fru-6-P) increased in lines HK4 and HK38 (although Glc-6-P concomitantly decreased). These were coupled to a decrease in the levels of ATP in lines HK4 and HK38 and an increase in the level of ADP in line HK4 and corresponding decreases in the ATP to ADP ratios in these lines. Furthermore, the levels of the nucleosides ADP-Glc and UDP-Glc are significantly decreased in line HK4. The levels of other sugars such as Man, maltose, and trehalose also tend to decrease in the transgenic lines, in some instances being reduced to as little as 10% of those found in wild type. In marked contrast, the levels of amino acids in green fruits of the transgenics were generally severalfold higher than those found in the wild-type control. Particularly large increases were observed in homo-Ser, Met, Ile, Lys, and His of the Asp family, the aromatic amino acids Tyr, Ala, and Leu of the pyruvate family, Arg and Pro of the Glu family, and in Ser. The only amino acid that consistently decreased was the photorespiratory intermediate Gly. The levels of organic acids in the transgenic lines generally showed less variance than the other classes of compounds. A 2-fold increase in fumarate was observed in all lines, and increased levels of malate and succinate in the two strongest lines were the only consistent changes seen in the transformants. α-Ketoglutarate also increased 2-fold in lines HK37 and HK38 but was conversely reduced to approximately 40% of the wild-type level in the strongest line. In addition to these changes, we also observed decreased levels of ascorbate and increased levels of both shikimate and 6-phosphogluconate in the transformants with further changes in the levels of some 18 metabolites of unknown chemical structure (for details, see http://www.mpimp-golm.mpg.de).
Table IV.
Metabolite levels in wild type and hexokinase overexpressing fruits across development
Metabolites were determined from the same samples used to determine hexokinase activity. Data are normalized to the mean response calculated for the wild-type (WT) green fruit. Values are presented as the mean ± se of six independent determinations. Those that are significantly different to WT green or to their own respective WT are set in bold type. n.d. indicates compounds that were not detected. GABA, gamma-aminobutyric acid.
| Metabolite | Green Fruit Wild Type | HK4 | HK38 | HK37 | Orange Fruit Wild Type | HK4 |
|---|---|---|---|---|---|---|
| Ala | 1.00 ± 0.11 | 14.48 ± 0.04 | 14.92 ± 0.04 | 2.75 ± 0.14 | 0.52 ± 0.12 | 3.88 ± 0.17 |
| Allantoin | 1.00 ± 0.13 | 0.93 ± 0.16 | 1.04 ± 0.06 | 1.14 ± 0.05 | 0.38 ± 0.13 | 0.41 ± 0.25 |
| Arg | 1.00 ± 0.04 | 4.40 ± 0.14 | 6.74 ± 0.07 | 1.88 ± 0.06 | 3.68 ± 0.12 | 9.15 ± 0.19 |
| Asn | 1.00 ± 0.15 | 1.01 ± 0.08 | 0.93 ± 0.13 | 0.80 ± 0.11 | 2.59 ± 0.14 | 3.67 ± 0.10 |
| Asp | 1.00 ± 0.09 | 0.32 ± 0.15 | 0.23 ± 0.15 | 1.62 ± 0.40 | 1.44 ± 0.09 | 1.34 ± 0.05 |
| β-Ala | 1.00 ± 0.04 | 1.67 ± 0.06 | 2.09 ± 0.07 | 1.14 ± 0.07 | 0.60 ± 0.12 | 1.55 ± 0.14 |
| Cys | 1.00 ± 0.17 | 3.43 ± 0.19 | 1.50 ± 0.20 | 1.94 ± 0.09 | 2.33 ± 0.20 | 8.50 ± 0.12 |
| GABA | 1.00 ± 0.04 | 1.20 ± 0.05 | 1.37 ± 0.03 | 1.24 ± 0.07 | 0.78 ± 0.06 | 1.02 ± 0.06 |
| Glu | 1.00 ± 0.03 | 1.00 ± 0.10 | 0.98 ± 0.12 | 1.11 ± 0.08 | 3.40 ± 0.12 | 4.52 ± 0.06 |
| Gln | 1.00 ± 0.11 | 2.16 ± 0.08 | 2.31 ± 0.13 | 1.54 ± 0.13 | 3.53 ± 0.07 | 1.24 ± 0.29 |
| Gly | 1.00 ± 0.12 | 0.09 ± 0.22 | 0.43 ± 0.47 | 2.24 ± 0.09 | 0.57 ± 0.10 | 3.76 ± 0.08 |
| His | 1.00 ± 0.16 | 8.45 ± 0.15 | 13.30 ± 0.16 | 2.88 ± 0.34 | 1.46 ± 0.12 | 7.03 ± 0.17 |
| Homo-Ser | 1.00 ± 0.11 | 4.12 ± 0.18 | 11.38 ± 0.16 | 3.38 ± 0.25 | 0.57 ± 0.10 | 1.52 ± 0.17 |
| Ile | 1.00 ± 0.12 | 2.03 ± 0.10 | 2.74 ± 0.13 | 2.07 ± 0.13 | 0.53 ± 0.10 | 2.52 ± 0.16 |
| Leu | 1.00 ± 0.09 | 8.10 ± 0.13 | 12.93 ± 0.19 | 2.72 ± 0.16 | 0.96 ± 0.08 | 5.43 ± 0.18 |
| Lys | 1.00 ± 0.07 | 9.27 ± 0.11 | 15.70 ± 0.06 | 1.51 ± 0.31 | 1.88 ± 0.06 | 7.74 ± 0.08 |
| Meth | 1.00 ± 0.13 | 3.57 ± 0.12 | 5.22 ± 0.11 | 2.63 ± 0.17 | 1.37 ± 0.09 | 2.85 ± 0.15 |
| Ornithine | 1.00 ± 0.06 | 1.26 ± 0.13 | 1.83 ± 0.15 | 0.95 ± 0.16 | 1.56 ± 0.05 | 1.53 ± 0.09 |
| 5-oxo | 1.00 ± 0.07 | 1.70 ± 0.07 | 2.07 ± 0.09 | 1.31 ± 0.09 | 0.81 ± 0.11 | 1.41 ± 0.09 |
| Oxalate | 1.00 ± 0.13 | 1.13 ± 0.08 | 1.19 ± 0.17 | 1.06 ± 0.11 | 0.03 ± 0.07 | 0.02 ± 0.10 |
| Phe | 1.00 ± 0.15 | 0.50 ± 0.10 | 1.10 ± 0.29 | 1.91 ± 0.16 | 0.40 ± 0.14 | 0.53 ± 0.12 |
| Pro | 1.00 ± 0.26 | 5.38 ± 0.10 | 7.28 ± 0.27 | 1.07 ± 0.22 | 2.62 ± 0.31 | 8.09 ± 0.29 |
| Ser | 1.00 ± 0.08 | 4.85 ± 0.03 | 4.84 ± 0.04 | 1.80 ± 0.11 | 1.60 ± 0.12 | 5.83 ± 0.06 |
| Thre | 1.00 ± 0.08 | 1.99 ± 0.09 | 2.14 ± 0.03 | 1.33 ± 0.08 | 0.68 ± 0.13 | 2.20 ± 0.10 |
| Trp | 1.00 ± 0.11 | 1.62 ± 0.08 | 1.96 ± 0.10 | 2.16 ± 0.13 | 1.33 ± 0.13 | 2.77 ± 0.14 |
| Tyr | 1.00 ± 0.14 | 2.72 ± 0.18 | 6.76 ± 0.21 | 3.17 ± 0.24 | 0.46 ± 0.12 | 1.51 ± 0.21 |
| Val | 1.00 ± 0.08 | 2.69 ± 0.08 | 3.50 ± 0.03 | 1.67 ± 0.09 | 0.31 ± 0.13 | 2.15 ± 0.15 |
| 2-Aminoadipate | 1.00 ± 0.11 | 1.51 ± 0.12 | 2.77 ± 0.18 | 0.95 ± 0.10 | 0.95 ± 0.18 | 1.57 ± 0.11 |
| 5-Aminopentanoate | 1.00 ± 0.05 | 0.79 ± 0.05 | 1.27 ± 0.08 | 1.04 ± 0.05 | 1.11 ± 0.08 | 0.87 ± 0.06 |
| Ascorbate | 1.00 ± 0.07 | 0.62 ± 0.14 | 0.81 ± 0.03 | 1.02 ± 0.05 | 0.51 ± 0.41 | 0.06 ± 0.19 |
| Ascorbate derivate | 1.00 ± 0.05 | 0.55 ± 0.19 | 0.60 ± 0.17 | 1.11 ± 0.11 | 1.55 ± 0.09 | 1.64 ± 0.16 |
| Citramalate | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 14.83 ± 0.15 | 20.09 ± 0.16 |
| Citrate | 1.00 ± 0.04 | 1.14 ± 0.08 | 0.87 ± 0.07 | 1.11 ± 0.05 | 1.46 ± 0.05 | 1.26 ± 0.07 |
| Fumarate | 1.00 ± 0.06 | 2.45 ± 0.12 | 2.23 ± 0.15 | 2.19 ± 0.08 | 0.37 ± 0.05 | 0.48 ± 0.10 |
