Abstract
The polycotyledon mutant of tomato (Lycopersicon esculentum L. cv Ailsa Craig) showed altered development during embryogenesis and during vegetative and reproductive phases. The phenotype was pleiotropic and included the formation of extra cotyledons, changes in leaf shape, increased number of flowers (indeterminacy) with abnormal floral organs, the formation of epiphyllous structures, and altered gravitropism. The earliest defects were observed at the transition from the globular to the heart stage of embryogenesis with the formation of multiple cotyledons. Epidermal cells in the mutant embryo were smaller and less expanded compared with wild type. Examination of polar auxin transport (PAT) showed a striking enhancement in the case of the mutant. Increase in PAT did not appear to be caused by a decrease in flavonoids because the mutant had normal flavonoid levels. Application of 2,3,5-triiodobenzoic acid, an inhibitor of polar transport of auxin, rescued postgermination phenotypes of young seedlings. Our analysis reveals a level of control that negatively regulates PAT in tomato and its contribution to plant development and organogenesis.
In higher plants, phytohormone auxin (indole-3-acetic acid [IAA]) is transported basipetally from its site of synthesis at the shoot apex toward the roots by a process termed polar auxin transport (PAT). PAT provides directional information regulating several facets of plant development such as cell elongation, vascular differentiation, apical dominance, tropic movements, and organ development (Lomax et al., 1995). Physiological studies have indicated that PAT requires specific auxin influx and efflux carriers located on the plasma membrane of transporting cells. Biochemical studies support a “chemiosmotic model” of auxin transport that proposes that uncharged, protonated auxin can enter cells from the acidic apoplast either passively by diffusion or via energized uptake by specific influx carriers. In the cytosol because of the more basic pH, IAA is deprotonated and is trapped within the cell due to poor membrane permeability of anion. As a consequence, anionic IAA can leave the cell only by the action of auxin efflux carriers (Rubery and Sheldrake, 1974; Raven, 1975). The polarity of auxin transport presumably is maintained by localization of auxin efflux carrier molecules at the basal ends of transporting cells (Jacobs and Gilbert, 1983). The selective efflux of auxin anion from the basal ends of transporting cells and arrangement of these cells in a long file from the shoot apex to the root apex is the basis of PAT.
Much of our current knowledge about the nature of components participating in PAT comes from molecular genetic analysis of mutants of Arabidopsis that are defective in transport of auxin. The pin1 mutant has reduced PAT, characteristically develops a naked pin-like inflorescence, and shows morphological abnormalities in flowers and leaves. The PIN1 gene encodes a membrane protein that most likely functions as an auxin efflux carrier as suggested by its localization at the basal ends of xylem parenchyma cells in vascular bundles (Gälweiler et al., 1998). Another group of mutants defective in the pin2/agr1/eir1/wav6 locus displays agravitropic roots and reduced root growth and exhibits a defect in auxin transport in roots. The product of the EIR1/AGR1/PIN2/WAV6 gene shows similarities to PIN1 protein and is asymmetrically localized at the periclinal side of epidermal and cortical cells in the meristematic region and elongation zone of the root (Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998). Similar to pin1, the pid1 mutant also shows reduced PAT and produces a naked inflorescence devoid of floral buds (Bennett et al., 1995). PID1 encodes a Ser-Thr protein kinase that was initially proposed to have a signaling or regulatory function in auxin action (Christensen et al., 2000) and appears to act as a positive regulator of auxin transport (Benjamins et al., 2001). A similar link between auxin transport and protein phosphatase 2A is seen in the rcn1 mutant, which shows root curling in the presence of 1-naphthylphthalamic acid (NPA; Rashotte et al., 2001). The aux1 mutant is defective in auxin uptake and displays defects in gravitropic responses and resistance to exogenous auxin (Pickett et al., 1990). The AUX1 gene encodes an influx carrier of auxin that has characteristics of an amino acid permease-like protein (Bennett et al., 1996; Marchant et al., 1999).
The treatment of plants with inhibitors of auxin efflux carrier activity, 2,3,5-triiodobenzoic acid (TIBA) and NPA, influences many growth and developmental processes thought to be controlled by PAT. The pin1 phenotype could be copied in wild-type Arabidopsis by treatment with auxin transport inhibitors with alterations in vascular development and the formation of a pin-like inflorescence instead of floral buds (Okada et al., 1991; Gälweiler et al., 1998). It has been proposed that NPA acts by binding to a plasma membrane-associated protein called the NPA-binding protein (Muday et al., 1993; Bernasconi et al., 1996). Evidence suggests that NPA-binding protein is distinct from the auxin efflux carrier and plays an important role in cycling of auxin efflux carriers to plasma membranes (Geldner et al., 2001). The tir3 mutants that are resistant to the inhibitory action of NPA on root elongation show a pleiotropic phenotype, including reduced inflorescence height with few and short siliques, decreased petiole and root length, and reduced apical dominance (Ruegger et al., 1997). The TIR3 gene, renamed BIG, encodes a protein that might be essential for proper positioning of PIN1 at the plasma membrane (Gil et al., 2001).
Several lines of physiological evidence have suggested that a specific class of flavonoids may act as auxin transport inhibitors (Jacobs and Rubery, 1988). Flavonoids such as quercetin act as competitive inhibitors of NPA binding, suggesting both compounds may regulate auxin transport by binding to the same protein (Murphy et al., 2000). The flavonoid-deficient tt4 mutant shows reduced inflorescence length, reduced apical dominance, increased root branching, and stimulation of basipetal transport of auxin in inflorescence segments (Brown et al., 2001).
A distinct role for PAT has been noticed during plant embryogenesis, particularly in cotyledon development. It is observed that auxin transport is necessary for the establishment of bilateral symmetry during the transition from the globular to heart stage of embryogenesis leading to formation of cotyledons (Liu et al., 1993; Hadfi et al., 1998). The treatment of excised globular mustard (Brassica juncea) embryos with inhibitors of PAT caused fusion of cotyledons at the subsequent heart stage (Liu et al., 1993). During wild-type embryogenesis, PIN1 shows polar localization beginning at the mid-globular stage with localization narrowing down to vascular precursor cells in the cotyledonary primordia and embryo axis, whereas the gnom mutant that shows disorganized localization of PIN1 in globular embryos fails to develop cotyledonary primordia (Steinmann et al., 1999). Apparently, GNOM localizes to endosomes where it controls the polarized trafficking of PIN1 to the basal plasma membrane (Geldner et al., 2003). In the gnom mutant, PIN1 accumulates internally and fails to localize to the plasma membrane, causing the mutant phenotype.
Little information is available on mutants defective in auxin action/transport in species other than Arabidopsis. The dgt mutant of tomato (Lycopersicon esculentum L. cv Ailsa Craig) has been reported to be defective in auxin action, and its phenotype can be ascribed to reduced sensitivity of mutant tissues to auxin (Kelly and Bradford, 1986; Muday et al., 1995; Coenen et al., 2003). In this report, we show that the poc (polycotyledon) mutation in tomato enhances PAT. The mutant displays several developmental abnormalities throughout the life cycle of the plants, from embryonic to vegetative and reproductive phases, suggesting that these changes may be related to alteration of auxin transport in the mutant. We also show that the postgermination seedling phenotype of the mutant can be rescued by treatment with TIBA consistent with the view that this is due to enhanced PAT.
