Abstract
A cDNA encoding cytosolic glutamine synthetase (GS) from Lotus japonicus was fused in the antisense orientation relative to the nodule-specific LBC3 promoter of soybean (Glycine max) and introduced into L. japonicus via transformation with Agrobacterium tumefaciens. Among the 12 independent transformed lines into which the construct was introduced, some of them showed diminished levels of GS1 mRNA and lower levels of GS activity. Three of these lines were selected and their T1 progeny was further analyzed both for plant biomass production and carbon and nitrogen (N) metabolites content under symbiotic N-fixing conditions. Analysis of these plants revealed an increase in fresh weight in nodules, roots and shoots. The reduction in GS activity was found to correlate with an increase in amino acid content of the nodules, which was primarily due to an increase in asparagine content. Thus, this study supports the hypothesis that when GS becomes limiting, other enzymes (e.g. asparagine synthetase) that have the capacity to assimilate ammonium may be important in controlling the flux of reduced N in temperate legumes such as L. japonicus. Whether these alternative metabolic pathways are important in the control of plant biomass production still remains to be fully elucidated.
Nitrogen (N) is one of the major limiting factors for plant growth. However, an excessive external supply of N causes major problems in agriculture and the environment, mostly due to nitrate leaching into underground water (Benes et al., 1989), not only polluting the aquatic environment but also resulting in a high nitrate content in food and drinks (Moller et al., 1990). Paradoxically, dinitrogen is 80% of the atmosphere, but it can only be assimilated by prokaryotic symbiotic or free-living diazotrophic organisms possessing nitrogenase activity (Dilworth, 1974). In higher plants, nitrate and ammonium are the two major inorganic N compounds that can be directly assimilated (Beevers, 1976). They are provided either artificially by the external supply of fertilizers, bacterial nitrification, or atmospheric reduction of dinitrogen during lightning, or naturally through biological N fixation. Therefore, regulation of inorganic N assimilation and incorporation of inorganic N into organic matter is of major importance not only in maintaining a sustainable agriculture but also protecting the environment.
Over the past few years, attention has been particularly focused on the enzyme Gln synthetase (GS; E.C. 6.3.1.2) because of its central role in N metabolism and its diverse metabolic and developmental regulation in different plant species and organs (Cren and Hirel, 1999). Two major isoforms exist for the GS enzyme: cytosolic GS, occurring in the cytoplasm of leaves and non photosynthetic organs, and chloroplastic GS, present only in the chloroplasts of photosynthetic tissues and the plastids of roots or etiolated plants. The relative proportions of the cytosolic and plastidial GS activity may vary within different organs of the same plant or within different plant species depending on their photosynthetic type (McNally et al., 1983). The occurrence of two distinct GS isoforms located in two different cellular compartments led to the proposal that each GS isoenzyme had a specific function in assimilating or reassimilating ammonium derived from a variety of processes such as nitrate reduction, photorespiration, or N recycling (Lea and Ireland, 1999). In addition, a number of studies have shown that GS is encoded by a multigene family, and each isoenzyme may be constituted by one or several gene products (Cren and Hirel, 1999). The expression of each gene appears to be tissue specific or regulated by external factors such as light, the N source, and the symbiotic association with Rhizobium loti (Cren and Hirel, 1999). Moreover, it has been demonstrated recently that in addition to organ- or tissue-specific expression, a number of GS genes can be differentially regulated by several metabolites, including amino acids and soluble carbohydrates (Oliveira and Coruzzi, 1999).
These recent findings suggest that the metabolic status of individual organs and/or tissues may be as important as developmental regulation in efficiently controlling the pathway of ammonium assimilation in the plant kingdom. The pattern of expression of the various cytosolic GSs has been most thoroughly investigated in legumes where GS is actively synthesized in root nodules to assimilate the large supply of ammonium produced by the symbiotic N-fixing bacteroids (Robertson et al., 1975; Lara et al., 1983). Although different from one legume species to the other (temperate legumes versus tropical legumes forming either determinate or indeterminate nodules), it has been shown clearly that metabolic and/or developmental events control the expression of some members of the nodule GS multigene family in a tissue-specific manner. Moreover, each of these genes is differentially transcribed and translated to allow the synthesis of a polypeptide component of the native GS enzymes (Cren and Hirel, 1999). In all legume species studied so far, it has been found that compared with roots or shoots, higher cytosolic GS activity was always present in nodules where ammonium resulting from N2 fixation is diffused out from the bacteroids to the host cytoplasm. However, the exact role of various GS isoenzymes identified in the different nodules cell types is still not fully understood. In particular, recent findings have shown that final nodule GS activity is highly regulated and subjected to a multiple step coordinated process, starting at the transcriptional level up to the control of the holoenzyme turnover (Ortega et al., 1999). This complexity seems to be consistent with the fact that most legumes have a greater potential to assimilate major amounts of N in their root system whether or not they are under atmospheric N-fixing conditions. However, in these species, the efficiency of root N assimilation has been questioned because of a possible competition with shoot inorganic N assimilation (Oaks, 1992).
