Abstract
The arl2 mutants of Arabidopsis display altered root and hypocotyl gravitropism, whereas their inflorescence stems are fully gravitropic. Interestingly, mutant roots respond like the wild type to phytohormones and an inhibitor of polar auxin transport. Also, their cap columella cells accumulate starch similarly to wild-type cells, and mutant hypocotyls display strong phototropic responses to lateral light stimulation. The ARL2 gene encodes a DnaJ-like protein similar to ARG1, another protein previously implicated in gravity signal transduction in Arabidopsis seedlings. ARL2 is expressed at low levels in all organs of seedlings and plants. arl2-1 arg1-2 double mutant roots display kinetics of gravitropism similar to those of single mutants. However, double mutants carrying both arl2-1 and pgm-1 (a mutation in the starch-biosynthetic gene PHOSPHOGLUCOMUTASE) at the homozygous state display a more pronounced root gravitropic defect than the single mutants. On the other hand, seedlings with a null mutation in ARL1, a paralog of ARG1 and ARL2, behave similarly to the wild type in gravitropism and other related assays. Taken together, the results suggest that ARG1 and ARL2 function in the same gravity signal transduction pathway in the hypocotyl and root of Arabidopsis seedlings, distinct from the pathway involving PGM.
Gravity is one of the environmental cues that guides plant organs' growth. Most plant organs are characterized by a specific gravity set point angle, which defines their preferential growth vector relative to gravity (Firn and Digby, 1997). In young Arabidopsis seedlings, shoots grow upward, displaying negative gravitropism, whereas roots grow downward, toward the center of gravity (positive gravitropism; Bullen et al., 1990; Boonsirichai et al., 2002).
Gravity perception by dicot organs involves primarily the sedimentation of amyloplasts within specialized cells (statocytes) located in the columella region of the root cap and in the starch sheath, which constitutes the endodermis of hypocotyls and inflorescence stems (Kiss et al., 1996; Kuznetsov and Hasenstein, 1996; Blancaflor et al., 1998; Weise et al., 2000). In shoots, sedimentable amyloplasts and the curvature response to gravistimulation occur along the elongation zone (for review, see Masson et al., 2002). After amyloplast sedimentation, signals are likely transduced within the endodermal cells, and physiological signals are transported laterally to affect elongation of cortical and epidermal cells. In roots, sites of gravity perception and curvature response may be physically separated (Poff and Martin, 1989). Hence, physiological signals resulting from activation of the gravity signal transduction pathway should be transported from the root cap columella to the elongation zones where the gravitropic curvature is initiated (for review, see Boonsirichai et al., 2002).
Auxin is a physiological signal that has been shown to mediate the gravitropic response (for review, see Masson et al., 2002). In gravistimulated roots, auxin is redistributed asymmetrically across the root cap and transmitted to the elongation zones where it promotes a gravitropic curvature. This redistribution of auxin appears modulated, at least partly, by the relocation of PIN3-containing auxin efflux machinery, from a symmetrical distribution along the plasma membrane of columella cells toward an accumulation at their new physical bottom (Friml et al., 2002). This process is accompanied by rapid alkalinization of the statocytes' cytoplasm and acidification of the cell wall surrounding the statocytes (Scott and Allen, 1999; Fasano et al., 2001). Both processes appear essential for full graviresponsiveness.
Polar auxin transport machineries, including auxin influx and efflux carriers that are partly made of the AUX1 and AGR1/EIR1/PIN2/WAV6 gene products, respectively, modulate the transmission of the gravity-induced lateral auxin gradient from the root cap to the elongation zones (Bennett et al., 1996; Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998; Utsuno et al., 1998; Swarup et al., 2001). There, an auxin response machinery converts the auxin gradient signal into a differential cellular growth responsible for the gravitropic curvature (for review, see Masson et al., 2002), although an auxin gradient-independent phase may contribute to the early curvature response that occurs in the root distal elongation zone (Evans and Ishikawa, 1997; Wolverton et al., 2002).
Little is known about the molecular mechanisms that underlie gravity signal transduction in root cap cells, leading to asymmetric redistribution of auxin. Ca2+ may serve as a second messenger in this pathway (Plieth and Trewavas, 2002). On the other hand, experiments carried out in cereal pulvini suggest that inositol 1,4,5-trisphosphate might also contribute to gravity signal transduction, at least in this system (Perera et al., 1999).
Only a few genes have been uncovered that affect gravity signal transduction in roots and hypocotyls. Mutations in two Arabidopsis genes, including SGR2 (which encodes a putative phospholipase A1 localized in the membranes of vacuoles and small organelles) and ZIG/SGR4 (which encodes the AtVTI11 SNARE protein), result in altered hypocotyl and shoot gravitropism, along with misshapen seeds and seedlings (Kato et al., 2002; Morita et al., 2002). The shoot gravitropic phenotype of sgr2 and zig/sgr4 mutants could be rescued by expressing the corresponding wild-type genes in the endodermis. These two proteins may be involved in a vacuolar membrane system that participates in the early events of gravity signal transduction specific to shoots (Kato et al., 2002; Morita et al., 2002).
Mutations in the ARG1 gene of Arabidopsis result in altered root and hypocotyl gravitropism without pleiotropic phenotypes. Mutant roots and hypocotyls contain starch in their statocytes and respond like wild type to phytohormones, polar auxin transport inhibitors, and lateral light stimulation (Fukaki et al., 1997; Sedbrook et al., 1999). The ARG1 gene encodes a DnaJ-like protein that carries a coiled-coil domain with similarity to coiled coils found in several cytoskeleton binding proteins. Hence, it was postulated that ARG1 might mediate gravity signal transduction by promoting the folding, targeting, or degradation of gravitropic regulators in the vicinity of the cytoskeletal network in statocytes (Sedbrook et al., 1999).
Ninety-one genes encode DnaJ-like proteins in Arabidopsis (Arabidopsis Genome Initiative, 2000; Miernyk, 2001). However, phylogenetic studies indicate that only two of these genes, named ARG1-LIKE1 (ARL1, also called AtDjB16 or At1g24120) and ARG1-LIKE2 (ARL2, also called AtDjC39 or At1g59980), encode proteins with high-level similarity to ARG1 throughout their lengths (Sedbrook et al., 1999; Miernyk, 2001). Nothing is known about the function of ARL1 and ARL2 in plant growth and development. In this manuscript, we report on the isolation and phenotypic characterization of allelic mutations in these two genes.
RESULTS
13-8 Mutation Affects Root and Hypocotyl Gravitropism
To identify Arabidopsis mutants affected in root and hypocotyl gravitropism, we subjected 103,000 fast neutron-mutagenized Estland (Est) seedlings to an on-agar reorientation assay. Three hundred and twenty four seedlings displayed an altered gravitropic response. Their progeny were retested for altered gravitropism, 50 of which again showed a diminished curvature response to gravistimulation. 13-8 was one of them. This mutant was backcrossed seven times against the parental wild-type (Est) to remove unlinked mutations.
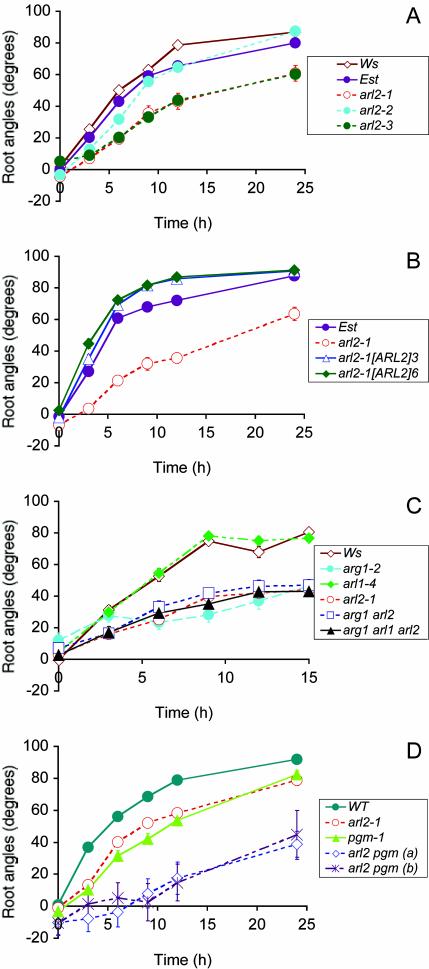
To better characterize the gravitropic phenotype of 13-8, we subjected wild-type and 13-8 mutant seedlings to an in-agar reorientation assay in darkness. The root and hypocotyl tip angles from the horizontal were measured at regular time points after gravi-stimulation. Figures 1A and 2A show that 13-8 (arl2-1) mutant roots and hypocotyls displayed slower kinetics of gravitropism compared with wild type. They also showed increased variation in root and hypocotyl tip angles compared with wild type at each time point (F test probabilities below 0.05).