RESULTS
Genetic Characterization of the Polycot Mutant
The polycot mutants were initially identified based on their abnormal seedling phenotype highlighted by the presence of extra cotyledons. The mutants were male sterile due to the lack of dehiscence of the anther sac; nevertheless the anther sacs contained viable pollens (75%-80% germination). As a consequence, the mutant was maintained in homozygous state by manual self-pollination. The crosses with wild type yielded normal F1 seedlings with the wild-type phenotype in seedling stage and during vegetative and reproductive development and were self-pollinating with normal setting of seeds. The genetic segregation analysis of F2 seedlings showed that the mutant phenotype was inherited as a monogenic Mendelian trait (119 seedlings, wild type; 42 seedlings, polycot). The reciprocal crosses with wild type also showed 3:1 segregation of wild type and poc phenotype in F2 generation. The above segregation analysis suggests that the poc mutant contains a monogenic, recessive, and nuclear mutation. Though we obtained nine mutant lines, the results of complementation analysis using homozygous poc lines showed that all these lines were allelic, showing only the poc phenotype in the F1 generation. This was further supported by the results of crossing with heterozygous poc lines. The F1 progeny of these lines segregated in typical 3:1 ratio of wild-type and poc seedlings (data not shown). Given the ratio of M2:M1 plants (50,000:1,500), these results are consistent with the view that the above nine lines of poc mutants likely represent siblings. One line was named poc1-1 and backcrossed twice for further characterization. The crossing of poc mutants with the Ds transposon-tagged dem mutant (Keddie et al., 1998), which also has a variable number of cotyledons, revealed lack of allellism between these two mutants (M. Kavitha, M.S. Sharada, P. Janila, S. Negi, R. Sharma, unpublished data).
The poc Mutant Shows Pleiotropic Developmental Defects
Nearly all poc mutant seedlings (98.5%, P > 0.0001) showed extra cotyledons with approximately one-half of the seedlings being tetracot and about 35% seedlings being tricot. In the remaining seedlings, the cotyledons showed varied extent of fusion yielding tricot or dicot seedlings (Fig. 1A). The fused cotyledons could be distinguished from a normal single cotyledon by the presence of two midveins in the blade pointing toward the respective tips and running in parallel in the petiole (Fig. 1B). Though a minor number (1.5%) of seedlings showed a dicot phenotype, these could be distinguished from the wild type by their round-shaped cotyledons (Fig. 1C). The roots of poc seedlings were distinctly shorter than the wild type and showed earlier appearance of lateral roots, and in adult mutant plants, the roots were bushier than the wild type.
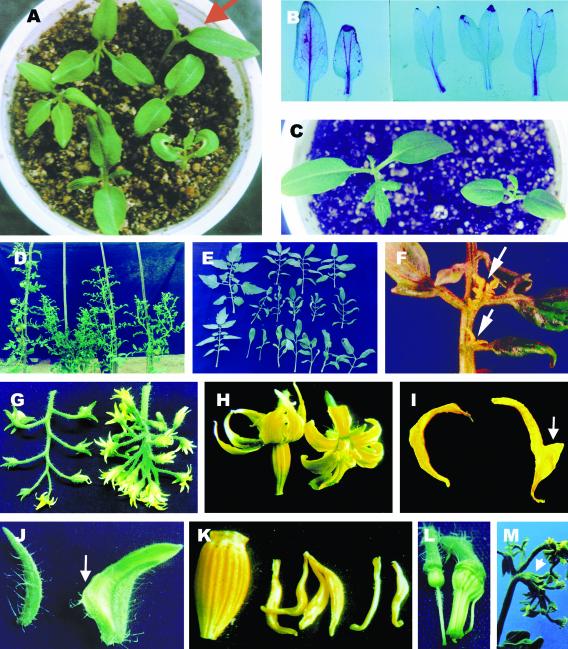
Figure 1.
Morphology of poc plants throughout life cycle. A, poc seedlings (clockwise from top right): wild-type seedlings (arrow); dicot [1+(2)], note two midveins; dicot with two halves of curled cotyledons; dicot [1+(2)] with a partially fused cotyledon; tetracot; and tricot. B, Fused poc cotyledons showing two midveins running parallel in the petiole. Wild type (left). C, poc dicot seedling showing smaller and more rounded cotyledons. Wild type (left). D, Phenotype of 3.5-month-old plants. Wild type (left), poc A (second from left), poc B (third from left), poc C (right). E, Adult leaves (sixth node onwards) of 3-month-old wild type and poc mutant. In poc mutant, the leaf abnormalities vary from the simple lanceolate leaf to leaf with variable number of leaflets, reduction in size, and loss of lobing in leaflets. Wild type (upper and lower left), poc B (upper row), poc A (middle row), poc C (lower row). Wild-type leaves (upper and lower left). F, Epiphyllous structures (arrows) on poc leaves appearing near the junction of petiolule to the rachis. G, poc inflorescence (right) showing multiple blooming flowers with abnormal phyllotactic arrangement. H, poc flower (right) showing the absence of anther cone and shorter petals. I, poc petal (right) with an appendage on stamen-facing side (arrow). J, poc sepal (right) with a petal-like sector. Note the absence of trichomes (arrow). K, Twisted and short stamens of poc mutant lacking fusion to form anther cone. Wild type (left). L, Fusion of stamens of poc to the carpel. M, Appearance of a new inflorescence from inside of a fully differentiated flower of poc mutant (arrow).
Nearly all poc mutant plants showed abnormalities in the shape and size of leaves. Based on the leaf abnormalities, we classified poc plants under three classes (Fig. 1D). The class A plants showed a short and bushy phenotype. Leaves of class A were shorter in size and bore smaller, narrow, and curled leaflets (Fig. 1E). A distinct feature of this class is the appearance of epiphyllous structures with a nearly 100% frequency resembling leaves and shoots on rachis normally near the junction to petiolules (Fig. 1F). The class B plants were only somewhat shorter than the wild type. Though the leaves of class B appeared similar to class A, the leaf size was intermediate between wild type and class A, and the leaflets were less curled than class A (Fig. 1E). Also, no epiphyllous structures were seen on class B leaves. The most severe abnormalities were observed in leaves of class C. The leaf lacked the small and minor leaflets, and the characteristic lobing of the blade was absent in the major leaflets (Fig. 1E). In extreme cases, the leaf lacked both small and minor leaflets and formed a lanceolate leaf. The loss of lobing in the leaflet margin was associated with an altered venation pattern of the leaflets with veins headed toward the leaflet tip. The formation of first inflorescence in class C is delayed, and it appears only after formation of the 16th node, whereas in wild type and in the other two classes of poc mutant, inflorescence forms between the eighth and 10th node.
In wild-type plants, the inflorescence consists of about seven to 14 flowers that are organized in a simple cyme pattern. This pattern of organization in the inflorescence was disrupted in poc mutants, with appearance of multiple branches on the main axis of the inflorescence, which either terminated in a flower or dichotomously branched further to give additional flowers. The number of flowers in poc inflorescences ranged between 20 and 60, with about 50 flowers being more common (Fig. 1G). Markedly, in the poc inflorescence, most flowers bloomed at the same time.
The poc mutation showed incomplete penetrance for the observed floral abnormalities (Tables I and II); however, the penetrance for male sterility was nearly 100%. The poc mutants grown in the greenhouse or net house did not produce any fruit unless these were self-pollinated by hand. Several abnormalities were observed in poc floral organs such as increase in number, decrease in size, and fusion of organs (Fig. 1H). Many flowers also showed occasional formation of mosaic organs (Table II). The number of petals in poc mutant was higher, and petals were relatively short with petals of class C being smallest in size. In nearly one-half of the flowers, the petals developed an appendage of tissue facing toward stamens (Fig. 1I). In many flowers, this appendage penetrated between anther filaments, causing separation of stamens. Nearly two-thirds of flowers of the class C mutant flowers contained a patch of green tissue resembling sepals in the center of petals. Similarly, the sepals of poc flowers showed an increase in number and about 5% to 8% of flowers contained petaloid sepals with a sector of tissue resembling petals (Fig. 1J). Occasionally, the sepals were fused with neighbors, with highest fusion (94%) in the flowers of class C.
Table I.
