Abstract
l-Mimosine, a plant amino acid, can reversibly block mammalian cells at late G1 phase and has been suggested to affect translation of mRNAs such as p27, the CDK inhibitor. However, the mechanism of this effect is not known. Regulation of translation generally occurs at the initiation step that, in mammalian cells, is a complex process that requires multiple eukaryotic initiation factors (eIFs) and ribosome. The effects of mimosine on initiation factors or regulators consequently will influence translation initiation. P170, a putative subunit of eIF3, has been suggested to be nonessential for eIF3 function to form preinitiation complexes and it may function as a regulator for translation of a subset of mRNAs. In this article, we tested this hypothesis and investigated whether eIF3 p170 mediates mimosine effect on mRNA translation. We found that p170 translation was dramatically reduced by mimosine due to its iron-chelating function. The decreased expression of p170 by mimosine caused diminished de novo synthesis of tyrosinated α-tubulin and elevated translation of p27 before cell cycle arrest. These observations suggest that p170 is likely an early response gene to mimosine treatment and a mediator for mimosine effect on mRNA translation. The effect of p170 on the synthesis of tyrosinated α-tubulin and p27 in a reciprocal manner also suggests that p170 functions as a regulator for mRNA translation.
INTRODUCTION
l-Mimosine, a plant amino acid derived from seeds of Leucaena leucocephala or Mimosa pudica, can specifically and reversibly block mammalian cells at late G1 phase (Lalande, 1990; Mosca et al., 1992). Mimosine is commonly used as a synchronizing agent for investigating cell cycle progression in mammalian cells (Wang et al., 1995; Hughes and Cook, 1996). However, the molecular mechanism of mimosine action is ill-defined. It has been reported that the cells treated with mimosine lack proteins required for initiation and elongation of DNA replication (Kalejta and Hamlin, 1997). Although mimosine does not affect mRNA synthesis in general (Tsai and Ling, 1971), a subset of mRNAs was found to relocate to the mono-ribosome pool at the expense of polyribosome fraction after mimosine treatment. On mimosine removal, they reappeared in poly-ribosome pool, indicating that the mimosine effect is reversible (Hanauske-Abel et al., 1995). A recent study showed that expression of the cyclindependent kinase inhibitor p27 was elevated at the translational level by mimosine (Wang et al., 2000). These findings suggest that mimosine may modulate the synthesis of some proteins, which in turn causes inhibition of DNA replication and cell cycle arrest. One possible mechanism for such effect is that mimosine affects the synthesis and/or the function of proteins controlling mRNA translation.
The rate-limiting step in mRNA translation is the initiation step. In eukaryotes, 11 factors have been identified that participate in the translation initiation step (Hershey and Merrick, 2000). Among these factors, eIF3 is the largest and the most complex one with a molecular weight of ∼550–700 kDa (Trachsel et al., 1977; Merrick, 1979; Hershey et al., 1996; Hershey and Merrick, 2000). It plays a key role in the initiation of translation by binding to 40S subunit to prevent the reassociation of the 60S to the 40S subunit before formation of the 43S preinitiation complex and by stabilizing the MettRNA, eIF2, and the GTP ternary complex. Mammalian eIF3 consists of ∼11 putative subunits: p170, p116, p110, p66, p48, p47, p44, p40, p36, p35, and p28 (Hershey and Merrick, 2000). The p170 subunit is the largest and thought to be the major subunit of eIF3 complex purified initially from rabbit reticulocyte lysates (Benne and Hershey, 1976). However, the functional importance of p170 in translational control and in the eIF3 complex is not clear. The findings that p170 interacts with other subunits of eIF3 such as p44 (Block et al., 1998) and p116 (Methot et al., 1997) and also with eIF4B (Methot et al., 1996) as well as with RNA (Block et al., 1998; Buratti et al., 1998) support a role of p170 in eIF3 function and translation initiation. However, eIF3 preparations rich in p170 did not differ from preparations that essentially lacked this protein in assays of preinitiation complex formation (Chaudhuri et al., 1997). This finding suggests that eIF3 p170 may not be essential for the formation of preinitiation complex in the initiation of general translation.
Recently, overexpression of eIF3 p170 has been associated with several human cancers, including cancers of breast (Bachmann et al., 1997), cervical (Dellas et al., 1998), esophageal (Chen and Burger, 1999), and lung (Pincheira et al., 2001b). We recently also found that the expression of eIF3 p170 oscillates with cell cycle. That is, its expression level is low in G1 and high in S phase (Dong, Pincheira, and Zhang, unpublished observation). Thus, p170 may play some role in modulating cell proliferation and cell cycle. In the present study, we investigated the effect of mimosine on the expression of eIF3 p170. We found that mimosine treatment of HeLa cells decreased the expression of p170 at translational level. Moreover, experimental manipulations of p170 expression recapitulate the effect of mimosine on translation of mRNAs of p27 and α-tubulin.
MATERIALS AND METHODS
Materials
Monoclonal antibodies against p27 and tyrosinated α-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Accurate Chemical & Scientific (Westbury, NY), respectively. Polyclonal antibody against hPrt 1 (eIF3 p116) was a kind gift from Dr. Max M. Burger (Friedrich Miescher Institute, Basel, Switzerland). The enhanced chemiluminescence system for Western blot analysis was from Amersham Biosciences (Piscataway, NJ). N1-Guanyl-1,7-diaminoheptane (GC-7) was a kind gift of Dr. Myung Hee Park (National Institute of Dental and Craniofacial Research, National Institutes of Health). Mimosine, deferoxamine mesylate (DFO), and ferric ammonium citrate were from Sigma-Aldrich (St. Louis, MO). [35S]Methionine was purchased from PerkinElmer Life Sciences (Boston, MA). Sequi-Blot polyvinylidene difluoride membrane and concentrated protein assay dye reagents were from Bio-Rad (Hercules, CA). Cell culture media and reagents were obtained from Invitrogen (Carlsbad, CA). All other reagents were of molecular biology grade and were purchased from Sigma-Aldrich or Fisher Scientific (Chicago, IL).
Engineering of the p170 Expression Constructs
The p170 cDNA insert (4.7 kb) was released from pGEM4Z vector (Pincheira et al., 2001b) by NotI digestion and ligated into a NotI-linearized pTracer-CMV2 vector (Invitrogen). The orientation of the cDNA in the vector was determined by restriction mapping and confirmed by double-strand DNA sequencing. The construct with cDNA in an antisense orientation was named pTracer-CMV2-P170AS, which was used to generate antisense p170 mRNA to inhibit p170 expression. The 4.7-kb insert was then released from pTracer-CMV2-P170AS by double digestion with NotI and KpnI and cloned into a pCβA vector linearized with the same enzymes. The resulting plasmid with p170 in sense orientation was named pCβA-p170. All constructs were confirmed by DNA sequencing.
Cell Culture, Treatment, and Transfection
HeLa cells were maintained in modified Eagle's medium supplemented with 10% fetal calf serum and in humidified atmosphere of 5% CO2 at 37°C. NIH3T3 cells were cultured in modified Eagle's medium containing 10% donor calf serum and maintained in humidified atmosphere of 10% CO2 at 37°C. HeLa cells were seeded at 6 × 105 cells in 100-mm dishes and grow for 3 d before treated with 600 μM mimosine, 150 μM GC-7, 200 μM DFO, or 400 μM ferric ammonium citrate.
For transient transfection, 1.5 × 106 cells were seeded into 10-cm cell culture dishes and grow for 24 h. The cells were then transfected with 4–10 μg of pCβA-p170 or pCβA vector, pTracer-CMV2-P170AS, or pTracer-CMV2 vector by using LipofectAMINE/Plus reagent. Cells were harvested 36–48 h after transfection for further analysis.