Therefore, considering both the economical and ecological importance of atmospheric N-fixing symbiosis (Shantharam and Mattoo, 1997) and the unique developmental and molecular events associated with nodule development, a large number of studies have been performed to unravel the regulatory mechanisms controlling ammonium assimilation both in the bacterium and the host (Waters et al., 1998). However, despite the significant progress made in understanding the physiological and molecular mechanisms involved during the establishment of the symbiosis, there has been little success in either enhancing biological N fixation in legumes or transferring important biological N fixation traits to non-N-fixing organisms (Shantharam and Mattoo, 1997). As a consequence, alternative physiological approaches have been proposed for improving mobilization, redistribution, and utilization of N-fixed and stored N reserves within the host plant rather than N2 fixation itself. This prompted a number of groups to modify ammonium assimilation in legumes by the means of genetic manipulation. This approach was at the same time a means of assessing the role of the different nodule GS genes and isoenzymes in response to the massive supply of ammonium resulting from N2 fixation. In addition, the impact of such genetic manipulation was examined to determine if the reaction catalyzed by GS was one of the limiting factors in terms of N use efficiency (NUE) and yield.
The original idea of modulating GS activity in legumes arose from work published by Knight and Langston-Unkefer (1988), where nodulated alfalfa (Medicago sativa) plants were infested with Pseudomonas syringae pv tabaci, a bacteria living at the root surface of many plants and releasing tabtoxin-β-lactam, an irreversible inhibitor of GS. After the infection, an approximate doubling of plant growth, total N, nodulation, and overall dinitrogen fixation was observed. This spectacular effect on plant biomass production was explained by the selective action of the inhibitor on the root-specific cytosolic GS isoform, reducing by one-half the total GS activity in the nodules. Although detailed physiological analysis of the infested plants was not presented, the authors proposed that alternative routes of ammonium assimilation may be more efficient in assimilating the ammonium produced as the result of N fixation. In addition, the important decrease in the Gln pool (a potent feedback inhibitor of nitrogenase activity) in nodules of infested plants could explain the enhancement of N fixation, thus being beneficial to the overall N metabolism.
To investigate the implication of cytosolic GS in determining the biomass production of a legume, we have developed an antisense technology to diminish the quantity of cytosolic GS in the nodules of the model legume, Lotus japonicus. L. japonicus was chosen for this study because, like alfalfa, it is an amide producer. In addition, its high transformation efficiency allows a sufficient number of primary transformants, usually required when developing a transgenic approach, to be obtained (Handberg and Stougaard, 1992). The physiological impact of reduced GS activity in the nodules of transgenic L. japonicus plants was analyzed both through plant biomass production and the accumulation of carbon (C) and N metabolites in roots, shoots, and nodules when plants were placed under symbiotic N-fixing conditions.