The no. and length of floral organs in wild type (WT) and poc mutant
The sample size was 50 flowers from each genotype WT, poc A, poc B, and poc C. The P values (WT versus mutant) were calculated using a two-tailed Student's t test as described in “Materials and Methods.” The P values for all mutant samples were less than 0.0001.
| Organ
|
No. of Organs
|
Length of Organs (mm)
|
||||||
|---|---|---|---|---|---|---|---|---|
| WT | poc A | poc B | poc C | WT | poc A | poc B | poc C | |
| Sepals | 6.32 ± 0.12 | 7.42 ± 0.1 | 7.26 ± 0.14 | 7.44 ± 0.15 | 6.84 ± 0.18 | 5.1 ± 0.12 | 6.06 ± 0.15 | 5.86 ± 0.22 |
| Petals | 5.98 ± 0.099 | 7.14 ± 0.097 | 6.85 ± 0.14 | 6.92 ± 0.14 | 11.32 ± 0.21 | 9.72 ± 0.195 | 10.82 ± 0.22 | 8.48 ± 0.22 |
| Stamens | 6.04 ± 0.09 | 7.04 ± 0.08 | 6.84 ± 0.13 | 5.4 ± 0.16 | 8.28 ± 0.14 | 7.38 ± 0.14 | 7.9 ± 0.13 | 6.08 ± 0.18 |
| Carpels | 2.12 ± 0.15 | 2.5 ± 0.08 | 2.62 ± 0.12 | Not observed | 7.26 ± 0.13 | 6.4 ± 0.14 | 7.56 ± 0.12 | 5.2 ± 0.18 |
Table II.
The frequency of floral organ abnormalities in the flowers of different classes of poc mutant (n = 150-200)
| Phenotypic Abnormalities
|
Mutant Class
|
||
|---|---|---|---|
| poc A | poc B | poc C | |
| % | |||
| Organ fusion | |||
| Fusion of sepals | 5 | 5 | 94 |
| Lack of fusion of stamens | 85 | 75 | 100 |
| Stamens fused to carpels | 0 | 0 | 89.4 |
| Petals and stamens fused to carpels | 0 | 0 | 3 |
| Identity change | |||
| Petaloid sepals | 7.5 | 7.5 | 5.3 |
| Outgrowth in petals | 52.5 | 22.5 | 60 |
| Sepaloid petals | 0 | 2.5 | 65.8 |
| White-green petals | 0 | 2.5 | 5.3 |
The characteristic fusion of stamens resulting in the formation of a narrow-necked anther tube was nearly absent in the poc flowers (Fig. 1K). The flowers of the poc mutant were male sterile because the anthers lacked dehiscence. In class B and C flowers, the stamens were twisted, distorted, and also variable in size. In class C, the stamen number was reduced, and stamens were fused to the carpels (Fig. 1L). Despite these severe abnormalities, the gynoecium of these flowers remained functional. Occasionally, from within a fully differentiated poc flower, either an inflorescence bearing flowers (Fig. 1M) or a shoot developed. Several of these phenotypes are indicative of the floral meristem retaining features of the inflorescence and may reflect an incomplete transition from the inflorescence to a floral meristem.
Multiple Cotyledons Initiate at the Heart Stage in Mutant Embryos
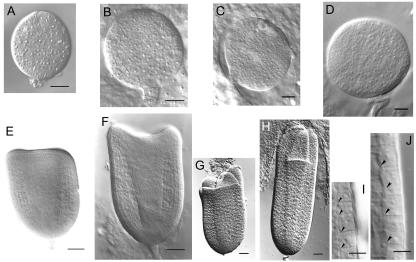
The appearance of multiple cotyledons in poc seedlings is a manifestation of the alteration of embryo development in the mutant. Growth of the embryo in the poc mutant was slower than in the wild type. At 10 d after pollination (DAP), the globular embryo of the mutant was approximately 60% the size of the wild-type embryo (Fig. 2, A and B). The poc embryo was smaller at the late globular stage (Fig. 2, C and D). The slower development of poc embryos was apparent at the triangular stage, where the poc embryo was shorter and squatter in shape relative to the wild type (Fig. 2, E and F). The central procambium region of the poc embryo was also broader at this stage (Fig. 2, E and F). A comparison of cell shape in the medial epidermis of the poc embryo revealed that the cells were more square in shape in comparison with wild type, in which cells were more rectangular and elongated along the anticlinal axis (Fig. 2, I and J). In fact, poc embryos do not show a typical heart stage because in place of two cotyledons, three or four cotyledons form in the embryo (Fig. 2, G and H).
Figure 2.
Comparison of the development of embryo in poc mutant (A, C, E, G, and I) and wild-type plants (B, D, F, H, and J). A and B, Globular stage 10 DAP. C and D, Late globular stage (13 DAP). E and F, Triangular/early heart stage (15 DAP). G and H, Late heart/torpedo stage (18 DAP). I and J, Magnification of medial epidermal cells from the right-hand margin region of embryos shown in E and F, respectively. Arrowheads, Cell boundaries in an epidermal cell file. Scale bars: A to D, 10 μm; E to G, 20 μm; and I and J, 5 μm.
Cell Size Is Altered in the poc Mutant
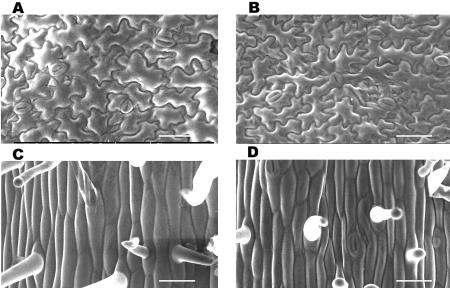
A decrease in size of cells in the poc mutant was noticed for developing embryos at the triangular stage. Scanning electron microscopy (SEM) examination of the surface of cotyledons and hypocotyls showed a similar change in cell size in the mutant seedlings. The increase in cotyledon number was associated with a reduction in size of epidermal cells of cotyledons and hypocotyls for poc. The abaxial epidermal cells of wild-type cotyledons (Fig. 3A) were larger in size (2,396 ± 107.3 μm2) than the poc mutant cells (2,050 ± 74.3 μm2, P = 0.0380; Fig. 3B). A similar difference in width was also visible in epidermal hypocotyl cells of light-grown plants, between wild type (23.94 ± 0.7 μm) and poc (15.13 ± 0.61 μm, P = 0.0007; Fig. 3, C and D).
Figure 3.
Scanning electron micrograph of epidermal cells of light-grown seedlings. A and B, One-week-old cotyledons showing that the poc (B) has smaller cells. C and D, One-week-old hypocotyls showing that poc (D) have shorter and less broad cells. Scale bars in A to D: 50 μm.
PAT Is Enhanced in the poc Mutant
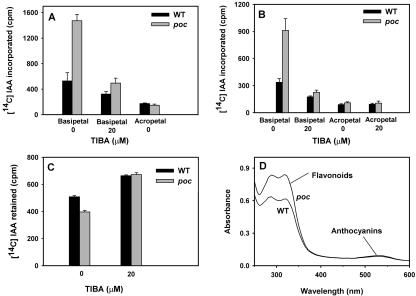
The transport of [14C] IAA was monitored in stem segments in both the acropetal and basipetal directions using two different methods outlined by Okada et al. (1991) and Daniel et al. (1989; Fig. 4, A and B). Using both of these methods, it was found that the polar transport of auxin in poc mutant was significantly higher than the wild-type control. In the acropetal direction, transport of auxin was minimal and about the same in wild type and mutant. In contrast, a significant increase in auxin flow was observed in the basipetal direction with the poc mutant displaying nearly 2.5- to 3-fold higher transport than the wild type. The inclusion of TIBA reduced the magnitude of transport in both mutant and wild type. In an assay for retention of auxin, the poc mutant displayed decreased accumulation of auxin as evident by less retention of [14C] IAA as compared with wild type (Fig. 4C). The fact that inclusion of TIBA increased the amount of retention of [14C] IAA indicates the process to be mediated by PAT. The possibility that enhanced PAT could be due to a reduction in flavonoid levels is argued against by the observation that both poc mutant (0.70 ± 0.11, A330/shoot, P = 0.7071) and wild type (0.64 ± 0.10, A330/shoot) plants have similar levels and spectra of flavonoids (Fig. 4D).
