Abstract
Ubiquitin-mediated proteolysis by the proteasome is a critical regulatory mechanism controlling many biological processes. In particular, SKP1, cullin/CDC53, F-box protein (SCF) complexes play important roles in selecting substrates for proteolysis by facilitating the ligation of ubiquitin to specific proteins. In plants, SCF complexes have been found to regulate auxin responses and jasmonate signaling and may be involved in several other processes, such as flower development, circadian clock, and gibberellin signaling. Although 21 Skp1-related genes, called Arabidopsis-SKP1-like (ASK), have been uncovered in the Arabidopsis genome, ASK1 is the only gene that has been analyzed genetically. As a first step toward understanding their functions, we tested for expression of 20 ASK genes using reverse transcription-polymerase chain reaction experiments. Also, we examined the expression patterns of 11 ASK genes by in situ hybridizations. The ASK genes exhibit a spectrum of expression levels and patterns, with a large subset showing expression in the flower and/or fruit. In addition, the ASK genes that have similar sequences tend to have similar expression patterns. On the basis of the expression results, we selectively suppressed the expression of a few ASK genes using RNA interference. Compared with the ask1 mutant, the strong ASK1 RNA interference (RNAi) line exhibited similar or enhanced phenotypes in both vegetative and floral development, whereas ASK11 RNAi plants had normal vegetative growth but mild defects in flower development. The diverse expression patterns and distinct defects observed in RNAi plants suggest that the ASK gene family may collectively perform a range of functions and may regulate different developmental and physiological processes.
Selective proteolysis of proteins has been recognized as a very important mechanism for regulating many cellular events (Hershko and Ciechanover, 1998; Zheng et al., 2002). A major pathway for controlled protein destruction is the ubiquitin-mediated proteolysis by the proteasome (Hershko and Ciechanover, 1998; del Pozo and Estelle, 2000). Three types of enzymes (E1, E2, and E3) are responsible for ubiquitination of proteins. E1 is known as the ubiquitin-activating enzyme, E2 is the ubiquitin-conjugating enzyme, and E3 is the ubiquitin-protein ligase, which acts as a factor for substrate recognition (Koepp et al., 1999; Pickart, 2001). Both E1 and E2 are less specific than E3. The SKP1, cullin/CDC53, F-box protein (SCF) complexes are the largest and best studied family of E3 ubiquitin-protein ligases and are known to control cell cycle regulation, signal transduction, transcription, and other biological events (Bai et al., 1996; Hershko and Ciechanover, 1998; Schulman et al., 2000; Shen et al., 2002; Zheng et al., 2002).
Among the subunits of the SCF complex, SKP1 acts as an adapter that links cullin to one of the F-box proteins, which are highly variable (Willems et al., 1999; Schulman et al., 2000; Zheng et al., 2002). X-ray crystallography (Zheng et al., 2002) revealed that the human cullin, Cul1, is elongated and has a long stalk domain that binds to SKP1 and a globular domain that associates with Rbx1, a recently identified fourth subunit that contains a RING finger domain (Kamura et al., 1999; Seol et al., 1999). F-box proteins bind to SKP1 through the conserved F-box motif that is 40 to 50 amino acids long and is named for the human cyclin F (Skowyra et al., 1997; Winston et al., 1999; del Pozo and Estelle, 2000). Several SCF complexes have been identified that function in a myriad of vital biological processes in yeast and mammals. Some examples are SCFSkp2 (Zhang et al., 1995; Schulman et al., 2000) and SCFhCdc4 (Strohmaier et al., 2001), where the superscript indicates the specific F-box protein.
Recent studies suggest that plants make extensive use of SCF complexes to regulate multiple biological processes. Several SCF complexes have been characterized in Arabidopsis. For example, the SCFTIR1 complex regulates auxin response (Ruegger et al., 1998; Gray et al., 1999, 2001; Schwechheimer et al., 2001), and the SCFCOI1 complex is involved in jasmonate responses (Xie et al., 1998; Xu et al., 2002). Most recently, another complex called SCFAtSKP2 was found to play a role in controlling cell division (del Pozo et al., 2002). The characterization of mutants affecting genes encoding F-box proteins further indicates that SCF complexes may regulate a variety of processes in plants, such as flower development (Ingram et al., 1995; Levin and Meyerowitz, 1995; Samach et al., 1999; Zhao et al., 1999, 2001), circadian clock (Mizoguchi and Coupland, 2000; Somers et al., 2000), gibberellin signaling (Sasaki et al., 2003), light signaling (Dieterle et al., 2001), defense response (Kim and Delaney, 2002), and leaf senescence (Woo et al., 2001).
In both yeast and human, there is only one known functional SKP1 gene (Yu et al., 1998; Kipreos and Pagano, 2000). In contrast, fruitfly (Drosophila melano-gaster) and Caenorhabditis elegans both have multiple SKP1 homologs. C. elegans has at least 21 SKP1-related (SKR) genes that exhibit different expression patterns (Yamanaka et al., 2002). The SKR proteins also exhibit differences in their association with both cullins and F-box proteins (Nayak et al., 2002; Yamanaka et al., 2002). Loss-of-function studies using RNA interference (RNAi) show that SKR genes are differentially involved in regulating cell proliferation, meiosis, and morphogenesis (Nayak et al., 2002; Yamanaka et al., 2002).
The Arabidopsis genome contains 21 predicted ASK genes (Arabidopsis Genome Initiative, 2000; Farras et al., 2001), but only ASK1 has been analyzed genetically. The ask1-1 mutant is defective in both vegetative growth and reproductive development (Yang et al., 1999; Zhao et al., 1999). Compared with wild type, it has smaller rosette leaves and shorter stature. Furthermore, the mutant is defective in petal and stamen development. ASK1 genetically interacts with UFO to regulate B function genes AP3 and PI, probably through SCF-mediated ubiquitination and proteolysis (Samach et al., 1999; Zhao et al., 2001). In addition, the ASK1 gene is required for homolog separation in male meiosis (Yang et al., 1999) and is also involved in auxin response (Gray et al., 1999). The pleiotropic functions of ASK1 are consistent with its broad expression in both vegetative and reproductive tissues (Porat et al., 1998). Mutational studies of other ASK genes have not been reported; moreover, for most predicted ASK genes, it is not known whether they are active genes or pseudogenes. To learn about possible functions of the ASK gene family, we conducted reverse transcription-PCR (RT-PCR) and in situ hybridization experiments to characterize their expression patterns. Furthermore, we selectively suppressed the expression of a few ASK genes to investigate their possible functions. Our results show that the ASK genes have a variety of expression profiles and transgenic plants with different ASK RNAi constructs have distinct phenotypes, suggesting that members of the ASK gene family may have diverse functions in plant development and physiology.
RESULTS
Expression Analysis by RT-PCR
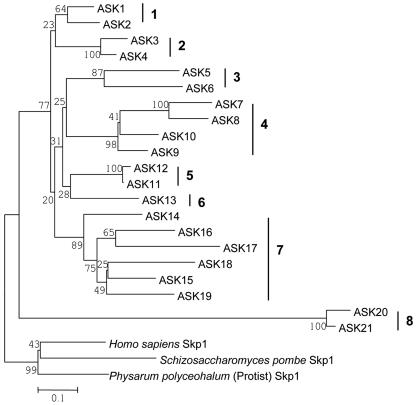
For the convenience of description and discussion, we divide the genes into several groups based on their sequence similarity: group 1, ASK1 and ASK2; group 2, ASK3 and ASK4; group 3, ASK5 and ASK6; group 4, ASK7 through ASK10; group 5, ASK11 and ASK12; group 6, ASK13; group 7, ASK14 through ASK19; and group 8, ASK20 and ASK21 (Fig. 1). Although the ASK genes share higher levels of amino acid sequence similarity within each group than between groups, the levels of similarity within different groups are not uniform.
Figure 1.
Phylogenetic relationships of the ASK genes. This neighbor joining (NJ) tree was generated in MEGA2.1 based on amino acid sequence analysis, with the “complete deletion” option selected and the poisson correction for distance estimates. The number below (or above) each clade indicates the bootstrap supports in 1,000 replicates (H. Kong and H. Ma, unpublished data). ASK genes have been classified into eight groups based on this tree.