Figure 1.
Gravitropic phenotype of wild-type, arl2, and arl1 mutant seedlings in darkness (A and C) or in light (B and D). Average root tip angles from the horizontal are shown at each time point. In A to D, genotypes of tested seedlings are indicated in a legend box. A, Root gravitropism of wild-type Est, Wassilewskija (Ws), and arl2-1, arl2-2, and arl2-3 mutant seedlings. This graph shows the results of two independent experiments, one involving Est and arl2-1, the other one involving Ws, arl2-2, and arl2-3. Wild-type Ws seedlings were included in both experiments, where they showed almost identical kinetics of root gravitropism (data not shown). Hence, we show only the Ws response for the second experiment. n = 33 to 84 for the first experiment and 38 to 120 for the second experiment. B, Root gravitropism of wild-type Est, untransformed arl2-1 mutant seedlings, and progeny of two independent arl2-1 transformants carrying the p35S-His6::ARL2 construct (arl2-1[ARL2]3 and arl2-1[ARL2]6, respectively; n = 41-53). C, Root gravitropism of wild-type Ws, single mutants arl1-4, arg1-2, and arl2-1, double mutant arg1-2 arl2-1, and triple mutant arg1-2 arl2-1 arl1-4 (n = 28-61). D, Root gravitropism of wild type (WT), single mutants arl2-1 and pgm-1, and double mutant arl2-1 pgm-1 seedlings. All wild-type and mutant lines tested in this experiment were derived from individual segregating F2 progeny from a cross between arl2-1 and pgm-1. Two independent arl2-1 pgm-1 double-mutant lines (a and b) were analyzed (n = 14-40). In A to D, vertical bars representing ses are shown at each time point. However, they are often masked by the curve symbols.
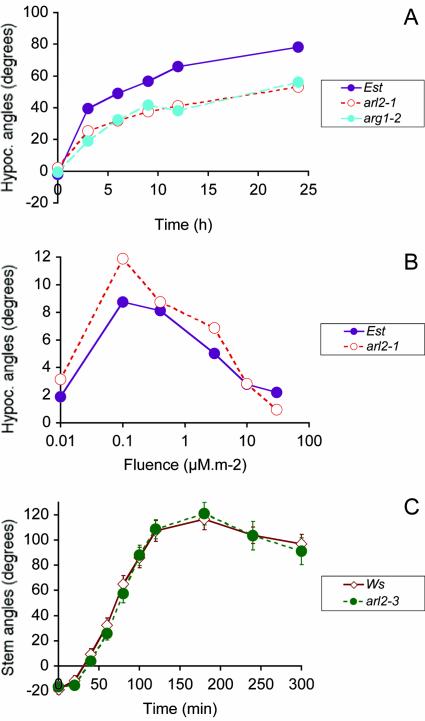
Figure 2.
Tropic phenotypes of arl2 shoots. A, Kinetics of hypocotyl gravitropism in darkness for wild-type Est, arg1-2, and arl2-1 mutant seedlings (n = 111-168). B, Hypocotyl phototropism of 42-h-old seedlings exposed to a single pulse of blue light (450 nm) at a fluence rate of 0.15 μm m-2 s-1. Hypocotyl curvatures were measured 30 min after phototropic stimulation (n = 58-104). C, Kinetics of primary inflorescence stem gravitropism in darkness for 3- to 3.5-week-old wild-type and arl2-3 mutant plants (n = 15-16). As in Figure 1, ses are shown by vertical bars that are often masked by the curve symbols.
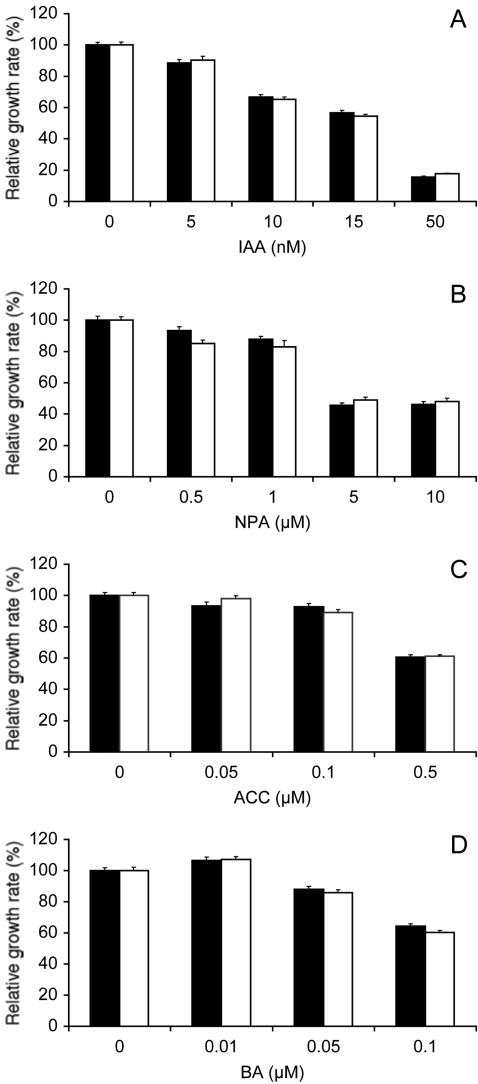
The gravitropic phenotype of 13-8 mutant seedlings was very similar to that of arg1-2 mutants (Figs. 1C and 2A), which displayed no pleiotropic phenotypes (Sedbrook et al., 1999). Therefore, we set up experiments aimed at determining if 13-8 also affected specifically gravitropism, without resulting in pleiotropic phenotypes. Results indicated that 13-8 mutant roots grew at wild-type rates in the absence of phytohormones or polar auxin transport inhibitors in the medium (Student's t test probability = 0.48; n = 207-215). They also displayed wild-type root growth sensitivity to auxins indole-3-acetic acid (IAA; Fig. 3A), 1-naphthaleneacetic acid (1-NAA), and 2,4-dichlorophenoxyacetic acid (2,4-D; data not shown), to a polar auxin transport inhibitor (NPA; Fig. 3B), to ACC (a precursor of ethylene biosynthesis; Fig. 3C), and to BA (Fig. 3D). In the experiment shown in Figure 3B, a slight but statistically meaningful difference in root growth sensitivity to 0.5 μm NPA was observed between wild-type and mutant roots (Student's t test P value of 0.01). However, this difference was not reproducible in other experiments (data not shown).
Figure 3.
Root growth sensitivity to phytohormones and a polar auxin transport inhibitor. A to D, Relative root growth rate of wild-type Est (black bars) and arl2-1 mutant seedlings (white bars) in the presence of varying concentrations of IAA (A), naphthylphthalamic acid (NPA; B), 1-aminocyclopropane-1-carboxylic acid (ACC; C), or N6-benzyladenine (BA; D). Four-day-old seedlings were transferred onto fresh germination medium (GM) containing 0.1% (v/v) ethanol (A and D), 0.05% (v/v) dimethyl sulfoxide (B), or 0.05% (v/v) isopropanol (C) with the indicated concentrations of IAA, NPA, ACC, or BA. Root growth was measured over a period of 2 d. Average root growth rates were determined for each compound concentration and divided by the corresponding growth rate in absence of the compound (control). ses are represented by vertical bars. The numbers of seedlings tested in these experiments were 36 to 52 (A), 27 to 43 (B), 44 to 91 (C), and 81 to 95 (D). Average growth rates in the absence of added compounds were: A, 5.2 ± 0.09 (Est) and 4.5 ± 0.08 (arl2-1) mm d-1; B, 4.6 ± 0.12 (Est) and 4.5 ± 0.12 (arl2-1) mm d-1; C, 4.4 ± 0.09 (Est) and 5.09 ± 0.1 (arl2-1) mm d-1; and D, 5.2 ± 0.09 (Est) and 5.0 ± 0.1 (arl2-1) mm d-1.
We also tested the mutant's ability to accumulate starch in the statocytes. Results indicated that 13-8 mutant roots accumulated starch in the columella cells of their root cap similarly to wild-type roots, whereas pgm-1 mutant roots, which are defective in the phosphoglucomutase enzyme involved in starch biosynthesis (Caspar and Pickard, 1989), did not (data not shown). Hence, it appears that the 13-8 mutation affects root and hypocotyl gravitropism, without altering root growth rate, root growth sensitivity to phytohormones or inhibitors of polar auxin transport, or starch accumulation in the statocytes.
