Abstract
Dehydroepiandrosterone (D) is biosynthesized in the brain by a pathway different from that existing in the adrenal cortex. C6 rat glioma tumor cells in culture biosynthesize both pregnenolone (P) and D. They possess the mRNA, protein, and side-chain cleavage activity of P450scc. On the other hand, P450c17 was not detected. Adding FeSO4 to C6 cells increased the synthesis of both P and D. Even in the presence of aminoglutethimide, an inhibitor of P450scc, FeSO4 increased the synthesis of both steroids, indicating that the Fe2+-sensitive process does not involve P450scc. Likewise, the FeSO4-induced formation of D was not blocked by the P450c17 inhibitor, SU-10603. These results suggest that the FeSO4-induced synthesis of D as well as of P in C6 cells may be due to the fragmentation of in situ-formed tertiary hydroperoxides. It is likely, however, that the effect of the Fe2+ is not limited to this one reaction. When exogenous P was added to C6 microsomes, along with FeSO4, the amount of D formed was greater than control values, indicating that Fe2+ facilitated the conversion of P to D. Unlike the constituents that are converted by Fe2+ to P, the precursor of D in C6 cells is not soluble in a 1:1 mixture of ether and ethylacetate. Treatment of C6 cells with KI, NaBH4, or HIO4 resulted in an increase in D synthesis. From this it seems clear that a precursor of the D produced in C6 cells is a steroid where both C-17 and C-20 are oxygenated.
The specific interactions of steroids with binding sites in the brain (1) together with the rapid effects of various steroids on neuronal function (2) have prompted the investigation of the steroidogenic potential of central nervous system structures. The pioneering work of Baulieu and Robel demonstrated that pregnenolone (P) and dehydroepiandrosterone (D) accumulate in the brain independently of the supply by peripheral endocrine organs (3). In addition, these authors demonstrated that glial cells can convert cholesterol to P and thus make possible the availability of steroid metabolites as potential modulators of neuronal function. It has been shown that oligodendrocytes (4), a glioma cell line (5), and Schwann cells (6) express P450scc and have the ability to metabolize cholesterol to P. In other endocrine glands, P450c17 is the enzyme responsible for the conversion of P to D. D was the first neurosteroid to be described (7) and is one of the main neuroactive steroids found in brain (7, 8).
Despite these initial findings and numerous subsequent studies, the data available to date on the synthesis of neurosteroids does not account for the mechanisms responsible for their synthesis. First, the levels of the P450scc enzymatic activity, immunoreactivity, and mRNA are not consistent with each other (3, 9). Second, neither P450c17 protein nor its activity have been detected in brain (10) or glioma cells (11). Only a transient expression of the mRNA for this enzyme during embryonic life was reported (12), and contradictory data on the presence of its mRNA in the adult has been presented (9, 13, 14). Thus, the pathway by which D is synthesized in the brain is unknown, and it seems that brain steroid synthesis may not fit the well accepted scheme for adrenal and gonadal steroidogenesis and that alternate pathways may exist.
In 1994, Prasad and colleagues (15) presented evidence that showed that organic extracts of rat brain contain precursors which, upon treatment with various chemicals, especially FeSO4, liberate P and D. In the present paper, we report that in rat tumor glioma cells in culture, which do not possess the P450c17 enzyme, D biosynthesis may be mediated by alternative mechanisms.
MATERIALS AND METHODS
Materials.
[7-3H(N)]P (specific activity, 21.1 Ci/mmol; 1 Ci = 37 GBq), [1,2,6,7-3H(N)]progesterone (specific activity, 92 Ci/mmol), and [1,2,6,7-3H(N)]dehydroepiandrosterone (D) (specific activity, 89.2 Ci/mmol) were obtained from DuPont/New England Nuclear. Trilostane was a gift from Stegram Pharmaceuticals (Sussex, U.K.), and SU-10603 was from CIBA–Geigy. Aminogluthetamide (AMG) was obtained from Research Biochemicals. Cell culture supplies were purchased from GIBCO, and cell culture plasticware was from Corning. Electrophoresis reagents and materials were supplied from Bio-Rad. Sep-Pak Silica cartridges were purchased from Waters. Organic solvents were of HPLC grade purchased from Fluka and Fisher Scientific. All other chemicals were of analytical quality and were obtained from Sigma.
Cell Culture and Treatments.