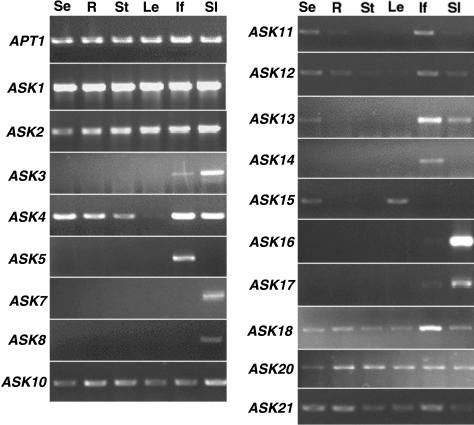
To learn whether predicted ASK genes are expressed during normal development and to estimate the relative abundance of ASK messages in different organs, we performed RT-PCR using gene specific primers (Table I). To control for specificity, PCR products from genomic DNA and cDNA templates were sequenced directly, and their sequences match known sequences of the corresponding genes (except ASK9), other than polymorphisms between the Landsberg erecta (Ler) and Columbia (Col) ecotypes. PCR using primers matching the Col ASK9 gene yielded products that were most similar to ASK8. Thus, ASK9 expression was not further analyzed. Expression of the 20 ASK genes was tested using RT-PCR, and the expression of 18 genes was detected in one or more part(s) of plants grown under normal conditions (Fig. 2; Table II). However, the sequence of the ASK15 RT-PCR product matched the known ASK15 genomic sequence, including a predicted 50-bp intron, suggesting that ASK15 may be a pseudogene. The expression of ASK6 and ASK19 was not detected. As a control for RNA extraction and RT-PCR for different tissues, we detected uniformly strong RT-PCR products in all RNA samples for the APT1 gene encoding adenine phosphoribosyltransferase (Moffatt et al., 1994).
Table I.
Primers for RT-PCRs
| Gene Name and Identification No.a | OMC No. | Primer Sequenceb 5′ to 3′ | Gene Name and Identification No.a | OMC No. | Primer Sequence 5′ to 3′ |
|---|---|---|---|---|---|
| APT1 | 571 | TCCCAGAATCGCTAAGATTGCC | ASK13 | 589 | TCAGAAAGCACACTAACGTCGAAA |
| 572 | CCTTTCCCTTAAGCTCTG | At3g60010 | 590 | CAAAAATCGCCACCACAAAAAGA | |
| ASK1 | 221 | AAGGTGATCGAGTATTGCAAGAG | ASK14 | 609 | CATCACCCAAAATCTCAAAAACGA |
| At1g75950 | 383 | GAAGATAGTCATGATTCATGAAG | At2g03170 | 610 | TCATCAAAACCCTAAAACCGACAA |
| ASK2 | 420 | ATCCGAAACCACGGCCGAT | ASK15 | 601 | CACAGCGAAACACAAAGCTAGGG |
| At5g42190 | 593 | AAATGGGTCGAGGACATGAC | At3g25650 | 602 | TCGTTTAACACACAAAGCAAACAAA |
| ASK3 | 575 | CAAACGATGGCAGAAACGAAGA | 746c | TCTCGAACTTCCTCTGGCGTCT | |
| At2g25700 | 576 | AGCCGGGATTGAATGAAGAAAA | 747c | TGTTGACTAGTTCCGATGGCGA | |
| ASK4 | 622 | CCATCCTCGCCAAGGTTATTGAG | ASK16 | 607 | CAAAACAAACAATGTCTTCGAACAAG |
| At1g20140 | 574 | AACACCCTTCAGATCCGATC | At2g03190 | 608 | CATCGAGATCAACACTTAGAAAACGGA |
| ASK5 | 591 | TCAACAACTTTGATGATCCGCTCTC | ASK17 | 605 | TCGAGATCAACACTTAGACAACGGA |
| At3g60020 | 592 | CGCTCCATAACAATGTCGACGAA | At2g20160 | 606 | CAGCAAAACAAACAAAATGTCTTCG |
| ASK6 | 617 | ATCGTGTTGACAAGCTCCGATGA | ASK18 | 581 | GCCTTATAACAATGGCTTCTTCTTCCG |
| At3g53060 | 618 | TCGCATCAAGAAAACCGAAAACA | At1g10230 | 582 | TCGAAACGAAATTCTGCAAACCC |
| ASK7 | 616 | TCCCCCACAAAGAAAACAACAATG | ASK19 | 922 | GAAGGAGATGATGAAGCTGAGGA |
| At3g21840 | 666 | AGCCCATTTATTGTCGTTGACC | At2g03160 | 923 | CATTGCGAATTGCTTCTTCTTCT |
| ASK8 | 603 | GAAAGAAAGCTGTTTAGGTTTTGA | ASK20 | 641 | ATGTCAGAAGGTGATTTGGCCGT |
| At3g21830 | 604 | TCCCCCACAAAGAAAACAACAATG | At2g45950 | 642 | AGAGCCTCTTTGTGTCCATTCGG |
| ASK10 | 643 | CATTGATTCTCCCTACGAACCGC | ASK21 | 832 | TTCCACCATCGTCACTCATTGCT |
| At3g21860 | 628 | AATCCCGCTTCCAGAAGTGACAG | At3g61415 | 833 | CTGGATTGACTCGCTGTGGAAGA |
| ASK11 | 838 | AATCCCTCTTGCAAACGTGGAAA | |||
| At4g34210 | 839 | TCGATCAAGACGTTAAAAAGAAAACA | |||
| ASK12 | 838 | AATCCCTCTTGCAAACGTGGAAA | |||
| At4g34470 | 665 | TCTACATTATTGATCAAGAAGTTGC | |||
| 583c | GCTAGTTAGGTTTTTGATTCATGGG |
a The gene identification number is provided below the gene name.
b The primers for each gene are listed next to the gene name and identification number, except those noted in c.
c Primers used for nested PCR for the gene immediately above.
Figure 2.
Analysis of ASK gene expression using RT-PCR. The APT1 gene was used as an internal control, The RT-PCR results were from one round of 35 cycles, except that those for ASK11, ASK12, and ASK15 were from two rounds of PCR (see “Materials and Methods”). Se, Seedling; R, root; St, stem; Le, leaf; If, inflorescence; Sl, silique.
Table II.
Relative RT-PCR band intensities of ASK genes
The abbreviation for the tissues is the same as in Figure 2. The number of + stands for the relative amount of PCR products; more + symbols stand for a higher expression level.
| Gene Name | Se | R | St | Le | If | Sl | Gene Name | Se | R | St | Le | If | Sl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASK1 | +++ | +++ | +++ | +++ | +++ | +++ | ASK12 | + | + | + | + | + | + |
| ASK2 | +++ | +++ | +++ | +++ | +++ | +++ | ASK13 | + | ++ | + | |||
| ASK3 | + | ++ | ASK14 | + | |||||||||
| ASK4 | ++ | ++ | + | +/− | +++ | +++ | ASK15 | + | + | ||||
| ASK5 | + | ASK16 | ++ | ||||||||||
| ASK6 | ASK17 | + | ++ | ||||||||||
| ASK7 | + | ASK18 | + | + | + | + | ++ | + | |||||
| ASK8 | + | ASK19 | |||||||||||
| ASK10 | + | + | + | + | + | + | ASK20 | + | + | + | + | + | + |
| ASK11 | + | + | + | + | ASK21 | + | + | + | + | + | + |
RT-PCR produced nearly uniformly strong bands for ASK1 and ASK2 in all tissues tested: young seedlings, roots, leaves, floral stems, inflorescences, and siliques. ASK3 seems highly expressed in the silique. ASK4 expression seems to be at a higher level in inflorescence and siliques than in seedlings, roots, and stems. For group 4, the expression of ASK7 and ASK8 was detected only in the silique, whereas ASK10 expression was found in all tissues tested. In group 5, ASK11 and ASK12 had similar expression patterns, with perhaps a slightly higher level in the inflorescence than in other tissues.