To further investigate the possibility that the 13-8 mutation might affect more generally the ability of plant organs to curve in response to environmental parameters, we characterized the curvature response of hypocotyls to varying fluences of lateral light stimulation (phototropism). Wild-type and 13-8 mutant hypocotyls displayed similar phototropic dose response curves to lateral light stimulation, with peak responses at 0.1 μm m-2 radiance. However, 13-8 hypocotyls responded more strongly than wild type to lateral light stimulation at most fluences tested (Fig. 2B; Student's t test p value of 0.0083 for seedlings exposed to 0.1 μm m-2 lateral light).
13-8 Affects ARL2, an ARG1 Paralog
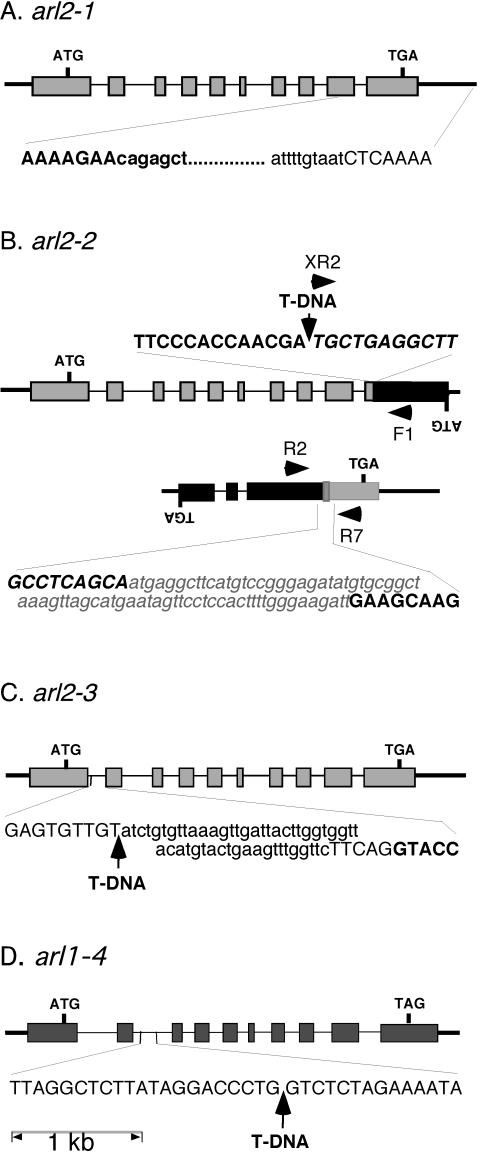
The results described above indicated that the 13-8 phenotype was similar to that of arg1 mutants (Sedbrook et al., 1999). However, genetic complementation studies suggested that the two mutations were not allelic, even though both were recessive and mapped on the bottom arm of chromosome 1, between the nga111 and ATHGENEA simple sequence length polymorphism (SSLP) markers (data not shown; Bell and Ecker, 1994; Sedbrook et al., 1999). Interestingly, one of the ARG1 paralogs, ARL2, is located within 10 map units of ARG1 on chromosome 1 (Arabidopsis Genome Initiative, 2000; Miernyk, 2001). Therefore, we decided to determine if the 13-8 mutation affects ARL2. We PCR amplified, cloned, and sequenced a piece of genomic DNA surrounding ARL2 in wild-type and 13-8 mutant plants. Results shown in Figure 4A indicated that the 3′ end of ARL2 is deleted in 13-8 mutant plants as part of an 857-bp deletion. This deletion eliminates the carboxyl end of the predicted protein, including the conserved region predicted to form a coiled-coil structure in ARG1 and ARL2 (Sedbrook et al., 1999). The next predicted gene found within the same T2K10 bacterial artificial chromosome genomic clone (At1g59990) is located 6.7 kb away from ARL2, on its 3′ side (Arabidopsis Genome Initiative, 2000). Hence, it appeared likely that the 13-8 mutation affects the ARL2 gene. This mutation was named arl2-1.
Figure 4.
Genomic structure of arl2-1 (A), arl2-2 (B), arl2-3 (C), and arl1-4 (D). Exons are represented by rectangles, introns by thin bars, and intergenic chromosomal regions by thick bars. ATG, Translation initiation codons; TGA or TAG, stop codons, ARL2 and At2g20050 or ARL1. Initiation and stop codons for lower strand open reading frames (ORFs; B) are indicated by inverted letters. The nucleotide sequence of a segment of DNA flanking the mutation site in each allele is indicated at the bottom or top of the corresponding diagram. The nucleotide sequence of an ARL2 or ARL1 exon fragment is represented in bold and uppercase characters, whereas that of an intronic or intergenic region is represented by uppercase characters. The sequence of At2g20050 exon fragments (B) is represented by italic uppercase characters. Sequences deleted within a specific allele are represented by lowercase characters, whereas new sequences added at a specific site (translocation breakpoint between the ARL2 and At2g20050 3′end fragments in arl2-2) are represented by a gray box, and written in bold, italicized, lowercase gray characters. T-DNA inserts are represented by arrows. For arl2-2 (B), the two ARL2 sequence-containing loci derived from a reciprocal translocation between chromosomes 1 and 2 are shown, along with the position of primers (black arrowheads) used in diagnostic PCR reactions (Fig. 5).
We used a reverse genetic strategy to screen two collections of T-DNA-mutagenized plants for individuals carrying other mutations in ARL2 (Young et al., 2001). Plants carrying two independent T-DNA insertion alleles of ARL2 were identified and characterized. Molecular analysis revealed that arl2-2 contains a T-DNA insertion within the predicted ARL2 ORF at position 2,550 of the gene (Fig. 4B). Southern-blot analysis confirmed the existence of one T-DNA in this line. However, it also indicated that the ARL2 DNA normally present 3′ to the T-DNA insertion site in wild-type DNA was physically separated from the T-DNA insertion and ARL2 5′ fragment in this mutant (data not shown). Therefore, we used an inverse PCR strategy to clone and characterize genomic DNA located upstream of the 3′ end ARL2 fragment in this line. Results indicated that this arl2-2 3′ end fragment was flanked by a 71-bp DNA sequence of unknown origin (sharing no homologies with sequences present in the databases), itself followed by DNA derived from the 3′ end of a putative protein phosphatase 2C gene located on chromosome 2 (At2g20050; Fig. 4B). The recombination breakpoint within At2g20050 was located 537 bp downstream of the A residue of the predicted initiation codon. Both genes were in opposite orientations within the recombined structure (Fig. 4B).
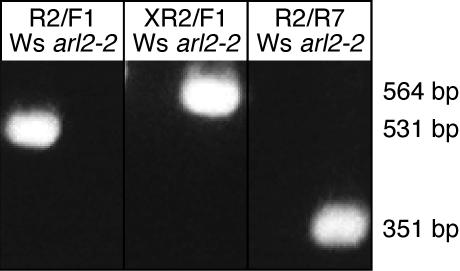
To determine if this recombined structure was strictly associated with arl2-2, we tested the ability of At2g20050-specific PCR primers (PP2C-F1 and PP2CR2; Table I; Fig. 4B) to amplify At2g20050 DNA overlapping the recombination breakpoint described in the previous paragraph from wild-type and arl2-2 mutant seedlings. Results shown in Figure 5 indicated that these primers allowed amplification of a 531-bp fragment from wild-type Ws DNA but not from mutant arl2-2 DNA. On the other hand, a 351-bp DNA fragment could be amplified from arl2-2 DNA when a combination of ARL2-specific (ARL2-R7) and At2g20050-specific (PP2C-R2) primers was used in the reaction (Fig. 5). No fragments could be amplified from wild-type DNA with this primer pair (Fig. 5). Taken together, these data suggest that arl2-2 resulted from the combination of a T-DNA insertion within ARL2 and a reciprocal translocation between chromosomes 1 and 2 involving breakpoints within the ARL2 and At2g20050 genes.
Table I.