RESULTS
Expression of pLBCASGS3 in Primary Transformants and F1 Progeny
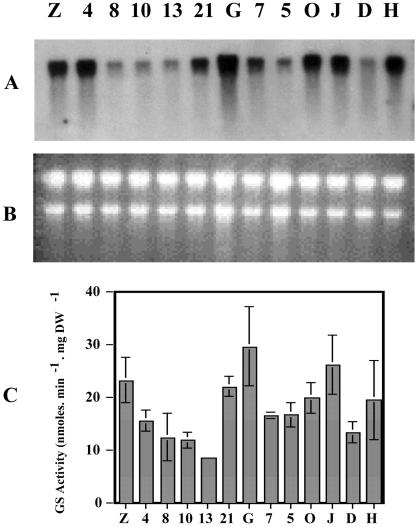
The binary vector LBC3-LjGS1As containing the soybean (Glycine max) LBC3 promoter upstream of the cytosolic LjGS1 cDNA placed in the antisense orientation was introduced into L. japonicus Gifu via Agrobacterium tumefaciens-mediated transformation, and the transgenic plants derived were analyzed. After plant transformation and regeneration, PCR analysis allowed the screening and selection from the primary transformants, 12 independent transformed lines in which the construct was expressed (data not shown). Northern-blot analysis using a 32P-labeled DNA fragment of L. japonicus cDNA Ljgln1 was used to measure the levels of GS1 mRNA in nodules of transformed plants. An important reduction in GS1 transcripts in several of the transformed lines in comparison with the controls can be seen (Fig. 1A). Figure 1C shows the results of the analysis of GS activity in the nodules. When control and transformed lines were compared, a clear decrease in GS activity of up to 50% was observed. Except for line 4, a rather good correlation between the level of GS1 transcripts and the corresponding GS activity was observed. Three of these lines (lines 5, 7, and 8), exhibiting reduced expression of both GS1 mRNA and GS activity in the nodules, were selected because of their ability to develop nodules and set seeds. Most of the other lines either did not develop seeds, did not nodulate, or if they did so, developed abnormal nodules. Moreover, some transgenic lines exhibiting a level of nodule GS activity similar to that of the untransformed control were sterile, indicating that there was no relationship between plant sterility and reduced GS activity in the nodules. For analysis of the T1 generation, control plants were either untransformed “Gifu” plants or the line 21, which had undergone the transformation by A. tumefaciens but did not show significant changes in mRNA level or GS activity. The three independent transformed lines were self-pollinated, and the resulting grain was germinated on kanamycin-containing media to check that the construct had not been lost. When these plants were sufficiently large, cuttings were taken, from which all further experimental results presented were obtained. This allowed the experiments to be performed on plants that were genetically identical.
Figure 1.
Expression of GS in nodules of primary transformants of L. japonicus expressing a GS1 antisense RNA under the control of the leghemoglobin promoter. A, Northern-blot analysis of GS1 mRNA in nodules of LBC3-LjGS1As L. japonicus primary transformants (10 μg of total RNA was loaded into each lane and probed with a 32P-labeled DNA fragment of the L. japonicus cDNA Ljgln1). B, Ethidium bromide-stained gel to show equal RNA loading in all lanes. C, GS activity of nodules of LBC3-LjGS1As L. japonicus primary transformants. Numbers and letters refer to the 12 transgenic lines that have been generated. G, Parental line Gifu used for transformation. Plants were grown under symbiotic N-fixing conditions for 4 weeks before harvest. Values are the mean ± se of three individual plants.
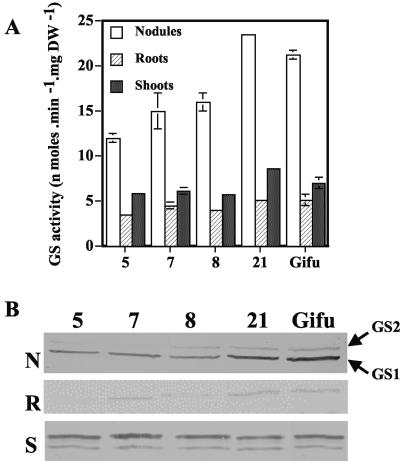
The activity of GS was again verified in the nodules, shoots, and roots of the T1 plants (Fig. 2A) and the GS protein content (Fig. 2B). As observed for the T0 lines, there was a significant reduction (up to 50%) in the GS activity of the nodules in the transformed lines (Fig. 2A) and in the quantity of GS1 protein (Fig. 2B). Leaves and roots from transformed plants showed GS activities that were also slightly decreased in comparison with the control lines (Fig. 2A). Cytosolic GS protein levels of roots and cytosolic and plastidial GS content of leaves were only very slightly altered as a result of the transformation.
Figure 2.
Expression of GS in three T1 transformants of L. japonicus expressing a GS1 antisense RNA in the nodules. The three T1 generation plants are lines 5, 7, and 8, and the controls are lines Gifu and 21. Plants were grown for 4 weeks under symbiotic N-fixing conditions before harvest. A, GS activity in nodules, roots, and shoots. Values are the mean ± se of eight individual plants. Bars for the se are not presented when they are below the thickness of the line. B, Western-blot analysis of GS subunits in nodules (N), roots (R), and shoots (S). GS1, Cytosolic GS subunits (molecular mass = 41 kD); GS2, Plastidic GS subunits (molecular mass = 45 kD). Ten micrograms of soluble protein was loaded into each lane.