| Glucuronate | 1.00 ± 0.06 | 0.09 ± 0.10 | 0.09 ± 0.19 | 0.08 ± 0.13 | 0.51 ± 0.03 | 1.64 ± 0.16 |
| 3-Hydroxypropanate | 1.00 ± 0.08 | 1.43 ± 0.06 | 1.37 ± 0.03 | 1.11 ± 0.06 | 0.60 ± 0.03 | 0.99 ± 0.08 |
| Glycerate | 1.00 ± 0.08 | 1.12 ± 0.10 | 0.13 ± 0.14 | 1.12 ± 0.12 | 0.66 ± 0.68 | 0.79 ± 0.10 |
| Isocitrate | 1.00 ± 0.13 | 0.58 ± 0.15 | 0.03 ± 0.21 | 4.05 ± 0.77 | 1.38 ± 0.11 | 0.85 ± 0.13 |
| α-Ketoglutarate | 1.00 ± 0.10 | 0.35 ± 0.18 | 2.22 ± 0.12 | 1.71 ± 0.07 | 1.98 ± 0.08 | 1.33 ± 0.31 |
| Malate | 1.00 ± 0.12 | 2.66 ± 0.13 | 2.06 ± 0.09 | 1.28 ± 0.04 | 1.16 ± 0.07 | 1.39 ± 0.14 |
| 3-PGA | 1.00 ± 0.03 | 1.08 ± 0.18 | 1.54 ± 0.06 | 1.33 ± 0.17 | 0.91 ± 0.15 | 0.52 ± 0.16 |
| 6-P-Gluconate | 1.00 ± 0.27 | 3.07 ± 0.34 | 6.86 ± 0.14 | 2.63 ± 0.21 | 2.51 ± 0.14 | 3.01 ± 0.24 |
| Phosphate | 1.00 ± 0.04 | 1.64 ± 0.09 | 2.60 ± 0.07 | 1.56 ± 0.04 | 0.99 ± 0.07 | 1.64 ± 0.12 |
| Pyrrol-2-carboxylate | 1.00 ± 0.32 | 4.47 ± 0.22 | 9.87 ± 0.28 | 0.79 ± 0.21 | 1.05 ± 0.22 | 3.93 ± 0.46 |
| Pyruvate | 1.00 ± 0.23 | 0.89 ± 0.11 | 1.49 ± 0.06 | 1.36 ± 0.07 | 1.27 ± 0.14 | 0.72 ± 0.20 |
| Guinate | 1.00 ± 0.08 | 0.96 ± 0.13 | 1.02 ± 0.40 | 1.17 ± 0.09 | 2.54 ± 0.05 | 1.34 ± 0.06 |
| Saccharate | 1.00 ± 0.13 | 0.66 ± 0.08 | 0.75 ± 0.06 | 0.81 ± 0.05 | 0.62 ± 0.12 | 0.57 ± 0.10 |
| Shikimate | 1.00 ± 0.23 | 2.83 ± 0.11 | 4.25 ± 0.20 | 2.06 ± 0.07 | 2.83 ± 0.18 | 2.23 ± 0.09 |
| Succinate | 1.00 ± 0.09 | 1.45 ± 0.07 | 4.28 ± 0.16 | 2.35 ± 0.13 | 1.20 ± 0.04 | 1.56 ± 0.12 |
| Threonate | 1.00 ± 0.10 | 0.87 ± 0.08 | 1.51 ± 0.06 | 2.09 ± 0.13 | 0.66 ± 0.08 | 0.63 ± 0.16 |
| Fuc | 1.00 ± 0.04 | 1.15 ± 0.07 | 1.11 ± 0.07 | 1.07 ± 0.06 | 0.88 ± 0.07 | 1.11 ± 0.07 |
| Galacitol/sorbitol | 1.00 ± 0.03 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.58 ± 0.14 | 1.22 ± 0.18 | 1.36 ± 0.10 |
| Galactose | 1.00 ± 0.07 | 9.38 ± 0.17 | 0.28 ± 0.08 | 0.88 ± 0.08 | 1.42 ± 0.07 | 1.53 ± 0.07 |
| Glycerol | 1.00 ± 0.05 | 0.92 ± 0.06 | 0.90 ± 0.07 | 2.25 ± 0.58 | 1.33 ± 0.06 | 0.98 ± 0.03 |
| Inositol | 1.00 ± 0.09 | 0.66 ± 0.05 | 0.82 ± 0.07 | 0.97 ± 0.08 | 1.02 ± 0.12 | 0.77 ± 0.22 |
| Inositol like | 1.00 ± 0.04 | 0.86 ± 0.05 | 0.95 ± 0.07 | 1.16 ± 0.06 | 1.18 ± 0.07 | 0.99 ± 0.11 |
| Maltitol | 1.00 ± 0.14 | 0.25 ± 0.12 | 0.23 ± 0.14 | 0.87 ± 0.10 | 1.90 ± 0.14 | 0.89 ± 0.20 |
| Maltose | 1.00 ± 0.29 | 0.31 ± 0.15 | 1.39 ± 0.06 | 2.12 ± 0.25 | 43.48 ± 0.31 | 1.24 ± 0.36 |
| Mannitol | 1.00 ± 0.08 | 1.10 ± 0.06 | 1.43 ± 0.04 | 2.16 ± 0.50 | 1.31 ± 0.09 | 1.43 ± 0.10 |
| Man | 1.00 ± 0.10 | 0.25 ± 0.24 | 0.13 ± 0.08 | 0.80 ± 0.09 | 1.25 ± 0.12 | 1.15 ± 0.10 |
| Suc | 1.00 ± 0.08 | 0.63 ± 0.11 | 1.19 ± 0.07 | 1.11 ± 0.08 | 2.24 ± 0.14 | 1.38 ± 0.27 |
| Trehalose | 1.00 ± 0.09 | 0.21 ± 0.21 | 0.29 ± 0.30 | 0.89 ± 0.07 | 1.90 ± 0.11 | 0.62 ± 0.15 |
| Starch | 331.18 ± 34.29 | 208.35 ± 32.32 | 222.77 ± 31.25 | 348.09 ± 43.06 | 65.83 ± 11.75 | 13.74 ± 1.58 |
| Glc | 76.26 ± 11.63 | 30.31 ± 4.96 | 23.72 ± 4.02 | 54.24 ± 7.89 | 403.37 ± 28.43 | 269.28 ± 21.85 |
| Fru | 381.23 ± 24.09 | 215.99 ± 21.51 | 179.06 ± 27.73 | 383.22 ± 33.30 | 371.21 ± 17.16 | 273.09 ± 17.32 |
| Glc-6-P | 289.94 ± 24.85 | 192.09 ± 15.75 | 201.10 ± 11.28 | 243.99 ± 59.5 | 467.1 ± 15.78 | 287.82 ± 19.04 |
| Glc-1-P | 85.67 ± 13.97 | 101.43 ± 14.5 | 108.68 ± 63.26 | 113.68 ± 20.77 | 237.99 ± 36.24 | 351.28 ± 98.14 |
| Fru-6-P | 90.91 ± 6.11 | 141.46 ± 18.09 | 174.63 ± 6.61 | 169.74 ± 21.95 | 237.47 ± 55.55 | 153.40 ± 0.70 |
| ATP | 40.05 ± 2.72 | 12.84 ± 3.93 | 25.14 ± 2.25 | 32.32 ± 2.49 | 39.29 ± 6.38 | 17.58 ± 2.88 |
| UTP | 61.87 ± 1.58 | 54.93 ± 1.44 | 57.72 ± 0.51 | 58.54 ± 0.80 | 55.55 ± 1.00 | 62.14 ± 0.72 |
| ADP | 12.77 ± 2.12 | 7.18 ± 2.33 | 19.76 ± 1.02 | 16.86 ± 1.57 | 8.64 ± 1.37 | 10.29 ± 1.10 |
| UDP | 16.61 ± 0.91 | 13.74 ± 0.86 | 16.49 ± 0.32 | 19.04 ± 1.03 | 14.48 ± 0.93 | 14.12 ± 0.24 |
| ADP-Glc | 2.62 ± 0.25 | 1.44 ± 0.34 | 3.08 ± 0.52 | 2.18 ± 0.19 | 2.12 ± 0.31 | 1.76 ± 0.18 |
| UDP-Glc | 61.80 ± 3.52 | 31.07 ± 6.96 | 61.04 ± 4.35 | 48.69 ± 4.89 | 34.16 ± 4.23 | 20.82 ± 3.00 |
| ATP/ADP | 3.51 ± 0.43 | 2.01 ± 0.24 | 1.27 ± 0.07 | 2.16 ± 0.53 | 4.66 ± 0.56 | 3.33 ± 0.20 |
| HK38 | HK37 | Red Fruit WT | HK4 | HK38 | HK37 | |
|---|---|---|---|---|---|---|
| μmol g fresh wt | ||||||
| Ala | 11.23 ± 0.06 | 0.70 ± 0.06 | 0.91 ± 0.19 | 2.88 ± 0.19 | 1.84 ± 0.38 | 0.62 ± 0.05 |
| Allantoin | 0.29 ± 0.09 | 0.41 ± 0.18 | 0.57 ± 0.17 | 0.67 ± 0.29 | 0.54 ± 0.24 | 0.22 ± 0.18 |
| Arg | 15.89 ± 0.17 | 4.90 ± 0.22 | 15.52 ± 0.10 | 19.86 ± 0.20 | 19.56 ± 0.18 | 10.43 ± 0.17 |
| Asn | 4.16 ± 0.08 | 3.62 ± 0.08 | 2.82 ± 0.06 | 3.45 ± 0.09 | 3.33 ± 0.07 | 2.13 ± 0.08 |
| Aspartate | 0.98 ± 0.11 | 1.33 ± 0.05 | 2.43 ± 0.02 | 2.43 ± 0.12 | 2.63 ± 0.06 | 2.19 ± 0.05 |
| β-Alanine | 1.66 ± 0.06 | 0.74 ± 0.07 | 0.32 ± 0.19 | 0.90 ± 0.07 | 0.60 ± 0.08 | 0.23 ± 0.19 |
| Cys | 6.17 ± 0.11 | 2.77 ± 0.16 | 4.01 ± 0.23 | 8.45 ± 0.16 | 3.85 ± 0.19 | 1.37 ± 0.13 |
| GABA | 1.19 ± 0.05 | 0.82 ± 0.03 | 0.67 ± 0.08 | 0.97 ± 0.09 | 0.94 ± 0.02 | 0.69 ± 0.11 |
| Glu | 4.63 ± 0.07 | 3.18 ± 0.08 | 7.04 ± 0.02 | 7.26 ± 0.11 | 7.66 ± 0.06 | 6.08 ± 0.07 |
| Gln | 3.13 ± 0.14 | 3.09 ± 0.10 | 5.29 ± 0.03 | 3.66 ± 0.21 | 5.86 ± 0.09 | 5.02 ± 0.03 |
| Gly | 8.80 ± 0.05 | 1.06 ± 0.10 | 0.47 ± 0.20 | 2.56 ± 0.14 | 1.66 ± 0.33 | 0.37 ± 0.12 |
| His | 15.14 ± 0.10 | 3.38 ± 0.23 | 3.52 ± 0.05 | 12.45 ± 0.10 | 11.47 ± 0.13 | 2.48 ± 0.11 |
| Homo-Ser | 2.12 ± 0.09 | 0.86 ± 0.34 | 0.93 ± 0.09 | 1.86 ± 0.13 | 1.87 ± 0.12 | 0.65 ± 0.15 |
| Ile | 3.90 ± 0.04 | 1.06 ± 0.13 | 0.45 ± 0.15 | 1.60 ± 0.06 | 1.18 ± 0.19 | 0.52 ± 0.07 |
| Leu | 12.33 ± 0.06 | 1.75 ± 0.15 | 0.93 ± 0.13 | 3.73 ± 0.18 | 2.20 ± 0.20 | 0.76 ± 0.06 |