Primers used for PCR amplifications of ARG1, ARL1, and ARL2 sequences
All primers were ordered from Integrated DNA Technologies, Inc. (Coraville, IA).
| Primer Name | Primer Sequence |
|---|---|
| ARG1-F | 5′-ATCCTCCTTTCTTCATCGTTTCTT-3′ |
| ARG1-R | 5′-CATTGTCATAGTGCCTTCTCTTTT-3′ |
| ARL1-F1 | 5′-TTCAATAGGGCACAATATGTTTAAGAGAACT-3′ |
| ARL1-F4 | 5′-GGAGGTCACGTTTTCTTACAAC-3′ |
| ARL1 nestr1 | 5′-CAATTTCCGGTAAGCGCTC-3′ |
| ARL1 nestr2 | 5′-GACACAGCTTGTCCAAGTAC-3′ |
| ARL1-R1 | 5′-CCAAGTCGGAGAAGAAGGATGCTGATAAG-3′ |
| ARL2-F | 5′-GGAGAATAAAGACGCCGGAGAAGAAGATGA-3′ |
| ARL2-F6 | 5′-TGGTCTATGGTTTATCTCTTTGCACACTTTG-3′ |
| ARL2-F7 | 5′-CACACTTTGCCTGATTCATCTCTTGCTA-3 |
| ARL2GFP2f2 | 5′-TCCCCCGGGATGGCCACTCATTCATC-3′ |
| ARL2KOFU3 | 5′-GGACAAACTGGCTGTAGGGAAAGA-3′ |
| ARL2KOFL1 | 5′-CGAAACGATAAACAGGGAAGCCGA-3′ |
| ARL2-R | 5′-GTAAGCCGCACATATCTCATTCCTCCTCT-3′ |
| ARL2-R2 | 5′-TAACTAAACCAACAAAACCAAAGGGAT-3′ |
| ARL2-r3 | 5′-CAGCTCCTAAGCTTGACAG-3′ |
| ARL2-R6 | 5′-ATATTGTACCATTTCTTCTTCTTGACTCCA-3′ |
| ARL2-R7 | 5′-CACCTTCTTCCCTAACTGCGACTTCTCCTTC-3′ |
| ARL2-SL2 | 5′-TTCAACGCCTCCCCCAATAAAT-3′ |
| ARL2-SU1 | 5′-AGCTCATCTTCTTCTCCGGCGTCTTTATT-3′ |
| ARL2-SU3 | 5′-GCTGGTTCTCATGTCTTTGCTGTT-3′ |
| GFPPAr | 5′-CATGCTTAACGTAATTCAAC-3′ |
| 6HisOEf | 5′-TGCCATGGATGAACTATACAAAG-3′ |
| JL202 | 5′-CATTTTATAATAACGCTGCGGACATCTAC-3′ |
| JL270 | 5′-TTTCTCCATATTGACCATCATACTCATTG-3′ |
| PP2C-F1 | 5′-GATGCGGTGGATGATGTAGATAACGACGAA-3′ |
| PP2C-R2 | 5′-AGCTAAAACCGCCCTGGAATC-3′ |
| pCB35SPf | 5′-GCCTTCAGTTGAGCTCCATGG-3′ |
| pCB6Hisr2 | 5′-CTGGTCACCTCTAGACACGTG-3′ |
| pCBNOSPAf | 5′-CCGATCGCTCGAGCATTTGGC-3′ |
| pCBNOSPAr | 5′-CACTGGTACCTTAATTCCCGA-3′ |
| T3 | 5′-AATTAACCCTCACTAAAGGG-3′ |
| T7 | 5′-AATACGACTCACTATAG-3′ |
| XR2 | 5′-TGGGAAAACCTGGCGTTACCCAACTTAAT-3′ |
Figure 5.
arl2-2/At2g20050 recombinant DNA fragments can be PCR amplified from arl2-2 genomic DNA but not from wild-type DNA. Primers used to PCR amplify wild-type ARL2 or recombinant arl2-2/At2g20050 fragments from Ws or arl2-2 DNAs are shown by horizontal arrowheads on the diagram shown in Figure 4B and marked F1 (PP2C-F1), R2 (PP2C-R2), XR2, and R7 (ARL2-R7) on that diagram and at the top of the gel. Their respective sequences are provided in Table I. The DNA used as a template in diagnostic PCR reactions is indicated above each electrophoretic lane. The sizes of amplified fragments, as determined by comparison with Mr markers loaded on the same gel, are shown on the right of the gel.
Molecular analysis of arl2-3 revealed that this allele derived from insertion of an 8-kb T-DNA within the first predicted intron of the gene. This insertion involved a T-DNA flanked by two left borders and was accompanied by a small 42-bp deletion of predicted intronic sequences 3′ of this insertion site (Fig. 4C).
We isolated homozygous arl2-2 and arl2-3 plants and tested the gravitropic phenotype of their progeny. Results shown in Figure 1A demonstrated that the roots of arl2-2 and arl2-3 seedlings display altered gravitropism, similarly to arl2-1. Mutant hypocotyls also showed similar alterations in their gravitropic response, whereas mutant inflorescence stems displayed wild-type kinetics of gravitropism (Fig. 2C; data not shown).
A cross between homozygous 13-8 (arl2-1) and arl2-2 plants yielded trans-heterozygous progeny that displayed a gravitropic defect in both roots and hypocotyls. Because these mutations were isolated independently in two separate backgrounds (Est and Ws) and both were recessive (data not shown), the data strongly support a role for ARL2 in root and hypocotyl gravitropism. To verify this conclusion, we transformed arl2-1 mutant plants with the p35S-His6::ARL2 construct and analyzed the gravitropic phenotype of the progeny of two independent transformants. Results shown in Figure 1B indicated that seedlings carrying the transgene developed a stronger root curvature response to gravistimulation than untransformed mutant seedlings or than wild-type seedlings of the corresponding ecotype (Est). The gravitropic defect of arl2-1 hypocotyls was also rescued by the p35S-His6::ARL2 construct (data not shown).
Null Mutation in ARL1, Another ARG1 Paralog, Does Not Affect Gravitropism
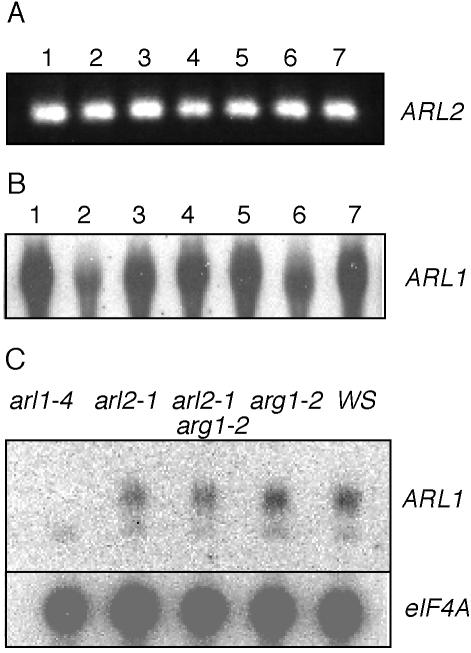
ARL2 is one of two genes that encode ARG1-like proteins in Arabidopsis (Fig. 6; Sedbrook et al., 1999; Arabidopsis Genome Initiative, 2000). To investigate the role of the other ARG1 paralog, named ARL1, in plant growth and development, we isolated the arl1-4 mutation, which carries a T-DNA insertion within the second predicted intron of ARL1 (Fig. 4D). Although ARL1 is expressed in all organs of wild-type plants (Fig. 7B), no ARL1 mRNA could be detected in homozygous arl1-4 mutant plants (Fig. 7C), indicating that this mutation is an RNA null. When tested for gravitropism, homozygous arl1-4 mutant seedlings displayed wild-type kinetics of root and hypocotyl gravitropism, suggesting that ARL1 is not necessary for full gravitropism (Fig. 1C). arl1-4 mutants also appeared wild type in general plant growth and morphology and in experiments aimed at testing germination, seedling growth responses to light, phytohormones, and polar auxin transport inhibitors (data not shown). Their roots also appeared to wave like wild type when grown on tilted hard-agar surfaces. Hence, so far, we have not been able to associate a specific phenotype with the arl1-4 mutation.
Figure 6.
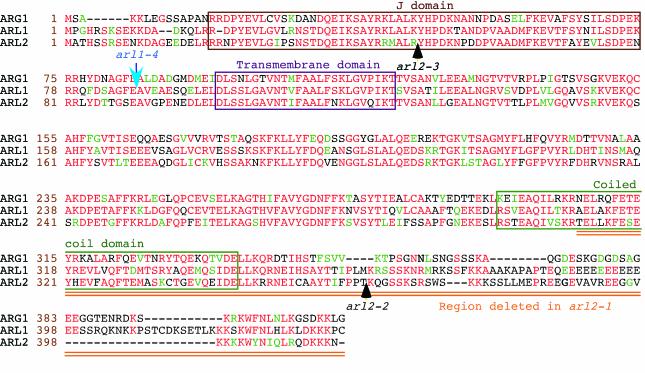
Amino acid sequence alignments of the ARG1 (top), ARL1 (middle), and ARL2 (bottom) proteins (Ws ecotype). Amino acid positions within each protein are shown at the left of each sequence (brown numbers). Protein names are indicated at the left of these numbers. Amino acids that are identical or conserved between at least two of the three proteins are shown in red and green characters, respectively. Gaps introduced within a protein to allow better alignment of flanking sequences are represented by dashes. The J domain, putative transmembrane domain, and predicted coiled-coil region are boxed. The arl2-1 mutation deletes the doubly underlined (orange) carboxyl end of the protein, whereas positions of T-DNA inserts within ARL2 and ARL1 alleles are indicated by black and blue arrows, respectively. Alignments were obtained by the Boxshade server (Institut Pasteur, Paris; http://bioweb.pasteur.fr/seqanal/interfaces/boxshade.html).