Physiology of the Transformed Plants
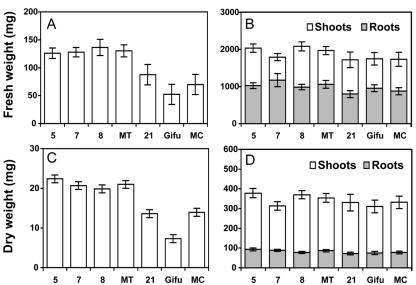
To determine the physiological impact of a reduction in GS activity in nodules, the ability of the plant to assimilate inorganic N was tested. Figure 3 shows the biomass accumulation of the lines. The mean fresh weight of nodules of transformed plants was 2-fold greater than the control lines (Fig. 3A), and the same was found when the nodule dry weight was determined (Fig. 3C). The fresh weights of roots and shoots were slightly higher in the transformed lines compared with the controls with an average increase of 20% (Fig. 3B). The increase in roots and shoots dry weights was not significantly different between the transgenic plants and the two controls (Fig. 3D). No significant differences were seen in the development of the plants between the transformed and the control lines, with time taken to flowering remaining unchanged (data not shown). When N fixation was measured using the acetylene reduction assay (Deroche et al., 1983), no significant differences were observed between the transformed plants and the two controls, the rate of N2 fixation being around 42 nmol h-1 mg nodules dry weight-1 in all of the lines.
Figure 3.
Fresh weight and dry weight accumulation in nodulated transgenic L. japonicus expressing a GS1 antisense RNA in the nodules. The three T1 generation plants are lines 5, 7, and 8, and the controls are lines Gifu and 21. A, Nodule fresh weight. B, Root and shoot fresh weight. C, Nodule dry weight D, Root and shoot dry weight. Plants were grown for 4 weeks under symbiotic N-fixing conditions before harvest. Values are the mean ± se of eight individual plants. MT is the mean of the three independent transformed plants. MC is the mean of the two control lines used in the study.
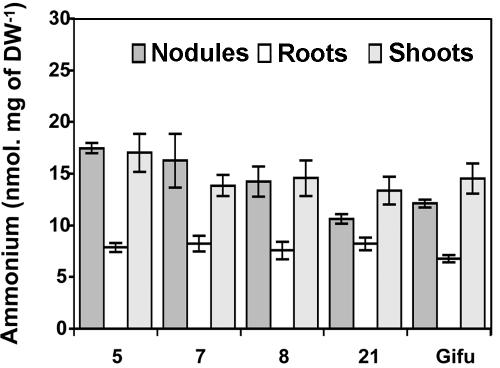
The effect of the reduction in GS activity on the level of free ammonium in the nodules was also investigated. Figure 4 shows that the reduction in GS activity in the three transformed lines resulted in an increase in the quantity of free ammonium in the nodules. The free ammonium content of the roots and shoots was similar (Fig. 4).
Figure 4.
Free ammonium concentration of the nodules, roots, and shoots of L. japonicus expressing a GS1 antisense RNA in the nodules. The three T1 generation plants are lines 5, 7, and 8 and the controls lines Gifu and 21. Plants were grown for 4 weeks under symbiotic N-fixing conditions before harvest. Values are the mean ± se of eight individual plants.
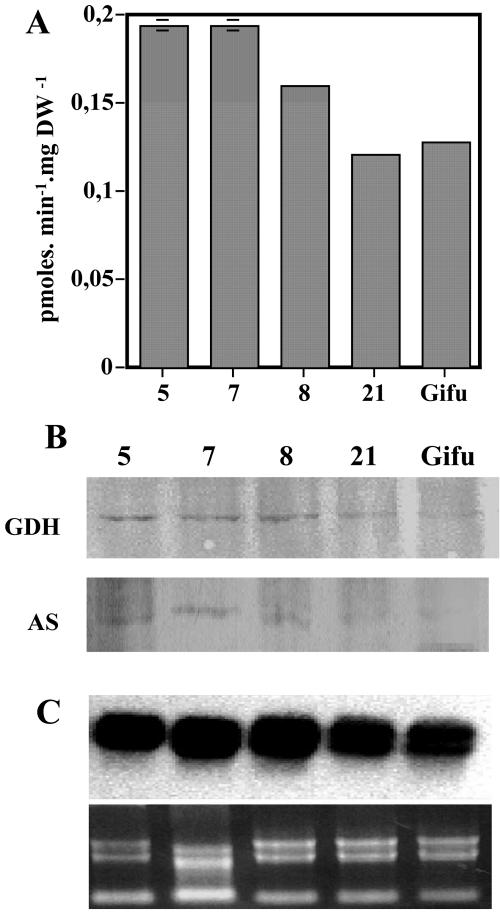
The activity of Glu dehydrogenase (GDH), which was once thought to be the principle route by which ammonium is assimilated in plants (Lea and Ireland, 1999), was also measured in the transformed plants. NADH-dependent GDH activity was found to be higher in the transformed plants compared with the control lines, with an average 30% to 40% increase (Fig. 5A). An increase in the amount of the corresponding protein was also observed (Fig. 5B). Probably due to its instability, we were not able to measure the activity of the enzyme AS (E.C. 6.3.5.4). However, western- and northern-blot analysis revealed that in nodules of transformed plants, the levels of AS protein (Fig. 5B) and the corresponding transcript (Fig. 5C) were enhanced.