Figure 4.
Auxin polar transport in the presence or absence of TIBA. A, Auxin polar transport was measured using the method of Okada et al. (1991) in stem sections of 5-week-old light-grown plants. The basal end of stem segments was submerged in a solution containing [14C] IAA in an Eppendorf tube (Eppendorf Scientific, Westbury, NY) and after 4 h, a 5-mm section from non-submerged end of segments was excised, and the amount of radioactivity was determined. Error bars = se of five replicates. B, Auxin polar transport was measured using the method of Daniel et al. (1989) in stem sections of 4-week-old light-grown plants. The stem segments were sandwiched on glass microscopic slides between the receiver and donor blocks of agar, and the setup was placed vertically in a humid chamber. After 4 h of incubation, the amount of radioactivity was counted in the receiver blocks. Error bars = se of five replicates. C, Auxin efflux rate was measured by the retention of the amount of [14C] IAA in the hypocotyls of 3-week-old light grown plants. The cut segments of hypocotyls were floated on a solution containing [14C] IAA either with or without TIBA. Then, segments were incubated for another 2 h in the same buffer without IAA/TIBA and counted for radioactivity. Error bars = se of five replicates. D, Absorption spectrum of flavonoids extracted from stems of 4-week-old light-grown poc and wild-type plants.
The Seedling Phenotype of poc Can Be Rescued by TIBA
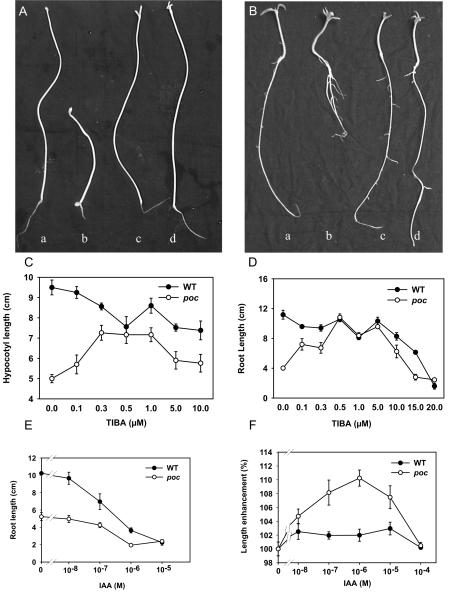
The hypocotyls of dark-grown poc seedlings were distinctly shorter than those of wild type (Fig. 5A). In view of enhanced PAT in the mutant, we reasoned that faster PAT might deplete auxin levels in hypocotyl cells, leading to the observed phenotype. In that case, a reduction in PAT by application of TIBA should rescue the phenotype of poc seedlings. The observed increase in length of hypocotyls of dark-grown poc seedlings at a low concentration of TIBA supports this presumption (Fig. 5A). TIBA at a wide range of concentrations tested (0.1-10 μm) brought about a stimulation of hypocotyl elongation in the mutant relative to the untreated control. At 0.3 μm concentration, TIBA promoted elongation of the poc hypocotyls by 43%, compared with a minor 10% reduction in wild type (Fig. 5C). At 10 μm, hypocotyls were 18% longer than untreated seedlings in the case of the mutant, whereas wild type showed a 22% reduction relative to the untreated control.
Figure 5.
Hypocotyl and root growth of seedlings in the presence of TIBA and IAA. A, Morphology of 9-d-old dark-grown wild-type (a and c) and poc (b and d) seedlings in the presence (c and d) or absence (a and b) of TIBA (0.5 μm). B, Morphology of 9-d-old light-grown wild-type (a and c) and poc (b and d) seedlings in the presence (c and d) or absence (a and b) of TIBA (0.5 μm). C, Effect of various concentrations of TIBA on elongation of hypocotyl length of 10-d-old dark-grown poc seedlings compared with the wild type. D, Effect of various concentrations of TIBA on elongation of root lengths of 9-d-old light-grown poc seedlings compared with wild type. E, Effect of various concentrations of IAA on root elongation of 9-d-old light-grown wild-type seedlings compared with poc. F, Effect of IAA in promoting elongation of hypocotyl segments of 5-d-old dark-grown poc seedlings compared with wild type.
The root length of light-grown poc seedlings was nearly 3 times less than the wild type (Fig. 5B), but root tip is normal as visualized by amyloplast staining (Benjamins et al., 2001). The possible relationship of the short root phenotype with enhanced PAT in the poc mutant was examined by application of TIBA. The inclusion of 0.5 μm TIBA could fully restore the poc root length to a level similar to that of the wild type. Interestingly, this low concentration of TIBA had no significant effect on the length of wild type roots (Fig. 5D). For roots, as for hypocotyls, TIBA-mediated stimulation of elongation was seen for a wide range of concentrations (0.1-10 μm).
The response to auxin was normal in roots of poc seedlings as evidenced by similarity to wild type for auxin-mediated inhibition of root elongation (Fig. 5E). The inhibitory action of auxin also indicates that reduced elongation of poc roots might be due to overaccumulation of auxin. The possibility that shorter hypocotyl length of dark-grown mutant seedlings resulted from auxin deficiency was examined by studying elongation of excised hypocotyls in the presence of auxin. Although auxin only slightly stimulated elongation of wild type, it significantly stimulated elongation of mutant hypocotyl (Fig. 5F), indicating a likely deficiency of auxin in the mutant tissue. Taken together, these data show that several of the phenotypes of the poc mutant can be ascribed to a depletion of auxin arising from increased PAT and not to a reduced sensitivity to auxin because the mutant retains substantial sensitivity to exogenously applied auxin.
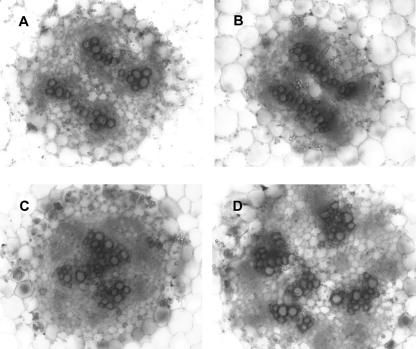
Examination of transverse sections of poc hypocotyls revealed alteration in anatomy, particularly with respect to vascular differentiation (Fig. 6). In wild-type seedlings, the vascular bundles were usually arranged as four bundles (two pairs of two bundles) with a central pith. A distinct feature of the poc mutant was that vascular bundles were arranged near each other due to a reduction in the central pith region (Fig. 6C). Chemical inhibition of the PAT in poc seedlings resulted in proliferation of xylem vessels with appearance of central pith and arrangement of bundles similar to untreated wild-type control (Fig. 6D). In contrast, in the presence of TIBA, the proliferation of xylem vessels in wild type led to fusion of adjacent bundles (Fig. 6B). These results indicate that changes in the vasculature of the poc mutant could be due to altered levels of auxin.
Figure 6.
Transverse sections showing the vasculature in hypocotyls of 15-d-old light-grown poc seedlings in the presence or absence of TIBA (10 μm). Sections were taken from the middle portion of hypocotyls and stained with safranine. A, Wild type; B, wild type + TIBA; C, poc mutant; D, poc mutant + TIBA. Note the difference in placement of the vascular bundles between mutant and wild type. Scale bars in A to D: 50 μm.