| Lys | 13.89 ± 0.05 | 3.66 ± 0.07 | 3.91 ± 0.07 | 9.81 ± 0.05 | 7.54 ± 0.12 | 3.12 ± 0.06 |
| Meth | 5.60 ± 0.05 | 1.89 ± 0.33 | 1.30 ± 0.10 | 3.19 ± 0.14 | 3.96 ± 0.15 | 1.02 ± 0.13 |
| Orn | 1.63 ± 0.05 | 1.12 ± 0.17 | 1.52 ± 0.07 | 2.42 ± 0.12 | 1.92 ± 0.04 | 1.67 ± 0.06 |
| 5-Oxo-Pro | 1.48 ± 0.04 | 1.19 ± 0.08 | 0.40 ± 0.02 | 0.55 ± 0.08 | 0.49 ± 0.05 | 0.37 ± 0.04 |
| Oxalate | 0.02 ± 0.08 | 0.02 ± 0.09 | 0.07 ± 0.13 | 0.20 ± 0.08 | 0.12 ± 0.08 | 0.07 ± 0.12 |
| Phe | 1.60 ± 0.06 | 0.77 ± 0.15 | 0.59 ± 0.20 | 0.63 ± 0.09 | 0.68 ± 0.18 | 0.54 ± 0.12 |
| Pro | 9.73 ± 0.14 | 1.00 ± 0.20 | 3.99 ± 0.12 | 4.69 ± 0.26 | 5.32 ± 0.22 | 1.93 ± 0.13 |
| Ser | 6.76 ± 0.04 | 2.24 ± 0.12 | 1.14 ± 0.09 | 4.96 ± 0.10 | 3.07 ± 0.29 | 0.93 ± 0.14 |
| Threo | 2.79 ± 0.04 | 1.17 ± 0.09 | 0.87 ± 0.06 | 1.95 ± 0.05 | 1.41 ± 0.11 | 0.83 ± 0.11 |
| Try | 5.21 ± 0.07 | 0.07 ± 0.09 | 3.78 ± 0.22 | 3.39 ± 0.08 | 2.97 ± 0.14 | 2.80 ± 0.10 |
| Tyr | 5.42 ± 0.12 | 1.13 ± 0.12 | 1.09 ± 0.28 | 1.30 ± 0.11 | 1.45 ± 0.26 | 1.07 ± 0.09 |
| Val | 3.54 ± 0.04 | 0.71 ± 0.11 | 0.19 ± 0.17 | 1.17 ± 0.16 | 0.62 ± 0.25 | 0.19 ± 0.10 |
| 2-Aminoadipate | 2.36 ± 0.03 | 1.39 ± 0.11 | 0.01 ± 0.00 | 1.82 ± 0.08 | 1.68 ± 0.13 | 0.93 ± 0.10 |
| 5-Aminopentanoate | 1.45 ± 0.04 | 1.05 ± 0.08 | 0.91 ± 0.05 | 0.95 ± 0.10 | 1.11 ± 0.05 | 1.12 ± 0.08 |
| Ascorbate | 0.27 ± 0.16 | 0.35 ± 0.26 | 0.13 ± 0.13 | 0.27 ± 0.22 | 0.34 ± 0.15 | 0.33 ± 0.20 |
| Ascorbate derivate | 1.42 ± 0.23 | 1.94 ± 0.18 | 4.67 ± 0.12 | 4.25 ± 0.28 | 4.94 ± 0.11 | 3.49 ± 0.13 |
| 2-Methylmalate | 29.42 ± 0.10 | 19.01 ± 0.16 | 34.43 ± 0.12 | 22.05 ± 0.20 | 23.66 ± 0.03 | 27.68 ± 0.08 |
| Citrate | 0.71 ± 0.14 | 1.33 ± 0.06 | 1.36 ± 0.07 | 1.08 ± 0.08 | 1.00 ± 0.17 | 1.15 ± 0.05 |
| Fumarate | 1.25 ± 0.22 | 0.40 ± 0.07 | 0.30 ± 0.07 | 0.41 ± 0.04 | 0.36 ± 0.04 | 0.36 ± 0.06 |
| Glucuronate | 1.90 ± 0.11 | 0.10 ± 0.13 | 1.30 ± 0.08 | 0.52 ± 0.13 | 0.53 ± 0.09 | 1.22 ± 0.15 |
| 3-Hydroxypropanate | 0.97 ± 0.06 | 0.73 ± 0.06 | 0.62 ± 0.07 | 0.92 ± 0.10 | 0.86 ± 0.07 | 0.64 ± 0.04 |
| Glycerate | 2.23 ± 0.10 | 0.70 ± 0.08 | 0.68 ± 0.02 | 0.76 ± 0.14 | 0.68 ± 0.12 | 0.62 ± 0.06 |
| Isocitrate | 0.05 ± 0.10 | 1.02 ± 0.11 | 0.66 ± 0.13 | 0.65 ± 0.13 | 0.76 ± 0.20 | 0.67 ± 0.05 |
| α-Ketoglutarate | 9.60 ± 0.27 | 1.17 ± 0.13 | 1.05 ± 0.21 | 0.74 ± 0.19 | 1.72 ± 0.38 | 1.04 ± 0.14 |
| μmol g fresh wt | ||||||
| Malate | 0.98 ± 0.25 | 0.93 ± 0.07 | 0.33 ± 0.31 | 0.40 ± 0.18 | 0.34 ± 0.25 | 0.38 ± 0.13 |
| 3-PGA | 0.82 ± 0.05 | 0.65 ± 0.11 | 0.31 ± 0.04 | 0.47 ± 0.14 | 0.55 ± 0.04 | 0.32 ± 0.14 |
| 6-P-Gluconate | 3.98 ± 0.11 | 3.96 ± 0.28 | 1.37 ± 0.16 | 4.46 ± 0.20 | 3.09 ± 0.15 | 1.99 ± 0.30 |
| Phosphate | 1.54 ± 0.07 | 1.30 ± 0.12 | 0.89 ± 0.02 | 1.07 ± 0.08 | 1.01 ± 0.05 | 0.82 ± 0.02 |
| Pyrrol-2-carboxylate | 4.38 ± 0.22 | 0.61 ± 0.25 | 2.27 ± 0.07 | 3.83 ± 0.44 | 4.33 ± 0.41 | 1.19 ± 0.18 |
| Pyruvate | 1.69 ± 0.26 | 0.58 ± 0.10 | 0.58 ± 0.16 | 0.52 ± 0.13 | 0.45 ± 0.10 | 0.69 ± 0.10 |
| Guinate | 1.21 ± 0.07 | 2.18 ± 0.06 | 3.84 ± 0.06 | 1.73 ± 0.12 | 2.26 ± 0.10 | 4.01 ± 0.08 |
| Saccharate | 0.52 ± 0.09 | 0.66 ± 0.10 | 1.47 ± 0.17 | 0.56 ± 0.19 | 0.62 ± 0.13 | 1.04 ± 0.10 |
| Shikimate | 2.34 ± 0.06 | 2.61 ± 0.05 | 3.38 ± 0.09 | 2.25 ± 0.13 | 3.25 ± 0.05 | 3.31 ± 0.18 |
| Succinate | 6.10 ± 0.30 | 1.81 ± 0.12 | 0.60 ± 0.10 | 1.26 ± 0.09 | 0.90 ± 0.04 | 1.11 ± 0.04 |
| Threonate | 0.73 ± 0.11 | 0.53 ± 0.08 | 0.61 ± 0.10 | 0.51 ± 0.06 | 0.52 ± 0.04 | 0.77 ± 0.11 |
| Fuc | 1.17 ± 0.05 | 0.90 ± 0.03 | 1.36 ± 0.04 | 1.34 ± 0.09 | 1.18 ± 0.06 | 1.22 ± 0.05 |
| Galactitol/sorbitol | 1.26 ± 0.12 | 1.01 ± 0.19 | 1.57 ± 0.14 | 1.28 ± 0.23 | 1.75 ± 0.12 | 2.05 ± 0.12 |
| Gal | 2.24 ± 0.09 | 1.80 ± 0.12 | 4.91 ± 0.32 | 3.18 ± 0.17 | 4.00 ± 0.15 | 3.76 ± 0.25 |
| Glycerol | 1.21 ± 0.09 | 0.85 ± 0.05 | 0.81 ± 0.05 | 1.56 ± 0.25 | 0.87 ± 0.06 | 0.68 ± 0.05 |
| Inositol | 0.72 ± 0.06 | 0.94 ± 0.09 | 1.39 ± 0.03 | 0.85 ± 0.20 | 0.71 ± 0.02 | 0.84 ± 0.04 |
| Inositol like | 0.80 ± 0.07 | 0.99 ± 0.05 | 1.54 ± 0.07 | 0.93 ± 0.15 | 1.01 ± 0.05 | 1.28 ± 0.08 |
| Maltitol | 1.03 ± 0.11 | 1.53 ± 0.11 | 2.57 ± 0.05 | 1.32 ± 0.29 | 1.91 ± 0.12 | 3.04 ± 0.10 |
| Maltose | 0.89 ± 0.23 | 8.48 ± 0.25 | 5.92 ± 0.07 | 2.05 ± 0.51 | 8.32 ± 0.31 | 8.04 ± 0.26 |
| Mannitol | 1.52 ± 0.07 | 1.25 ± 0.18 | 1.17 ± 0.08 | 1.48 ± 0.10 | 1.44 ± 0.10 | 1.09 ± 0.16 |
| Man | 4.47 ± 0.14 | 1.18 ± 0.12 | 2.79 ± 0.02 | 1.41 ± 0.16 | 2.13 ± 0.11 | 2.06 ± 0.16 |
| Suc | 1.16 ± 0.08 | 2.07 ± 0.14 | 1.40 ± 0.12 | 1.03 ± 0.46 | 1.41 ± 0.10 | 1.64 ± 0.17 |
| Trehalose | 0.91 ± 0.12 | 1.41 ± 0.13 | 3.09 ± 0.10 | 0.90 ± 0.25 | 1.35 ± 0.08 | 2.75 ± 0.10 |
| Starch | 0.97 ± 0.23 | 87.61 ± 29.42 | n.d. | n.d. | n.d. | n.d. |
| Glc | 259.09 ± 18.60 | 338.90 ± 26.34 | 485.79 ± 30.84 | 260.92 ± 34.94 | 333.21 ± 15.34 | 490.50 ± 11.52 |
| Fru | 261.75 ± 18.42 | 321.62 ± 21.23 | 456.32 ± 20.27 | 277.36 ± 23.47 | 331.71 ± 10.21 | 420.19 ± 10.15 |
| Glc-6-P | 253.80 ± 7.60 | — | 565.00 ± 45.31 | 340.71 ± 18.36 | 360.8 ± 38.51 | 654.61 ± 26.80 |
| Glc-1-P | 433.9 ± 81.81 | — | 208.82 ± 54.79 | 209.53 ± 17.94 | 944.88 ± 66.48 | 424.03 ± 99.59 |
| Fru-6-P | 138.3 ± 3.63 | — | 239.51 ± 48.88 | 148.75 ± 7.42 | 156.28 ± 14.52 | 266.52 ± 10.12 |
| ATP | 16.59 ± 4.01 | 38.24 ± 4.48 | 40.56 ± 5.29 | 23.82 ± 7.54 | 38.23 ± 4.16 | 31.31 ± 4.81 |
| UTP | 50.67 ± 0.67 | 52.71 ± 1.80 | 53.93 ± 1.36 | 51.10 ± 1.46 | 54.11 ± 1.83 | 51.02 ± 0.57 |
| ADP | 10.97 ± 1.15 | 11.52 ± 1.15 | 5.29 ± 1.40 | 12.03 ± 1.52 | 12.18 ± 1.79 | 14.35 ± 1.99 |
| UDP | 13.24 ± 0.90 | 13.73 ± 0.27 | 12.80 ± 0.34 | 14.00 ± 0.44 | 14.58 ± 0.75 | 14.53 ± 0.62 |