Figure 5.
Expression of Glu dehydrogenase (GDH) and Asn synthetase (AS) in nodules of transgenic L. japonicus expressing a GS1 antisense RNA in the nodules. The three T1 generation plants are lines 5, 7, and 8, and the controls lines are Gifu and 21. Plants were grown for 4 weeks under symbiotic N-fixing conditions before harvest. A, NADH-dependent GDH activity. Values are the mean ± se of eight individual plants. Bars for the se are not presented when they are below the thickness of the line B. Western-blot analysis of GDH and AS proteins in nodules. Twenty micrograms of soluble protein was loaded into each lane. C, Northern-blot analysis of AS mRNA in nodules of LBC3-LjGS1As L. japonicus transformants (10 μg of total RNA was loaded into each lane and probed with a 32P-labeled DNA fragment of the L. japonicus cDNA LJAS2; Waterhouse et al., 1996). Below is shown an ethidium bromide-stained gel to show equal RNA loading in all lanes.
The incorporation of the ammonium assimilated into free amino acids was assessed in leaves, roots, and nodules. A marked increase in the total amino acid content of the nodules of transformed plants was seen, which was due primarily to an increase in the Asn content (Table I). This increase was not at the expense of the other amino acids because its relative proportion (around 75%) remained very similar in both control and transgenic plants. In the nodules of transformed plants, the concentration of both Glu and Gln was practically unchanged compared with the untransformed control plants. Only in control line 21 was Glu content increased. A slight increase in the relative concentration of Asn was observed in leaves, whereas in roots, neither the amino acid content nor the relative concentrations of the individual amino acids were affected (Table I).
Table I.
Amino acid concentration in transgenic L. japonicus expressing a GS1 antisense RNA in the nodules
Amino acid concentration in the different organs of transgenic plants and control plants and their relative proportions (indicated in parentheses and expressed as percentage of the total) of T1 generation of the three lines 5, 7, and 8 and the two control lines 21 and Gifu. Plants were grown for 4 weeks under symbiotic N-fixing conditions. For each line, samples from height individual plants were pooled prior to ion exchange chromatography.
| 5 | 7 | 8 | 21 | Gifu | |
|---|---|---|---|---|---|
| μmole mg dry wt−1 | |||||
| Nodules | |||||
| Asp | 2.8 (1.6) | 3.9 (2.1) | 0.0 (0.0) | 0.9 (0.9) | 0.9 (0.7) |
| Asn | 136.1 (75.5) | 137.0 (74.6) | 108.9 (77.8) | 63.2 (62.5) | 95.2 (74.5) |
| Glu | 20.0 (11.1) | 20.2 (11.0) | 19.5 (10.9) | 17.5 (17.3) | 14.8 (11.6) |
| Gln | 3.7 (2.1) | 3.7 (2.0) | 3.2 (1.8) | 2.2 (2.2) | 2.8 (2.0) |
| Others | 17.7 (9.8) | 18.4 (1.0) | 17.1 (8.5) | 17.3 (17.1) | 14.1 (11.1) |
| Total | 180.3 | 183.4 | 178.7 | 101.0 | 127.6 |
| Leaves | |||||