The C6–2B clone of C6 rat glioma cell line (5) and MDA-231 human breast cancer cells (16) were maintained in DMEM supplemented with 10% fetal bovine serum/100 units/ml penicillin/100 μg/ml streptomycin at 37°C and 5% CO2 in 95% air. MA-10 mouse Leydig tumor cells were maintained in modified Waymouth’s MB752/1 medium containing 20 mM Hepes/1.2 g/liter NaHCO3/15% horse serum, pH 7.4, as previously described (17). Rat Leydig cells were isolated from 70-day-old Sprague–Dawley rats after enzymatic dissociation of the testes followed by discontinuous Percoll gradient centrifugation as we described (18). For all the experiments, glial, Leydig, as well as breast tumor cells each were cultured in 100-mm and 150-mm dishes. Cells were washed with serum-free medium to remove preexisting steroids and treated for 2 hr with the indicated concentrations of oxidizing and reducing agents (FeSO4, FeCl3, H2O2) in the presence or absence of 0.76 mM AMG, a specific inhibitor of P450scc (19). At the end of the incubation, the culture medium was quickly removed, and the remaining cells were collected by scraping and sonicated in 1 ml ice-cold deionized water. Aliquots of these sonicates were reserved for protein assay and the remainder were recombined with the culture medium to be processed for steroid extraction and isolation.
To determine whether a 17-hydroperoxide derivative is a likely precursor of D, C6 glial cells were treated with 1% solution of the reducing agent KI for 10 min at room temperature, then washed once with H2O2 (0.01%) to eliminate excess KI and twice with deionized water. Cells were then incubated with 20 mM aqueous solution of NaBH4 for 4 hr at 37°C, washed with 1% solution formaldehyde to destroy excess NaBH4, and subsequently treated with 20 mM aqueous solution of HIO4 for 2 hr at 37°C. The glial cells were processed as described above.
Steroid Isolation and Measurement.
Samples from cells treated and processed as described above were extracted with diethyl-ether/ethyl acetate (1:1, vol:vol); the organic phases were collected and then evaporated to dryness. In all samples, radiolabeled steroids were added to correct for the recovery of the extraction. The dried residues were resuspended in n-hexane and applied to Sep-Pak Silica cartridges, where the steroids of interest were eluted with n-hexane/isopropyl alcohol (95:5, vol:vol) as previously described (20). The steroids were then separated by HPLC (Beckman) by using a Beckman ultrasphere XL 3-μm Spherical 80 A pore column equilibrated with methanol (50%) in water and eluted with a 1 ml/min flow rate with a 50–100% gradient of methanol. Steroids were identified by their respective retention time (Rt) compared with radiolabeled steroid standards (Rt for P = 30 min, Rt for progesterone = 24 min; Rt for D = 18 min). In other experiments, unrelated to those described in this paper, this isolation procedure provided samples in which P, D, and progesterone were identified by GC coupled to MS.
Steroid contents were quantified by using specific RIAs. Antisera to P, D, and progesterone were obtained from ICN, and the assays were performed as described by the manufacturer. The sensitivity of the RIAs was 10 pg. The analysis of the RIA data was performed by using the IBM PC ria data reduction program (version 4.1) obtained from Jaffe and Associates (Silver Spring, MD).
Reverse Transcriptase (RT)–PCR.
Total cellular RNA from C6 glioma and MA-10 Leydig cells was isolated by acid-guanidium thiocyanate-phenol-chloroform extraction method (21) by using the RNAzol B reagent (Tel-Test, Friendswood, TX). Reverse transcription and PCR were carried out according to Strömsted and Waterman (13) by using the Gene Amp RNA PCR kit (Perkin–Elmer), 1 μg of total RNA as template, and 20 μM of specific primers. The primers used for PCR amplification were as follows: (i) for the P450scc, sense CAACATCACAGAGATGCTGGCAGG and antisense CTCAGGCATCAGGATGAG GTTGAA, and (ii) for the P450c17, sense CCCATCTATTCTCTTCGCCTGGGTA and antisense GCCCCAAAGATGTCTCCCACCGTG. PCR products were resolved on 1.5% agarose electrophoresis gels containing 1 μg/ml ethidium bromide. The amplified fragments were recovered and purified by using the Quiaquick gel extraction kit (Qiagen). The identity of the generated PCR products was confirmed by automatic sequencing, which was carried out by using the ABI Prism Dye Terminator Cycle Sequencing ready reaction kit (Perkin–Elmer). DNA sequencing was performed at the Lombardi Cancer Center Sequencing Core Facility (Georgetown University Medical Center).
D Synthesis in Microsomes.
Microsomes from C6 glioma cells were prepared by differential centrifugation as previously described (22). Briefly, the cells were homogenized with 0.25 M sucrose in a glass–Teflon homogenizer. Homogenates were centrifuged for 10 min at 1,000 × g, the pellets were discarded, and the supernatants were centrifuged for 10 min at 10,000 × g to eliminate the mitochondrial fraction of the cells. The supernatants from the mitochondrial fraction were collected and centrifuged for 60 min at 120,000 × g. Microsomal pellets were washed and suspended in 50 mM Tris-maleate buffer (pH 7.4) to a final protein concentration of 5 mg/ml.