| ADP-glc | 1.33 ± 0.21 | 1.64 ± 0.17 | 1.69 ± 0.49 | 2.07 ± 0.33 | 1.67 ± 0.21 | 1.43 ± 0.13 |
| UDP-glc | 19.99 ± 5.92 | 30.43 ± 3.75 | 35.31 ± 8.50 | 24.83 ± 5.07 | 40.92 ± 5.03 | 26.93 ± 4.41 |
| ATP/ADP | 1.59 ± 0.48 | 1.73 ± 0.23 | 14.61 ± 6.29 | 2.28 ± 0.37 | 3.24 ± 0.22 | 1.87 ± 0.38 |
As would be expected, the starch content of orange fruit was markedly reduced from the level found in green fruits. Furthermore, the starch levels of lines HK4 and HK38 were significantly lower than that found in the wild-type control. At this time point, the levels of Fru and Glc were also reduced in these lines (in line HK38, the level of Suc was also reduced) and Glc-6-P increased in lines HK4 and HK38, whereas Fru-6-P decreased. As was observed in the green fruit, these changes corresponded to decreases in the level of ATP and, consequently, in the ATP to ADP ratio. Line HK4, furthermore, exhibits a decreased level of UDP-Glc. The levels of other sugars such as Man, maltose, and trehalose again were observed to be lower in the transgenic lines because they were at the earlier stage of development; however, at this time point, the differences between the pool sizes (with the exception of maltose) in the transformants and the wild type were not as great. Likewise, a similar pattern of change was observed in the amino acid pool sizes of orange fruit to that documented above for green fruits. As was noted for the sugar measurements, however (with the exceptions of Ala, Ile, and Val, which are even more strikingly increased at this developmental stage), the relative difference between the size of the amino acid pools in the transgenic and wild-type lines is smaller than that observed at the earlier stage of development. Another exception to the previous statement is the level of Gly, which was decreased in the transformants in early fruit development but increases dramatically in these lines by the orange stage. The relative pattern of change in other compounds between green and orange stages is fairly conserved, and despite minor increases in the differences in the relative levels of succinate and fumarate during this period, differences in the relative levels of other important metabolites such as shikimate and 6-phosphogluconate got much smaller in the same time period. Intriguingly there are also far fewer differences in the levels of the unknown metabolites in the transgenic lines, with respect to wild type, at this developmental stage (for details, see http://www.mpimp-golm.mpg.de).
The metabolic profiling of the red fruit revealed even fewer differences in metabolite pool sizes between the transgenic and wild-type lines. Starch was undetectable at this stage, Glc and Fru were once again generally decreased (with the exception of the Glc level in line HK37), and Glc monophosphates generally increased, whereas Fru-6-P generally decreased in the transgenic lines. On the other hand ADP conversely increased in all lines, whereas the level of UDP was higher in line HK37 than it was in wild type at this developmental stage. With the exception of trehalose, which was depressed to lower levels in the transgenics, the levels of minor sugars and sugar alcohols were largely unchanged in the transformants.
Far fewer changes in the amino acids were observed across the genotypes at this developmental stage, and those that did occur were generally not of the same order of magnitude as those seen in either of the earlier developmental stages. Similarly, the organic acid levels of the transformants were very similar to those of the wild type at this time point. The levels of 3PGA and 6-phosphogluconate, however, were increased in red fruits of the transformants, although as was the case for orange fruits, there were also far fewer differences in the levels of the unknown metabolites in the transgenic lines, with respect to wild type, at this developmental stage than there were in green fruit (for details, see http://www.mpimp-golm.mpg.de).
Comparison of Relative Metabolite Levels in Transgenic and Wild-Type Tomato Leaves Harvested from 6-Week-Old Plants
A similar analysis for leaves taken from 6-week-old plants was also performed (the full data set can be viewed at http://www.mpimp-golm.mpg.de). In contrast to what was observed in the fruits, the starch content in leaves was unaltered; however, the Glc and Fru content was significantly reduced in line HK4, whereas the hexose phosphate pools were increased up to 5-fold (Table V). The leaves of the transgenic lines were also characterized by dramatic decreases in the levels of all adenylates (ATP, ADP, and ADP-Glc), but this was most pronounced in ATP rendering the deduced ATP to ADP ratio much reduced. The UTP to UDP ratio of these lines was reduced in a similar manner, but given that the level of UDP-Glc increased in the transgenics, the total uridinylate pool size was unaltered. Analysis of the data from the GC-MS analysis of leaf extracts revealed large changes in the levels of the majority of metabolites in lines HK4 and HK38. The pattern of change in the amino acid pool sizes was similar to that observed in the green fruit, with a massive increase in Asn concomitant to a decrease in the level of Asp and also large increases in the level of Lys, Thr, Ile, homo-Ser, Tyr, Trp, Leu, Orn, Arg, and Val. The levels of organic acids tended to decrease in the transgenic lines with the exception of fumarate, which was up to 7-fold increased, and ascorbate, which was up to 15-fold increased. Interestingly, the vast majority of the unidentified peaks were dramatically decreased in leaves from the hexokinase overexpressors (see http://www.mpimp-golm.mpg.de).
Table V.