In group 3, ASK5 is expressed in the inflorescence, as confirmed by in situ hybridization (see below). ASK13 expression was detected as a stronger PCR band for the inflorescence than those for the seedling and silique. In group 7, ASK14 expression was only detected in the inflorescence. ASK15 expression was difficult to detect after one round of PCR; a second PCR using nested primers found it to be expressed in the seedling and leaf (Fig. 2). ASK16 and ASK17 expressions were mainly detected in the silique (Fig. 2). For ASK18, ASK20, and ASK21, similar levels of expression were detected in all tissues examined (Fig. 2).
Expression Analysis by in Situ Hybridization
The expression patterns of 11 ASK genes were further characterized using RNA in situ hybridization experiments; these 11 genes were chosen in part based on their amino acid sequence similarities. ASK1 was included as a positive control; moreover, we wanted to compare ASK1 and ASK2 expression patterns because they have very similar sequences and RT-PCR results. ASK3 and ASK4 are highly similar to each other and, to a lesser extent, to ASK1 and ASK2. ASK14, ASK15, ASK18, and ASK19 are relatively similar in sequence, but differ in expression based on RT-PCR experiments. Finally, ASK5, ASK9, and ASK11 were chosen as representatives of three other distinct groups.
To obtain gene-specific probes, we used 3′-untranslated regions (UTRs) of the ASK genes. Previous in situ hybridization experiments indicated that probes of 400 bps or longer do not cross-hybridize to related sequence with 83% or less nucleotide sequence identity (Yanofsky et al., 1990; Ma et al., 1991; Flanagan and Ma, 1994). The ASK UTRs that were used here as templates for probes are all about 250 to 350 bps long, and most of them have less than 50% identities to other ASK genes. Only one exception is that the ASK9 sequence used for probe synthesis is 89% identical to ASK10, 89% to ASK8, and 86% to ASK7. Furthermore, in situ hybridization experiments using ASK1 probe on ask1 null mutant tissues, as a control, indicated that the probe was specific and did not detect expression of the other ASK genes (data not shown).
Expression Patterns of ASK1 and ASK2
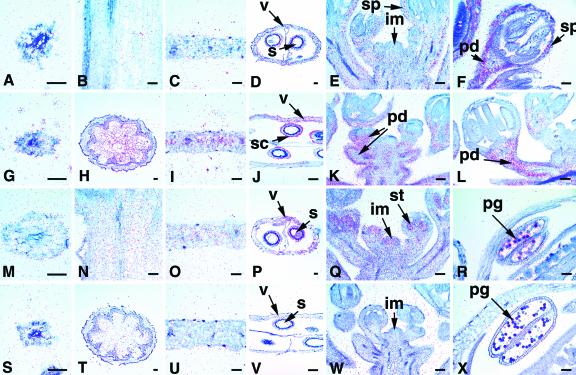
The ASK1 and ASK2 mRNAs were detected in all major organs examined, although ASK2 expression was at lower levels than those of ASK1 (Fig. 3). Both ASK1 and ASK2 mRNAs accumulate weakly in the cortical layer and epidermis of the root (Fig. 3, A and B) but are not detectable in the vascular tissue. In the young stem, ASK1 and ASK2 are expressed uniformly in the pith and vascular bundle (Fig. 3, C and D). No expression was observed for either ASK1 or ASK2 in the mature stem (data not shown). In the leaf, ASK1 is uniformly expressed at a higher level than that of ASK2 (Fig. 3, E and F). In the silique, ASK1 is expressed throughout the valve and developing seed (Fig. 3G), again at levels higher than those of ASK2 (Fig. 3H). ASK1 expression is strong in the inflorescence meristem (IM) and young floral bud (Fig. 3I), at high levels in all floral organ primordia; furthermore, it is expressed at a high level in the male meiocytes in a flower at approximately stage 9, when meiosis occurs (Fig. 3K). ASK1 expression remained high in the pollen grains (Fig. 3M). Compared with ASK1, ASK2 is expressed in the IM and flower at lower levels (Fig. 3J). ASK2 signal is also present in the male meiocytes and pollen grains (Fig. 3, L and N). These in situ hybridization results indicate that ASK1 and ASK2 have very similar expression patterns.
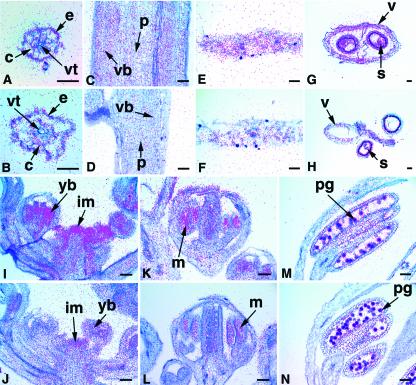
Figure 3.
In situ hybridizations with ASK1 and ASK2. A, C, E, G, I, K, and M, ASK1; B, D, F, H, J, L, and N, ASK2. A and B, Root cross sections with expression signal in the cortical layer (c) and epidermal cells (e), but not in the vascular tissue (vt). C and D, Longitudinal sections of a young stem with moderately expression in the pith (p) and vascular bundle (vb). E and F, Leaf cross sections showing that the ASK1 mRNA (E) is present at a higher level than the ASK2 mRNA (F). G and H, Silique cross sections showing expression in the valve (v) and developing seeds (s). I and J, Longitudinal sections of an inflorescence with signals in the IM (im) and young buds (yd). K and L, Two sections of a stage-9 bud with expression detected in male meiocytes (m). M and N, Anther sections of mature flowers showing expression in pollen grains (pg). Scale bar = 50 μm.
Expression Patterns of ASK3 and ASK4
The ASK3 and ASK4 genes also have similar expression patterns (Fig. 4). In the root, young stem, and young leaf, the signals of ASK3 (Fig. 4, A, C, and E) and ASK4 (Fig. 4, B, D, and F) are very low if detectable at all. Both ASK3 and ASK4 have high levels of expression in the silique (Fig. 4, G and H); ASK4 is very strongly expressed in the valve, septum, and developing seed (Fig. 4H). Weak signals of ASK3 were also detected in the inflorescence (Fig. 4I). No expression of ASK3 was detected in the IM, and ASK3 mRNA in the flower is mostly restricted to the sepal and pedicel (Fig. 4, I and K). ASK4 expression was detected throughout the inflorescence at a higher level in the IM than in the young flower (Fig. 4, J and L). Although ASK3 expression was not detected in pollen grains (Fig. 4M), ASK4 (Fig. 4N) is expressed in the pollen grains, but at a lower level than those of ASK1 and ASK2 (Fig. 3, M and N).
Figure 4.
In situ hybridizations with ASK3 and ASK4. A, C, E, G, I, K, and M, ASK3; B, D, F, H, J, L, and N, ASK4. A and B, Root cross sections with no ASK3 expression (A) and just detectable ASK4 expression (B). C and D, Young stem longitudinal sections with no ASK3 (C) and weak ASK4 expression (D). E and F, Cross sections of a leaf showing ASK3 (E) and ASK4 (F) mRNAs. G, Cross sections of a silique indicating that the ASK3 signal is present in the valve (v) and seed coat (sc). H, The ASK4 gene is very highly expressed in the valve, septum (sm), and all developing seeds (s). I, Longitudinal sections of an inflorescence showing low level ASK3 expression in the sepal (sp) and pedicel (pd) of a young bud but not in the IM (im). J, ASK4 expression is present in the IM but weak in young buds. K and L, Sections of an about stage-10 flower having ASK3 expression (K) in the sepal and weak uniform ASK4 expression (L). M and N, Sections of anther having ASK4 (N) but no ASK3 (M) expression in the pollen grains (pg). Scale bar = 50 μm.