Enhanced PAT Alters Gravitropic Curvatures
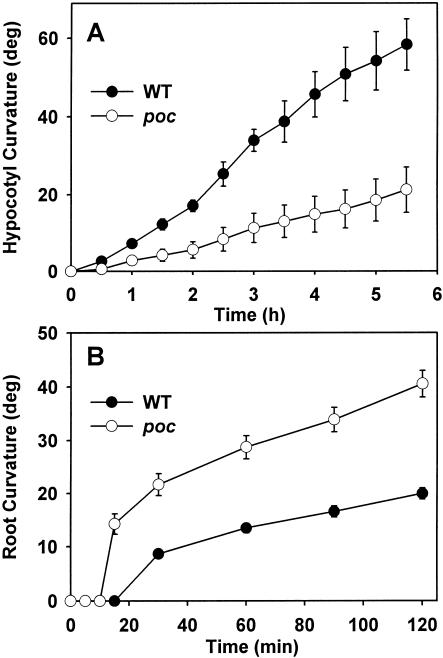
The experiments on the rescue of the poc phenotype and auxin action on organ elongation indicated that enhanced PAT may cause depletion of auxin in hypocotyl tissue and at the same time may cause over accumulation of auxin at the root tip. Such alteration in auxin level is expected to affect physiological processes such as gravitropic curvature that are related to auxin levels or distribution. Therefore, we compared gravitropic curvature of the hypocotyl and of the root in the poc mutant with that in wild type. The gravitropic curvature of the poc hypocotyl differed from wild type in two aspects (Fig. 7A). First, onset of curvature in poc seedlings could be seen only after a lag period of 1 h, which was almost twice that required for wild type. Second, at all time points, the magnitude of curvature was much lower than that for wild-type seedlings. In fact, some poc seedlings showed only a little curvature even after 5 h of gravistimulation. However, after 24 h, these poc seedlings showed significant curvature, albeit the extent of the curvature in poc seedlings was less than in the wild type.
Figure 7.
Analysis of gravitropic response in seedlings. A, Kinetics of gravitropic response in the hypocotyls of 7-d-old light-grown wild-type and poc seedlings. Seedlings grown in plastic cuvettes were reoriented 90° relative to the gravity vector. The curvature was determined by photographing the seedlings at the given time intervals using a PC camera. B, Gravitropic response in the roots of 1-d-old light-grown wild-type and poc seedlings. Seedlings grown on vertical agar plates were reoriented 90° relative to the gravity vector. The curvature was determined by photographing the roots at the given time intervals using a PC camera.
It is believed that curvature of the root tip on gravistimulation results from basipetal flow of auxin from the root tip into cortical and epidermal cells. In that case, accumulation of auxin at the root tip due to enhanced PAT in the mutant would be expected to increase the amount of basipetal flow of auxin leading to higher curvatures. For poc seedlings, a higher root gravitropic curvature was observed compared with wild type (Fig. 7B). Interestingly, the gravitropic curvature of roots of the poc mutant showed deviation from wild type in two aspects in an entirely opposite way from that seen for the hypocotyl. First, the lag period of curvature in poc was reduced to 10 min compared with a lag of 15 min in wild type. Second, for poc, a much higher degree of curvature was seen compared with wild type.
DISCUSSION
Phenotypes Exhibited by poc Mutant Are Most Likely Caused by an Increase in Polar Transport of Auxin
The strong pleiotropic effect of the poc mutation lasting throughout the plant life cycle likely signifies modification in some central physiological processes regulating plant development. The observed developmental abnormalities in the poc mutant such as increase in cotyledon numbers, change in shape and size of the leaf, loss of lobing in the leaflet, ectopic formation of organs on the rachis, increased number of flowers in the inflorescence, and fusion of floral organs are broadly reminiscent of alteration in phytohormone action. Examination of mutant seedlings revealed a 3-fold higher level of PAT. During postembryonic development, interference with PAT by mutation or exogenous application of PAT inhibitors results in defects in leaf morphology and venation, inflorescence architecture, vascular anatomy, and tropic movement of plants (Friml and Palme, 2002). Interestingly, several of the morphological and anatomical changes in the poc mutant are the reverse of those observed for Arabidopsis mutants such as pin1 (Okada et al., 1991; Gälweiler et al., 1998), tir3 (Ruegger et al., 1997), and ifl1 (Zhong and Ye, 2001) that display a reduction in PAT. The stronger alleles of these mutants develop a pin-shaped inflorescence devoid of floral primordia and abnormal leaves. In contrast, the poc mutant shows features such as extensive branching of the inflorescence with numerous abnormal flowers (Fig. 1).
Several lines of evidence indicate that developmentally regulated auxin levels and distribution specify vascular differentiation during normal plant organogenesis (Ye, 2002). Such a role for auxin as an inducer of vascular differentiation is seen in auxin-overproducing transgenic plants, wherein an excess of auxin levels causes increase in the amounts of vascular tissue (Klee et al., 1987). PAT is essential for the formation of spatially organized patterns of vascular tissue, and a reduction of PAT in ifl1 and pin1 mutants is accompanied by proliferation of xylem in the vascular bundles of inflorescence stems (Gälweiler et al., 1998; Mattsson et al., 1999; Tsiantis et al., 1999; Zhong and Ye, 2001). Because reduction in PAT increases vascular proliferation, increase in PAT is expected to reduce auxin levels in the stem and display the opposite effect. In fact, auxin-deficient transgenic plants show a reduction in vascular development (Romano et al., 1991). The anatomical alterations observed in poc, such as smaller epidermal cells and compact placement of vascular bundles with reduction of the central pith (Fig. 6), are in conformity with the view that these changes may reflect reduced auxin levels due to increase in PAT.
One way to ascertain a causal connection between the mutation and a physiological process is to phenocopy the mutation by external application of bio-active molecules. In several instances, phenotypes of mutants defective in PAT have been phenocopied in wild type by application of PAT inhibitors (Okada et al., 1991; Gälweiler et al., 1998). Although a lack of agonists that can stimulate PAT in plants precludes phenocopy of the poc mutation in wild type, we tested whether the application of PAT inhibitors to the poc mutant could rescue the mutant phenotype to wild type. Etiolated seedlings of the poc mutant characteristically show a shorter hypocotyl, whereas the light-grown mutant seedlings exhibit a shorter primary root than the wild type. A rescue of the phenotype is clearly seen in seedlings raised in the presence of TIBA (Fig. 5). TIBA increases the root and hypocotyl lengths of the mutant seedlings to close to that of the wild type. A similar rescue of phenotype by TIBA was also seen for anatomical alteration in the poc mutant. Taken together, we can reasonably speculate that the aberrant morphology of the poc mutant may be induced by an enhancement in PAT.
Several lines of evidence support the assumption concerning elevation in the rate of auxin transport in the poc mutants. First, direct quantification of basipetal transport shows that the rate of auxin transport in the poc mutant is nearly 3-fold higher than in the wild type, and the exogenous application of TIBA reduces the transport rate close to the wild-type control. Second, tomato stems preloaded with radiolabeled auxin show a higher rate of auxin efflux in the poc mutant compared with wild type. Again, in this case, TIBA reduces the efflux rate in the mutant to the levels observed for wild type. The auxin transport in inflorescence stems and hypocotyls of the flavonoid-deficient tt4 mutant is elevated over wild type by about 2-fold (Brown et al., 2001). The observed increase in PAT in the poc mutant cannot be explained by a general reduction in the levels of flavonoids because the mutant plants had nearly the same level of flavonoids as in the wild type. Together, these data strengthen the view that the poc mutation enhances the rate of auxin transport, and it acts at a step other than flavonoid biosynthesis.