Metabolite levels in wild type and hexokinase overexpressing leaves
Metabolites were determined from methanol extracts of the same samples used to determine hexokinase activity. Data are normalized to the mean response calculated for the wild type. Values are presented as the mean ± se of six independent determinations. Those that are significantly different to wild type are set in bold type. n.d. indicates compounds that were not detected.
| Metabolite | Wild Type | HK4 | HK38 | HK37 |
|---|---|---|---|---|
| μmol/g fresh wt−1 | ||||
| Ala | 1.00 ± 0.13 | 1.97 ± 0.32 | 2.30 ± 0.03 | 0.84 ± 0.10 |
| Arg | 1.00 ± 0.11 | 11.89 ± 0.13 | 10.02 ± 0.15 | 1.24 ± 0.24 |
| Asn | 1.00 ± 0.29 | 9.90 ± 0.16 | 4.13 ± 0.09 | 0.52 ± 0.15 |
| Asp | 1.00 ± 0.07 | 0.28 ± 0.06 | 0.60 ± 0.07 | 0.68 ± 0.14 |
| β-Ala | 1.00 ± 0.20 | 2.12 ± 0.06 | 1.65 ± 0.06 | 0.56 ± 0.19 |
| Cys | 1.00 ± 0.08 | 0.75 ± 0.34 | 1.04 ± 0.05 | 0.68 ± 0.12 |
| Glu | 1.00 ± 0.26 | 1.34 ± 0.20 | 1.24 ± 0.28 | 2.01 ± 0.06 |
| Gln | 1.00 ± 0.11 | 2.15 ± 0.18 | 2.75 ± 0.11 | 0.92 ± 0.11 |
| Gly | 1.00 ± 0.27 | 1.46 ± 0.06 | 4.13 ± 0.18 | 1.99 ± 0.19 |
| Homo-Cys | 1.00 ± 0.16 | 2.47 ± 0.12 | 1.98 ± 0.25 | 2.17 ± 0.18 |
| Homo-Ser | 1.00 ± 0.29 | 5.23 ± 0.09 | 6.50 ± 0.13 | 1.31 ± 0.29 |
| Ile | 1.00 ± 0.15 | 4.67 ± 0.06 | 3.28 ± 0.05 | 0.87 ± 0.14 |
| Leu | 1.00 ± 0.13 | 4.93 ± 0.09 | 3.39 ± 0.04 | 1.13 ± 0.13 |
| Lys | 1.00 ± 0.20 | 6.26 ± 0.08 | 3.73 ± 0.06 | 1.13 ± 0.18 |
| Meth | 1.00 ± 0.24 | 0.26 ± 0.07 | 0.66 ± 0.09 | 1.40 ± 0.40 |
| Orn | 1.00 ± 0.16 | 3.38 ± 0.08 | 3.87 ± 0.07 | 1.13 ± 0.17 |
| 5-Oxo-Pro | 1.00 ± 0.17 | 0.94 ± 0.29 | 2.04 ± 0.04 | 1.03 ± 0.10 |
| Phe | 1.00 ± 0.09 | 1.35 ± 0.05 | 1.64 ± 0.03 | 0.95 ± 0.14 |
| Ser | 1.00 ± 0.22 | 2.95 ± 0.06 | 3.60 ± 0.04 | 1.00 ± 0.18 |
| Thr | 1.00 ± 0.18 | 3.90 ± 0.06 | 4.27 ± 0.08 | 0.98 ± 0.13 |
| Trp | 1.00 ± 0.17 | 3.57 ± 0.11 | 3.57 ± 0.15 | 1.00 ± 0.27 |
| Tyr | 1.00 ± 0.28 | 5.23 ± 0.10 | 2.20 ± 0.05 | 1.03 ± 0.22 |
| Val | 1.00 ± 0.13 | 4.46 ± 0.06 | 2.45 ± 0.03 | 0.77 ± 0.12 |
| 2-Aminoadipate | 1.00 ± 0.31 | 4.82 ± 0.57 | 3.32 ± 0.10 | 0.69 ± 0.33 |
| 5-Aminopentanate | 1.00 ± 0.14 | 15.91 ± 0.87 | 0.55 ± 0.09 | 0.57 ± 0.15 |
| Ascorbate | 1.00 ± 0.13 | 4.43 ± 0.52 | 14.70 ± 0.03 | 1.14 ± 0.22 |
| Ascorbate derivate | 1.00 ± 0.36 | 1.16 ± 0.76 | 0.34 ± 0.11 | 0.41 ± 0.20 |
| Caffeate | 1.00 ± 0.09 | 0.65 ± 0.18 | 1.25 ± 0.05 | 1.17 ± 0.15 |
| Citramalate | 1.00 ± 0.09 | 7.94 ± 0.75 | 1.10 ± 0.01 | 1.08 ± 0.10 |
| Citrate | 1.00 ± 0.21 | 0.86 ± 0.24 | 0.71 ± 0.02 | 1.17 ± 0.08 |
| Fumarate | 1.00 ± 0.17 | 6.92 ± 0.25 | 3.87 ± 0.09 | 1.97 ± 0.22 |
| Gluconate | 1.00 ± 0.22 | 0.29 ± 0.17 | 0.39 ± 0.09 | 0.69 ± 0.39 |
| Glucuronate | 1.00 ± 0.23 | 0.21 ± 0.71 | 0.11 ± 0.13 | 0.67 ± 0.50 |
| Glycerate | 1.00 ± 0.14 | 1.42 ± 0.22 | 1.18 ± 0.09 | 0.92 ± 0.08 |
| 3-Hydroxypropanate | 1.00 ± 0.25 | 0.74 ± 0.27 | 0.99 ± 0.17 | 1.07 ± 0.13 |
| Isocitrate | 1.00 ± 0.14 | 1.45 ± 0.19 | 1.11 ± 0.10 | 0.60 ± 0.20 |
| α-Ketoglutarate | 1.00 ± 0.18 | 1.18 ± 0.59 | 0.47 ± 0.12 | 0.84 ± 0.18 |
| Malate | 1.00 ± 0.12 | 0.37 ± 0.30 | 0.69 ± 0.02 | 1.01 ± 0.09 |
| Oxalate | 1.00 ± 0.16 | 1.07 ± 0.12 | 0.88 ± 0.06 | 0.88 ± 0.21 |
| 6-P-Gluconate | 1.00 ± 0.22 | 2.15 ± 0.32 | 2.20 ± 0.09 | 1.20 ± 0.21 |
| Phosphate | 1.00 ± 0.20 | 0.71 ± 0.26 | 1.12 ± 0.06 | 1.34 ± 0.11 |
| Pyruvate | 1.00 ± 0.14 | 0.69 ± 0.27 | 0.61 ± 0.06 | 0.92 ± 0.20 |
| Guinate | 1.00 ± 0.14 | 0.18 ± 0.21 | 0.72 ± 0.06 | 0.85 ± 0.22 |
| Ribonate | 1.00 ± 0.15 | 0.29 ± 0.13 | 0.48 ± 0.07 | 0.63 ± 0.21 |
| Saccharate | 1.00 ± 0.16 | 0.05 ± 0.21 | 0.21 ± 0.09 | 0.57 ± 0.35 |
| Shikimate | 1.00 ± 0.18 | 9.84 ± 0.88 | 1.01 ± 0.04 | 1.23 ± 0.20 |
| Succinate | 1.00 ± 0.12 | 1.17 ± 0.11 | 1.32 ± 0.06 | 1.34 ± 0.08 |
| Threonate | 1.00 ± 0.07 | 0.22 ± 0.17 | 0.75 ± 0.04 | 0.80 ± 0.15 |
| Fru | 1.00 ± 0.11 | 1.19 ± 0.30 | 0.88 ± 0.17 | 0.83 ± 0.07 |
| Fuc | 1.00 ± 0.13 | 0.79 ± 0.13 | 0.99 ± 0.07 | 1.03 ± 0.22 |
| Gal | 1.00 ± 0.10 | 0.80 ± 0.07 | 1.07 ± 0.05 | 1.56 ± 0.22 |
| Glc | 1.00 ± 0.20 | 1.20 ± 0.06 | 1.03 ± 0.19 | 1.04 ± 0.18 |
| Inositol | 1.00 ± 0.05 | 1.48 ± 0.16 | 1.09 ± 0.03 | 1.39 ± 0.13 |
| Maltose | 1.00 ± 0.16 | 0.37 ± 0.06 | 0.91 ± 0.09 | 2.91 ± 0.23 |
| Mannitol | 1.00 ± 0.20 | 0.71 ± 0.13 | 0.77 ± 0.18 | 0.94 ± 0.14 |
| Man | 1.00 ± 0.11 | 0.93 ± 0.22 | 1.00 ± 0.05 | 1.09 ± 0.16 |
| Suc | 1.00 ± 0.07 | 1.14 ± 0.06 | 0.72 ± 0.06 | 0.70 ± 0.11 |
| Starch | 42.73 ± 7.75 | 40.22 ± 6.28 | 54.65 ± 10.32 | 55.31 ± 5.14 |
| Glc | 15.91 ± 1.24 | 6.27 ± 0.52 | 17.64 ± 0.58 | 12.61 ± 1.36 |
| Fru | 14.88 ± 1.53 | 9.20 ± 1.38 | 15.81 ± 0.99 | 11.93 ± 1.41 |
| Suc | 1.27 ± 0.11 | 2.48 ± 0.28 | 0.57 ± 0.07 | 0.89 ± 0.12 |
| Glc-6-P | 164.66 ± 10.40 | 456.38 ± 34.10 | 349.47 ± 82.58 | 192.67 ± 17.05 |
| Glc-1-P | 33.37 ± 6.25 | 152.95 ± 23.91 | 100.39 ± 23.53 | 83.76 ± 15.29 |
| Fru-6-P | 49.22 ± 7.88 | 78.30 ± 9.40 | 46.33 ± 12.11 | 50.24 ± 8.76 |
| ATP | 117.20 ± 6.37 | 47.37 ± 11.04 | 40.92 ± 11.80 | 52.95 ± 14.70 |
| UTP | 51.24 ± 3.21 | 28.74 ± 2.62 | 27.10 ± 4.16 | 34.98 ± 6.06 |
| ADP | 96.49 ± 1.62 | 61.90 ± 7.44 | 51.46 ± 10.64 | 61.30 ± 11.95 |
| UDP | 23.06 ± 1.13 | 18.64 ± 0.75 | 17.46 ± 1.82 | 19.20 ± 1.58 |
| ADP-Glc | 12.49 ± 0.82 | 8.17 ± 1.07 | 7.33 ± 0.70 | 9.83 ± 0.57 |
| UDP-Glc | 182.07 ± 4.46 | 239.30 ± 9.31 | 211.43 ± 25.88 | 214.01 ± 11.52 |
| ATP/ADP | 1.21 ± 0.04 | 0.73 ± 0.06 | 0.75 ± 0.06 | 0.81 ± 0.06 |
PCA of the Metabolic Complement of Developmental Changes in the Wild-Type and Transgenic Plants
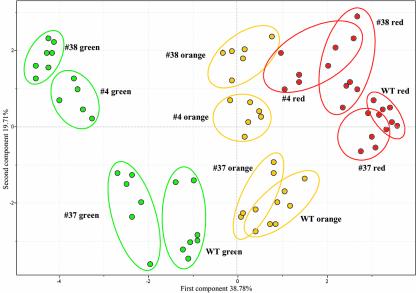
Given that one of the primary objectives of this study was to evaluate the relative influence of hexose phosphorylation during fruit development, we next applied the statistical tool PCA to our combined data set. Several distinct clusters were clearly observable with the various developmental stages of fruit from both wild-type and transgenic lines largely separating along the first component axis, whereas the transgenic lines largely separated from the wild-type lines along the second component axis (Fig. 3). Intriguingly, in keeping with the findings for specific metabolites described above, the clusters of the transgenics were most distinct at the early stage of fruit development, with the orange fruit samples from the transgenics converging somewhat and with the wild-type samples of the red fruit even more. Furthermore, when a PCA of the leaf data set was performed, a similar pattern of separation to that of the green fruit was observed (data not shown).