Cell Cycle Analysis by Using Flow Cytometry
Cells were harvested and washed twice with phosphate-buffered saline (PBS). The resuspended cells in PBS were fixed in 80% ethanol for 30 min at room temperature and stored at 4°C. The cells were then collected by centrifugation and stained with 50 μg/ml propidium iodide. The cells were then treated with 100 μg/ml RNase for 15 min at room temperature followed by analysis using a FACScan flow cytometer. Cell cycle distribution was analyzed with the Modfit LT program.
Sample Preparation, Western Blot, and Immunoprecipitation
Cells were lysed in TNN-SDS buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 0.1% SDS, and 2 mM phenylmethylsulfonyl fluoride) at 4°C for 30 min. Lysates were cleared by centrifugation (10,000 × g for 10 min at 4°C), and protein concentrations were determined using the Bradford method (Bradford, 1976) with reagents from Bio-Rad.
Western blot analyses were performed as described previously (Pincheira et al., 2001a). Briefly, cell lysates were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The blot was then probed with affinity-purified polyclonal antibody AbD (1:1000 dilution), actin-specific monoclonal antibody (mAb) (1:3000 dilution), tyrosinated α-tubulin–specific mAb YL1/2 (1:500 dilution), or p27 mAb (1:300 dilution) followed by reaction with horseradish peroxidase-conjugated secondary antibody. The signal was detected using enhanced chemiluminescence.
Immunoprecipitation was performed as follows. SDS and dithiothreitol were added to cell lysates of 200–500 μg of proteins to final concentrations of 0.5% and 10 mM, respectively. The samples were boiled for 15 min, diluted 10-fold with TNN buffer containing 2% bovine serum albumin but without SDS and dithiothreitol, and then mixed with 30 μl of 50% protein G-Sepharose 4B slurry. The mixture was incubated at 4°C for 1 h and centrifuged to remove Sepharose beads together with nonspecifically bound proteins. To the supernatant, 10 μl of affinity-purified AbD to p170 or antibodies to tyrosinated α-tubulin and p27 were added and incubated at 4°C for 3 h before mixing with 30 μl of 50% protein G-Sepharose 4B slurry. The mixture was further incubated for 3 h or overnight at 4°C with agitation. The immunoprecipitate was collected by centrifugation and washed six times with TNN-SDS buffer. The final pellet was solubilized in 15-μl sample buffer for SDS-PAGE.
Pulse and Pulse-Chase Labeling of Cells
Cells were washed twice with PBS and once with DMEM lacking methionine followed by incubation for 2 h in the same medium supplemented with 75 μCi/ml [35S]methionine. The pulse-labeled cells were then washed three times with PBS and harvested for cell lysate preparation and immunoprecipitation. To chase the labeling, the cells were washed twice with PBS and once with DMEM medium after the 2-h pulse labeling. The cells were then cultured in DMEM medium supplemented with 100 μg/ml cold methionine in the absence or presence of 600 μM mimosine up to 8 h. After washing three times with PBS, the cells were harvested for cell lysate preparation and immunoprecipitation.
RNA Extraction, Northern Blot, and Ribonuclease Protection Assay
Cells were harvested and total RNAs were extracted using RNeasy mini kit (QIAGEN, Valencia, CA). For Northern blot analysis, 20 μg of total RNAs was separated by electrophoresis in 1.2% agaroseformaldehyde gels, transferred to nitrocellulose membranes, and probed with cDNAs labeled with 32P by a Rediprime II random prime labeling system (Amersham Biosciences). The hybridization and washing conditions were as described previously (Dong et al., 1998). The blots were then stripped and reprobed with the probe of constitutively expressed human GAPDH gene. RNase protection assay was performed as previously described with 20 μg of total RNA (Dong et al., 1998) by using the RPA-III kit (Ambion, Austin, TX) according to the instructions of the supplier. The constitutively expressed human GAPDH gene obtained from Ambion was used as a control for RNase protection assay.
RESULTS
Effect of Mimosine Treatment on p170 Expression
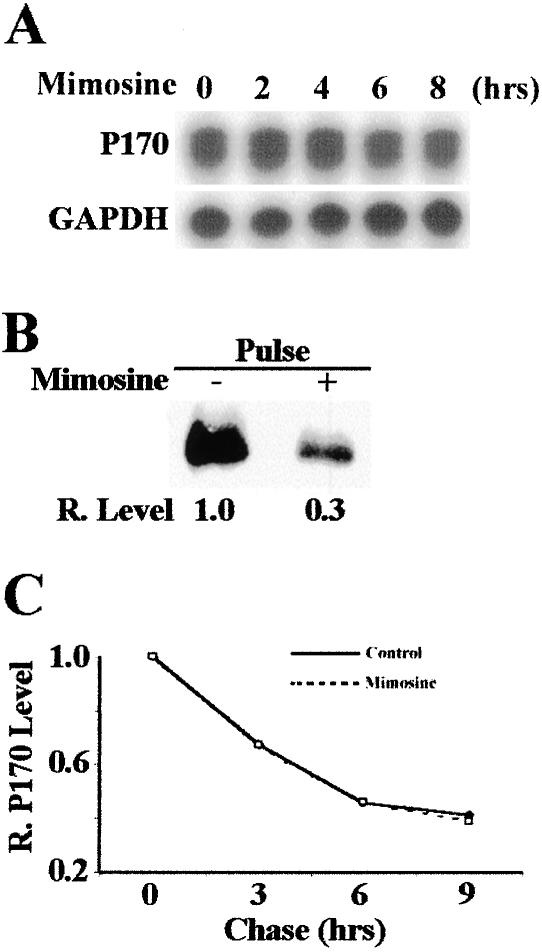
To determine whether cells arrested at G0/G1 phase by mimosine treatment have lower levels of p170, we treated HeLa cells with mimosine for 48 h, which increased the fraction of cells at G0/G1 (Figure 1) and determined p170 protein levels by Western blot analysis. As shown in Figure 1, p170 decreased to about one-third of the level in the untreated control cells after mimosine treatment. To determine whether the p170 decrease was due to G0/G1 arrest, we performed a time-course analysis of p170 level after mimosine treatment. As shown in Figure 2A, p170 level began to decrease at ∼2 h and reached plateau around 6 h after mimosine treatment. However, the cell cycle distribution did not change during the period of 8 h of mimosine treatment (our unpublished data). Thus, the decrease in p170 level occurred before and was not a result of cell cycle arrest. It is noteworthy that the expression level of another eIF3 subunit, hPrt1 (p116), remained the same during the 8-h period of mimosine treatment (Figure 2B), suggesting that mimosine effect does not extend to other eIF3 subunits. Interestingly, the total [35S]methionine incorporation after mimosine treatment began to decrease at 4 h after mimosine treatment and reached a maximum of 50% decrease at 6 h of treatment (Figure 2C). On the other hand, the total [35S]methionine incorporation was dramatically decreased at 2 h after cycloheximide (a known translation inhibitor) treatment and reached a maximum of 90% decrease around 4 h of treatment (our unpublished data). These results suggest that mimosine is not a general protein synthesis inhibitor. It is, thus, possible that mimosine inhibits expression of p170, which then mediates the cell cycle arrest by affecting the synthesis of other proteins.
Figure 1.