Figure 7.
The ARL2 and ARL1 genes are expressed ubiquitously in Arabidopsis seedlings and plants. A, 3′ ARL2 cDNA fragments can be reverse transcription-PCR amplified from mRNAs extracted from siliques (1), cauline leaves (3), rosette leaves (4), stems (5), and flowers (6) of mature plants, roots of 3-week-old liquid-grown plants (7), and cotyledon and leaves of 5-d-old seedlings (2). B, Northern-blot analysis of total RNAs extracted from the plant organs defined in A, using ARL1 cDNA as a probe. Twenty micrograms of total RNA was loaded in each lane. C, Northern-blot analysis of total RNAs extracted from arl1-4, arl2-1, arg1-2 arl2-1, arg1-2, and wild-type Ws seedlings, using ARL1 (upper) or eIF4A (lower; loading control) cDNA sequences as probes. In this experiment, the ARL1 probe detected a low-intensity, nonspecific signal in all RNAs tested (including arl1-4). However, the specific ARL1 signal was not detectable in RNAs extracted from arl1-4 seedlings. In each panel, the source of mRNA is indicated above each lane, whereas the probe is identified at the right of the panel.
ARL2 and ARL1 Encode ARG1-Like Proteins
We cloned full-length ARL2 cDNAs (GenBank accession no. AY226826) by RACE-PCR and sequenced them. Three of four cDNA 5′ ends were located within a 10-bp A-rich region. The fourth transcription start site was located 53 bp downstream of the first one (data not shown). Two polyadenylation sites were also found at the 3′ end, located within 28 bp of each other. The first one was located 136 bp downstream of the translation stop codon (TGA). ARL2 contains 10 exons and nine introns. We also cloned ARL1 cDNA (GenBank accession no. AY226825) and showed that ARL1 contains 10 exons and nine introns, similarly to ARL2. The position of each intron is conserved within these genes (Fig. 4). It is also conserved with the position of introns within ARG1, although the latter gene contains an additional intron within its 3′ region (Sedbrook et al., 1999). The seventh exon of ARL1 and ARL2 was not predicted in database annotations of the Arabidopsis genome (At1g24120 and At1g59980).
The first ATG of ARL2 predicted to constitute a good translation initiation site by the Netstart 1.0 program (http://www.cbs.dtu.dk/cgi-bin/) is located 284 bp downstream of the first transcription start site (Fig. 4A). It initiates a large ORF predicted to encode a 415-amino acid polypeptide whose sequence is similar to that of ARG1 and ARL1 throughout their entire lengths (Fig. 6). All three proteins contain a J domain at their N terminus, a potential transmembrane domain, and a putative coiled-coil domain at the C end (Fig. 6). The arl2-1 deletion eliminates all amino acids downstream of R311, deleting most of the coiled-coil domain in the protein. The T-DNA insertion and translocation found in arl2-2 disrupts the protein-coding region at T362, whereas the T-DNA insertion in arl2-3 disrupts the coding potential of ARL2 right at the middle of the J domain (Fig. 6).
ARL2 Is Expressed at Low Levels in All Organs of the Plant
The ARG1 gene was shown to be expressed ubiquitously in Arabidopsis, even though the phenotypic analysis of arg1 mutants suggested a specific involvement in gravity signal transduction (Sedbrook et al., 1999). Similarly, ARL1 is expressed in all organs of the plant (Fig. 7B), and its expression is neither affected by arl2-1 or arg1-2 mutations nor by arg1-2 arl2-1 double mutations (Fig. 7C). To investigate the pattern of ARL2 expression, we extracted RNAs from stems, rosette and cauline leaves, flowers, and siliques of mature plants, and from roots of 3-week-old liquid culture-grown seedlings, and subjected them to northern-blot analysis, using the ARL2 cDNA as a probe. Unfortunately, no signal was found using this procedure (data not shown). Therefore, we subjected the same RNA preparations to reverse transcription and RNA Ligase Mediated (RLM)-RACE-PCR. Figure 7A shows that ARL2 is expressed in all tissues tested, though at low levels, below the limit of detection by northern-blot procedures.
ARG1 and ARL2 May Function in a Common Genetic Pathway That Is Distinct from the Pathway Involving PGM
As illustrated in Figures 1, A through D, and 2A, the gravitropic phenotypes associated with mutations in either ARG1 or ARL2 were intermediate between wild-type gravitropism and a complete abolition of the gravitropic response. This afforded an opportunity to genetically test whether the two mutations affected parallel branches of the gravity signal transduction pathway. Homozygous arl2-1 and arg1-2 plants were crossed, and double heterozygous F1 progeny were recovered and shown to develop wild-type responses to gravistimulation (data not shown). arg1-2 arl2-1 double mutants were recovered from the segregating F2 progeny, and the gravitropic phenotype of their selfed progeny was analyzed. Figure 1C shows that arg1-2 arl2-1 double mutants displayed a gravitropic phenotype that was almost identical to the phenotype associated with single mutants.
Using similar procedures, we also isolated arl2-1 arl1-4 and arg1-2 arl1-4 double mutants and arl1-4 arl2-1 arg1-2 triple mutants. All double and triple mutants displayed kinetics of root gravitropism that were similar to those of single mutants (Fig. 1C; data not shown).
Because ARG1, ARL2, and PGM all appear to function in gravity perception and/or signal transduction (Kiss et al., 1989; Sedbrook et al., 1999), we also generated arl2-1 pgm-1 double mutants and tested the gravitropic phenotype of their progeny. Results shown in Figure 1D demonstrated that arl2-1 pgm-1 double mutant roots displayed much stronger gravitropic defects than single mutants or wild-type roots. Furthermore, the population of arl2-1 pgm-1 roots displayed enhanced deviation from the vertical compared with wild-type or single mutant populations in the absence of gravistimulation (Fig. 1D; F test P values below 0.05). Hypocotyls of the double mutants behaved similarly (data not shown).
DISCUSSION
In this paper, we demonstrate that three independently isolated mutations in the ARL2 locus, which encodes a DnaJ-like protein similar to ARG1 (Sedbrook et al., 1999), affect root and hypocotyl gravitropism of seedlings grown under light (Fig. 1, B and D) or darkness (Figs. 1, A and C, and 2A). All three mutations are recessive, and do not complement each other. We also show that the gravitropic phenotype associated with arl2-1 can be rescued by transformation with a construct that drives expression of a His-tagged version of the ARL2 protein (Fig. 1B). Together, these data demonstrate that ARL2 is involved in root and hypocotyl gravitropism in Arabidopsis.
The agravitropic phenotype associated with arl2-1 and arl2-3 is stronger than that associated with arl2-2 (Fig. 1A). This difference could reflect the relative strengths of these mutations. arl2-1 carries a 3′ deletion that eliminates most of a predicted coiled-coil domain at the C terminus of the ARL2 protein, potentially eliminating its ability to interact with target proteins. Similarly, arl2-3 carries a T-DNA insert within its first intron, possibly disrupting the coding potential of this gene within the J domain. These two alleles are likely to be null, although the low level of ARL2 expression in wild-type plants precludes careful verification of this prediction. arl2-2, on the other hand, contains a T-DNA insertion near the 3′ end of the gene's ORF, downstream of its coiled-coil-encoding domain (Figs. 4 and 6). Hence, unlike arl2-1 and arl2-3, arl2-2 could potentially encode a functional protein that may interact with its targets.
The arl2-2 mutation involved a complex recombination event that also disrupted a putative protein phosphatase 2C gene (At2g20050). In this line, the 3′ end of At2g20050 is associated with the 3′ end of arl2-2 and a 71-bp insertion of unknown origin. A second locus carrying the 5′ end of ARL2 associated with a T-DNA and the complementary 5′ end of At2g20050 was also found in this line (Figs. 4B and 5). No wild-type ARL2 or At2g20050 could be found in this mutant, strongly suggesting that this recombination involved a reciprocal translocation between chromosomes 1 and 2, with breakpoints located at positions 2,550 of ARL2 and 537 of At2g20050 (Fig. 4B). It is not unusual to find chromosomal rearrangements associated with T-DNA insertions in plants (Tax and Vernon, 2001). Unfortunately, the complex nature of this arl2-2 mutation also implies that the phenotypes found in this line could be associated with alterations in ARL2, in At2g20050, or in both genes. It is possible that the mutation in the putative protein phosphatase 2C At2g20050 gene could contribute to the gravitropic phenotype found in this line. For instance, mutations in RCN1, which encodes a protein phosphatase 2A subunit, also affect gravitropism (Rashotte et al., 2001). However, it is likely that the T-DNA insertion and 3′ end translocation found in arl2-2 also contribute to the gravitropic defect in this line. F1 progeny from a cross between homozygous arl2-1 and arl2-2 plants display a root gravitropism defect (data not shown).