Enhanced Auxin Polar Transport Likely Alters Auxin Distribution Pattern in the poc Mutant
Auxin distribution has been implicated as a key regulator of several developmental processes such as cell elongation and tropic curvatures. Mutations affecting PAT in several instances may elicit their phenotype by consequent disturbance of the auxin balance of the tissues (Sabatini et al., 1999). Etiolated hypocotyls of mdr1 mutants, defective in PAT, display larger gravitropic and phototropic curvatures probably due to accumulation of auxin (Spalding et al., 2002). On the contrary, increase in PAT may result in faster removal of auxin, reducing its lateral flow. One critical physiological assay for the lateral flow of auxin is the magnitude of gravitropic curvature that is related to its lateral distribution as per Cholodny and Went's hypothesis (Trewavas, 1992). The decrease in the lateral flow of auxin in the pin3 mutant also correlates with a reduction in the tropic curvature of Arabidopsis seedlings (Friml et al., 2002b). The observed delay in onset and reduction in magnitude of gravitropic curvature in poc hypocotyls is consistent with reduction in auxin levels in the tissue due to enhanced PAT. Reduced lateral flow of auxin also correlates with a reduction in the width of epidermal cells in poc hypocotyls. The paucity of auxin in hypocotyls is also indicated by greater stimulation of hypocotyl elongation by auxin in the poc mutant than wild type and is consistent with its depletion due to increased PAT.
One of the obvious consequences of enhanced PAT is the likelihood of channelization of most auxin to the root pole, causing its accumulation at the root tip. Three lines of evidence point to such an overaccumulation in the poc mutant based on current physiological models of auxin-mediated root growth and tropic curvature (Sabatini et al., 1999; Masson et al., 2002). First, a reduction in the root tip growth observed is consistent with an increased level of auxin because beyond a threshold level, auxin acts as an inhibitor of growth. In fact, such accumulation of auxin at the root tip in transgenic pinoid-overproducing plants leads to total collapse of the root meristem, and this could be rescued by application of PAT inhibitors (Benjamins et al., 2001). An increase in root length of poc seedlings by application of PAT inhibitors indicates that the increased accumulation of auxin may be responsible for the decreased root growth. Second, adult poc plants produce more lateral roots than the wild type (Reed et al., 1998). Third, an increased accumulation of auxin at the root meristem would also increase the “back flow” of auxin in the basipetal direction in epidermal and cortical cells of the root. Such increase in the basipetal flow of auxin in the root, as per the “fountain model” of auxin-mediated root gravitropism, would be predicted to stimulate the gravitropic root tip curvature in the poc mutant. Analysis of root gravitropic curvature broadly substantiates this view because gravistimulated poc root tips showed a much shorter lag period and nearly twice the curvature compared with wild type.
Is Polar Transport of Auxin Related to Initiation of Cotyledons in the Globular Embryo?
Auxin is considered as a principal regulator of patterning during embryogenesis. Because auxin is polarly transported and may act in a non-cell autonomous fashion, it can provide the necessary input for determination of pattern specificity (Souter and Lindsey, 2000; Berleth and Chatfield, 2002). Recent reports have brought some support to the role of auxin as a “morphogen” regulating cell differentiation in a concentration-dependent manner (Sabatini et al., 1999; Friml et al., 2002a). Current research on embryo development in Arabidopsis mutants has highlighted that the transition from the globular to the heart stage requires elements participating in auxin perception and distribution. A role for auxin in the progression of embryogenesis is indicated by the observation that a null mutation in the auxin-binding protein ABP1 arrests Arabidopsis embryogenesis at the globular stage (Chen et al., 2001). Similarly, failure to establish a properly localized distribution of auxin transporting proteins during the globular stage in the gnom mutant results in an abnormal embryo devoid of cotyledonary primordia (Mayer et al., 1993; Steinmann et al., 1999).
The appearance of cotyledonary poles on a developing embryo marks the end of the globular stage leading to establishment of bilateral symmetry. Available evidence indicates that development of the auxin transport system during the globular stage is related to the subsequent appearance of cotyledon primordia. In a range of plant species, disruption of PAT either by a mutation such as pin1 (Liu et al., 1993) or gnom (Mayer et al., 1993) or by using inhibitors of auxin transport during zygotic and also somatic embryogenesis (Hadfi et al., 1998; Liu et al., 1993) disrupts embryonic development with generation of embryos with fused or missing cotyledons. Thus, normal auxin transport appears to be one of the prerequisites for the radial globular embryo to progress to the bilaterally symmetrical heart stage embryo (Liu et al., 1993; Hadfi et al., 1998). However, our knowledge about the events leading to onset of cotyledon initiation during embryogenesis is limited. Embryonic fate mapping using chimeric tissue indicated that in Arabidopsis, first one cotyledon gets determined and that in turn directs the positioning of the other cotyledon at the opposite end. It is suggested that some kind of lateral inhibition is responsible for this process, directing the formation of new primordia at the site of lowest inhibition (Woodrick et al., 2000).
The association of defects in auxin transport with alterations in cotyledon initiation/separation in a number of instance points to the likelihood of auxin playing an important role in the initiation of cotyledon development. Based on reports that indicate association between auxin transport and cotyledon development, it seems possible that faster removal of auxin may result in initiation of additional cotyledons. In fact, based on their results on auxin and PAT inhibitor application to developing mustard embryos, Hadfi et al. (1998) proposed that polar transport removes auxin from the area between the two emerging cotyledon primordia causing separation of cotyledons. Therefore, the removal of auxin by enhanced polar transport could deplete auxin below a threshold level in cells of the apical dome, leading to initiation of additional cotyledon primordia. The appearance of multiple cotyledons in poc embryos at the transition from globular to heart stage is indicative of such a link between auxin transport and cotyledon formation. Likewise, the reduction in size of epidermal cells in poc embryos compared with wild type could be a consequence of increased PAT.
Though an association of increase in cotyledon number with increase in PAT is seen for the poc mutant of tomato, it is at variance with the pinoid mutant of Arabidopsis, which shows polycot seedlings but reduced PAT in inflorescence stems (Bennett et al., 1995). A recent report suggested that PINOID acts as a positive regulator of PAT (Benjamins et al., 2001), whereas our results indicate POC to be a negative regulator. It is proposed that PINOID may be required for proper positioning of cotyledonary primordia (Benjamins et al., 2001), whereas POC action might be related to separation of cotyledon primordia (Hadfi et al., 1998). In such a case, these two might affect different physiological processes and still show similar phenotypic effects. It could be equally plausible that enhanced PAT in Arabidopsis is not related to development of polycotyly. For example, the Arabidopsis tt4 mutant shows enhanced PAT but does not exhibit a polycot phenotype (Brown et al., 2001).
In summary, our results indicate that the poc mutation of tomato shows increased PAT and that this could be responsible for its pleiotropic phenotype. We have located the poc gene on chromosome nine of tomato (M. Kavitha, P. Bauer, M.S. Sharada, A.S.A. Al-Hammadi, P. Janila, S. Negi, R. Sharma, unpublished data) and are currently fine mapping it with an aim to isolate and determine its role in PAT.
MATERIALS AND METHODS
Plant Growth Conditions
Tomato (Lycopersicon esculentum L. cv Ailsa Craig) seeds were surface sterilized and sown on filter papers. After emergence of the radicle, seedlings were grown on vermiculite either under continuous white light (100 μmol m-2 s-1) or in darkness at 25°C. Unless otherwise mentioned, for all experiments the plant age was counted from the time point of emergence of the radicle. To test the viability, pollens were germinated on Brewbaker and Kwack's media (Brewbaker and Kwack, 1963), and the percentage pollen germination was calculated after staining with 1% (w/v) acetocarmine. Leaf and cotyledon venation was examined by depigmenting tissues in acidified methanol (2% [v/v] HCl) followed by clearing in a mixture of chloral hydrate:glycerol:water (8:1:2 [w/v]) and staining with p-rosaniline hydrochloride. Flavonoid content of 4-week-old light-grown plants (n = 10, three replicates) was estimated according to Harborne (1967). The shoots of these plants were excised and extracted in 10 mL of acidified (1% [v/v] HCl) methanol for 24 h in darkness with shaking. After centrifugation, the absorption spectra were recorded. The total flavonoid level is expressed as A330/shoot. The experimental data for the cell size and morphological variation was also analyzed by a two-tailed Student's t test, and P values were calculated.