To test the P450c17 enzymatic activity, 100 μg of the microsomal protein fraction was incubated in Tris-maleate buffer in the presence of 600 μM NADPH, 10 mM glucose/6-phosphate, 1,500 units/liter glucose-6-phosphate dehydrogenase together with the substrate P (50 μM) (23) in the presence or absence of the inhibitors of P450c17, SU-10603 (5 μM), and 3β-hydroxysteroid dehydrogenase, trilostane (5 μM). In addition, to test the hypothesis that a process responsible for D formation could occur in the microsomal fractions, microsomes were also incubated with 50 μM P or cholesterol in the presence of 10 mM FeSO4. All the incubations were carried out at 37°C in 5% CO2 atmosphere for 20 min and stopped by the addition of cold ethanol. Steroids were extracted by using diethyl ether, separated by HPLC, and quantified by RIA. GC/MS analysis of the samples was performed on an HP5890 GC coupled to an HP5988A mass spectrometer as previously described (24). The identity of D was established by its Rt on GC (8.8 mm), identical with that of an authentic sample, and the m/e values of diagnostically important ions, 288, 270, and 255. Quantification was obtained from the area under the ion peak (288) when compared with a standard curve.
Miscellaneous.
Protein concentration was determined by the protein–dye binding assay of Bradford (25), using BSA as standard. Statistical analysis of the data was performed by ANOVA followed by the Student–Newman–Kauls test by using the instat 2.04 package from GraphPad (San Diego).
RESULTS
C6 rat glioma cells contain P450scc immunoreactive protein (5) and have the ability to synthesize P from the precursor mevalonolactone (20). In addition, C6 glioma cell mitochondria incubated with the cholesterol derivative, (22R)-22-hydroxycholesterol, synthesize P in an AMG-sensitive manner, further suggesting the presence of an active P450scc (5). In the present studies, we isolated and measured P, progesterone, and D production by the C6 cells incubated under basal conditions. Table 1 shows that C6 cells produce measurable amounts of all three steroids. Because the presence of lipid-soluble constituents (peroxides and others) that may be precursors of P and D have been demonstrated (15), we used C6 glial cells to investigate this mechanism of neurosteroid synthesis. Treatment of glioma cells with various agents resulted in the detection of increased amounts of P and D (Table 1). Of those chemicals used, the reducing agent FeSO4 was the most revealing. FeSO4 induced a 3.6- and 4.9-fold increase in P and D formation, respectively, compared with basal levels. No effect of FeSO4 addition on progesterone formation was observed. MA-10 mouse Leydig cells were also used as a representative model of steroidogenesis. Treatment with FeSO4 induced a 2-fold increase in the amount of D found in the Leydig cells, without affecting P and progesterone formation. Both FeCl3 and H2O2 increased by 2-fold the amounts of P and D found in C6 cells, but these increments were less than that found by treatment with FeSO4.
Table 1.
Effect of various chemicals on steroid synthesis by C6 glioma and MA-10 Leydig cells
| Cell type | Treatment | P, fold increase | Progesterone | D | N (n) |
|---|---|---|---|---|---|
| C6 | Control | 1.0 | 1.0 | 1.0 | 4 (12) |
| FeSO4, 10 mM | 3.6 | 1.0 | 4.9 | 4 (18) | |
| FeCl3, 10 mM | 2.0 | 2.4 | 2.8 | 2 (4) | |
| H2O2, 10 mM | 2.0 | 1.0 | 2.0 | 1 (3) | |
| MA-10 | Control | 1.0 | 1.0 | 1.0 | 2 (5) |
| FeSO4, 10 mM | 1.2 | 1.0 | 2.0 | 2 (5) | |
| FeCl3, 10 mM | 1.1 | 1.0 | 1.8 | 2 (5) | |
| H2O2, 10 mM | 1.5 | 1.0 | 1.0 | 2 (5) |
Cell treatment, steroid isolation, and measurement were performed as described in Materials and Methods. Results shown are expressed as fold increase above control values. Basal values for C6 glioma cells are: P, 70 pg/mg protein; progesterone, 32 pg/mg protein; D, 4 pg/mg protein. Basal values for MA-10 Leydig cells are: P, 2,200 pg/mg protein; progesterone, 2,700 pg/mg protein; D, 7.4 pg/mg protein. N, number of experiments; n, number of samples.