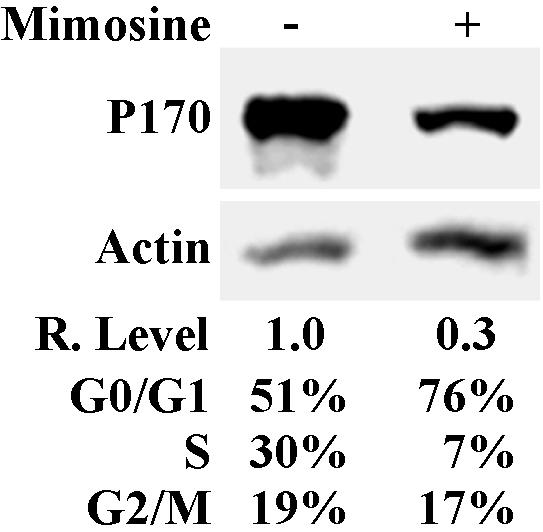
Expression of p170 after mimosine treatment. HeLa cells were treated with 600 μM mimosine for 48 h and were used to determine p170 expression and cell cycle distribution as described in MATERIALS AND METHODS. Actin was used as a loading control. The relative expression level of p170 was calculated based on the density of p170 and actin bands measured using a Scion Image software.
Figure 2.
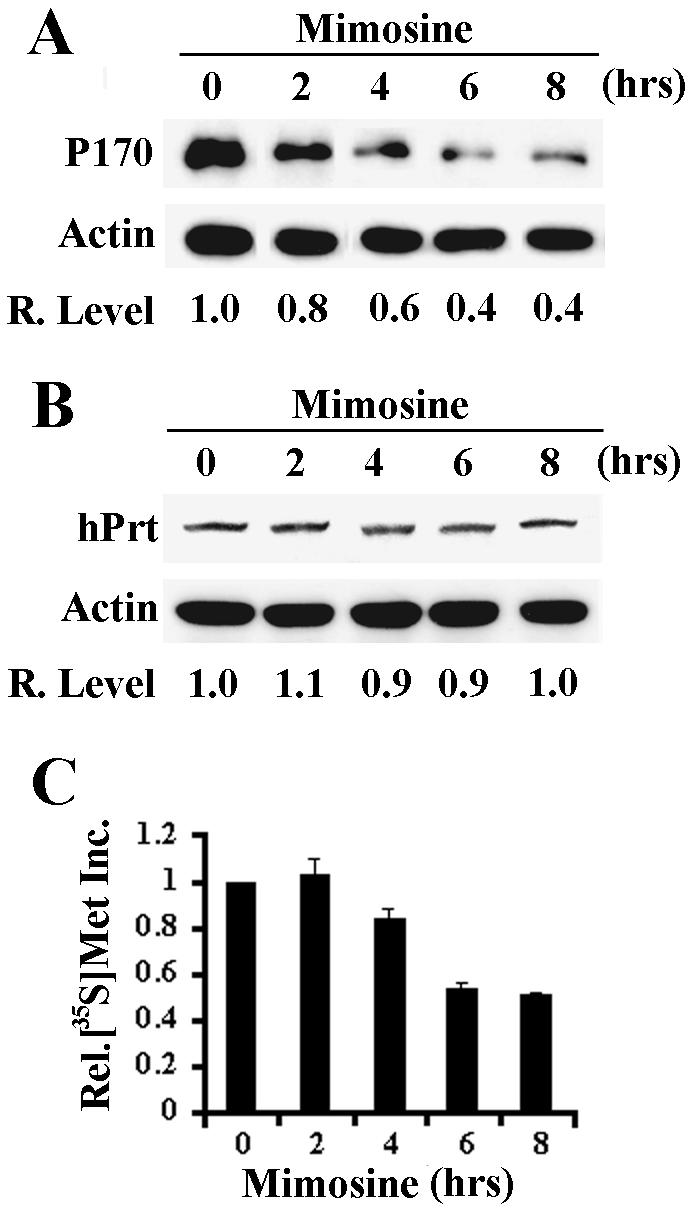
Time course of mimosine effect on protein synthesis. HeLa cells were treated with 600 μM mimosine for different times and then used for analysis of expression of p170 (A) and hPrt 1 (B) by Western blot, and global protein synthesis (C) by [35S]methionine incorporation. Actin was used as a loading control for Western blot. The relative expression levels of p170 in A and hPrt 1 in B were calculated based on the density of p170, hPrt 1 and actin bands measured using a Scion Image software.
Mimosine Inhibits the Synthesis of p170 Protein
To test the above-mentioned hypothesis and to determine at which level mimosine treatment decreases p170 expression, we first performed an RNase protection analysis of p170 mRNA at different time points after mimosine treatment. As shown in Figure 3A, within the 8-h period of mimosine treatment, the steady-state level of p170 mRNA remained the same. This observation suggests that the mimosine effect on p170 expression is not at the RNA level (e.g., transcription or degradation). To determine whether the synthesis of p170 protein is altered by mimosine, we performed a pulse labeling study with [35S]methionine followed by immunoprecipitation of p170. As shown in Figure 3B, p170 protein synthesis after the 8-h mimosine treatment is decreased to about one-third of the untreated cells. To further determine whether mimosine treatment affects the stability of p170 protein, we performed a pulse-chase and immunoprecipitation experiment. As shown in Figure 3C, the degradation rate of p170 protein is the same between cells chased in the absence or presence of mimosine with a half-life around 5 h. Together, these results suggest that mimosine treatment inhibits only the synthesis of p170 protein.
Figure 3.
Effects of mimosine on p170 synthesis. (A) Effect of mimosine on the level of p170 mRNA. HeLa cells were treated with 600 μM mimosine, and total RNAs were prepared for RNase protection analysis of p170 mRNA. GAPDH was used as a control. (B) Effect of mimosine on p170 synthesis. HeLa cells were treated with or without 600 μM mimosine for 6 h followed by a 2-h pulse labeling with [35S]methionine. Labeled p170 was then immunoprecipitated for SDS-PAGE and autoradiography analysis as described in MATERIALS AND METHODS. (C) Effect of mimosine on p170 degradation. HeLa cells were first pulse-labeled with [35S]methionine for 2 h followed by a chase in DMEM supplemented with 100 μg/ml unlabeled methionine in the presence or absence of 600 μM mimosine. P170 was then immunoprecipitated for SDS-PAGE and autoradiography followed by measurement of the p170 level with a Scion Image software.
Mimosine Inhibits p170 Protein Synthesis by Its Iron-chelating Effect
It has been reported that mimosine has two functions as an iron chelator and as an inhibitor of deoxyhypusyl hydroxylase (DOHH) (Hanauske-Abel et al., 1994; Andrus et al., 1998), an enzyme responsible for the second step of synthesis of the unique amino acid, hypusine, in eIF5A. Hypusine is critical for eIF5A function and is formed by a two-step posttranslational modification: synthesis and hydroxylation of deoxyhypusine catalyzed by deoxyhypusine synthase (DHS) and DOHH, respectively (Park et al., 1993a). To investigate whether the effect of mimosine on p170 synthesis is due to its effect on formation of hypusinated eIF5A or its iron-chelating function, we tested the effect on p170 expression of two other compounds: GC-7, a specific inhibitor of DHS (Jakus et al., 1993), and deferoxamine mesylate (DFO), a known iron chelator. As shown in Figure 4A, the 8-h treatment by GC-7 did not change the expression level of p170. Extended treatment for 28 h also did not affect p170 expression (our unpublished data). Thus, the effect of mimosine on p170 synthesis is not due to the inhibition of the hypusinated eIF5A formation. However, 8-h treatment by DFO decreased the expression level of p170 (Figure 4B), similar to that found with mimosine treatment (Figure 2A). Furthermore, addition of exogenous iron can overcome the inhibitory effect of mimosine on the expression of p170 (Figure 4C). Thus, it is likely that the mimosine effect on p170 synthesis is due to its iron-chelating activity.
Figure 4.