Expression Patterns of ASK14, ASK15, ASK18, and ASK19
Although the ASK14, ASK15, ASK18, and ASK19 genes are similar in sequence, they have different expression patterns (Fig. 5). In the root, there is no detectable expression of ASK14 and ASK19 (Fig. 5, A and S), and signals of ASK15 and ASK18 are barely detectable (Fig. 5, G and M). ASK15 and ASK18 are highly expressed in the pith and vascular bundle in the stem, with the levels of ASK15 greater than that of ASK18 (Fig. 5, H and N). In contrast, little or no expression was seen for ASK14 or ASK19 in either young or mature stem (Fig. 5, B and T; data not shown). Their expression in leaf varied in levels from least to greatest: ASK14, ASK19, ASK15, and ASK18 (Fig. 5, C, U, I, and O). Barely detectable expression of ASK14 was observed in the silique (Fig. 5D). In the silique, ASK15 and ASK18 mRNAs are higher in inner epidermis of the valve than elsewhere (Fig. 5, J and P), and ASK19 signals are detectable in the valve and seed coat (Fig. 5V). ASK14, ASK15, ASK18, and ASK19 are all expressed in the inflorescence but with different spatial patterns. There is no expression of ASK14 in the IM or pollen grains (Fig. 5E; data not shown). However, ASK14 is expressed in the male meiocytes and even more in the tetrads (Fig. 5F; data not shown). The expression of ASK15 and ASK18 was found in the pedicel of young buds, with lower expression elsewhere in the flower (Fig. 5, K, Q, and R). ASK15 is also expressed in the inner epidermis of the carpel and pedicel in the mature flower (Fig. 5L). The level of ASK19 expression is moderate and nonspecific in the IM and flower (Fig. 5W). In the mature flower, ASK19 expression was seen in the sepal, petal, and filament of the stamen (Fig. 5X).
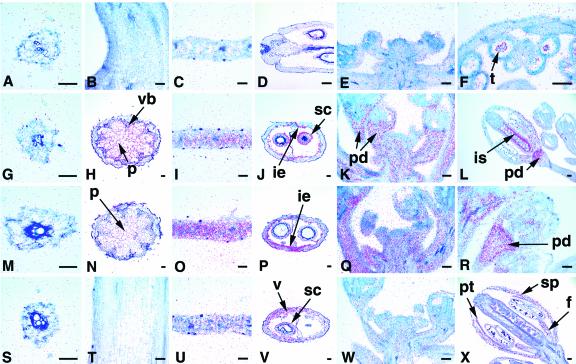
Figure 5.
In situ hybridizations with ASK14, ASK15, ASK18, and ASK19. A to D, Sections showing no ASK14 expression in the root (A) and young stem (B), but very weak expression in the leaf (C) and silique (D). E, Low level uniform ASK14 expression in the inflorescence. F, ASK14 expression in tetrads (t). G, No ASK15 expression in the root. H, ASK15 gene is highly expressed in the pith (p) and vascular bundle (vb) of a stem. I, ASK15 expression in the leaf. J, ASK15 is present in the inner epidermis (ie) of the valve and seed coat (sc). K, In the inflorescence, ASK15 is more restricted to the pedicel (pd) but much less expressed in other parts of the flower. L, ASK15 expression in the inner surface (is) of the carpel and pedicel. M, Barely detectable ASK18 expression in the root. N, Low ASK18 expression in the stem. O, ASK18 is very strongly expressed in the leaf. P, ASK18 is present in the valve; more expression in the inner epidermis of the silique. Q and R, In the inflorescence (Q) and a young bud (R), ASK18 is restricted to the pedicel and is minimally expressed in other parts of the flower. S to W, ASK19 had no expression in the root (S) and young stem (T), low level expression in the leaf (U), moderate expression in the valve and seed coat (V), and low and uniform expression in the inflorescence (W). X, ASK19 expression in the sepal (sp), petal (pt), and filament (f) of mature flower. Scale bar = 50 μm.
Expression Patterns of ASK5, ASK9, and ASK11
Our results showed that the expression patterns of ASK5 and ASK11 were different (Fig. 6). The expression of ASK5 was not found in the root, stem, and leaf (Fig. 6, A-C), was barely detectable in the silique (Fig. 6D), and was observed at a very low level in the inflorescence except for the IM (Fig. 6E). In the young bud, the ASK5 mRNA is restricted to the sepal and pedicel (Fig. 6F). ASK11 has a very weak expression in the root, stem, leaf, and silique (Fig. 6, M-P). However, ASK11 has a slightly higher expression in the IM and young bud, particularly in the stamen (Fig. 6Q). We also detected the expression of ASK11 in the pollen grains (Fig. 6R). As a negative control, sense probes detected very low levels of signals that matched backgrounds seen with our experimental probes (e.g. Fig. 6, S-X; data not shown). Because the ASK9 UTR used for probe synthesis has high identity to UTRs of ASK7, ASK8, and ASK10, the signals detected here probably represent the combined expression of all four genes. The ASK9-hybridizing signal was detected in every tissue, very weakly in root (Fig. 6G), at moderate levels in the pith and vascular bundle of the young stem and in the leaf blade (Fig. 6, H and I), and at moderate levels in the valve and seed coat in silique (Fig. 6J). Signals are also present in the inflorescence except for the IM (Fig. 6K) and are especially high in the pedicel of the young flower (Fig. 6L).
Figure 6.
In situ hybridizations with ASK5, ASK9, and ASK11. A to D, No ASK5 expression in the root (A), young stem (B), and leaf (C), but just detectable expression in the silique (D). E, Very low ASK5 expression in flowers, but no expression in the IM (im). F, ASK5 expression in both the sepal (sp) and pedicel (pd). G to J, ASK9-hybridizing signal is present at a very low level in the root (G), at a moderate level in the stem (H) and leaf (I), and at low levels in the valve (v) and a seed coat (sc; J). K and L, In the inflorescence (K) and young bud (L), ASK9-hybridizing signal is present mostly in the pedicel but much less in other parts of the flower. M to P, ASK11 was just detected in the root (M), and expressed in the stem (N), leaf (O), and valve and seed (P) at low levels. Q, ASK11 was more highly expressed in the IM and stamen (st) in young buds. R, ASK11 is present in pollen grains (pg). S to X, Sections hybridized with ASK9 sense probe only showing signals at the background level in the root (S), stem (T), leaf (U), silique (V), inflorescence (W), and anther (X). Scale bar = 50 μm.
Analysis of ASK Functions Using RNAi
To begin investigating the function of ASK genes, we selectively suppressed the expression of a few ASK genes using double-stranded RNA interference (RNAi) method. ASK1 was chosen as a positive control because the ask1-1 null mutant phenotypes have been described. In addition, we also wanted to know whether ASK1 shares redundant functions with ASK2, or possibly other ASK genes, by comparing RNAi plants with the ask1-1 mutant. ASK11 was chosen to test the hypothesis that it plays some specific roles, because it has a more restricted expression pattern.
Phenotypes from ASK1 RNAi Plants
We generated approximately 200 independent ASK1 RNAi lines; more than 80% of the transgenic lines showed some degrees of floral defects similar to those of the ask1-1 mutant, including reduced fertility, indicating that the suppression is very efficient. ASK1 RNAi lines with a weak phenotype usually had normal vegetative growth, normal stature, mild defects in flower development, and normal or partially reduced fertility. In contrast, lines with a strong phenotype had abnormal vegetative growth, short stature, and severe defects in flower development, and were usually male sterile. A strong (ASK1-Line 3) line and relative weak (ASK1-Line 11 and 14) lines were further examined.
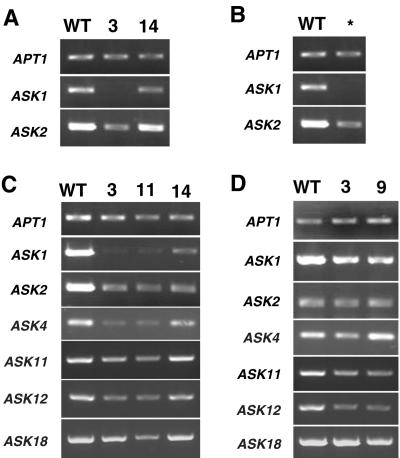
During vegetative development, we observed a slight reduction of leaf size and plant stature in the ask1-1 mutant, similar to previous findings (Fig. 7, B and F; Zhao et al., 1999). The strong ASK1-Line 3 showed dark-green, wrinkled leaves with a very short leaf petiole, whereas the width of the leaf was not affected much (Fig. 7C). In addition, the plant was much shorter in stature than the ask1-1 mutant (Fig. 7G). RT-PCR analysis showed that ASK1 expression was not detectable in the rosette leaf of ASK1-Line 3, and ASK2 expression was also reduced (Fig. 8A). In the weak lines ASK1-Line 11 and ASK1-Line 14, vegetative development was generally normal (Fig. 7H). RT-PCR analysis further confirmed only a slight reduction of ASK1 expression and nearly normal level of ASK2 expression in rosette leaf in ASK1-Line 14 (Fig. 8A). In addition to these lines, we also observed several dwarf, dark-green plants that died before flowering, one of which is shown in Figure 7D. RT-PCR results indicate that ASK1 expression was not detected in this plant, and ASK2 expression was reduced (Fig. 8B).