Figure 3.
PCA of the metabolic profiles of fruits from hexose kinase overexpressing tomato plants at different stages of development. The distances between samples were determined as detailed in “Materials and Methods” by using the log-transformed, normalized data of the single measurements from which the mean values presented in Table IV were derived. PCA vectors span a 10-dimensional space to afford best sample separation. Vectors 1 and 2 including 58.5% of the metabolic variance are presented here.
DISCUSSION
This study illustrates the potential of comprehensive metabolic analysis coupled with statistical clustering methods for the analysis of the relative influence of an enzyme activity throughout development. We have used previously the techniques described in this paper to phenotype transgenic lines exhibiting enhanced Suc mobilization (Roessner et al., 2001a) and restricted starch metabolism (Roessner et al., 2001b), demonstrating their power in phenotyping. In addition, we demonstrated that we were able to faithfully phenocopy genetic manipulations through alterations in environmental conditions (Roessner et al., 2001b). Here, we take a range of approaches to profile the primary metabolic complement of transgenic tomato lines overexpressing an Arabidopsis hexokinase AtHK1 (Dai et al., 1999), with particular focus on distinct phases of fruit development. Although many studies have followed developmental changes of a restricted number of parameters across diverse genotypes (for example, see Chen et al., 2002; Borisjuk et al., 2003; Hajirezaei et al., 2003; Kühn et al., 2003), and one unbiased analysis of metabolite change across strawberry (Fragaria uesca) fruit development has been carried out (Aharoni et al., 2002), to our knowledge this study represents the first comprehensive analysis of metabolic change during fruit development across diverse genotypes.
Adaptation of the GC-MS method that we routinely use for analysis of metabolites from potato tissue allowed the detection of 92 metabolites in tomato leaf tissue and 62 metabolites in fruit tissue, of which 58 and 59 were of known chemical structure, respectively.
For this study, we decided to concentrate on these metabolites alone because when they are augmented by the parallel analysis of nucleotides, starch, hexose phosphates, and sugars, a fairly complete picture of primary metabolism is achieved. To check the applicability of our potato method for tissues of tomato, we determined the absolute concentrations of a range of compounds including sugars, organic acids, and amino acids in wild-type tomato leaf, green fruit, and red fruit extracts (Table II). The absolute values we obtained for these metabolites were very similar to those previously reported for these tissues (for example, see Boggio et al., 2000; Chen et al., 2001). In addition, we demonstrated that the vast majority of metabolites identified in tomato tissue extracts could be quantitatively recovered after recombination experiments with potato tissue extracts. When taken together, these experiments provide a robust validation of the utility of the method in the determination of metabolites from tissues of tomato.
Next, we turned our attention to confirming the genetic identity of the transgenic lines used in this study. Perhaps surprising is that the increase in activity observed in fruit was greatest in later stages of development. However, this is most probably explained by the fact that hexokinase activity in the wild type decreases to vanishingly low levels, nearing the point of detection (for example, see Schaffer and Petreikov, 1997). Similarly, the level of expression of both isoforms of tomato hexokinase is also dramatically reduced in later stages of fruit development (Menu et al., 2001; Dai et al., 2002).
Although the major purpose of this study was to combine multiparallel metabolite analysis with bioinformatic tools for data analysis in the evaluation of the influence of hexokinase on fruit metabolism, the comprehensive analysis afforded by metabolic profiling alone allowed some important conclusions to be made. In addition to analysis of fruit metabolism, we performed a parallel analysis of leaf samples because it is highly possible that changes in the metabolite pools of the leaves could influence those of the fruit. Despite this cautionary note, several of the changes observed in the leaves are probably the result of different factors in the leaf and the fruit. One such example is the changes in the adenylate pool sizes: Although both leaf and fruit tissue are characterized by a reduction in the adenylate pools, the reasons behind this are likely to be, at least partially, different. The initial characterization of the hexokinase transformants revealed an inhibition of photosynthesis in these lines, which could explain why the drop in adenylates and in the ATP to ADP ratio was more severe in the leaf tissue. However, it should be noted that although reduced photosynthesis can correlate with reduction in the adenylate pool size (for example, see Lytovchenko et al., 2002b), this is not always the case (for example, see Lytovchenko et al., 2002a). In contrast, the less dramatic changes observed in the adenylate pool sizes of the fruit most probably result merely from the increased demand for ATP imposed by the expression of the transgene.
Although the changes in the transgenics largely correlate to the level of expression of the transgene (being far more predominant in the cases of lines HK4 and HK38), this is not exclusively the case, implying that some of the metabolic changes are secondary consequences of the genetic manipulation. Although the exact reasons underlying this remain unclear, the previous study of these plants also documented that they were characterized by a reduction in fruit growth (Dai et al., 1999), a trait that was also observed in our current study (data not shown). Although our results cannot provide the exact mechanism by which this is brought about, the metabolite data allows us to speculate that fruit growth is restricted in one of two ways. It is conceivable that growth may be inhibited by the reduced availability of nucleotides and nucleotide sugars within the fruit. Because the transgenic fruits are characterized by a marked reduction in UDP-Glc level, which is a major precursor of matrix polysaccharides (Feingold and Avigad, 1980; Reiter and Vanzin, 2001), it follows that cell wall biosynthesis may be compromised. However, it is worth noting that UDP-Glc levels are not regarded to be so crucial in cellulose synthesis, where Suc levels are believed to be more important (for review, see Haigler et al., 2001). Alternatively, the restriction of growth may be due to a limited availability of carbon for polymer synthesis. There are several lines of evidence that support this hypothesis. First, the accumulation of UDP-Glc in leaves of plants that have been characterized to display a reduced photosynthesis is consistent with a restriction of Suc synthesis and, by implication, the rate of Suc export from the leaf. When Suc transport was inhibited in potato plants using antisense technology, a similar yet more drastic reduction in tuber yield was observed (Riesmeier et al., 1994). However, even if the Suc transport is unaltered in these lines, there are three indications that the carbon availability for polymer biosynthesis in the fruit is restricted. First, the combined levels of Suc, Glc, and Fru are markedly reduced in the transgenic lines. Second, a large amount of carbon is partitioned toward amino acids. Finally, starch content is also markedly reduced in the transgenic lines.
As mentioned above, the hexokinase overexpressors were also characterized by a dramatic increase in the levels of several key amino acids both in leaf and fruit tissue. Because the level of protein is unaltered in either tissue (data not shown), it seems likely that in both instances these changes are the result of an elevated carbon partitioning through glycolysis toward the amino acid pool. However, we cannot directly rule out that changes in the amino acid composition of the fruit are due to increased import of amino acids from the leaves. Nevertheless, this possibility seems unlikely because the pattern of change in amino acids in the fruits is distinct from that observed in leaves. Furthermore, characterization of key activities of glycolysis revealed an induction of this pathway occurs in green fruit of the transgenics similar to that observed on induction of glycolysis in potato tubers exhibiting enhanced Suc mobilization (Trethewey et al., 1998, 2001).