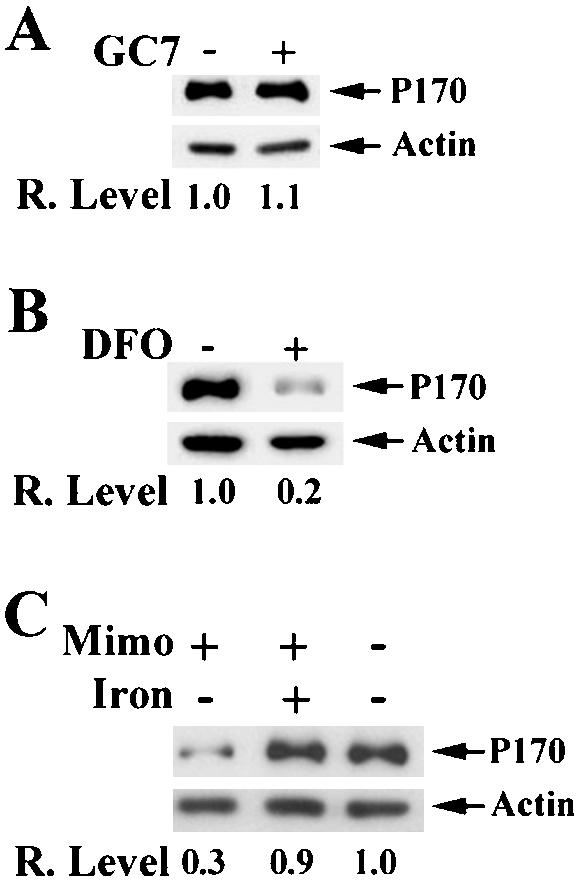
Effect of iron level on p170 expression. HeLa cells were cultured with or without 150 μM GC-7 (A), 200 μM DFO (B), or 400 μM ferric ammonium citrate in combination with mimosine (C) followed by determination of p170 level by using Western blot as described in MATERIALS AND METHODS. Actin was used as a loading control. The relative expression levels of p170 were calculated based on the density of p170 and actin bands measured using a Scion Image software.
P170 Mediates the Effect of Mimosine on α-Tubulin Expression
To test the hypothesis that p170 is an early mediator for the effect of mimosine on cell cycle arrest, we tested whether the changed level of p170 expression will affect the synthesis of other proteins. It has been shown previously that the human p170 and its yeast analog bind to microtubule (Hasek et al., 2000; Shanina et al., 2001) and that human p170 coprecipitates with tyrosinated α-tubulin (our unpublished data). These observations suggest that p170 may interact with tyrosinated α-tubulin, the primary product translated from α-tubulin mRNA.
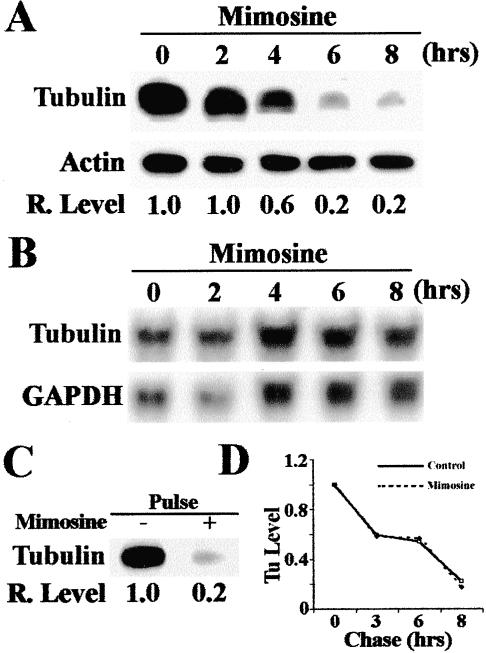
It has been shown previously that the translation of α-tubulin is regulated by a feedback mechanism (Gonzalez-Garay and Cabral, 1996). Overexpression of α-tubulin inhibits its own translation. This finding implies that α-tubulin may interact with translation apparatus that in turn may regulate the translation of its own mRNA. The binding of α-tubulin to p170 may play a role in regulating α-tubulin translation. Thus, we examined the expression of α-tubulin to determine whether it can be influenced by mimosine, and thus by expression of p170. Because only the tyrosinated α-tubulin represents the newly synthesized α-tubulin, we examined only tyrosinated α-tubulin in this study. As shown in Figure 5, the level of tyrosinated α-tubulin, unlike p170, began to decrease at 4 h after mimosine treatment (Figure 5A, also compare to Figure 2 for p170). Similar to p170, the mRNA level of α-tubulin did not change after mimosine treatment (Figure 5B). Thus, the effect of mimosine on tyrosinated α-tubulin level must be at the protein level.
Figure 5.
Effect of mimosine on the expression of tyrosinated α-tubulin. (A) Time course of the effect of mimosine on the expression of tyrosinated α-tubulin. HeLa cells were treated with 600 μM mimosine for 0–8 h and then lysed for determination of the expression of tyrosinated α-tubulin by Western blot. Actin was used as loading control. (B) Northern blot analysis of mRNA level of α-tubulin after mimosine treatment. HeLa cells were treated with 600 μM mimosine and total RNAs were prepared for Northern blot analysis of α-tubulin. GAPDH was used as a loading control. (C) Effect of mimosine on the synthesis of tyrosinated α-tubulin. HeLa cells were treated with or without 600 μM mimosine for 6 h followed by a 2-h pulse labeling with [35S]methionine. Labeled tyrosinated α-tubulin was then immunoprecipitated for SDS-PAGE and autoradiography analysis as described in MATERIALS AND METHODS. (D) Effect of mimosine on the degradation of tyrosinated α-tubulin. HeLa cells were first pulse-labeled with [35S]methionine for 2 h followed by a chase in DMEM supplemented with 100 μg/ml unlabeled methionine in the presence or absence of 600 μM mimosine. Tyrosinated α-tubulin was then immunoprecipitated for SDS-PAGE and autoradiography followed by measurement of tyrosinated α-tubulin level with a Scion Image software.
We next examined whether the mimosine effect is on generation or degradation of tyrosinated α-tubulin by performing pulse and pulse-chase experiments as described above. Tyrosinated α-tubulin can be generated by de novo synthesis and retyrosination of the existing detyrosinated α-tubulin. However, only the de novo synthesized tyrosinated α-tubulin will be labeled by the pulse and pulse-chase labeling procedure. Degradation of tyrosinated α-tubulin reflects both detyrosination and degradation. As shown in Figure 5C, the pulse labeling of tyrosinated α-tubulin in the presence of mimosine is much less than that in the absence of mimosine. However, the degradation of tyrosinated α-tubulin is the same in the absence or presence of mimosine (Figure 5D). Thus, the effect of mimosine on the expression of tyrosinated α-tubulin is likely at the translational level.