Figure 7.
Phenotypes of ASK1 and ASK11 RNAi transgenic plants. A to C, Seedlings of the wild type, ask1-1 mutant, and strong ASK1-Line 3 at the age of 3 weeks. D, Two-week-old seedlings of the wild type and a strong ASK1 RNAi plant (inset), which was dwarf and dark-green and died before flowering. E to H, Wild-type, ask1-1 mutant, ASK1-Line 3, and the weak ASK1-Line 14 plants at the age of 4 weeks. The plant from Line 3 had a much shorter stature than the ask1 mutant (G), whereas the plant from Line 14 had a normal stature and normal vegetative growth compared with that of wild type (H). I, Wild-type flower. J, An ask1 flower showing a staminoid petal (arrow) and short stamens. K to M, Flowers from the strong ASK1-Line 3 exhibiting down-curved sepals, reduced petal identity, short stamens (K), carpel-like structures in outer whorl (L, arrow), and four carpels (M). N to P, Flowers from weak ASK1 RNAi plants having fused organ between petals, petal and stamen (N, Line 11; P, Line14), stamens (O, line 11). Q, Five-week-old wild-type (left) and ASK11 RNAi plants (right) with a normal vegetative growth and seed setting. R to T, Flowers from the ASK11 RNAi plants showing a petaloid stamen (R), fused stamen (S), and filament-like structure or abnormal anther (T). One petal in T was removed. Scale bars = 0.5 cm (A-C, I-P, and R-T), 1 mm (D); 0.75 cm (E-H); and 2 cm (Q).
Figure 8.
RT-PCR analysis of the RNAi transgenic plants. A, Leaf tissue from the wild type, ASK1-Line 3, and Line 14. B, Seedling from the wild type and a strong ASK1 RNAi plant (*). C, Inflorescences from the wild type, ASK1-Line 3, and weak Line 11 and Line 14. D, Inflorescences from the wild-type and ASK11 RNAi transgenic lines.
The ask1-1 mutant flowers had a slightly reduced number of petals, staminoid petals, and short filaments (Fig. 7J; Zhao et al., 1999). In ASK1-Line 3, stamens were similar to those in ask1-1 flowers, whereas petal number was further reduced. The most distinctive phenotype in this line is that the outer whorl has sepals that usually curved downward (Fig. 7, K and L), with occasional carpel-like structures (Fig. 7L). In some plants, the number of carpels was increased (Fig. 7M). In weak ASK1 RNAi lines, sepal and carpels were normal, and the reduction of filament length was not as much as that of the ask1-1 mutant. Staminoid petals were also frequently observed. In addition, fused petals, fused stamens, or petal-stamen chimeras were frequently found (Fig. 7, N-P). Similar to the ask1-1 mutant, the strong ASK1-Line 3 was male sterile, whereas the weak lines (ASK1-Line 11 and ASK1-Line 14) were partially sterile. Consistent with the phenotypes of the transgenic plants, ASK1 expression was suppressed to a greater extent in the strong line than in weak lines (Fig. 8C). In addition, ASK2, and possibly ASK4, expression levels also seemed to be suppressed (Fig. 8C). The expression of ASK11, ASK12, and ASK18 was detected only after increased numbers of PCR cycles; therefore, it is not certain whether they are also affected by the ASK1 RNAi transgene.
Phenotypes from ASK11 RNAi Plants
ASK11 is almost identical to ASK12 in the coding region; in addition, our RT-PCR results for ASK11 and ASK12 were very similar. Therefore, ASK11 and ASK12 may play very similar roles and might be functionally redundant. Consequently, the phenotype observed in ASK11 RNAi plants, if any, might be the result of a reduction of both ASK11 and ASK12 functions. Approximately 100 independent transgenic ASK11 RNAi lines were generated, and no obvious phenotype was observed during vegetative development. All transgenic lines had normal fertility (Fig. 7Q). In other words, none of the ASK11 RNAi plants had vegetative or fertility defects that were observed in typical ASK1 RNAi plants.
However, we observed slight defects in early flowers during reproductive development, although more severe phenotypes were observed in the flowers and inflorescences at late stages. Flowers of ASK11 RNAi plants had normal sepals, petals, and carpels. The only defects were petaloid or fused stamens in the third whorl of about 15% of the first 15 flowers (Fig. 7, R and S). Occasionally filament or abnormal anthers were also found in the transgenic plants, whereas other stamens in the same flower still had normal length and produced viable pollen (Fig. 7T). The phenotypes of the ASK11 RNAi plants differ from the ASK1 RNAi plants in that even weak ASK1 RNAi plants exhibit defects in both second and third whorls, as do the ask1-1 mutant flowers. Comparison of the RT-PCR results between the control APT1 gene and various ASK genes suggested that the expression of ASK11 and ASK12 was slightly reduced in the transgenic plants. In addition, ASK1 expression was reduced slightly, but ASK2, ASK4, and ASK18 expression was close to normal (Fig. 8D).
DISCUSSION
ASK Genes Exhibit Several Different Expression Patterns
Our RT-PCR and RNA in situ hybridization experiments indicate that nearly all of the ASK genes predicted from genomic sequences are expressed at different levels and exhibit a variety of spatial patterns. In addition, highly similar genes within each of several groups tend to have similar expression levels and patterns, although members of some groups show different expression patterns. In particular, ASK1 and ASK2 are both highly expressed in all of the tissues that were tested. In situ hybridization experiments further indicate that these two genes share very similar spatial patterns of expression throughout the plant, suggesting that they may be functionally redundant. This is further supported by the results that both ASK1 and ASK2 proteins interact with one of several F-box proteins in vitro and/or in yeast two-hybrid assays (Gray et al., 1999; Samach et al., 1999; Gagne et al., 2002; Xu et al., 2002). Also, similar expression patterns for ASK3 and ASK4 in the silique and the inflorescence suggest possible redundancy. Furthermore, whereas the group 4 genes (ASK7, 8 and 10) are preferentially expressed in the silique, the group 5 genes (ASK11 and 12) are more abundant in inflorescence. The highly conserved sequences and very similar expression patterns suggest that the members of each of groups 1, 2, 4, and 5 are the result of relatively recent gene duplication events. These ideas are supported by the fact that group 4 genes form a tandem array of direct repeats on chromosome 3 and ASK11 and ASK12 are closely linked to each other on chromosome 4 (see below for more discussion of ASK11 and ASK12 functions).
In contrast with the above ASK genes, those in groups 3, 6, 7, and 8 exhibit greater divergence in sequence and/or in expression pattern, suggesting that they may have evolved to have different functions and/or lost their original functions. As mentioned above, no ASK6 expression was detected. This and the fact that its sequence predicts a truncated protein lacking parts of both the highly conserved N- and C-terminal domains strongly suggest that it is a pseudogene. In addition, ASK15 mRNA was found to contain a predicted 50-bp intron, making it a possible pseudogene.
A moderate expression level of ASK15 and ASK19 was detected from our in situ experiments, whereas RT-PCR results suggest that they are expressed at very low levels. One explanation for this is that the tissues used for those two experiments were not identical; alternatively, the primers for ASK19 RT-PCR may be inefficient.