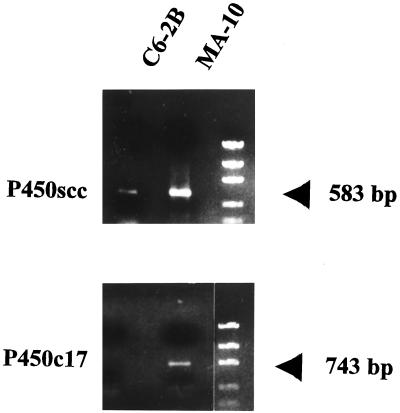
To examine the possibility that D formation was due to P450c17 activity we investigated the mRNA expression of this enzyme by RT-PCR using primers designed from the published rat P450scc and P450c17 nucleotide sequences. Fig. 1 shows that, as expected, P450scc mRNA is present in both the C6 glioma and MA-10 Leydig tumor cells. RT-PCR amplification did not result in the detection of P450c17 mRNA in glial cells, although P450c17 mRNA was found in the Leydig cells. The amplified P450scc fragments from both the glial and Leydig cells, and P450c17 fragment from the Leydig cells had the expected size of 583 bp and 743 bp, respectively, and their identity was confirmed by sequencing.
Figure 1.
Ethidium bromide-stained P450scc and P450c17 DNA fragments generated by RT-PCR from C6 and MA-10 total cell RNA. Conditions for RNA isolation, cDNA preparation, and amplification using specific primers are described in Materials and Methods. Standards shown have molecular sizes of 2,000, 1,200, 800, 400, and 200 bp.
Based on the above data we then examined the effects of FeSO4 on glioma and Leydig cell steroidogenesis. Fig. 2A and B shows P and D synthesis by the C6 cells after treatment with increasing concentrations of FeSO4. The amounts of the steroids measured were increased by FeSO4 in a dose-dependent manner. Maximal increases were obtained at a concentration of 10 mM FeSO4, where P and D formation was increased by 10-fold. Treatment of the cells with FeSO4 in the presence of the P450scc inhibitor AMG partially blocked the FeSO4-dependent P formation but had little effect on the FeSO4-induced D production. Because the addition of AMG obliterated P formation in the control glial cells (Fig. 2A), the P formed in the presence of FeSO4 appears to have been derived from a FeSO4-stimulated pathway where the effect of AMG is questionable. In contrast to the brain tumor cells, P formation in the Leydig cells was not increased by FeSO4. Although AMG abolished basal P formation by the MA-10 cells, FeSO4 increased P levels in the presence of AMG (Fig. 2C). A comparison of the amount of P found when both AMG and FeSO4 were added with that found when only AMG was present (Fig. 2C) suggests that the former could have come from a P450scc-independent pathway. D levels were also increased after treatment with 10 mM FeSO4 (Fig. 2D). This effect was not altered by AMG in the MA-10 Leydig cells, suggesting that D formed in the presence of both AMG and FeSO4 was derived from a pathway that did not involve P synthesized by the P450scc enzyme.
Figure 2.
FeSO4-induced P (A and C) and D (B and D) formation by C6 glioma (A and B) and MA-10 Leydig (C and D) cells. Cells were treated for 2 hr with the indicated concentrations of FeSO4 or with 10 mM FeSO4 in the presence of 0.76 mM AMG. Steroids produced were extracted, isolated, and quantified as described in Materials and Methods. Data shown are means ± SD from an experiment performed in triplicate. Similar results were obtained in three other independent experiments. t = incubation time of 2 hr; a, statistics performed compared with control; b, statistics performed compared with AMG treatment alone; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
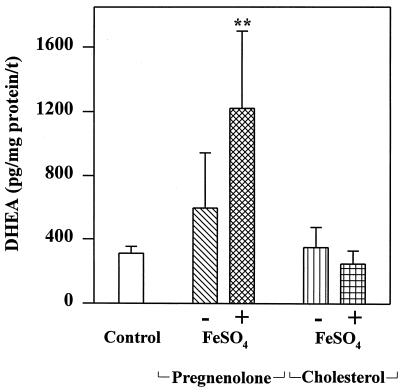
Microsomes prepared from C6 glial cells were found to contain the constituents that are stimulated by Fe2+ to produce D. Microsomal fractions treated with FeSO4 in the presence of the known inhibitor of the 3β-hydroxysteroid dehydrogenase, trilostane (26), and SU-10603, an inhibitor of P450c17 (19), make as much D (1,221 ± 478 pg D/mg protein per 20 min; n = 3) as they did in their absence (1,235 ± 542 pg D/mg protein per 20 min; n = 3). As shown in Fig. 3, addition of both P and FeSO4 to microsomes from C6 cells in the presence of both trilostane and SU-10603 resulted in a statistically significant increase in D formation over the control. Addition of cholesterol had no such effect. Fractions considered to be D from many experiments were pooled. The pooled sample had 4 ng D as determined by RIA. This was then submitted to GC/MS for qualitative and quantitative analysis and found to contain 3 ng D, thus validating the assay used. Ordinarily this finding would be taken to suggest that P is the precursor of the D found in the control; however, such an in vitro experiment reveals only what is possible but does not prove what actually occurs in situ. If D was in fact formed from P, the data shown in Fig. 2 indicate that that P was synthesized by a process not catalyzed by P450scc.