Interestingly, as was also observed in these potato transgenics (Roessner et al., 2001), fruits (but not leaves) of the hexokinase plants displayed large increases in the levels of Ala and succinate. When considered alongside the changes in the energy levels seen in these fruits, this suggests that the fruit may tend toward hypoxia (Geigenberger, 2003).
When the changes in the metabolite pools of the transgenics at the independent time points are assessed in the context of fruit development, with a few notable exceptions, the pattern of change in largely reflects that observed for the wild type, although the magnitude of the changes between the different stages is on occasion much larger. Although many of the metabolic changes during development have been reported previously (for example, see Schaffer and Petreikov, 1997; Boggio et al., 2000; Chen et al., 2001), the comprehensive nature of the GC-MS analysis presented here means that several of these are novel. Of special note are the sugar trehalose and the organic acid ascorbate that currently receive a large amount of research attention (for example, see Smirnoff and Wheeler, 2000; Paul et al., 2001; Eastmond et al., 2002). It is possible that the pattern of change in these metabolites could be used, in combination with additional genomic tools, to further elucidate genetic components involved in the regulation of these important metabolites. Interestingly, trehalose levels closely mirror those of Glc, a fact that we previously observed in potato tubers (Roessner et al., 2001a). In fact, in the tuber, trehalose is only detected in transgenic lines overexpressing a yeast invertase (and, consequently, accumulating large amounts of Glc). Although very many interesting results emerged from point-by-point analysis and from looking at changes of specific metabolites over developmental time, perhaps the clearest insight was that gained using PCA. This revealed two important conclusions: first, that tomato fruits of different developmental stage can be distinguished from one another on the basis of their metabolic complement; and second, because the separation of the transgenics is much greater in the early developmental stage, it follows that the influence of hexokinase on the metabolic complement is much greater at this stage.
In conclusion, the influence of hexokinase on primary metabolism diminishes markedly over developmental time. Thus, the results presented in this study support our earlier postulate that the influence of hexokinase in metabolism is highly dependent on the developmental and/or environmental situation. Furthermore, although these results do not preclude a role for hexokinase-mediated sugar sensing, they can all be rationalized purely on the basis of the enzymatic activity of the protein.
MATERIALS AND METHODS
Plant Material
Three independent transgenic tomato (Lycopersicon esculentum) lines overexpressing the Arabidopsis hexokinase AtHXK1 (for description, see Dai et al., 1999) were grown in parallel (alongside wild-type controls) with a minimum of 250 μmol photons m-2 s-1 at 22°C in the greenhouse. Leaf samples were taken 6 h into the light period from mature fully developed leaves of 6-week-old plants. Fruit samples were taken exclusively from pericarp tissue after rapid removal of the epidermis.
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemie GmbH (Deisenhofen, Germany), with the exception of the starch determination kit and biochemical enzymes (Boehringer Mannheim, Mannheim, Germany), N-methyl-N-[trimethylsilyl]trifluoroacetamide (Macherey-Nagel GmbH & Co. KG, Düren, Germany), and radiolabel (Amersham International, Braun-schweig, Germany).
mRNA Extraction and Northern-Blot Analysis
Total RNA was isolated from leaves and developing tomato fruits as described by Hughes and Galau (1988). Standard conditions were used for the transfer of RNA to membranes and for the subsequent hybridization (Sambrook et al., 1989). Loading was standardized relative to total RNA levels. Transcript levels of AtHK1 were determined using the full-length clone as a probe.
Enzyme Analysis
Leaf or fruit pericarp samples were rapidly frozen in liquid N2, and enzymes were extracted and desalted according to Geigenberger and Stitt (1993). Hexokinase activity was measured as described by Dai et al. (1999), enolase activity according to Fernie et al. (2001a), fructokinase and phospho-Glc isomerase activities as described by Fernie et al. (2001b), whereas ADP-Glc pyrophosphorylase, aldolase, phosphoglucomutase, pyruvate kinase, phosphofructokinase, glyceraldehyde-3-phosphate dehydrogenase, and triose-phosphate isomerase activities were determined according to Lytovchenko et al. (2002b).
Determination of Starch, Soluble Sugars, Hexosephosphates, Nucleotides, and Nucleotide Sugars
Leaf or fruit pericarp samples were rapidly frozen in liquid N2 and extracted in either ethanol (for starch and sugar measurements) or in trichloroacetic acid (for hexosephopshates, nucleotide, and nucleotide sugar measurements). Starch and sugars were determined spectrophotometrically as described by Fernie et al. (2001a), and hexose phosphates were determined spectrophotometrically as detailed by Tauberger et al. (2000). Nucleotides and nucleotide sugars were separated by HPLC on a Partisil-SAX anion-exchange column (P10SAX-250; Hichrom, Reading, UK) as described by Fernie at al. (2001a). Eluted nucleotides were detected by their A254 and identified and quantified by cochromatography with authentic nucleotide standards. The recoveries of small representative amounts of metabolites throughout the extraction, storage, and assay procedures have been documented previously (Fernie et al., 2001a).
Extraction, Derivatization, and Analysis of Tomato Leaf and Fruit Metabolites Using GC-MS
Metabolite analysis by GC-MS was carried out by a method modified from that described by Roessner et al. (2000). Tomato leaf tissue (250 mg) was homogenized using a ball mill precooled with liquid nitrogen and extracted in 1,400 μL of methanol, and 60 μL of internal standard (0.2 mg ribitol mL-1 water) was subsequently added as a quantification standard. The mixture was extracted for 15 min at 70°C and mixed vigorously with 1 volume of water. To separate polar and nonpolar metabolites, 750 μL of chloroform was then added to the mixtures. After centrifugation at 2,200g, the upper methanol/water phase was taken and reduced to dryness in vacuo. For tomato fruit tissue, the same procedure was used with the exception that 300 mg of tissue was taken, and the extraction mixture was comprised entirely of methanol.
Residues after reduction were redissolved in and derivatized for 90 min at 37°C (in 40 μL of 20 mg mL-1 methoxyamine hydrochloride in pyridine) followed by a 30-min treatment with 60 μL of N-methyl-N-[trimethylsilyl]trifluoroacetamide at 37°C. Eight microliters of a retention time standard mixture (0.029% [v/v] n-dodecane, n-pentadecane, n-nonadecane, n-docosane, n-octacosane, n-dotracontane, and n-hexatriacontane dissolved in pyridin) was added before trimethylsilylation. Sample volumes of 1 μL were then injected onto the GC column using a hot needle technique.
The GC-MS system used comprised an AS 2000 autosampler, a GC 8000 gas chromatograph, and a Voyager quadrupole mass spectrometer (ThermoFinnigan, Manchester, UK). The mass spectrometer was tuned according to the manufacturer's recommendations using Tris-(perfluorobutyl)-amine (CF43). GC was performed on a 30-m Rtx_5Sil MS column with 0.25-μm film thickness with a 10-m Integra precolumn (Restek, Bad Homburg, Germany). The injection temperature was set at 230°C, the interface at 250°C, and the ion source adjusted to 200°C. Helium was used as the carrier gas at a flow rate of 1 mL min-1. The analysis was performed under the following temperature program; 5 min of isothermal heating at 70°C, followed by a 5°C min-1 oven temperature ramp to 350°C, and a final 5-min heating at 330°C. The system was then temperature equilibrated for 1min at 70°C before injection of the next sample. Mass spectra were recorded at 2 scan s-1 with a mass-to-charge ratio of 50 to 600 scanning range. Both chromatograms and mass spectra were evaluated using the MASSLAB program (ThermoQuest, Manchester, UK), and the resulting data were prepared and presented as described by Roessner et al. (2001a). The absolute concentrations of several metabolites were determined by comparison with calibration standard curve response ratios of various concentrations of standard substance solutions, including the internal standard ribitol, which were derivatized concomitantly to tissue samples. Recombination experiments were carried out by assessing the recovery of tomato metabolites of defined quantity in a mixed tomato-potato (Solanum tuberosum) extract containing defined quantities of potato metabolites.
Statistical Analysis
PCA was carried out exactly as detailed by Roessner et al. (2001a). If two observations are described in the text as different, this means that their difference was determined to be statistically significant (P < 0.05) by the performance of Student's t tests.
Acknowledgments
We are especially grateful to Lothar Willmitzer for supporting our work. We also thank Dr. Oliver Kreft for the determination of Glu in tomato fruit extracts using HPLC. We thank Dr. Joachim Kopka for helpful discussion concerning data mining and Dr. Markus Pauly for discussions concerning cell walls.