Our results clearly indicate that some ASK genes are widely expressed, suggesting that they may have pleiotropic functions, whereas others have more restricted expression and perhaps specific functions. Sequence divergence and expression differences between some groups of ASK genes also suggest that genes of these different groups may have evolved to have distinct functions. Yeast two-hybrid results showed that ASK1, ASK2, ASK11, and ASK19 can interact with about half or more of the 23 F-box proteins tested, and ASK4 interacts with several F-box proteins, whereas ASK5, ASK16, and ASK18 can interact only with a few of the F-box proteins (Gagne et al., 2002). If these interactions reflect in vivo activities, then they also support the ideas that ASK1, ASK2, ASK11, ASK13, and perhaps ASK4, have more basic or broad functions, whereas the others may have more restricted functions. In addition, ASK9 and ASK17 cannot interact with any of F-box proteins tested (Gagne et al., 2002), suggesting that they may interact with F-box proteins other than those tested and have divergent functions from other ASK proteins. Tissue-specific expression was also found in some of the F-box protein genes (Ingram et al., 1995; Kuroda et al., 2002). It is expected that only the ASK proteins and F-box proteins that are expressed in the same plant cells can interact in vivo to confer a biological function. Thus it would be very informative to compare the expression of the ASK genes and genes encoding F-box proteins.
Many ASK Genes May Function during Reproductive Development
Our results show that most of the ASK genes are either expressed in all tissues tested or are preferentially expressed in the inflorescence and/or fruit. This is consistent with the fact that flowers and fruits are more complex structures than roots or leaves. It is possible that reproductive development and physiology require many regulatory proteolytic pathways, not only those found in vegetative tissues, but also those specific to reproductive structures. For example, the expression of ASK1, ASK2, ASK4, and ASK11 in the shoot apical meristem suggests that they are involved in regulating cellular events in the meristem. Their expression in the pollen at relatively high levels suggests that they may also play a role in pollen development. In addition, the fact that ASK15 and ASK18 were expressed in the pedicel of young flowers suggests roles during pedicel development. ASK1 is known to regulate male meiosis; the detection of ASK2 expression in meiotic cells suggests that it may also be involved in male meiosis. Furthermore, the ASK14 mRNA is present at a high level in the microspores but is absent in the pollen grains, suggesting that the ASK14 gene may function in the newly formed microspores.
The expression of most ASK genes was detected in the silique, suggesting that they play important roles during embryo, seed, or fruit development. However, the details of the expression patterns were different. For example, the ASK4 gene is expressed at high levels in several tissues, including the valve, septum, and developing seed, but ASK1 expression is higher in the embryo than other parts. The signals of ASK3 and ASK19 were more restricted to the seed coat, whereas, most interestingly, the ASK15 and ASK18 mRNAs are mostly present in the inner epidermis of the valve. Therefore, different ASK genes may have distinct functions in the development of seed and/or fruit. Recently, a mutation in the AtCUL1 gene was found to cause an arrest of embryo and endosperm development before either the first or second mitosis of the zygote/embryo (Shen et al., 2002). Therefore, ubiquitin-dependent proteolysis is likely to be critical for embryogenesis, possibly involving one or more of the ASK genes.
ASK1 May Participate in Multiple Pathways Regulated by SCF Complex and Share Redundant Function with Other ASK Genes
ASK1 is strongly expressed in all tissues tested, consistent with previous reports on its expression (Porat et al., 1998). Previous studies of the ask1-1 mutant, which is a null mutant with a Ds insertion, indicate that it is required for male meiosis and is important for flower development and auxin response (Gray et al., 1999; Yang et al., 1999; Zhao et al., 1999). Other studies demonstrated that ASK1 was able to interact with one of several F-box proteins in yeast two-hybrid and in vitro-binding assays (Gray et al., 1999; Samach et al., 1999; Dieterle et al., 2001; Woo et al., 2001; del Pozo et al., 2002; Gagne et al., 2002; Xu et al., 2002). Therefore, ASK1 is a likely component of multiple SCF complexes that regulate many important pathways. Further support for ASK1 having a more general function comes from a structure and evolution analysis of the SKP1 gene family, which showed that ASK1 is more conserved and may evolve more slowly than other ASK genes (H. Kong and H. Ma, unpublished data).
On the other hand, the relatively weak vegetative and floral phenotypes observed in the ask1-1 null mutant indicate that other ASK genes also play a role and may share redundant functions with ASK1; one likely candidate is the ASK2 gene, as suggested by its expression pattern (see above). This is supported by the enhanced phenotypes in the strong ASK1 RNAi line together with a reduction in expression of both ASK1 and ASK2 and perhaps that of other ASK genes.
The dwarf phenotype and the dark-green leaves of the strong ASK1 RNAi transgenic plant are similar to those found in GA-deficient or -insensitive mutants (Koornneef and van der Veen, 1980; Koornneef et al., 1985). It has been shown that putative transcription repressors (DELLA proteins), such as RGA and GAI, regulate GA signal transduction through transcriptional regulation (Olszewski et al., 2002). Recent studies have shown that the rice (Oryza sativa) GID2 gene encodes an F-box protein that controls the degradation of the rice DELLA protein SLR1 through GA-dependent phosphorylation (Sasaki et al., 2003). In Arabidopsis, a mutation in SLY1, a GID2 homolog, causes a deficiency in GA response and an accumulation of RGA protein (Steber et al., 1998; McGinnis et al., 2003). Our results suggest that part of the GA signaling in Arabidopsis might be mediated through the degradation of DELLA proteins by SCF complexes that involve ASK1, ASK2, and perhaps other ASK proteins.
Previous studies in our lab have demonstrated that ASK1 is required for the regulation of B function of the ABC model in flower development through interacting with an F-box protein UFO (Zhao et al., 1999, 2001). In addition to the severe reduction of B function in the strong ASK1 RNAi line, we also observed various defects in both sepals and carpels. The presence of carpel-like structures in outer whorls suggests the expansion of C function. In addition, fused organs between petals and stamens indicate that ASK1 and other ASK genes are also required for normal activity of the flower meristem, probably through the regulation of cell division.
ASK11 and ASK12 Might Be Involved in Flower Development
ASK11 and ASK12 are extremely similar in both sequence and expression patterns, suggesting that they have similar functions. ASK11 was able to interact with a similar set of F-box proteins as ASK1 and ASK2 in yeast two-hybrid assays (Gagne et al., 2002). Because ASK11 and ASK12 expression seems to be at low levels during normal development, their functions might not be very critical for development. We found that the growth of ASK11 RNAi plants was generally normal, but we did observe some defects in the stamen of the transgenic plants, which is consistent with the expression of ASK11/12 in stamen primordia, as observed in RNA in situ hybridization experiments.
Although the expression of ASK11 and ASK12 was not eliminated in the RNAi lines, weaker RT-PCR bands were reproducibly obtained from the RNAi lines than from the wild-type plants. Because additional RT-PCR cycles were needed to detect expression, the RT-PCR results suggest a reduction of ASK11/12 expression from already-low wild-type levels. It is possible that the RNAi construct with the 35S promoter was not able to completely eliminate weak expression. However, because the reduction was observed after an increased number of PCR cycles, the interpretation of a role of ASK11/12 in stamen development must be regarded as tentative. More definitive understanding of the function of these genes will require the analysis of mutations in these genes.
We have also noticed that ASK1 expression seems to be reduced slightly in the ASK11 RNAi plants, suggesting that the reduction of ASK1 might have contributed to the observed phenotypes. Nevertheless, the ASK11 RNAi flowers were different from flowers of ASK1 RNAi transgenic lines, supporting a possible distinct role for ASK11 (ASK12) in flower development.
CONCLUSIONS
Clearly, the ASK gene family contains a spectrum of actively expressed members that exhibit a variety of patterns. The encoded proteins can potentially form a multitude of SCF complexes that regulate plant development and physiology at different stages. It has been shown that SKP1 may form a complex lacking cullin, and results from C. elegans indicate that some SKP1 homolog do not interact with cullins (Yamanaka et al., 2002). Therefore, some ASK proteins could function by forming complexes different from SCF. In summary, ASK genes may play a wide range of important regulatory roles, and future studies using reverse genetics, biochemistry, and cell biology promise to uncover additional functions for this important regulatory gene family.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis plants were of the Ler ecotype. Except otherwise noted, they were grown on Metro-Mix 360 (Scotts-Sierra Horticultural Products, Maryville, OH) at 22°C (16 h of light and 8 h of dark).