Figure 3.
D formation by C6 glioma cell microsomes incubated in the presence of the substrate P (50 μM) or cholesterol (50 μM) and inhibitors of P450c17, SU-10603 (5 μM), and 3β-hydroxysteroid dehydrogenase, trilostane (5 μM). Microsomes were treated with or without 10 mM FeSO4. D was extracted, isolated, and measured as described in Materials and Methods. Data shown are means ± SD from an experiment performed in triplicate. Similar results were obtained in two other separate experiments. Incubation time was 20 min; ∗∗, P < 0.01.
We probed the nature of the precursor of D by using successive treatments with reducing and oxidizing agents as described under Materials and Methods. Table 2 shows data from an experiment where an in situ precursor was reduced by KI followed by successive reduction with NaBH4 and oxidation with HIO4. These probes increased the amount of D measured above that which existed in untreated cells. D in control dishes presumably was reduced to the diol by NaBH4. P was not formed in this experiment. Treatment of the cells with only one of the three reagents (either KI, NaBH4, or HIO4) did not produce any increment in the amount of D formed over the control (data not shown).
Table 2.
D formation by cells treated with the reducing agent KI followed by successive reduction and oxidation with NaBH4 and HIO4
| Cell type | Control, pg per dish | Treated, pg per dish |
|---|---|---|
| C6 | 1.4 | 20 |
| MA-10 | 9.2 | 17 |
Results shown represent the means from a representative experiment (n = 3). Similar results were obtained in a second, separate experiment.
Because the generation of oxygen-free radicals by the addition of Fe2+ to cells is a well known phenomenon (27, 28), we examined the possibility that the Fe2+-sensitive alternative pathway reported here is a nonspecific mechanism not related to steroidogenesis. Were this the case, this nonspecific mechanism should be present in nonsteroid synthesizing tissues. MDA-231 human breast cancer cells were used to test this hypothesis. Using similar techniques, we were unable to detect any steroid formation by these cells either under basal conditions or after FeSO4 treatment. In addition, we also used purified rat Leydig cells, which have the ability to synthesize androgens, as a control. FeSO4 treatment failed to induce the formation of androgens (D and testosterone) by these cells (data not shown). Also, FeSO4 treatment did not affect the inhibitory activity of the enzyme inhibitors, trilostane and SU-10603, when examined in a cell system (normal rat Leydig cells) possessing both the 3β-hydroxysteroid dehydrogenase and P450c17 activities (data not shown).
We also examined the possibility that the precursor of P and D can be found in organic extracts of the cells as previously reported by Prasad et al. (15) for extracts of lyophilized rat brains. Organic extracts of C6 cells treated with FeSO4 liberated P (69 pg/mg protein vs. 33 pg/mg protein control), but, surprisingly, the Fe2+-sensitive precursor of D was not detected in organic extracts of C6 cells.
DISCUSSION
The levels of D in brain are distinct from those in plasma, and its function as neuroactive steroid at the type A γ-aminobutyric acid and N-methyl-d-aspartate receptor level has been well established (2, 8). Understanding the mechanism of D formation in brain, however, is paramount to all further speculation and hypotheses about D and its role in normal and pathologic brain function. The findings reported in this paper indicate that rat tumor glioma cells, which do not contain the enzyme P450c17, are nonetheless able to produce D through alternative pathways. The same pathway also exists in MA-10 Leydig tumor cells. However, in Leydig tumor cells this process accounts for a small part of the steroids produced, suggesting that in this steroidogenic tissue, the principal pathway by means of which D is biosynthesized involves P450c17. The absence of any steroid formation by human breast cancer cells and by purified androgen-synthesizing normal rat Leydig cells by this alternative process suggests that the phenomenon is tissue-specific. At present, we identified this process only in rat glioma and Leydig tumor cells. In view of the fact that the P450c17 enzyme protein and activity have not yet been found in rat and guinea pig brain (8, 9), where high levels of D were measured, it may be possible that this D arises from a similar alternative process. We have yet to examine normal brain tissue.