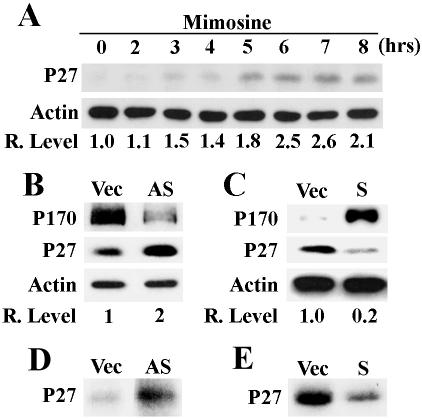
To determine whether p170 mediates the effect of mimosine on tyrosinated α-tubulin expression, we took advantage that the p170 expression level can be altered by transient transfection of antisense or sense p170 cDNA. Previously, it has been found that p170 expression level is high in tumor cell lines such as HeLa (Pincheira et al., 2001b) and low or undetectable in nontumorigenic cells such as NIH3T3 (Bachmann et al., 1997). In this study, the p170 level was up-regulated in NIH3T3 cells and down-regulated in HeLa cells by transient transfection of sense and antisense p170 cDNA, respectively, and the effect of these alterations on the level of tyrosinated α-tubulin was determined. As shown in Figure 6A, down-regulating p170 expression in HeLa cells by antisense p170 also decreased the level of tyrosinated α-tubulin. On the other hand, up regulating p170 expression in NIH3T3 cells also increased the level of tyrosinated α-tubulin (Figure 6B). However, it is noteworthy that the level of effect of antisense p170 cDNA on p170 expression is different from mimosine effect. In addition, the pulse labeling experiment showed that the synthesis of tyrosinated α-tubulin decreased dramatically in HeLa cells by down-regulating p170 expression (Figure 6C), while it increased dramatically in NIH3T3 cell by up-regulating p170 expression (Figure 6D). Thus, it is likely that the effect of mimosine on the de novo synthesis of tyrosinated α-tubulin is mediated by p170. We also determined the effect of p170 level on global protein synthesis and found that down regulating the expression of p170 in HeLa cells by antisense p170 cDNA caused 15–20% decrease in the global protein synthesis, whereas up-regulating p170 expression in NIH3T3 cells by sense p170 cDNA caused 40–50% increase in global protein synthesis. These results imply that p170 may be only responsible for the synthesis of some proteins and not essential for global protein synthesis, consistent with the previous report that p170 may not be essential for the formation of preinitiation complex in the initiation of general translation (Chaudhuri et al., 1997).
Figure 6.
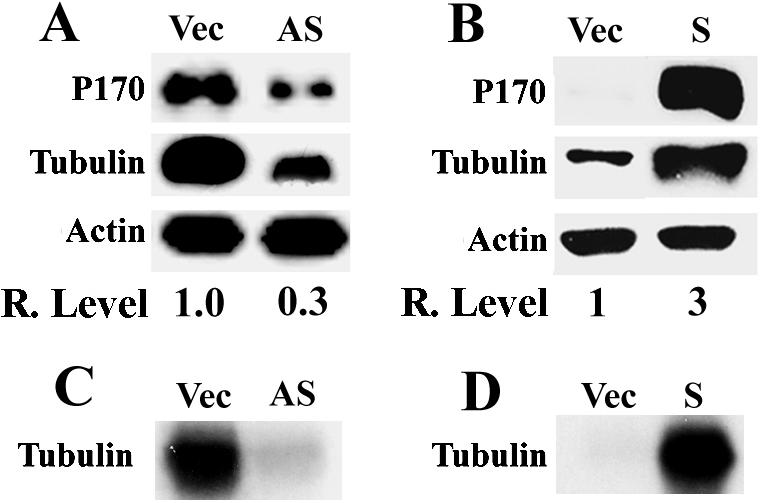
Effect of altering p170 expression level on the expression of tyrosinated α-tubulin. Down-regulating p170 expression in HeLa cells (A and C) and up-regulating p170 expression in NIH3T3 cells (B and D) were achieved by transient transfection with antisense (AS) and sense (S) p170 cDNA, respectively. The effect of the change in p170 expression on the expression level (A and B) and synthesis (C and D) of tyrosinated α-tubulin was determined using Western blot and pulse labeling, respectively, as described in MATERIALS AND METHODS. Vector-transfected cells (Vec) were used as controls for transfection. Actin was used as a loading control for Western blot. The relative expression levels of tyrosinated α-tubulin (A and B) were calculated based on the density of tyrosinated α-tubulin and actin bands measured using a Scion Image software.
P170 Mediates the Effect of Mimosine on p27 Expression
To rule out the possibility that the up- and down-regulation of p170 expression up- and down-regulate the general translation, respectively, and, thus, the synthesis of tyrosinated α-tubulin, we tested whether p170 can change the synthesis of proteins in opposite directions. That is, up- or down-regulation of p170 would down- and up-regulate, respectively, the synthesis of other proteins. For this purpose, we searched the literature and found that mimosine treatment increased the level of p27 expression at the translational level (Wang et al., 2000) and decided to use p27 as a model. As shown in Figure 7A, mimosine treatment increased the level of p27 protein. It seems that the increase in p27 level occurs around 3 h after the treatment.
Figure 7.
Effect of mimosine and p170 on p27 expression. (A) Time course of the effect of mimosine on p27 expression. HeLa cells were treated with 600 μM mimosine for 0–8 h and then lysed for determination of p27 expression using Western blot. (B–E) Effect of p170 level on p27 expression (B and C) and synthesis (D and E). Down-regulating p170 expression in HeLa cells (A and C) and up-regulating p170 expression in NIH3T3 cells (B and D) were achieved by transient transfection with antisense (AS) and sense (S) p170 cDNA, respectively. The effect of the change of p170 level on p27 expression (B and C) and synthesis (D and E) was determined using Western blot and pulse labeling, respectively, as described in MATERIALS AND METHODS. Vector-transfected cells (Vec) were used as controls for transfection. Actin was used as a loading control for Western blot. The relative expression levels of p27 (A–C) were calculated based on the density of p27 and actin bands measured using a Scion Image software.
We next tested whether the mimosine effect on p27 synthesis is also mediated by p170 by using the transient transfection strategy as described above. As shown in Figure 7B, 48 h after transfection, the level of p170 was significantly reduced, whereas that of p27 increased approximately two-fold in the antisense p170-transfected HeLa cells in comparison with the vector control. Similarly, p170 level increased while p27 level decreased in the p170-overexpressing NIH3T3 cells compared with the vector control (Figure 7C). Furthermore, using the pulse-labeling study we found that the synthesis of p27 protein increased in HeLa cells by down-regulating the expression level of p170 (Figure 7D), whereas the global protein synthesis decreased ∼15–20%. On the other hand, up-regulating p170 in NIH 3T3 cells decreased the synthesis of p27 protein (Figure 7E), whereas the global protein synthesis increased ∼40–50%. These observations suggest that mimosine effect on p27 synthesis is likely mediated by p170 and that p170, rather than as a general translation initiation factor, may function as a regulator for protein synthesis.
Effect of DFO on Expression of p27 and α-Tubulin
The above-mentioned studies showed that p170 can mediate the effect of mimosine on the synthesis of α-tubulin and p27 and that mimosine down-regulates the expression level of p170 by its iron-chelating activity. We next investigated the effect of iron chelator DFO on the expression of α-tubulin and p27. As shown in Figure 8, treatment of HeLa cells with DFO for 8 h decreased the expression of α-tubulin, whereas it increased the expression of p27. This effect is similar to that of mimosine and consistent with results reported previously that DFO-arrested cells in G1 phase as mimosine (Yoon et al., 2002). Therefore, one of the possible mechanisms for mimosine and DFO to regulate protein synthesis and cell cycle progression is by down-regulating the expression level of p170 through iron chelation, which then influences the expression of other genes, such as p27 and α-tubulin.
Figure 8.
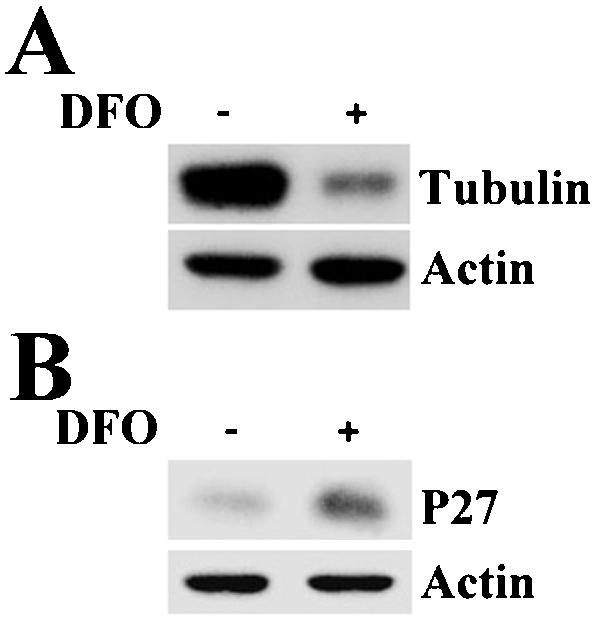
Effect of DFO on α-tubulin and p27 expression. HeLa cells were treated with or without 200 μM DFO for 8 h and then harvested for determination of the expression level of tyrosinated α-tubulin (A) and p27 (B) by using Western blot.