The hypothesis that mutations in ARL2 are responsible for the gravitropic phenotype found in these mutant lines is supported by the results of transformation experiments in which arl2-1 mutant seedlings were transformed with the p35S-His6::ARL2 construct. The transgenic progeny of independent transformants displayed a strong root gravitropic response relative to untransformed arl2-1 seedlings (Fig. 1B). It is interesting to note, however, that arl2-1 roots overexpressing ARL2 displayed enhanced gravitropism compared with wild-type Est roots (Fig. 1B). This result suggests that ARL2, or a component of the ARL2 pathway, might be limiting for the gravitropic response in the Est background.
Loss-of-function mutations in ARL2 affect root and hypocotyl gravitropism without altering inflorescence stem gravitropism (Figs. 1A and 2, A and C). This result is in agreement with previous observations indicating that the gravitropic responses of different organs are genetically separable (Bullen et al., 1990). Interestingly, arl2 mutations do not affect root growth rate, root growth resistance to phytohormones and polar auxin transport inhibitors, or starch accumulation in root statocytes. Hypocotyl phototropism, on the other hand, is slightly enhanced in the arl2-1 mutant compared with the wild type, at least within the first positive phase of the response (Fig. 2B). This slight enhancement of phototropism may be the consequence of the gravitropic defect associated with this mutation. In wild-type seedlings, gravitropism interferes with phototropism in directing organ tip curvature in response to lateral light stimulation (Correll and Kiss, 2002). In any case, the physiological data shown here strongly support a role for ARL2 in early phases of root and hypocotyl gravitropism, possibly in gravity signal transduction.
It is interesting to note that ARG1, an ARL2 paralog, was also proposed to function in gravity signal transduction, based on a similar combination of phenotypes (Sedbrook et al., 1999; K. Boonsirichai and P.H. Masson, unpublished data). Therefore, we analyzed the gravitropic defect associated with arg1-2 arl2-1 double mutants. As expected, arg1-2 arl2-1 double mutants displayed a gravitropic defect that was not exacerbated compared with single mutants (Fig. 1C). This result supports an involvement of ARG1 and ARL2 in the same genetic pathway.
Our analysis of arl2-1 pgm-1 root gravitropism revealed a strong phenotypic enhancement compared with single mutants (Fig. 1D). Similar observations were recently made with arg1-2 pgm-1 double mutants (K. Boonsirichai and P.H. Masson, unpublished data). These striking results strongly suggest that ARL2/ARG1 and PGM function in distinct branches of the gravity signal transduction pathway. Several physiological data support the existence of a secondary gravity perception mechanism, in addition to amyloplast sedimentation within the statocytes (Ishikawa and Evans, 1990; Blancaflor et al., 1998; Fasano et al., 2001; Wolverton et al., 2002), and it is possible that ARL2 and ARG1 function in this alternative pathway. Alternatively, ARL2 could directly or indirectly regulate the activity of specific step(s) in the gravity signal transduction pathway controlled by amyloplast sedimentation in the statocytes. Experiments are underway to test these models.
As components of HSP70-containing macromolecular chaperone complexes, DnaJ-like proteins interact with HSP70 through their J domain and with specific targets through a more divergent protein interaction domain (Zuber et al., 1998; Miernyk, 2001). Because the coiled-coil domain of ARG1 displays similarity to coiled coils found in a number of proteins that bind to cytoskeletal elements, we previously postulated that ARG1 might interact with the cytoskeleton, or modulate the formation of macromolecular signal transducing complexes in vicinity of the cytoskeleton (Sedbrook et al., 1999). Recent biochemical and immunocytological experiments appear to support this model (K. Boonsirichai and P.H. Masson, unpublished data). Because ARG1 and ARL2 share substantial sequence similarity within the putative coiled-coil region (Fig. 6), we hypothesize that ARL2 might also function in gravitropism through some interaction with cytoskeletal elements. Current work focuses on identifying and characterizing proteins that interact physically or genetically with the C-terminal ends of ARG1 and/or ARL2 and studying their involvement in gravitropism.
Like ARG1, ARL2 is expressed in all tissues of the plant, though at much lower levels (Fig. 7). This result appears to contradict a model postulating a specific involvement of ARL2 in gravity signal transduction. However, as previously discussed for ARG1 (Sedbrook et al., 1999), ARL2 may be involved in other processes that we have not yet analyzed. Alternatively, it is possible that other ARL2 functions are masked by functional redundancy.
Finally, we want to emphasize that not all dnaJ-like proteins are needed for root gravitropism. The Arabidopsis genome contains 91 genes encoding DnaJ-like proteins, many more than any other organism whose genome has now been sequenced (Miernyk, 2001; data not shown). Among these DnaJ-like proteins, only ARL2 and ARL1 are highly similar to ARG1 along their entire lengths (Fig. 6). Interestingly, an RNA-null allele of ARL1 (arl1-4) did not affect root or hypocotyl gravitropism (Fig. 1C; data not shown). Furthermore, incorporation of arl1-4 in arg1-2 and/or arl2-1 mutant backgrounds did not affect their gravitropic phenotype (Fig. 1C; data not shown). Therefore, ARL1 does not appear to function in gravitropism, and experiments are underway to define its function in plant growth, development, or response to the environment.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
To generate a collection of fast neutron-mutagenized seeds, approximately 250,000 seeds of the Est ecotype were exposed to 6,000-Rad fast-neutron radiation by Dr. H. Brunner (Plant Breeding Unit, International Atomic Energy Agency, Vienna). Mutagenized M1 seeds were separated into 29 subpopulations. M2 seeds were harvested in bulk for each subpopulation and examined separately in the gravitropism mutant screen described below.
T-DNA insertional mutants were obtained through the Arabidopsis Knockout Facility (University of Wisconsin Biotechnology Center, Madison) from populations of T-DNA-transformed Arabidopsis plants (Ws ecotype; Krysan et al., 1999). pgm-1 mutant seeds (Columbia ecotype) were obtained from Tim Caspar (DuPont Co., Wilmington, DE).
All plant manipulations, including surface sterilization of seeds, plating on agar-based media, growth conditions, transplantation of seedlings from plates to soil, pollination, and harvesting were as previously described (Rutherford and Masson, 1996; Sedbrook et al., 1999). GM contained one-half-strength Murashige and Skoog salts with 1.5% (w/v) Suc and macro- and micronutrients (Invitrogen, Carlsbad, CA).
Quantification of Root and Hypocotyl Gravitropism
Gravitropic Reorientation Assay
Approximately 30 to 80 surface-sterilized seeds were either embedded in or placed on the surface of GM medium containing 0.8% (w/v) agar, in in-agar or on-agar reorientation assays, respectively. Plated seeds were placed for 3 to 5 d in darkness at 4°C and germinated vertically at 22°C for 2 to 3 d, and then rotated 90°, as described (Sedbrook et al., 1999). Pictures were taken at regular time intervals (defined in the text) with a COOL-PIX800 Digital Camera (Nikon Inc., Melville, NY).
To quantify the gravitropic response of primary inflorescence stems, wild-type Ws and arl2-3 mutant plants were grown in soil for 3 to 3.5 weeks. At that age, the young plants carried 4- to 8-cm-long primary inflorescence stems. They were positioned horizontally in a dark growth chamber, and the inflorescence stems were photographed at regular time intervals, as described above.
Root, hypocotyl, and inflorescence stem curvature responses to gravistimulation were determined on digital images using the National Institutes of Health Image Analysis Software version 1.62 (http://rsb.info.nih.gov/nih-image/).
Root Wave Assay
Root waving on tilted surfaces were tested as described in Rutherford and Masson (1996).
Quantification of Hypocotyl Phototropism
Hypocotyl phototropism was characterized using the procedures described in Steinitz and Poff (1986). Dark-grown seedlings were exposed to a single pulse of 450-nm blue light at the fluence rate of 0.15 μm m-2 s-1, provided by a projector equipped with a 300-W ELH tungsten halogen lamp (Osram Sylvania, Inc., Danvers, MA) and a 450-nm interference filter with a half bandwidth of 10 nm (PTR Optics, Waltham, MA). The duration of light exposure was adjusted to generate a fluence response curve in the range of first positive phototropism, using a Uniblitz shutter (Vincent Associates, Rochester, NY; Janoudi and Poff, 1990). After the curvature was allowed to develop for 30 min in darkness, seedlings were mounted on clear tape, and analyzed as described (Steinitz and Poff, 1986).