One alternative pathway appears to involve hydroperoxides or peroxides because the reducing agent FeSO4, could react with the endogenous precursor(s), present in C6 glial cells and to a lesser extent in MA-10 Leydig cells, to form the ketones, P and D. These findings are in accord with those of Prasad et al. (15), who observed that treatment of organic extracts of rat brains with FeSO4 produced larger amounts of P, as estimated by mass spectrometric analysis, than were present in untreated extracts. Thus, P could be formed in brain either by the pathway (4, 5, 9), mediated by the P450scc, or by an alternative pathway from an as yet unknown cholesterol metabolite, which is present in organic extracts of the cells, or by both.
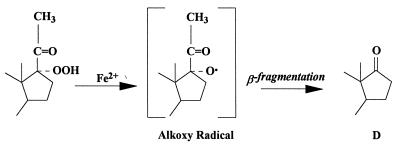
Addition of FeSO4 directly to glial cells in culture resulted in a 5- to 10-fold increase in D production. We have interpreted the results obtained with the addition of FeSO4 as indicating that this increase in D is due to the fragmentation of an in situ-formed tertiary hydroperoxide initiated by Fe2+. It has been shown that hydroperoxides react with FeSO4 to yield ketones (29). The reaction probably involves reduction of the hydroperoxide to the intermediate alkoxy radical sufficiently caged to allow it to fragment by β-scission to the ketone group (Fig. 4). In contrast to the precursor of P, the D precursor was not found in the organic extracts of glial cells (this work), and in fact it was present in limited amounts in the organic extracts of rat brain (15). These data suggest that the process leading to the formation of D need not necessarily be associated with that producing peroxy precursors of P, some of which are organic soluble.
Figure 4.
Mechanism of ketone formation from hydroperoxides. This scheme illustrates how the addition of FeSO4 might reduce the 17-hydroperoxide of P to an intermediate alkoxy radical (29). Cleavage of the two-carbon side chain by β-fragmentation results in the formation of D.
Evidence already exists that mammalian tissues contain enzymes that catalyze the fragmentation of peroxy constituents to steroid ketones. Larroque and van Lier (30) found that when 20-hydroperoxycholesterol was incubated with purified P450scc for only 30 sec at 0°C, it was readily converted to P. The heme content of the P450scc used was about 9 nmol/mg protein. Because the theoretical value of the heme content of P450scc is about 17 nmol/mg protein, the exact enzyme affecting the conversion is uncertain, but the results show that tissue constituents can fragment peroxy substrates. Moreover, as early as 1975, Tan and Rousseau (31) showed that the rat testis microsomal fraction could convert 17-hydroperoxyprogesterone to androstenedione when incubated in the presence of oxygen and NADPH. The cofactor was essential, but oxygen was not, because argon could substitute for it. These authors also reported that the 17-hydroperoxide could be trapped when progesterone was incubated with adrenal homogenates in the presence of the hydroxylase inhibitor, p-hydroxymercuri-benzoate (31).
Adding exogenous P along with FeSO4 to C6 microsomes resulted in a large increase in the amount of D formed. This example, perhaps the first, of a mammalian brain cell converting P to D indicates that Fe2+ can activate the conversion process, which may, in fact, involve hydroperoxylation at C-17 of the added P.
Although the Fe2+ ion is a pleiotropic agent in the central nervous system, the specificity of its reaction with endogenous precursors to form D is characterized by the following observations: (i) the effect is specific for the formation of D because no effect on progesterone production is seen; (ii) the effect appears to be tissue-specific; (iii) its action is found in the microsomal fraction; (iv) its effect is dose-dependent but not in a stoichiometric manner; and (v) the effect of FeSO4 could not be replicated to the same extent when using FeCl3 or H2O2.
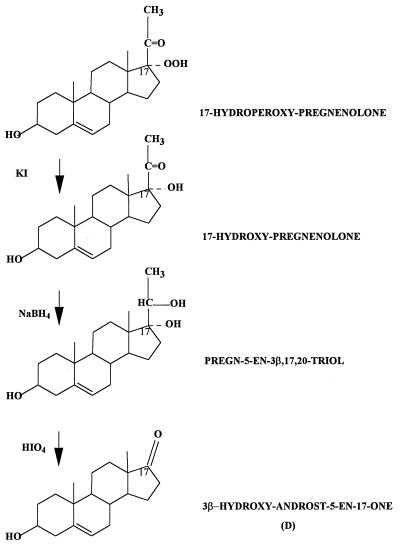
In an effort to obtain information about the endogenous peroxy precursors of D, C6 cells were treated with KI (to reduce peroxy compounds to their corresponding alcohols), then with NaBH4 (to reduce ketones, including preexisting D, to alcohols), and finally with HIO4 (to oxidize glycols to carbonyl products). Assuming that the reagents (KI, NaBH4, and HIO4) react as proposed, the newly formed D resulting from this sequence of reactions suggests that a C-17,C-20 glycol was the proximal precursor of this D. Treatment of the cells with only one of the three reagents (either KI, NaBH4, or HIO4) did not produce any increment in the amount of D formed over the control. The steroid glycol that is most obvious is pregn-5-ene-3β,17,20-triol, in which case its peroxy precursor is 17-hydroperoxide of P. It is noteworthy that treatment with the above-mentioned reagents did not result in the formation of P. As mentioned before, the formation of P by Fe2+ treatment in the presence of the inhibitor AMG indicates that this P is not produced by a process involving P450scc. The failure to produce P by the above sequence of reactions is particularly noteworthy because it supports the notion that the biosynthetic pathways for P and D, resulting from Fe2+ treatment, are not necessarily connected. Moreover, the evidence presented does not exclude other peroxy precursors, particularly one derived from a sterol, like cholesterol, such as a 17,20-dioxygenated derivative. Fig. 5 shows an alternative pathway suggested by the results obtained by using KI, NaBH4, and HIO4 in tandem. This alternative is illustrated by using the 17-hydroperoxide of P as a model.