DISCUSSION
In this study, we showed that the expression level of eIF3 p170 can be modulated by mimosine at the translational level. The mimosine effect on p170 synthesis is likely due to its iron-chelating activity. P170 seems to be an early response protein to the effect of mimosine and is likely a mediator of mimosine effect on the synthesis of other proteins and on cell cycle control. Our findings that p170 exerted opposite effects on the synthesis of p27 versus tyrosinated α-tubulin suggest that p170 is an important regulator for protein synthesis.
It has been suggested previously that mimosine can modulate the expression of specific genes at the translational level (Hanauske-Abel et al., 1995; Kalejta and Hamlin, 1997) and this modulation may be due to its inhibition on formation of hypusinated eIF5A (McCaffrey et al., 1995). The hypusine residue of eIF5A is critical to its function (Park et al., 1993a,b). Mimosine is an inhibitor of DOHH, essential for hypusine formation, and consequently inhibits the formation of hypusine and thus the function of eIF5A (Hanauske-Abel et al., 1994; Andrus et al., 1998), which has been reported to be critical for cell growth and survival (Schnier et al., 1991; Park et al., 1998). Interestingly, there was only 30% decrease in global protein synthesis in yeast with eIF5A depleted (Kang and Hershey, 1994). Thus, eIF5A may be responsible for controlling the translation of a subset of mRNAs (Park et al., 1993a; Hanauske-Abel et al., 1994; Park et al., 1997). However, we found that GC7, a specific inhibitor of DHS, another essential enzyme for hypusine formation (Jakus et al., 1993), did not alter the expression level of p170. Therefore, it seems that the inhibition of p170 translation by mimosine is not due to the inhibition of formation of hypusinated eIF5A.
Treatment of cells with high concentrations of mimosine inhibits global protein synthesis by up to 50%. Conversely, p27 synthesis increased after mimosine treatment. Thus, mimosine is not a general protein synthesis inhibitor and the decrease in p170 synthesis after mimosine treatment is less likely due to a global inhibition effect. It also seems that the decreased expression of p170 after mimosine treatment was not secondary to G0/G1 cell cycle arrest because p170 levels decreased before cell cycle arrest.
Mimosine is also an iron chelator (Kontoghiorghes and Evans, 1985; Kulp and Vulliet, 1996). Iron chelators, such as DFO, have been reported to inhibit cell growth by modulating gene expression and causing G1 arrest (Yoon et al., 2002). Therefore, one possible mechanism of mimosine inhibition of p170 synthesis may be related to its iron chelation function. We found that DFO indeed inhibited p170 expression similarly to mimosine. Furthermore, addition of exogenous iron into culture medium overcame the mimosine inhibitory effect on p170 expression. These observations suggest that iron is important for the translation of p170 mRNA. Although it is currently unknown how iron is involved in regulating the translation of p170 mRNA, it is tempting to propose that the 5′-untranslated region (UTR) sequence of p170 mRNA contains an iron-responsive sequence. However, analysis of the 5′-UTR sequence of p170 mRNA (Pincheira et al., 2001b) showed no consensus iron response loop sequence as described for ferritin mRNA (Rouault et al., 1996), although this does not rule out a potentially different iron response stem-loop structure in the 5′-UTR sequence as described for the 5′-UTR sequence of Alzheimer's amyloid precursor protein transcript (Rogers et al., 2002). Clearly, further investigations are needed to understand the role of iron in regulating p170 translation and to elucidate the mechanism of mimosine action.
From the time-course study, it seems that p170 expression was changed before tyrosinated α-tubulin and p27. This suggests that p170 mRNA may be an early mimosine response gene, which in turn regulates the translation of other mRNAs. This hypothesis is supported by the observation that experimentally modulating p170 expression by transient transfection of p170 cDNA changed the synthesis of tyrosinated α-tubulin and p27. It has also been shown previously that the synthesis of p27 protein, but not the mRNA, in HL60 cells was increased after TPA treatment (Millard et al., 1997). We likewise found that the expression level of p170 deceased after TPA treatment (our unpublished observation). The correlation of decreased p170 expression concomitant with increased p27 synthesis after TPA treatment is consistent with the conclusion that p170 may directly regulate p27 synthesis.
Although p170 has been suggested to be a major subunit of eIF3 complex, its role in initiation of general translation is not clear. EIF3 preparations rich in p170 did not differ from preparations that essentially lacked this protein in specific activity (Chaudhuri et al., 1997), suggesting that p170 may not be essential for the formation of preinitiation complex in the initiation of general translation. One potential function of p170 is to act as a regulator for translation of a specific subset of mRNAs as proposed for eIF5A (Park et al., 1993a; Hanauske-Abel et al., 1994; Park et al., 1997). Our findings that experimentally altering p170 levels directly affected synthesis of p27 and tyrosinated α-tubulin in a reciprocal manner supports the above-mentioned hypothesis and argues against the possibility that p170 plays a general role in translation initiation. It is also noteworthy that mimosine treatment inhibits global protein synthesis by 50%, whereas severe depletion of eIF5A decreased global protein synthesis by 30% (Kang and Hershey, 1994). Because mimosine affects both the formation of hypusinated eIF5A and the expression of p170, it is tempting to propose that p170 is responsible for regulating 20% of global protein synthesis. Consistently, we found that decreasing p170 expression in HeLa cells by antisense p170 cDNA decreased global protein synthesis by 15–20%.
The results from this study also suggest that mimosine arrests cell cycle through at least two pathways by regulating translation of mRNAs. In addition to the known pathway by inhibiting the formation of hypusinated eIF5A, which in turn possibly inhibits translation of a subset of mRNAs, mimosine also inhibits p170 synthesis, which also regulates translation of a subset of mRNAs. The observation that prolonged treatment of cells with mimosine only inhibits 50% of global protein synthesis supports the above-mentioned argument. The subsets of mRNAs regulated by mimosine through eIF5A and eIF3 p170 potentially encode proteins, such as p27, important for cell cycle progression. However, how eIF3 p170 and eIF5A regulate specifically the translation of these subsets of mRNAs is not clear. It is possible that p170 can bind to the 5′-UTR of mRNAs and different secondary structures of the 5′-UTR sequence have different responses to p170 level. Although p170 does not have an obvious RNA-binding motif, it has been shown that p170 can bind to mRNAs (Block et al., 1998; Buratti et al., 1998). It has also been reported that the translation of p27 mRNAs is regulated (Agrawal et al., 1996; Hengst and Reed, 1996; Millard et al., 1997), and the U-rich element in the 5′-UTR sequence is necessary for the translational regulation (Millard et al., 2000). The proteins that directly interact with this U-rich element have been identified by cross-linking experiments to be HuR and hnRNP C1/C2 (Millard et al., 2000). Whether p170 is involved in binding to this U-rich sequence in the hnRNP complex remains to be determined. It is also possible that p170 indirectly controls p27 translation by regulating translation of mRNAs of the hnRNP complex.