Analysis of Root Growth Sensitivity to Phytohormones and Polar Auxin Transport Inhibitors
Assays aimed at testing root growth in the presence of phytohormones or polar auxin transport inhibitors were carried out as described by Sedbrook et al. (1999). IAA, 2,4-D, 1-NAA, ACC, and BA were obtained from Sigma (St. Louis). NPA was obtained from Pfaltz & Bauer, Inc. (Waterbury, CT). Stock solutions were prepared in ethanol (IAA, 2,4-D, 1-NAA, and BA), isopropanol (ACC), and dimethyl sulfoxide (NPA). All compounds were added to the pH-buffered medium at the appropriate concentrations, and the solvent concentrations were corrected to the same value for all plates in each assay.
Statistical Analysis of the Data
Data derived from gravitropic and phototropic assays, and root growth responses to added compounds were subjected to statistical analysis (Excel, Microsoft Corporation, Redmond, WA), using Student's t tests to compare mean values between wild-type and mutant populations and F tests to compare deviations from the mean value between genotypes. Differences were assumed to be insignificant when the P values associated with these tests exceeded 0.05.
Detection of Starch within the Columella Cells of Wild-Type and Mutant Root Caps
Seedlings were grown on vertically oriented 0.8% (w/v) agar-based GM medium in the light for 3 d. They were then grown for 1 more d in darkness, before being exposed for 3 h to an I2:KI solution (Sedbrook et al., 1999). They were cleared for 1 h in Hoyer's solution (Newman et al., 2002) and analyzed as described by Sedbrook et al. (1999).
Mapping of arl2-1
Two mapping populations were generated by crossing arl2-1 mutant plants with wild-type plants of the Landsberg erecta or Columbia ecotypes, respectively. Their progeny were propagated by self-fertilization. F3 progeny were subjected to a root wave assay to determine their gravitropic phenotype. This progeny typing allowed a determination of the ARL2 genotype of the corresponding F2 parent. Two pools of F3 progeny derived from 15 homozygous wild-type and 15 homozygous mutant F2 plants, respectively, were generated and used to map arl2-1 by bulked segregant analysis (Michelmore et al., 1991). DNA was extracted from each pool and subjected to PCR amplification, using primer pairs from the MapPairs kit (Research Genetics Inc., Huntsville, AL). Linkage between ARL2 and specific SSLPs was confirmed by typing the linked SSLPs in individual F3 families.
Molecular Cloning and Characterization of ARL2
DNA and RNA Isolation
Large-scale isolation of total DNA from plants was accomplished as described by Dellaporta et al. (1985). Genomic DNA for PCR amplification was isolated from individual cotyledons (Klimyuk et al., 1993), whereas RNA was extracted from seedlings or plant organs (Chang et al., 1993).
Basic Molecular Biology Procedures
Enzymes used in the manipulation of DNA were purchased from New England Biolabs (Beverly, MA). Recombinant DNA techniques, random priming to radiolabel the DNA, Southern- and northern-blot analyses, and PCR amplifications were carried out as described by Ausubel et al. (1994). DNA sequencing was carried out by the University of Wisconsin Biotechnology Center DNA Sequencing Facility (Madison).
Cloning of ARL2 Genomic and cDNA Sequences
A piece of genomic DNA carrying the predicted ARL2 gene (At1g59980: Arabidopsis Genome Initiative, 2000) was PCR amplified from Ws genomic DNA, using the ARL2-F and ARL2-R primers (Table I), the ExTaq polymerase (TaKaRa Shuzo Co., LTD, Otsu, Japan), and thermocycle conditions dictated by the Lasergene PrimerSelect program (DNASTAR Inc., Madison, WI). A PCR amplification approach was also used to clone ARL2 cDNA from the CD4-6 (flower cDNA), CD4-12 (silique cDNA), and CD4-22 (cDNA from 3-day-old etiolated seedlings) cDNA phage libraries, obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus), using T7 and T3 primers along with either the ARL2-F or ARL2-R primers (Table I). PCR-amplified genomic and cDNA products were cloned into a pZERO vector (Invitrogen) and sequenced.
The 5′ end of the ARL2 mRNA was mapped on the sequenced genomic DNA using the Ambion First-Choice RLM-RACE PCR kit, as recommended by the supplier (Ambion, Inc., Austin, TX). Primers ARL2-SL2 and ARL2-r3 (Table I) were used in these assays, along with the Ambion kit primers, in nested PCR amplifications. PCR-amplified cDNAs were cloned into pBluescript (Stratagene Co., Cedar Creek, TX), and sequenced.
A 3′-RACE procedure was also used to detect ARL2 transcripts in total RNAs extracted from plant organs (Chang et al., 1993), using primers ARL2-SU1 and ARL2-F6 (Table I), along with Ambion First-Choice RLM-RACE PCR kit primers.
Identification and Characterization of arl2-2 and arl2-3
Two T-DNA-insertional mutants (arl2-2 and arl2-3; Ws ecotype) were isolated through the Arabidopsis Knockout Facility, using the T-DNA-specific JL202 and ARL2-specific ARL2KOFU3 and ARL2KOFL1 primers (Table I; Krysan et al., 1999). PCR-amplified T-DNA-flanking genomic fragments were sequenced. arl2-2 was derived from the ALPHA T-DNA collection, whereas arl2-3 was isolated from the BASTA collection (http://www.biotech.wisc.edu/Arabidopsis/).
To investigate the structure of arl2-2, DNA was isolated from homozygous plants, cleaved with NsiI or BglII, and subjected to Southern-blot analysis. Membranes were hybridized successively with PCR-amplified ARL2-specific probes corresponding to sequences flanking the arl2-2 T-DNA on the 5′ side, on the 3′ side, and to neomycin phosphotransferase II- or β-glucuronidase-specific T-DNA probes (Rutherford et al., 1998).
Genomic DNA associated with the arl2-2 3′ end segment was isolated by inverse PCR of HindIII-digested genomic DNA after self-ligation (Ochman et al., 1988). Nested PCR amplification was performed to amplify ARL2-flanking sequences, using the ARL2-F6 and ARL2-R6 primers, and then the ARL2-F7 and ARL2-R7 primers (Table I). The Elongase DNA polymerase (Invitrogen) was used in these reactions. PCR products were sequenced.
To characterize the arl2-3 mutation, arl2-3 genomic DNA was cleaved with NcoI and NdeI and subjected to Southern-blot analysis using ARL2 cDNA and T-DNA left-border probes (Ausubel et al., 1994). The analysis allowed us to verify that arl2-3 plants contain only one T-DNA insert and that this T-DNA disrupts the ARL2 gene (data not shown).
Complementation of arl2-1
To verify that the gravitropic defect is truly associated with mutations in ARL2, we cloned a His-tagged ARL2 cDNA into the pCAMBIA1302 binary vector (CAMBIA, Canberra City, Australia), under the control of the CaMV 35S promoter and NOS terminator sequences. This construct was generated as follows. The 35S::GFP::His6 DNA cassette carried by pCAMBIA1302 was PCR amplified with primers pCB35SPf and pCB6Hisr2 (Table I) and cloned between the SacI and XbaI sites of pBluescript II KS(+). A NOS terminator sequence was then amplified from pCAMBIA1302, using primers pCBNOSPAf and pCBNOSPAr (Table I), and inserted into this plasmid, between the XhoI and KpnI sites, generating the nGFP2a plasmid. The ARL2 cDNA, obtained by PCR amplification with the ARL2GFP2f2 and T3 primers (Table I), was inserted between the EcoRI and SmaI sites of nGFP2a. A His6::ARL2 cassette was amplified from this recombinant plasmid with primers 6HisOEf and GFPPAr (Table I), digested with NcoI and EcoRV, and cloned into pCAMBIA1302, between the NcoI and PmlI sites, thereby replacing the original GFP::His6 cassette of this plasmid. The resulting plasmid was called p35S-His6::ARL2.
We introduced the p35S-His6::ARL2 construct in wild-type and mutant plants by in planta transformation (Bent, 2000). Transformants were isolated on GM plates containing 20 μg mL-1 hygromycin (Calbiochem, San Diego) and 0.8% (w/v) agar, transferred to soil, and allowed to self-pollinate. The progeny of two of these transgenic plants were subjected to an in-agar reorientation assay under constant light. Individual seedlings carrying a T-DNA insert were identified by PCR amplification, using the 6HisOEf and GFPPAr primers (Table I), and root gravitropism was quantified on these seedlings.
Molecular Cloning and Characterization of ARL1
Procedures used to clone and characterize the ARL1 cDNA were as described above for ARL2, except that primers ARL1-F1 and ARL1-R1 (Table I) were used in the PCR reactions, along with T3 or T7 primers (Table I). The 5′ end of the ARL1 cDNA was mapped on the genomic DNA by RLM-RACE amplification, using the ARL1nestr2 and ARL1nestr1 primers along with Ambion First-Choice RLM-RACE PCR kit primers (Table I; Ambion, Inc).