References
- Aharoni A, de Vos CH, Verhoeven HA, Maliepaard CA, Kruppa G, Bino R, Goodenowe DB (2002) Nontargeted metabolome analysis by use of fourier transform ion cyclotron mass spectrometry. OMICS A J Integrative Biol 6: 217-234 [DOI] [PubMed] [Google Scholar]
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas LHW (1997) Developmental changes of enzymes involved in conversion of sucrose to hexose-phosphate during early tuberization of potato. Planta 202: 220-226 [Google Scholar]
- Boggio SB, Palatnik JF, Heldt HW, Valle EM (2000) Changes in amino acid composition and nitrogen metabolizing enzymes in ripening fruits of Lycopersicon esculentum Mill. Plant Sci 159: 125-133 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Wobus U, Weber H (2003) Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds. J Exp Bot 54: 503-512 [DOI] [PubMed] [Google Scholar]
- Chen GP, Wilson ID, Kim SH, Grierson D (2001) Inhibiting expression of a tomato ripening-associated membrane protein increases organic acids and reduces sugar levels of fruit. Planta 212: 799-807 [DOI] [PubMed] [Google Scholar]
- Chen L, Auh C, Chen F, Cheng XF, Aljoe H, Dixon RA, Wang ZY (2002) Lignin deposition and associated changes in anatomy, enzyme activity, gene expression, and ruminal degradability in stems of tall fescue at different developmental stages. J Agric Food Chem 50: 5558-5565 [DOI] [PubMed] [Google Scholar]
- Copeland L, Morrell M (1985) Hexose kinases from the plant cytosolic fraction of soybean Glycine max cultivar Williams nodules. Plant Physiol 79: 114-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland L, Tanner GJ (1988) Hexose kinases of avocado. Physiol Plant 74: 531-536 [Google Scholar]
- Dai N, Kandel-Kfir M, Petreikov M, Hanael R, Levin I, Ricard B, Rothan C, Schaffer AA, Granot D (2002) The tomato hexokinase LeHXK1 cloning, mapping, expression pattern and phylogenetic relationships. Plant Sci 163: 581-590 [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11: 1253-1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust MA, Yelle S, Nguyen-Quoc B (1999) Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell 11: 2407-2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demnitz-King A, Ho LC, Baker DA (1997) Activity of sucrose hydrolyzing enzymes and sugar accumulation during tomato fruit development. Plant Growth Regul 22: 193-201 [Google Scholar]
- Doehlert DC (1989) Separation and characterization of four hexokinases from developing maize kernels. Plant Physiol 89: 1042-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Zamir D (1994) Introgression from Lycopersicon penellii can improve the soluble solids yield of tomato hybrids. Theor Appl Genet 88: 891-897 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, van Dijken AJH, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JDG, Smeekens SC, Graham IA (2002) Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 29: 225-235 [DOI] [PubMed] [Google Scholar]
- Feingold DS, Avigad G (1980) Sugar nucleotide transformations in plants. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants: A Comprehensive Treatise, Vol. 3. Academic Press, New York, pp 101-170 [Google Scholar]
- Fernie AR, Roessner U, Trethewey RN, Willmitzer L (2001b) The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 213: 418-426 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ (2001a) Fructose 2, 6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212: 250-263 [DOI] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L (2000a) Metabolite profiling for plant functional genomics. Nat Biotechnol 18: 1157-1157 [DOI] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Trethewey RN, Willmitzer L (2000b) Identification of uncommon plant metabolites based on calculation of elemental compositions using gas chromatography and quadrupole mass spectrometry. Anal Chem 72: 3573-3580 [DOI] [PubMed] [Google Scholar]
- Galina A, Reis M, Alberquerque MC, Puyou AG, Puyou MTG, de Meis L (1995) Different properties of the mitochondrial and cytosolic hexokinases in maize roots. Biochem J 309: 105-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P (2003) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6: 247-256 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 201: 502-518 [DOI] [PubMed] [Google Scholar]
- Haigler CH, Ivanova-Datcheva I, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47: 29-51 [PubMed] [Google Scholar]
- Hajirezaei MR, Bornke F, Peisker M, Takahata Y, Lerchl J, Kirakosyan A, Sonnewald U (2003) Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). J Exp Bot 54: 477-488 [DOI] [PubMed] [Google Scholar]
- Hughes DW, Galau GA (1988) Preparation of RNA from cotton leaves and pollen. Plant Mol Biol Rep 6: 253-257 [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee M, Finger FL (1992) NADP+-malic enzyme and organic acid levels in developing tomato fruits. J Am Soc Hortic Sci 117: 799-801 [Google Scholar]
- Kruger NJ (1997) Carbohydrate synthesis and degradation. In DT Dennis, DH Turpin, DD Lefebrve, DB Layzelle, eds, Plant Metabolism. Longmann Harlow, Essex, UK, pp 83-104
- Kühn C, Hajirezaei MR, Fernie AR, Roessner-Tunali U, Czechowski T, Hirner B, Frommer WB (2003) The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiol 131: 102-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytovchenko A, Bieberich K, Willmitzer L, Fernie AR (2002a) Carbon assimilation and metabolism in potato leaves deficient in plastidial phosphoglucomutase. Planta 215: 802-811 [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Sweetlove L, Pauly M, Fernie AR (2002b) The influence of cytosolic phosphoglucomutase on photosynthetic carbohydrate metabolism. Planta 215: 1013-1021 [DOI] [PubMed] [Google Scholar]
- MacDougall AJ, Parker R, Selvedran RR (1995) Nonaqueous fractionation to access the ionic composition of the apoplast during fruit ripening. Plant Physiol 108: 1679-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Nunez JM, Ortiz A, Cerda A (1994) Changes in amino acid and organic acid composition in tomato and cucumber plants in relation to salinity and nitrogen nutrition. J Plant Nutr 17: 1359-1368 [Google Scholar]
- Martinez-Barajaz E, Randall DD (1998) Purification and characterisation of a glucokinase from young tomato (Lycopersicon escullentum Mill) fruit. Planta 205: 567-573 [DOI] [PubMed] [Google Scholar]
- Menu T, Rothan C, Dai N, Petreikov M, Etienne C, Destrac-Irvine A, Schaffer A, Granot D, Ricard B (2001) Cloning and characterization of a cDNA encoding hexokinase from tomato. Plant Sci 160: 209-218 [DOI] [PubMed] [Google Scholar]
- Miernyk JA, Dennis DT (1983) Mitochondrial, plastid and cytosolic isozymes of hexokinase from developing endosperm of Ricinus communis. Arch Biochem Biophys 226: 458-468 [DOI] [PubMed] [Google Scholar]
- Miron D, Petreikov M, Carmi N, Shen S, Levin I, Granot D, Zamski E, Schaffer AA (2002) Sucrose uptake, invertase localisation and gene expression in developing fruit of Lycopersicon esculentum and the sucrose-accumulating Lycopersicon hirsutum. Physiol Plant 115: 35-47 [DOI] [PubMed] [Google Scholar]
- Paul M, Pellny T, Goddijn O (2001) Enhancing photosynthesis with sugar signals. Trends Plant Sci 6: 197-200 [DOI] [PubMed] [Google Scholar]
- Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Geigenberger P, Kossmann J (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20: 1256-1260 [DOI] [PubMed] [Google Scholar]
- Reiter WD, Vanzin GR (2001) Molecular genetics of nucleotide sugar interconversion in plants. Plant Mol Biol 47: 95-113 [PubMed] [Google Scholar]
- Renz A, Stitt M (1993) Substrate specificity and product inhibition of different forms of fructokinases and hexokinases in developing potato tubers. Planta 190: 166-175 [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13: 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001a) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13: 11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 23: 131-142 [DOI] [PubMed] [Google Scholar]
- Roessner U, Willmitzer L, Fernie AR (2001b) High-resolution metabolic phenotyping of genetically and environmentally diverse potato tuber systems. Identification of phenocopies. Plant Physiol 127: 749-764 [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schaffer AA, Petreikov M (1997) Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113: 139-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C (1990). Characterization and compartmentation, in green leaves, of hexokinases with different specificities for glucose, fructose and mannose and for nucleotide triphosphates. Planta 181: 249-255 [DOI] [PubMed] [Google Scholar]
- Sindelar L, Sindelarova M, Burketova L (1998) Hexokinases of tobacco leaves: influence of plant age on particulate and soluble isozyme composition. Biol Plant 40: 469-474 [Google Scholar]
- Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19: 267-290 [DOI] [PubMed] [Google Scholar]
- Tauberger E, Fernie AR, Emmermann M, Renz A, Kossmann J, Willmitzer L, Trethewey RN (2000) Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J 23: 43-53 [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Fernie AR, Bachmann A, Fleische-Notter H, Geigenberger P, Willmitzer L (2001) Expression of a bacterial sucrose phosphorylase in potato tubers results in a glucose-independent induction of glycolysis. Plant Cell Environ 24: 357-365 [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15: 109-118 [DOI] [PubMed] [Google Scholar]
- Turner JF, Chensee QJ, Harrison DD (1977) Glucokinase of pea seeds. Biochim Biophys Acta 480: 367-375 [DOI] [PubMed] [Google Scholar]
- Turner JF, Copeland L (1981) Hexokinase II of pea seeds. Plant Physiol 68: 1123-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veramendi J, Fernie AR, Leisse A, Willmitzer L, Trethewey RN (2002) Potato hexokinase 2 complements transgenic Arabidopsis plants deficient in hexokinase 1 but does not play a key role in tuber carbohydrate metabolism. Plant Mol Biol 49: 491-501 [DOI] [PubMed] [Google Scholar]
- Veramendi J, Roessner U, Renz A, Willmitzer L, Trethewey RN (1999) Antisense repression of hexokinase 1 leads to an overaccumulation of starch in leaves of transgenic potato plants but not significant changes in tuber metabolism. Plant Physiol 121: 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola R, Roberts AG, Haupt S, Gazzani S, Hancock RD, Marmiroli N, Machray GC, Oparka KJ (2001) Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell 13: 385-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Sefkow M, Kopka J (2003) Construction and application of a mass spectral and retention time index database generated from plant GC/EI-TOF-MS metabolite profiles. Phytochemistry 62: 887-900 [DOI] [PubMed] [Google Scholar]
- Wiese A, Groner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flugge UI, Weber A (1999) Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett 461: 13-18 [DOI] [PubMed] [Google Scholar]
- Yelle S, Hewitt JD, Robinson NL, Damon S, Bennett AB (1988) Sink metabolism in tomato fruit: III Analysis of carbohydrate assimilation in a wild species. Plant Physiol 87: 737-740 [DOI] [PMC free article] [PubMed] [Google Scholar]