The finding that p170 regulates the de novo synthesis of tyrosinated α-tubulin is interesting. It has been postulated that eIF3 p170 binds to tubulin (Bachmann et al., 1997; Scholler and Kanner, 1997). Indeed, it has recently been shown that the yeast homolog of mammalian eIF3 p170, Rpg1p, bound to microtubules and coimmmunoprecipitated with α-tubulin (Hasek et al., 2000) and to actin-associated protein Sla2p (Palecek et al., 2001). Mammalian eIF3 p170 was also found in microtubule preparations (Shanina et al., 2001). We also found that human p170 could coimmunoprecipitate with tyrosinated α-tubulin (our unpublished observations). In addition, mammalian eIF3 p170 has been found to interact with membrane-bound microfilament (Pincheira et al., 2001a), intermediate filament protein K7 (Lin et al., 2001), and nerve growth factor receptor tyrosine kinase TrkA (MacDonald et al., 1999). These findings suggest that p170 may have functions in addition to its putative role in translation initiation. One possible function of p170 may be in controlling localized synthesis of proteins. For example, regulated synthesis of tyrosinated α-tubulin on microtubules will provide immediate control of α-tubulin level for microtubule formation. α-Tubulin is known to have a feedback inhibition mechanism of its de novo synthesis (Gonzalez-Garay and Cabral, 1996). P170, by binding to tyrosinated α-tubulin that normally exists as dimers and not found in microtubule, may mediate the feedback mechanism to regulate the synthesis and, thus, the level of α-tubulin and microtubule.
Currently, it is not known what other mRNAs are under p170 control for translation. However, translation of some mRNAs known to be affected by mimosine may be mediated by p170. It has been reported that mimosine inhibited the duplication of centrosomes in mammalian cells (Matsumoto et al., 1999). Because γ-tubulin is a conserved component of the centrosome and is required for the initiation of microtubule assembly (Jeng and Stearns, 1999; Schiebel, 2000) and because p170 contains a centrosomin domain (Pincheira et al., 2001b), it is possible that p170 also controls the translation of γ-tubulin. Recently, P-glycoprotein expression was found to increase after mimosine treatment of prostate cancer cells (Wartenberg et al., 2002). Thus, the translation of P-glycoprotein mRNA may be under p170 control.
Acknowledgments
We thank Dr. Myung Hee Park for GC7 and Dr. Max M. Burger for the polyclonal antibody against hPrt 1. Technical assistance from Min Choi and proofreading of this manuscript and suggestions by Dr. Martin Smith are very much appreciated. This work was supported in part by the National Institutes of Health grants CA-64539 and GM-59475 and by the Department of Defense grant DAMD17-02-1-0073. J.-T.Z. is a recipient of a Career Investigator Award from the American Lung Association.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–12–0784. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0784.
References
- Agrawal, D., Hauser, P., McPherson, F., Dong, F., Garcia, A., and Pledger, W.J. (1996). Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol. Cell. Biol. 16, 4327–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrus, L., Szabo, P., Grady, R.W., Hanauske, A.R., Huima-Byron, T., Slowinska, B., Zagulska, S., and Hanauske-Abel, H.M. (1998). Antiretroviral effects of deoxyhypusyl hydroxylase inhibitors: a hypusine-dependent host cell mechanism for replication of human immunodeficiency virus type 1 (HIV-1). Biochem. Pharmacol. 55, 1807–1818. [DOI] [PubMed] [Google Scholar]
- Bachmann, F., Banziger, R., and Burger, M.M. (1997). Cloning of a novel protein overexpressed in human mammary carcinoma. Cancer Res. 57, 988–994. [PubMed] [Google Scholar]
- Benne, R., and Hershey, J.W. (1976). Purification and characterization of initiation factor IF-E3 from rabbit reticulocytes. Proc. Natl. Acad. Sci. USA 73, 3005–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, K.L., Vornlocher, H.P., and Hershey, J.W. (1998). Characterization of cDNAs encoding the p44 and p35 subunits of human translation initiation factor eIF3. J. Biol. Chem. 273, 31901–31908. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Buratti, E., Tisminetzky, S., Zotti, M., and Baralle, F.E. (1998). Functional analysis of the interaction between HCV 5′UTR and putative subunits of eukaryotic translation initiation factor eIF3. Nucleic Acids Res. 26, 3179–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, J., Chakrabarti, A., and Maitra, U. (1997). Biochemical characterization of mammalian translation initiation factor 3 (eIF3). Molecular cloning reveals that p110 subunit is the mammalian homologue of Saccharomyces cerevisiae protein Prt1. J. Biol. Chem. 272, 30975–30983. [DOI] [PubMed] [Google Scholar]
- Chen, G., and Burger, M.M. (1999). p150 expression and its prognostic value in squamous-cell carcinoma of the esophagus. Int. J. Cancer 84, 95–100. [DOI] [PubMed] [Google Scholar]
- Dellas, A., Torhorst, J., Bachmann, F., Banziger, R., Schultheiss, E., and Burger, M.M. (1998). Expression of p150 in cervical neoplasia and its potential value in predicting survival. Cancer 83, 1376–1383. [DOI] [PubMed] [Google Scholar]
- Dong, Z., Wang, X., Zhao, Q., Townsend, C.M., Jr., and Evers, B.M. (1998). DNA methylation contributes to expression of the human neurotensin/neuromedin N gene. Am. J. Physiol. 274, G535–G543. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garay, M.L., and Cabral, F. (1996). alpha-Tubulin limits its own synthesis: evidence for a mechanism involving translational repression. J. Cell Biol. 135, 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauske-Abel, H.M., Park, M.H., Hanauske, A.R., Popowicz, A.M., Lalande, M., and Folk, J.E. (1994). Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim. Biophys. Acta 1221, 115–124. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel, H.M., Slowinska, B., Zagulska, S., Wilson, R.C., Staiano-Coico, L., Hanauske, A.R., McCaffrey, T., and Szabo, P. (1995). Detection of a sub-set of polysomal mRNAs associated with modulation of hypusine formation at the G1-S. boundary. Proposal of a role for eIF-5A. in onset of DNA replication. FEBS Lett. 366, 92–98. [DOI] [PubMed] [Google Scholar]
- Hasek, J., Kovarik, P., Valasek, L., Malinska, K., Schneider, J., Kohlwein, S.D., and Ruis, H. (2000). Rpg1p, the subunit of the Saccharomyces cerevisiae eIF3 core complex, is a microtubule-interacting protein. Cell Motil. Cytoskeleton 45, 235–246. [DOI] [PubMed] [Google Scholar]
- Hengst, L., and Reed, S.I. (1996). Translational control of p27Kip1 accumulation during the cell cycle. Science 271, 1861–1864. [DOI] [PubMed] [Google Scholar]
- Hershey, J.W., Asano, K., Naranda, T., Vornlocher, H.P., Hanachi, P., and Merrick, W.C. (1996). Conservation and diversity in the structure of translation initiation factor EIF3 from humans and yeast. Biochimie 78, 903–907. [DOI] [PubMed] [Google Scholar]
- Hershey, J.W.B., and Merrick, W.C. (2000). Pathway and mechanism of initiation of protein synthesis. In: Translational Control of Gene Expression, ed. N. Sonenberg, J.W.B. Hershey, and M. B. Mathews, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 33–88.