Mutants Screening and Genetic Analysis
The ethyl methanesulfonate (EMS) mutagenesis was performed essentially following the procedure of Koornneef et al. (1990). Approximately 5,000 seeds of tomato cv Ailsa Craig were soaked in a solution of 60 mm EMS for 24 h in the dark at room temperature. Thereafter, the seeds were washed with distilled water, and M1 generation plants were grown in the field. From fruits of a population of approximately 1,500 M1 plants, M2 seeds were harvested in bulk. A population of approximately 50,000 EMS-mutagenized M2 seedlings was screened for putative cotyledon mutants. One-week-old seedlings were screened for altered cotyledon number and size. Of 25 putative mutants showing multiple cotyledons, only nine mutants survived. Because the mutants were male sterile, these were rescued by manually pollinating with wild-type pollens, and F1 heterozygous seeds were obtained. Subsequently, mutants were also manually self-pollinated, and homozygous seeds were obtained. The complementation analysis using isolated mutant lines indicated that all nine mutants were allelic. For all experiments, seeds multiplied from the mutant line poc1-1 that was back-crossed twice were used.
Embryogenesis
Both the wild-type and poc mutant flowers were emasculated and manually self-pollinated. The developing fruits were harvested at regular intervals starting 10 DAP. Pistils were removed from the developing fruits and fixed in ethanol:acetic acid (6:1 [v/v]) overnight. After several washes in 100% ethanol followed by washing in 70% ethanol, the pistils were preserved in 70% ethanol. The pistils were then cleared in a mixture of chloral hydrate:glycerol:water (8:1:2 [w/v]) overnight (Berleth and Jurgens, 1993) and dissected under a dissection microscope to reveal the ovules, which were then further dissected to remove embryos. The embryos were observed using a Zeiss Axiophot microscope under DIC optics (Zeiss, Oberkochen, Germany) and photographed using black and white film (15-25 ASA; Siddiqi et al., 2000).
PAT Assay
The polar transport of auxin was assayed in the stem segments of light-grown plants using two different protocols outlined by Okada et al. (1991) and Daniel et al. (1989) with some modifications. The height and the diameters of the stems of both poc and wild-type plants were nearly similar. For the Okada et al. (1991) protocol, 10 stem segments (2.5 cm) were cut 2 mm below the cotyledonary node from 5-week-old light-grown plants. The segments were first floated for 2 h in 5 mm phosphate buffer (pH 5.8) containing either 1 μm IAA or 20 μm TIBA. To avoid a gravitropic response during that period, this pre-incubation took place on a shaker. Thereafter, either the apical or basal end of the segments (for basipetal or acropetal transport, respectively) was submerged in 50 μL of 5 mm phosphate buffer (pH 5.8) with 27 nCi mL-1 (specific activity = 4.2 mCi mmol-1 of [1,2-14C] IAA; BRIT, Mumbai, India) and 1 μm IAA (unlabeled) in a 1.5-mL Eppendorf tube. After 4 h, a 5-mm section from the non-submerged end of segments was excised and floated overnight in 3 mL of Bayer's solution. The radioactivity was counted in a Beckman liquid scintillation counter. Similar to Brown et al. (2001), we also found that a 4-h transport period was sufficient to measure PAT in tomato, unlike 18 h used by Okada et al. (1991).
For the Daniel et al. (1989) protocol using agar blocks, stem segments (1.2 cm) of 4-week-old tomato plants were excised 2 mm below the cotyledonary node and incubated for 2 h in 5 mm phosphate buffer (pH 5.8) containing either 1 μm IAA or 20 μm TIBA on a shaker. These segments were sandwiched on glass microscopic slides between receiver (1.5% [w/v] agar in water, 2.5 × 0.4 × 0.4 cm) and donor blocks (1.5% [w/v] agar in 5 mm phosphate buffer [pH 5.8] containing 1 μm IAA and approximately 17 nCi mL-1 [14C-IAA], 2.5 × 0.8 × 0.4 cm). Twelve segments for each of the donor receiver block units were placed either in basipetal (apical end toward donor block) or in acropetal direction (with basal end toward donor). The donor receiver block units were placed vertically in a humid chamber at 25°C ± 2°C, and the system was inverted to prevent drainage of [14C IAA] onto the receiver blocks. After 4 h of incubation, the receiver blocks were removed and placed in 3 mL of liquid scintillation cocktail. The samples were shaken at 100 rpm for 2 h and left overnight at 25°C ± 2°C before counting in a scintillation counter.
Auxin Efflux Assay
Auxin efflux in the hypocotyls was assayed according to the procedure of Bernasconi (1996). Twelve hypocotyls of 3-week-old light-grown seedlings were cut into segments (4 mm). The segments were floated with shaking for 2 h in 5 mm phosphate buffer (pH 5.8) with 1% (w/v) Suc containing 0.1 μCi mL-1 [14C] IAA either with or without 20 μm TIBA. Then, the segments were rinsed and incubated for another 2 h in the same buffer without IAA/TIBA. After a final rinse, the segments were placed in Bayer's solution and counted for radioactivity as described above.
Effect of TIBA on Organ Elongation
Surface-sterilized seeds of tomato were germinated in the dark. After the emergence of the radicle, seeds were transferred either onto petri plates or germination boxes containing 1.7% (w/v) agar support prepared with one-tenth-strength Murashige and Skoog media (inorganic salts only, Murashige and Skoog, 1962). For studying the effect of TIBA on root length, seeds were sown on agar containing different concentrations of TIBA in the range of 0 to 20 μm. The petri plates were vertically oriented after 9-d seedlings were removed, and the root lengths were measured. To study the effect of TIBA on hypocotyl length, seeds were sown on 1.7% (w/v) agar in transparent germination boxes containing different concentrations of TIBA in the range of 0 to 20 μm. The germination boxes were kept in the dark for 10 d. On the 10th d, the hypocotyl lengths were measured.
Effect of Auxin on Organ Elongation
The method of estimating elongation of hypocotyls was essentially similar to that described earlier by Kelly and Bradford (1986), with the variation that the hypocotyls were used immediately after excision and were not depleted of endogenous auxin. One-centimeter segments of hypocotyls were excised from 5-d-old dark-grown seedlings and floated in 5 mm phosphate buffer (pH 5.8) containing 1% (w/v) Suc with and without 1 μm IAA. The length of each segment was recorded after 4 h. For study on root length, seedlings were grown on agar with or without IAA in the light on vertical petri plates, and the root lengths were measured after 9 d.
SEM
The middle region from hypocotyls and cotyledons of 7-d-old light-grown seedlings was excised and mounted on stubs using a double-sided adhesive tape. In the case of cotyledons, the abaxial side was examined for epidermal cell shape. The stubs were plunged in liquid nitrogen, and the frozen organs were immediately examined in a environmental scanning electron microscope (Philips, Eindhoven, The Netherlands).
Light Microscopy
Free-hand sections were cut from the hypocotyls of 15-d-old light-grown seedlings. At this age, hypocotyls were approximately 3.5 cm long. The sections were cut from at least 10 seedlings from the central region of hypocotyls at a distance of 1.5 to 2 cm from the cotyledonary node. The cut sections were immediately stained in safranine and were destained in water. The sections were mounted on slides in water and were photographed in a Zeiss Axiophot microscope using DIC optics.