Figure 5.
Schematic representation of an alternative pathway by using the precursor 17-hydroperoxide of P as a model.
The observation that the peroxy precursor of D is not soluble in organic solvents suggests the potential requirement for a cellular component for activity. The effect of Fe2+ on the formation of D may be mediated by a proteinaceous microsomal component associated with iron reduction. Such a possibility was recently suggested by Tampo and Yonaha (32), who presented evidence indicating that a heat-labile component of the rat liver microsomal fraction was responsible for NADPH-supported lipid peroxidation. Although the peroxy precursor(s) of D made in C6 cells was not soluble in organic solvents, treatment of the intact cells with FeSO4 nevertheless produced 5–10 times as much D as was in the control. From this it appears that the process for making D in C6 cells is more complicated than that which simply involves fragmentation of peroxy compounds. Fe2+ ions undoubtedly affect many cellular processes, including those that stimulate oxygenases and hydroxylases. It is also conceivable that Fe2+ forms complexes with constituents within the C6 cells that are able to mimic the catalytic oxidative behavior of a P450. Previous reports (33, 34) suggest that the level of activation of O2 is similar to that for Fenton reagents and P450 hydroxylases (34), and thus it can affect oxygen insertion at a C17 bond. Therefore, it is not possible now to be certain of the mechanism(s) by which Fe2+ evokes an increase in D production by C6 cells. But the absence in these glial cells of P450c17 activity, protein, and mRNA indicates that whatever the process leading to the formation of D in these cells is, it is different from that involving this steroidogenic enzyme. Even if Fe2+ merely stimulates an existing enzyme to produce D, the precursor of that D also appears to be different from the precursor customarily assumed to be used in adrenals.
In conclusion, these results demonstrate that D is synthesized in the brain by P450c17-independent pathways. Thus, the identification of this new process for D biosynthesis may provide the answer to the mystery surrounding the biosynthesis of this neuroactive steroid in brain.
Acknowledgments
The authors thank Dr. M. Ascoli (University of Iowa) for providing the MA-10 cells, Stegram Pharmaceuticals for the gift of trilostane, and CIBA–Geigy for the gift of SU-10603. This work was supported by a grant from the National Science Foundation, IBN-9409551, to V.P. V.P. was supported by a Research Career Development Award (HD-01031) from the National Institute of Child Health and Human Development, National Institutes of Health.