The arl1-4 allele was identified in the BASTA collection of T-DNA insertion lines (Arabidopsis Knockout Facility), using the ARL1-F1 and T-DNA left-border (JL202) primers. Amplified DNA was subjected to nested PCR with JL270 and ARL1nestr2 and sequenced.
ARL1 expression was analyzed by northern-blot analysis of total RNA extracted from seedling and mature plant organs (Fig. 7), using 32P-labeled ARL1 cDNA as a probe (Ausubel et al., 1994).
Analysis of Possible Genetic Interactions between arg1-2, arl1-4, arl2-1, and pgm-1
Plants carrying both the arl2-1 and either arg1-2 or pgm-1 mutations at the homozygous state were obtained by crossing single mutants and selfing the corresponding F1 progeny. A cotyledon was excised from each F2 plant and prepared for PCR amplification (Klimyuk et al., 1993). The arg1-2 frameshift mutation destroys a BamHI restriction site within the ARG1 locus (Sedbrook et al., 1999), creating a BamHI RFLP that can be resolved by BamHI digestion of PCR-amplified ARG1 product (ARG1-F and ARG1-R primers; Table I). On the other hand, the ARL2-SU3 and ARL2-R2 primers were used to PCR amplify the ARL2 sequence that surrounds the 857-bp deletion present in arl2-1. Homozygosity at ARL2 was confirmed by a Southern-blot analysis. Plants homozygous for pgm-1 were identified by their starchless phenotype, using the I2:KI staining protocol described above. F2 seedlings with the specified genotype were grown and selfed. Their progeny were subjected to an in-agar reorientation assay.
arg1-2 arl2-1 arl1-4 triple mutants were obtained by crossing an arg1-2 arl2-1 double mutant with an arl1-4 single mutant and selfing the corresponding F1 progeny. F2 plants were genotyped as described above for the presence of arg1-2 and arl2-1 at the homozygous state. PCR amplification was also used to identify the arl1-4 allele in DNA extracted from individual cotyledons, using primers ARL1-nestr2 and JL270. Presence of the wild-type ARL1 allele was analyzed by PCR amplification, using the ARL1-F4 and ARL1-nestr2 primers. Individual F2 plants with a specified genotype were grown and selfed. Their progeny were subjected to an in-agar reorientation assay.
Acknowledgments
We thank the Arabidopsis Knockout Facility (University of Wisconsin, Madison) for providing the arl1-4, arl2-2 and arl2-3 mutants. We also thank Jessica Will and Nicole Ammerman for excellent technical assistance.
This work was supported in part by the National Science Foundation (grant nos. MCB-9905675 and MCB-0240084), by the National Aeronautic and Space Administration (grant nos. NAG2-1336 and NAG2-1602 to P.H.M.), by the National Science Foundation/Department of Energy/U.S. Department of Agriculture (Training Grant fellowship no. BIR 92-2033 to E.S.R.), and by the Thai Government (fellowship to K.B.). This is manuscript no. 3611 from the Laboratory of Genetics, University of Wisconsin (Madison).
References
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796-814 [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current Protocols in Molecular Biology: Updates 1994-2002. John Wiley and Sons, New York.
- Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137-144 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948-950 [DOI] [PubMed] [Google Scholar]
- Bent AF (2000) Arabidopsis in planta transformation: uses, mechanisms, and prospects for transformation of other species. Plant Physiol 124: 1540-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116: 213-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsirichai K, Guan C, Chen R, Masson PH (2002) Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Physiol Plant Mol Biol 53: 421-447 [DOI] [PubMed] [Google Scholar]
- Bullen BL, Best TR, Gregg MM, Barsel S-E, Poff KL (1990) A direct screening procedure for gravitropism mutants in Arabidopsis thaliana (L.) Heynh. Plant Physiol 93: 525-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Pickard BG (1989) Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta 177: 185-197 [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113-116 [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH (1998) The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA 95: 15112-15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll MJ, Kiss JZ (2002) Interactions between gravitropism and phototropism in plants. J Plant Growth Regul 21: 89-101 [DOI] [PubMed] [Google Scholar]
- Dellaporta S, Wood J, Hicks JB (1985) Maize DNA miniprep. In R Malmberg, J Messing, I Sussex, eds, Molecular Biology of Plants: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 36-37
- Evans ML, Ishikawa H (1997) Cellular specificity of the gravitropic motor response in roots. Planta 203: S115-S122 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD, Digby J (1997) Solving the puzzle of gravitropism: has a lost piece been found? Planta 203: S159-S163 [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806-809 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M (1997) The RHG gene is involved in root and hypocotyl gravitropism in Arabidopsis thaliana. Plant Cell Physiol 38: 804-810 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML (1990) Gravity-induced changes in intracellular potentials of elongating cortical cells of mung bean roots. Plant Cell Physiol 31: 457-462 [PubMed] [Google Scholar]
- Janoudi AK, Poff KL (1990) A common fluence threshold for first positive and second positive phototropism in Arabidopsis thaliana. Plant Physiol 94: 1605-1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14: 33-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177: 198-206 [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T (1996) Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant 97: 237-244 [DOI] [PubMed] [Google Scholar]
- Klimyuk VI, Carroll BJ, Colwyn MT, Jones JDG (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J 3: 493-494 [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283-2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov OA, Hasenstein KH (1996) Intracellular magnetophoresis of amyloplasts and induction of root curvature. Planta 198: 87-94 [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson PH, Tasaka M, Morita M, Guan C, Chen R, Boonsirichai K (2002) Arabidopsis thaliana: a model for the study of root and shoot gravitropism. In E Meyerowitz, C Somerville, eds, The Arabidopsis Book. American Society of Plant Biology, Rockville, MD. doi/10.1199/tab.0043 [DOI] [PMC free article] [PubMed]
- Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88: 9828-9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk JA (2001) The J-domain proteins of Arabidopsis thaliana: an unexpectedly large and diverse family of chaperones. Cell Stress Chaperones 6: 209-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M (2002) Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14: 47-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903-6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KL, Fernandez AG, Barton MK (2002) Regulation of axis determinacy by the Arabidopsis PINHEAD gene. Plant Cell 14: 3029-3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Gerber AS, Hartl DL (1988) Genetic applications of an inverse polymerase chain reaction. Genetics 120: 621-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IL, Heilmann I, Chang SC, Boss WF, Kaufman PB (1999) Transient and sustained increases in inositol-1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA 96: 5838-5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Trewavas AJ (2002) Reorientation of seedlings in the Earth's gravitational field induces cytosolic calcium transients. Plant Physiol 129: 786-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff KL, Martin HV (1989) Site of graviperception in roots: a reexamination. Physiol Plant 76: 451-455 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response and lateral root growth. Plant Cell 13: 1683-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R, Gallois P, Masson PH (1998) Mutations in Arabidopsis thaliana genes involved in the tryptophan biosynthesis pathway affect root waving on tilted agar surfaces. Plant J 16: 145-155 [DOI] [PubMed] [Google Scholar]
- Rutherford R, Masson PH (1996) sku mutants of Arabidopsis thaliana show surface dependent alteration of root growth vector. Plant Physiol 111: 987-998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AC, Allen NS (1999) Changes in cytosolic pH within Arabidposis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol 121: 1291-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Chen R, Masson PH (1999) ARG1 (Altered Response to Gravity) encodes a novel DnaJ-like protein which potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA 96: 1140-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz B, Poff KL (1986) A single positive phototropic response induced with pulsed light in hypocotyls of Arabidopsis thaliana seedlings. Planta 168: 305-315 [DOI] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15: 2648-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax FE, Vernon DM (2001) T-DNA-associated duplication/translocations in Arabidopsis: implications for mutant analysis and functional genomics. Plant Physiol 126: 1527-1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuno K, Shikanai T, Yamada Y, Hashimoto T (1998) AGR, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol 39: 1111-1118 [DOI] [PubMed] [Google Scholar]
- Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ (2000) Curvature in Arabidopsis inflorescence stems is limited to the region of amyloplast displacement. Plant Cell Physiol 41: 702-709 [DOI] [PubMed] [Google Scholar]
- Wolverton C, Mullen JL, Ishikawa H, Evans ML (2002) Root gravitropism in response to a signal originating outside of the cap. Planta 215: 153-157 [DOI] [PubMed] [Google Scholar]
- Young JC, Krysan PJ, Sussman MR (2001) Efficient screening of Arabidopsis T-DNA insertion lines using degenerate primers. Plant Physiol 125: 513-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber U, Buchberger A, Laufen T, Bukau B (1998) DnaJ proteins. In AL Fink, Y Goto, eds, Molecular Chaperones in the Life Cycle of Proteins: Structure, Function and Mode of Action. Marcel Dekker, Inc., New York, pp 241-273