RT-PCR
For RT-PCR experiments, RNA was isolated from 3- to 4-d-old seedlings, roots of 2-week-old plants, leaves of 3- to 4-week-old plants, inflorescence stems and inflorescences of 5-week-old plants, and 2- to 10-d-after-pollination siliques. Total RNA was isolated using the RNeasy mini kit (Qiagen USA, Valencia, CA) and was treated with DNase I (Invitrogen, Carlsbad, CA). One microgram of RNA from different tissues was reverse transcribed into cDNA with oligo(dT) (16-mer) using Super Script II reverse transcriptase (Invitrogen). A fraction (about 1/20) of the first strand cDNAs was used as a template for PCR with gene-specific primers (Table I). PCR was carried out under standard conditions using 10 pmol of each primer and 27 (for APT1 gene) or 35 cycles (for most ASK genes) of 94°C for 30 s, 56°C to 66°C for 40 s, and 72°C for 60 s. A second PCR of 12 cycles (for ASK11 and ASK15) or 20 cycles (for ASK12) was performed with the same or nested primers (Table I). Control PCRs without reverse transcriptase did not produce any PCR bands. Fifty microliters of PCR products was separated on 1.5% (w/v) agarose gels containing ethidium bromide and visualized by UV light.
In Situ Hybridization
Samples of root, leaf, inflorescence stem, inflorescence, and silique were isolated from 3- or 4 week-old plants and were immediately fixed in an formaldehyde-acetic acid fixative. RNA in situ hybridizations with radioactive probes were performed as previously described (Drews et al., 1991; Flanagan and Ma, 1994). To avoid cross-hybridization, the sequences downstream of the stop codon were amplified by PCR with gene-specific primers (Table III) and cloned into pGEM-T vector (Promega, Madison, WI) for synthesizing probes.
Table III.
Primers for cloning in situ hybridization probes
| Gene Name | OMC No. | Primer Sequence 5′ to 3′ | Clone pMC No. |
|---|---|---|---|
| ASK1 | 438 | AAAAGCAGCAAGCAACCAGT | 2391 |
| 439 | TTAGCATTCTGTGGCGATTG | ||
| ASK2 | 440 | GTCATGTCCTCGACCCATTT | 2392 |
| 441 | CAATCTCTCGTGTGTGCTTGT | ||
| ASK3 | 659 | TTGGAAAAGAATTTAAGAACATTTGA | 2581 |
| 660 | CTCGGGCAAACATGTTATTGTA | ||
| ASK4 | 442 | GATCGGATCTGAAGGGTGTT | 2406 |
| 443 | TCCATTCGTTGTAACCAGCA | ||
| ASK5 | 456 | TCATCAAAGTTGTTGATGCAAA | 2398 |
| 457 | TCTGTTTCGGATTAGAAAACTCAA | ||
| ASK9 | 452 | GCGGATTATCAAAACCTAAACA | 2580 |
| 453 | AACAAGCCATAACCTGTCAGAAA | ||
| ASK11 | 444 | GCCCATGAATCAAAACCCTA | 2393 |
| 445 | CTCCCAAGAGGTGATATGCAG | ||
| ASK14 | 448 | TTGTCGGTTTTAGGGTTTTGA | 2395 |
| 449 | GGCCAATCATTTAGTTCCCA | ||
| ASK15 | 458 | TGTGTTTCGCTGTGTTGTTTC | 2399 |
| 459 | CGAAAATTTTGTTTGCTCTTCA | ||
| ASK18 | 450 | ATTTCGTTTCGAATTGCACA | 2396 |
| 451 | CATACACGCGTCGATCATTC | ||
| ASK19 | 446 | TCCTTTTACATGTATGATTTGTTTCTG | 2394 |
| 447 | TTTTTAACGGCGGCGACT |
Plasmid Constructs and Arabidopsis Transgenic Lines
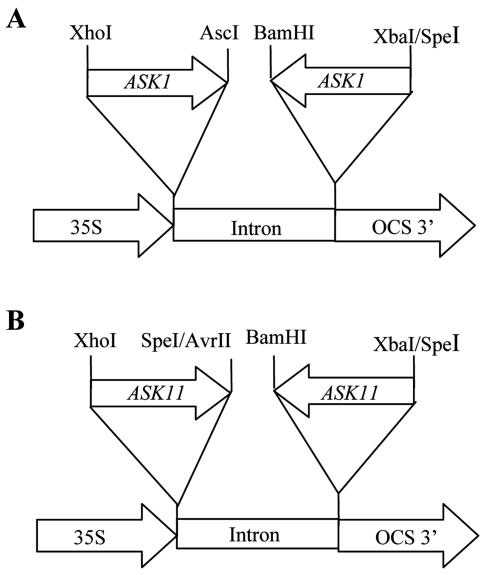
The RNAi vector pRR2222 was kindly provided by Dr. Ramesh Raina (Pennsylvania State University). It was derived from another RNAi vector pFGC1008 (kindly provided by Dr. Carolyn Napoli [University of Arizona, Tucson]) by replacing the β-glucuronidase fragment flanked by AscI and BamHI restriction sites in pFGC1008 with the first intron of the Arabidopsis GPA1 gene. To generate the ASK1 RNAi construct, the ASK1 coding region was amplified from the ASK1 cDNA with primers oMC529 (5′-TCACTAGTGAGCTCATAACCATGTCTGCGAAGAA-3′) and oMC 530 (5′-TCGGATCCGGCGCGCCGATAGTCATGATTCATGAAG-3′; an AscI site is underlined). The PCR product was cloned into the pCRII-TOPO vector (Invitrogen), resulting in the pMC2550 plasmid. The cloned ASK1 cDNA was then digested with XhoI (in ASK1 coding region) and AscI (introduced by oMC530) and was cloned into the XhoI and AscI sites of pRR2222 in the sense orientation. Finally, pMC2550 was digested with BamHI (introduced by oMC530) and XbaI (from pCRII-TOPO) and cloned in the antisense orientation into the BamHI and SpeI site of the pRR2222-derived plasmid with the sense ASK1 fragment to produce pMC2570 (Fig. 9A).
Figure 9.
ASK1 and ASK11 RNAi constructs.
The ASK11 RNAi vector was constructed in a similar way. The ASK11 cDNA was amplified from inflorescence of Ler ecotype using primers oMC 611 (5′-CCTCCTCCACAAGGAACACACAATG-3′) and oMC612 (5′-GCTAGTTAGGGTTTTGATTCATGGG-3′) and cloned into the pCRII-TOPO vector, resulting the plasmid pMC2569. The cloned ASK11 cDNA was then digested with XhoI and SpeI and cloned in the sense orientation into the XhoI and AvrII sites of pMC2560, a vector modified from pRR2222, yielding pMC2594. The pMC2560 plasmid was derived from pRR2222 by replacing the fragment flanked by XhoI and AscI restriction enzyme sites with the 3′-UTR of ASK1 gene amplified by oMC438 (Table III) and oMC577, the latter of which contains a same sequence with oMC439 (Table III) plus AvrII and AscI sites. The antisense ASK11 fragment was cloned into pMC2594 in the same way as that for ASK1, yielding pMC2600 (Fig. 9B). All constructs described above were confirmed by sequencing and were introduced into Arabidopsis plants by Agrobacterium tumefaciens-mediated in planta transformation (Clough and Bent, 1998). All plants were grown in the same condition, and the first 15 flowers were used for analysis.
To detect the expression of ASK genes in the transgenic plant, RT-PCR was carried out, with 25 cycles for ASK1 and ASK2, 28 cycles for ASK4, and 25 cycles followed by another 20 cycles for ASK11 and ASK12, and 35 cycles for ASK18. For each reaction, three replications were performed. Primers for ASK1 were oMC529 (describe above) and oMC799 (5′-GAGTAAGAAACATTGGTTCTTG-3′); primers for other genes are shown in Table I.