Analysis of Gravitropism
Gravity response in the hypocotyls was measured using 7-d-old light-grown seedlings grown vertically in plastic cuvettes filled with vermiculite. At the time of gravistimulation, seedlings were turned 90°, and the cuvettes were arranged in a rack. Seedlings were photographed using an Ezonics (Ezcam) PC camera (Ezonics Corporation, Pleasanton, CA) at every 15-min interval initially and later at every 30 min for 6 h. For each experiment, five poc and five wild-type seedlings were used and the experiment was repeated six to eight times. The angles were measured using Image Tool program after subtracting the zero time point image from the image at required time points.
For root gravitropism, seeds with just emerged radicles were sown on 1.7% (w/v) agar in petri plates, and plates were kept vertically under continuous white light. After 24 h, when roots elongated to 5 to 6 mm, the plates were rotated by 90°, and images were recorded at every 5 min for 2 h using an Ezonics PC camera. Each petri plate had three poc and three wild-type seedlings, and the experiment was repeated about 10 times.
Acknowledgments
We wish to thank Dr. Bernie Carroll (University of Queensland, Australia) for providing us the dem mutant seeds, Dr. Shashi Singh (Centre for Cellular and Molecular Biology, Hyderabad, India) for light microscopy, and Dr. Manjunath (Central Instruments Laboratory, University of Hyderabad, Hyderabad, India) for SEM.
This work was supported by the government of Yemen (fellowship to A.S.A.A.-H.), by Council of Scientific and Industrial Research (CSIR; fellowships to Y.S. and S.N. and grant to R.S.), by International Atomic Energy Agency (IAEA; grant to R.S.), and by Department of Science and Technology (DST; grant to R.S.).
References
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057-4067 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease like regulator of root gravitropism. Science 273: 948-950 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505-520 [Google Scholar]
- Berleth T, Chatfield S (2002) Embryogenesis: pattern formation from a single cell. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, http://www.aspb.org/downlaods/arabidopsis/berleth.pdf [DOI] [PMC free article] [PubMed]
- Berleth T, Jurgens G (1993) The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development 118: 575-587 [Google Scholar]
- Bernasconi P (1996) Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol Plant 96: 205-210 [Google Scholar]
- Bernasconi P, Bhavesh CP, Reagan JD, Subramanian MV (1996) The N-1-naphthylphthalamic acid-binding protein is an integral membrane protein. Plant Physiol 111: 427-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50: 859-865 [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-G, Ullah H, Young JC, Sussman MR, Jones AM (2001)ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 15: 902-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH (1998) The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA 95: 15112-15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100: 469-478 [DOI] [PubMed] [Google Scholar]
- Coenen C, Christian M, Lüthen H, Lomax TL (2003) Cytokinin inhibits a subset of diageotropica-dependent primary auxin responses in tomato. Plant Physiol 131: 1692-1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SG, Rayle DL, Cleland RE (1989) Auxin physiology of tomato mutant diageotropica. Plant Physiol 91: 804-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Palme K (2002) Polar auxin transport-old questions new concepts? Plant Mol Biol 49: 273-284 [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G et al. (2002a) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108: 661-673 [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benková E, Mendgen K, Palme K (2002b) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806-809 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226-2230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219-230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425-428 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985-1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfi K, Speth V, Neuhaus G (1998) Auxin-induced developmental patterns in Brassica juncea embryos. Development 125: 879-887 [DOI] [PubMed] [Google Scholar]
- Harborne JB (1967) Comparative Biochemistry of Flavonoids. Academic Press, London
- Jacobs M, Gilbert SF (1983) Basal localization of the presumptive auxin transport carrier in pea stem cells. Science 220: 1297-1300 [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH (1988) Naturally occurring auxin transport regulators. Science 241: 346-349 [DOI] [PubMed] [Google Scholar]
- Keddie JS, Carroll BJ, Thomas CM, Reyes MEC, Klimyuk V, Holtan H, Gruissem W, Jones JDG (1998) Transposon tagging of the defective embryo and meristems gene of tomato. Plant Cell 10: 877-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MO, Bradford KJ (1986) Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol 82: 713-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL (1987) The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev 1: 86-96 [Google Scholar]
- Koornneef M, Bosma TDG, Hanhart CJ, Van der Veen JH, Zeevaart JAD (1990) The isolation and characterization of gibberellin-deficient mutants in tomato. Theor Appl Genet 80: 852-857 [DOI] [PubMed] [Google Scholar]
- Liu C-M, Xu Z-H, Chua N-H (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5: 621-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH (1995) Auxin transport. In PJ Davies, ed, Plant Hormones, Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 509-530
- Luschnig C, Gaxiola RA, Grisafi P (1998) Fink GR EIR1 a root-specific protein involved in auxin transport is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066-2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson PH, Tasaka M, Morita MT, Guan C, Chen R, Boonsirichai K (2002) Arabidopsis thaliana: a model for the study of root and shoot gravitropism. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, http://www.aspb.org/downloads/arabidopsis/masson.pdf [DOI] [PMC free article] [PubMed]
- Mattsson J, Sung ZR, Berleth T (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126: 2979-2991 [DOI] [PubMed] [Google Scholar]
- Mayer U, Buettner G, Jurgens G (1993) Apical-basal pattern formation in the Arabidopsis embryo: studies on the role of the gnom gene. Development 117: 149-162 [Google Scholar]
- Muday GK, Brunn SA, Haworth P, Subramanian M (1993) Evidence for a single naphthylphthalamic acid binding site on the zucchini plasma membrane. Plant Physiol 103: 449-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL (1995) Characterization of the growth and auxin physiology of roots of the tomato mutant diageotropica. Planta 195: 548-553 [DOI] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903-6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 493-497 [Google Scholar]
- Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211: 315-324 [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport gravity response and lateral root growth. Plant Cell 13: 1683-1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J (1975) Transport of indoleacetic acid in plant cells in relation to pH and electric potential gradients, and its significance for polar IAA transport. New Phytol 74: 163-172 [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Hein MB, Klee HJ (1991) Inactivation of auxin in tobacco transformed with the indole acetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev 5: 438-446 [DOI] [PubMed] [Google Scholar]
- Rubery PH, Sheldrake AR (1974) Carrier mediated auxin transport. Planta 118: 101-121 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745-757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463-472 [DOI] [PubMed] [Google Scholar]
- Siddiqi I, Ganesh G, Grossniklaus U, Subbaiah V (2000) The dyad gene is required for progression through female meiosis in Arabidopsis. Development 127: 197-207 [DOI] [PubMed] [Google Scholar]
- Souter M, Lindsey K (2000) Polarity and signalling in plant embryogenesis. J Exp Bot 51: 971-983 [DOI] [PubMed] [Google Scholar]
- Spalding E, Murphy A, Noh B (2002) Larger and faster tropisms in mdr mutants lacking polar auxin transport. American Society of Plant Biologists, Minisymposium: Tropism. Abs #28003. http://abstracts.aspb.org/pb2002/public/M16/1053.html
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jurgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316-318 [DOI] [PubMed] [Google Scholar]
- Trewavas AJ (1992) FORUM: what remains of the Cholodny-Went Theory? Plant Cell Environ 15: 759-794 [PubMed] [Google Scholar]
- Tsiantis M, Brown MIN, Skibinski G, Langdale JA (1999) Disruption of auxin transport is associated with aberrant leaf development in maize. Plant Physiol 121: 1163-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrick R, Martin PR, Birman I, Pickett FB (2000) The Arabidopsis embryonic shoot fate map. Development 127: 813-820 [DOI] [PubMed] [Google Scholar]
- Ye Z-H (2002) Vascular tissue differentiation and pattern formation in plants. Annu Rev Plant Biol 53: 183-202 [DOI] [PubMed] [Google Scholar]
- Zhong R, Ye Z-H (2001) Alteration of auxin polar transport in the Arabidopsis ifl1 mutants. Plant Physiol 126: 549-563 [DOI] [PMC free article] [PubMed] [Google Scholar]