ABBREVIATIONS
- P
pregnenolone/3β-hydroxypregn-5-en-20-one
- D
dehydroepiandrosterone, 3β-hydroxyandrost-5-en-17-one (commonly called DHEA)
- AMG
aminoglutethimide
- RT
reverse transcriptase
References
- 1.Brann D, Henry L B, Mahesh V B. J Steroid Biochem Mol Biol. 1995;52:113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- 2.Paul S M, Purdy R H. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 3.Baulieu E E, Robel P. J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 4.Jung-Testas I, Hu Z Y, Baulieu E E, Robel P. Endocrinology. 1989;125:2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos V, Guarneri P, Krueger K E, Guidotti A, Costa E. Proc Natl Acad Sci USA. 1992;89:5113–5117. doi: 10.1073/pnas.89.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akwa Y, Schumacher M, Jung-Testas I, Baulieu E E. C R Acad Sci III (France) 1993;316:410–414. [PubMed] [Google Scholar]
- 7.Coprechot C, Robel P, Axelson M, Sjovall J, Baulieu E E. Proc Natl Acad Sci USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baulieu E E. J Clin Endocr Metab. 1996;81:3147–3151. doi: 10.1210/jcem.81.9.8784058. [DOI] [PubMed] [Google Scholar]
- 9.Mellon S H, Deschepper C F. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 10.Le Goascogne C, Sananes N, Gouezou M, Takemori S, Kominami S, Baulieu E E, Robel P. J Reprod Fertil. 1991;93:609–622. doi: 10.1530/jrf.0.0930609. [DOI] [PubMed] [Google Scholar]
- 11.Cascio C, Guarneri P, Li H, Brown R C, Amri H, Boujrad N, Kotoula M, Vidic B, Drieu K, Papadopoulos V. In: Neurosteroids: A New Regulatory Function in the Central Nervous System, Contemporary Endocrinology. Baulieu E E, Robel P, Schumacher M, editors. Clifton, NJ: Humana; 1997. , in press. [Google Scholar]
- 12.Compagnone N A, Bulfone A, Rubenstein J L R, Mellon S H. Endocrinology. 1995;136:5212–5223. doi: 10.1210/endo.136.11.7588260. [DOI] [PubMed] [Google Scholar]
- 13.Strömsted M, Waterman M R. Mol Brain Res. 1995;34:75–88. doi: 10.1016/0169-328x(95)00140-n. [DOI] [PubMed] [Google Scholar]
- 14.Sanne J-L, Krueger K E. Gene. 1995;165:327–328. doi: 10.1016/0378-1119(95)00536-f. [DOI] [PubMed] [Google Scholar]
- 15.Prasad V V K, Vegesna S R, Welch M, Lieberman S. Proc Natl Acad Sci USA. 1994;91:3220–3223. doi: 10.1073/pnas.91.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culty M, Shizari M, Thompson E W, Underhill C B. J Cell Physiol. 1994;160:275–286. doi: 10.1002/jcp.1041600209. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos V, Mukhin A G, Costa E, Krueger K E. J Biol Chem. 1990;265:3772–3779. [PubMed] [Google Scholar]
- 18.Papadopoulos V, Carreau S, Drosdowsky M A. FEBS Lett. 1985;188:312–316. doi: 10.1016/0014-5793(85)80393-8. [DOI] [PubMed] [Google Scholar]
- 19.Gower D B. J Steroid Biochem. 1974;5:501–523. doi: 10.1016/0022-4731(74)90051-x. [DOI] [PubMed] [Google Scholar]
- 20.Guarneri P, Papadopoulos V, Pan B, Costa E. Proc Natl Acad Sci USA. 1992;89:5118–5122. doi: 10.1073/pnas.89.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos V, Jia M, Culty M, Hall P, Dym M. In Vitro Cell Dev Biol. 1993;29A:943–949. doi: 10.1007/BF02634233. [DOI] [PubMed] [Google Scholar]
- 23.Albertson B D, Hill R B, Sprague K A, Wood K E, Nieman L K, Loriaux L D. Eur J Endocrinol. 1994;130:195–200. doi: 10.1530/eje.0.1300195. [DOI] [PubMed] [Google Scholar]
- 24.Mathur C, Prasad V V K, Raju V S, Welch M, Lieberman S. Proc Natl Acad Sci USA. 1993;90:85–88. doi: 10.1073/pnas.90.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Potts J O, Creange J E, Harding H R, Schane H P. Steroids. 1978;32:257–267. doi: 10.1016/0039-128x(78)90010-7. [DOI] [PubMed] [Google Scholar]
- 27.Gutteridge J M. Ann Neurol. 1992;32:S16–S21. doi: 10.1002/ana.410320705. [DOI] [PubMed] [Google Scholar]
- 28.Kumar U, Dunlop D M, Richardson J S. Life Sci. 1994;54:1855–1860. doi: 10.1016/0024-3205(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 29.Kharasch M S, Fono A, Nudenberg W. J Org Chem. 1950;15:763–774. [Google Scholar]
- 30.Larroque C, van Lier J E. J Biol Chem. 1986;261:1083–1087. [PubMed] [Google Scholar]
- 31.Tan L, Rousseau J. Biochem Biophys Res Commun. 1975;65:1320–1326. doi: 10.1016/s0006-291x(75)80374-3. [DOI] [PubMed] [Google Scholar]
- 32.Tampo Y, Yonaha M. Lipids. 1995;20:55–62. doi: 10.1007/BF02537042. [DOI] [PubMed] [Google Scholar]
- 33.Parton R F, Vankelecom I F, Casselman M J, Bezoukhanova C P, Uytterhoeven J B, Jacobs P A. Nature (London) 1994;370:541–544. doi: 10.1038/370541a0. [DOI] [PubMed] [Google Scholar]
- 34.Sawyer T T, Liu X, Redman C, Chong B. Bioorg Med Chem. 1994;2:1385–1395. doi: 10.1016/s0968-0896(00)82090-8. [DOI] [PubMed] [Google Scholar]