Acknowledgments
We thank E. Risseeuw and W.L. Crosby for kindly providing the ASK1, ASK2, and ASK4 cDNA clones and R. Raina for the generous gift of the pRR2222 vector. We also thank H. Kong for providing the ASK phylogenetic tree shown in Figure 1 and Y. Hu and R. Walsh for technical assistance with tissue section and in situ hybridization. In addition, we thank A. Omeis and J. Wang for plant care. We are grateful for helpful comments from C. Hendrix, W. Li, L.M. Zahn, G. Wang, H. Kong, and L. Timofejeva.
This work was supported by the National Science Foundation (grant nos. MCB-9896340 and MCB-0092075 to H.M.), by the National Institutes of Health (grant no. RO1 GM63871 to H.M.), and by Funds from the Department of Biology and the Huck Institute for Life Sciences at the Pennsylvania State University. T.H. was supported by China Scholarship Council and National Natural Science Foundation of China.
References
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796-815 [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ (1996) SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86: 263-274 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735-743 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Boniotti MB, Gutierrez C (2002) Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14: 3057-3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Estelle M (2000) F-box proteins and protein degradation: an emerging theme in cellular regulation. Plant Mol Biol 44: 123-128 [DOI] [PubMed] [Google Scholar]
- Dieterle M, Zhou YC, Schafer E, Funk M, Kretsch T (2001) EID1, an F-box protein involved in phytochrome A-specific light signaling. Genes Dev 15: 939-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews GN, Bowman JL, Meyerowitz EM (1991) Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65: 991-1002 [DOI] [PubMed] [Google Scholar]
- Farras R, Ferrando A, Jasik J, Kleinow T, Okresz L, Tiburcio A, Salchert K, del Pozo C, Schell J, Koncz C (2001) SKP1-SnRK protein kinase interactions mediate proteasomal binding of a plant SCF ubiquitin ligase. EMBO J 20: 2742-2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan CA, Ma H (1994) Spatially and temporally regulated expression of the MADS-box gene AGL2 in wild-type and mutant Arabidopsis flowers. Plant Mol Biol 26: 581-595 [DOI] [PubMed] [Google Scholar]
- Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD (2002) The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA 99: 11519-11524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitinligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271-276 [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425-479 [DOI] [PubMed] [Google Scholar]
- Ingram GC, Goodrich J, Wilkinson MD, Simon R, Haughn GW, Coen ES (1995) Parallels between UNUSUAL FLORAL ORGANS and FIMBRIATA, genes controlling flower development in Arabidopsis and Antirrhinum. Plant Cell 7: 1501-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O, Lane WS, Kaelin WG Jr, Elledge SJ, Conaway RC et al. (1999) Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284: 657-661 [DOI] [PubMed] [Google Scholar]
- Kim HS, Delaney TP (2002) Arabidopsis SON1 is an F-box protein that regulates a novel induced defense response independent of both salicylic acid and systemic acquired resistance. Plant Cell 14: 1469-1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos ET, Pagano M (2000) The F-box protein family. Genome Biol 1: REVIEWS3002.1-3002.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp DM, Harper JW, Elledge SJ (1999) How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97: 431-434 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rign L, Zeevaart JAD (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant 65: 33-39 [Google Scholar]
- Koornneef M, van der Veen JH (1980) Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Hehyn. Theor Appl Genet 58: 257-263 [DOI] [PubMed] [Google Scholar]
- Kuroda H, Takahashi N, Shimada H, Seki M, Shinozaki K, Matsui M (2002) Classification and expression analysis of Arabidopsis F-box-containing protein genes. Plant Cell Physiol 43: 1073-1085 [DOI] [PubMed] [Google Scholar]
- Levin JZ, Meyerowitz EM (1995) UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7: 529-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM (1991) AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev 5: 484-495 [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15: 1120-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Coupland G (2000) ZEITLUPE and FKF1: novel connections between flowering time and circadian clock control. Trends Plant Sci 5: 409-411 [DOI] [PubMed] [Google Scholar]
- Moffatt BA, McWhinnie EA, Agarwal SK, Schaff DA (1994) The adenine phosphoribosyltransferase-encoding gene of Arabidopsis thaliana. Gene 143: 211-216 [DOI] [PubMed] [Google Scholar]
- Nayak S, Santiago FE, Jin H, Lin D, Schedl T, Kipreos ET (2002) The Caenorhabditis elegans Skp1-related gene family: diverse functions in cell proliferation, morphogenesis, and meiosis. Curr Biol 12: 277-287 [DOI] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell Suppl 14: S61-S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503-533 [DOI] [PubMed] [Google Scholar]
- Porat R, Lu P, O'Neill SD (1998) Arabidopsis SKP1, a homologue of a cell cycle regulator gene, is predominantly expressed in meristematic cells. Planta 204: 345-351 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12: 198-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Klenz JE, Kohalmi SE, Risseeuw E, Haughn GW, Crosby WL (1999) The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant J 20: 433-445 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M et al. (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896-1898 [DOI] [PubMed] [Google Scholar]
- Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ER, Finnin MS, Elledge SJ, Harper JW, Pagano M, Pavletich NP (2000) Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature 408: 381-386 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng XW (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science 292: 1379-1382 [DOI] [PubMed] [Google Scholar]
- Seol JH, Feldman RM, Zachariae W, Shevchenko A, Correll CC, Lyapina S, Chi Y, Galova M, Claypool J, Sandmeyer S et al. (1999) Cdc53/cullin and the essential Hrt1 RING-H2 subunit of SCF define a ubiquitin ligase module that activates the E2 enzyme Cdc34. Genes Dev 13: 1614-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WH, Parmentier Y, Hellmann H, Lechner E, Dong A, Masson J, Granier F, Lepiniec L, Estelle M, Genschik P (2002) Null mutation of AtCUL1 causes arrest in early embryogenesis in Arabidopsis. Mol Biol Cell 13: 1916-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW (1997) F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91: 209-219 [DOI] [PubMed] [Google Scholar]
- Somers DE, Schultz TF, Milnamow M, Kay SA (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101: 319-329 [DOI] [PubMed] [Google Scholar]
- Steber CM, Cooney SE, McCourt P (1998) Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 149: 509-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI (2001) Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413: 316-322 [DOI] [PubMed] [Google Scholar]
- Willems AR, Goh T, Taylor L, Chernushevich I, Shevchenko A, Tyers M (1999) SCF ubiquitin protein ligases and phosphorylation-dependent proteolysis. Philos Trans R Soc Lond B Biol Sci 354: 1533-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW (1999) A family of mammalian F-box proteins. Curr Biol 9: 1180-1182 [DOI] [PubMed] [Google Scholar]
- Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG (2001) ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13: 1779-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091-1094 [DOI] [PubMed] [Google Scholar]
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14: 1919-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Yada M, Imaki H, Koga M, Ohshima Y, Nakayama KI (2002) Multiple Skp1-related proteins in Caenorhabditis elegans: diverse patterns of interaction with Cullins and F-Box proteins. Curr Biol 12: 267-275 [DOI] [PubMed] [Google Scholar]
- Yang M, Hu Y, Lodhi M, McCombie WR, Ma H (1999) The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc Natl Acad Sci USA 96: 11416-11421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, MeyerowitzEM (1990) The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346: 35-39 [DOI] [PubMed] [Google Scholar]
- Yu ZK, Gervais JL, Zhang H (1998) Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci USA 95: 11324-11329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kobayashi R, Galaktionov K, Beach D (1995) p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 82: 915-925 [DOI] [PubMed] [Google Scholar]
- Zhao D, Yang M, Solava J, Ma H (1999) The ASK1 gene regulates development and interacts with the UFO gene to control floral organ identity in Arabidopsis. Dev Genet 25: 209-223 [DOI] [PubMed] [Google Scholar]
- Zhao D, Yu Q, Chen M, Ma H (2001) The ASK1 gene regulates B function gene expression in cooperation with UFO and LEAFY in Arabidopsis. Development 128: 2735-2746 [DOI] [PubMed] [Google Scholar]
- Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M et al. (2002) Structure of the Cul1-Rbx1-Skp1-F box Skp2 SCF ubiquitin ligase complex. Nature 416: 703-709 [DOI] [PubMed] [Google Scholar]