- Hughes, T.A., and Cook, P.R. (1996). Mimosine arrests the cell cycle after cells enter S-phase. Exp. Cell Res. 222, 275–280. [DOI] [PubMed] [Google Scholar]
- Jakus, J., Wolff, E.C., Park, M.H., and Folk, J.E. (1993). Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J. Biol. Chem. 268, 13151–13159. [PubMed] [Google Scholar]
- Jeng, R., and Stearns, T. (1999). gamma-Tubulin complexes: size does matter. Trends Cell Biol. 9, 339–342. [DOI] [PubMed] [Google Scholar]
- Kalejta, R.F., and Hamlin, J.L. (1997). The dual effect of mimosine on DNA replication. Exp. Cell Res. 231, 173–183. [DOI] [PubMed] [Google Scholar]
- Kang, H.A., and Hershey, J.W. (1994). Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J. Biol. Chem. 269, 3934–3940. [PubMed] [Google Scholar]
- Kontoghiorghes, G.J., and Evans, R.W. (1985). Site specificity of iron removal from transferrin by alpha-ketohydroxypyridine chelators. FEBS Lett. 189, 141–144. [DOI] [PubMed] [Google Scholar]
- Kulp, K.S., and Vulliet, P.R. (1996). Mimosine blocks cell cycle progression by chelating iron in asynchronous human breast cancer cells. Toxicol. Appl. Pharmacol. 139, 356–364. [DOI] [PubMed] [Google Scholar]
- Lalande, M. (1990). A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp. Cell Res. 186, 332–339. [DOI] [PubMed] [Google Scholar]
- Lin, L., Holbro, T., Alonso, G., Gerosa, D., and Burger, M.M. (2001). Molecular interaction between human tumor marker protein p150, the largest subunit of eIF3, and intermediate filament protein K7. J. Cell. Biochem. 80, 483–490. [DOI] [PubMed] [Google Scholar]
- MacDonald, J.I., Verdi, J.M., and Meakin, S.O. (1999). Activity-dependent interaction of the intracellular domain of rat trkA with intermediate filament proteins, the beta-6 proteasomal subunit, Ras-GRF1, and the p162 subunit of eIF3. J. Mol. Neurosci. 13, 141–158. [DOI] [PubMed] [Google Scholar]
- Matsumoto, Y., Hayashi, K., and Nishida, E. (1999). Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 9, 429–432. [DOI] [PubMed] [Google Scholar]
- McCaffrey, T.A., et al. (1995). Specific inhibition of eIF-5A. and collagen hydroxylation by a single agent. Antiproliferative and fibrosuppressive effects on smooth muscle cells from human coronary arteries. J. Clin. Investig. 95, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick, W.C. (1979). Purification of protein synthesis initiation factors from rabbit reticulocytes. Methods Enzymol. 60, 101–108. [DOI] [PubMed] [Google Scholar]
- Methot, N., Rom, E., Olsen, H., and Sonenberg, N. (1997). The human homologue of the yeast Prt1 protein is an integral part of the eukaryotic initiation factor 3 complex and interacts with p170. J. Biol. Chem. 272, 1110–1116. [DOI] [PubMed] [Google Scholar]
- Methot, N., Song, M.S., and Sonenberg, N. (1996). A region rich in aspartic acid, arginine, tyrosine, and glycine (DRYG) mediates eukaryotic initiation factor 4B (eIF4B) self-association and interaction with eIF3. Mol. Cell. Biol. 16, 5328–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard, S.S., Vidal, A., Markus, M., and Koff, A. (2000). A U-rich element in the 5′ untranslated region is necessary for the translation of p27 mRNA. Mol. Cell. Biol. 20, 5947–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard, S.S., Yan, J.S., Nguyen, H., Pagano, M., Kiyokawa, H., and Koff, A. (1997). Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J. Biol. Chem. 272, 7093–7098. [DOI] [PubMed] [Google Scholar]
- Mosca, P.J., Dijkwel, P.A., and Hamlin, J.L. (1992). The plant amino acid mimosine may inhibit initiation at origins of replication in Chinese hamster cells. Mol. Cell. Biol. 12, 4375–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek, J., Hasek, J., and Ruis, H. (2001). Rpg1p/Tif32p, a subunit of translation initiation factor 3, interacts with actin-associated protein Sla2p. Biochem. Biophys. Res. Commun. 282, 1244–1250. [DOI] [PubMed] [Google Scholar]
- Park, M.H., Joe, Y.A., and Kang, K.R. (1998). Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 273, 1677–1683. [DOI] [PubMed] [Google Scholar]
- Park, M.H., Lee, Y.B., and Joe, Y.A. (1997). Hypusine is essential for eukaryotic cell proliferation. Biol. Signals 6, 115–123. [DOI] [PubMed] [Google Scholar]
- Park, M.H., Wolff, E.C., and Folk, J.E. (1993a). Hypusine: its posttranslational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors 4, 95–104. [PubMed] [Google Scholar]
- Park, M.H., Wolff, E.C., and Folk, J.E. (1993b). Is hypusine essential for eukaryotic cell proliferation? Trends Biochem. Sci. 18, 475–479. [DOI] [PubMed] [Google Scholar]
- Pincheira, R., Chen, Q., Huang, Z., and Zhang, J.T. (2001a). Two subcellular localizations of eIF3 p170 and its interaction with membrane-bound microfilaments: implications for alternative functions of p170. Eur. J. Cell Biol. 80, 410–418. [DOI] [PubMed] [Google Scholar]
- Pincheira, R., Chen, Q., and Zhang, J.T. (2001b). Identification of a 170-kDa protein over-expressed in lung cancers. Br. J. Cancer 84, 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, J.T., et al. (2002). An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J. Biol. Chem. 277, 45518–45528. [DOI] [PubMed] [Google Scholar]
- Rouault, T.A., Klausner, R.D., and Harford, J.B. (1996). Translational control of ferritin. In: Translational Control, ed. J.W.B. Hershey, M.B. Mathews, and N. Sonenberg, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 335–362.
- Schiebel, E. (2000). gamma-Tubulin complexes: binding to the centrosome, regulation and microtubule nucleation. Curr. Opin. Cell Biol. 12, 113–118. [DOI] [PubMed] [Google Scholar]
- Schnier, J., Schwelberger, H.G., Smit-McBride, Z., Kang, H.A., and Hershey, J.W. (1991). Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 3105–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler, J.K., and Kanner, S.B. (1997). The human p167 gene encodes a unique structural protein that contains centrosomin A homology and associates with a multicomponent complex. DNA Cell Biol. 16, 515–531. [DOI] [PubMed] [Google Scholar]
- Shanina, N.A., Ivanov, P.A., Chudinova, E.M., Severin, F.F., and Nadezhdina, E.S. (2001). [Translation initiation factor eIF3 is able to bind with microtubules in mammalian cells]. Mol. Biol. 35, 638–646. [PubMed] [Google Scholar]
- Trachsel, H., Erni, B., Schreier, M.H., and Staehelin, T. (1977). Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J. Mol. Biol. 116, 755–767. [DOI] [PubMed] [Google Scholar]
- Tsai, W.C., and Ling, K.H. (1971). Toxic action of mimosine. I. Inhibition of mitosis and DNA synthesis of H. Ep-2 cell by mimosine and 3,4-dihydroxypyridine. Toxicon 9, 241–247. [DOI] [PubMed] [Google Scholar]
- Wang, G., Miskimins, R., and Miskimins, W.K. (2000). Mimosine arrests cells in G1 by enhancing the levels of p27(Kip1). Exp. Cell Res. 254, 64–71. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Zhao, J., Clapper, J., Martin, L.D., Du, C., DeVore, E.R., Harkins, K., Dobbs, D.L., and Benbow, R.M. (1995). Mimosine differentially inhibits DNA replication and cell cycle progression in somatic cells compared to embryonic cells of Xenopus laevis. Exp. Cell Res. 217, 84–91. [DOI] [PubMed] [Google Scholar]
- Wartenberg, M., Fischer, K., Hescheler, J., and Sauer, H. (2002). Modulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by cell cycle inhibitors. Biochim. Biophys. Acta 1589, 49–62. [DOI] [PubMed] [Google Scholar]
- Yoon, G., Kim, H.J., Yoon, Y.S., Cho, H., Lim, I.K., and Lee, J.H. (2002). Iron chelation-induced senescence-like growth arrest in hepatocyte cell lines: association of transforming growth factor beta1 (TGF-beta1)-mediated p27Kip1 expression. Biochem. J. 366, 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]