| Asp | 0.1 (0.2) | 0.1 (0.1) | 5.2 (9.0) | 0.1 (0.2) | 0.0 (0.1) |
| Asn | 15.5 (46.5) | 22.3 (46.8) | 26.9 (46.6) | 13.7 (43.6) | 10.8 (34.6) |
| Glu | 8.4 (25.2) | 11.4 (25.0) | 12.7 (22.0) | 7.3 (23.4) | 9.5 (30.3) |
| Gln | 0.1 (0.4) | 0.2 (0.3) | 0.2 (0.3) | 0.0 (0.1) | 0.0 (0.0) |
| Others | 9.3 (27.7) | 11.8 (25.8) | 12.8 (22.1) | 10.3 (32.8) | 10.9 (34.9) |
| Total | 33.4 | 45.8 | 57.8 | 31.4 | 31.2 |
| Roots | |||||
| Asp | 0.6 (9.8) | 0.5 (9.4) | 0.4 (10.9) | 0.4 (10.0) | 0.4 (10.3) |
| Asn | 2.8 (46.4) | 2.3 (46.6) | 1.5 (37.0) | 1.7 (39.8) | 1.5 (40.3) |
| Glu | 0.9 (15.4) | 0.7 (14.5) | 0.7 (16.9) | 0.8 (19.3) | 0.7 (17.9) |
| Gln | 0.3 (5.0) | 0.2 (4.9) | 0.2 (5.7) | 0.2 (5.9) | 0.2 (4.7) |
| Others | 1.4 (23.3) | 1.2 (24.6) | 1.2 (29.4) | 1.1 (25.0) | 1.0 (26.8) |
| Total | 6.0 | 4.9 | 4.0 | 4.2 | 3.8 |
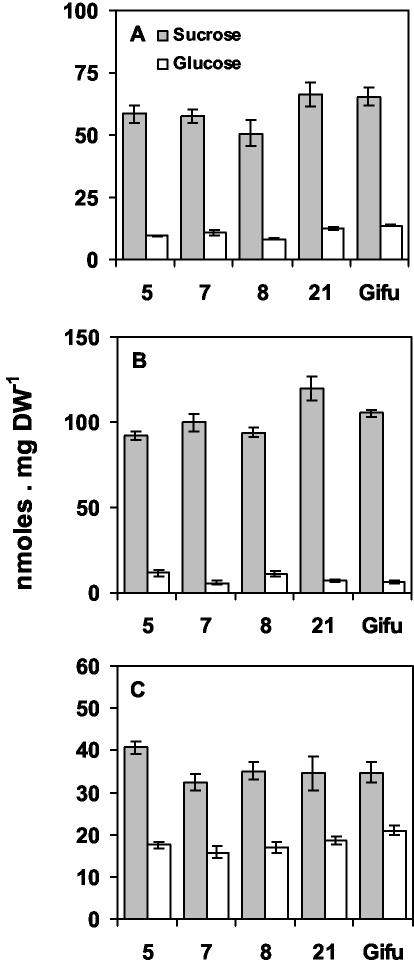
For effective incorporation of the assimilated ammonium, an adequate supply of carbohydrates is required. The relative amounts of the carbohydrates, Suc and Glc, in the different plant parts are shown in Figure 6. The main carbohydrate in all organs examined was Suc, whereas Glc represented less than 10% of the soluble carbohydrate content. The Suc content of roots and nodules was slightly decreased (10%) in the transformed lines in comparison with the control plants, whereas Glc content of the transformed nodules was also reduced by 30%.
Figure 6.
Carbohydrate content in transgenic L. japonicus expressing a GS1 antisense RNA in the nodules. Suc and Glc content of nodules (A), roots (B), and shoots (C) of lines 5, 7, and 8 and the two control lines 21 and Gifu. Plants were grown for 4 weeks under symbiotic N-fixing conditions before harvest. Values are the mean ± se of eight individual plants. Bars for the se are not presented when they are below the thickness of the line.
DISCUSSION
After transformation of L. japonicus with a vector expressing an antisense mRNA under the control of the nodule-specific LBC3 promoter, three primary transformants exhibiting reduced GS activity in the nodules that were able to set seeds and produce normal nodules were selected. The T1 progeny of three plants was then analyzed both for plant biomass production and for C and N metabolite content.
Our study on L. japonicus and that of Carvahlo et al. (2003) on Medicago truncatula are the first, to our knowledge, that report a reduction in GS1 gene expression leading to reductions in both protein level and enzyme activity. In a number of previous investigations using alfalfa as a model plant, several strategies were developed to down-regulate GS1 expression in the different organs of alfalfa (Temple et al., 1994, 1998). When the alfalfa GS cDNA was placed in the antisense orientation behind the acidic chitinase promoter from Arabidopsis, a decrease in the steady-state level of GS transcripts in all organs was observed. The amount of GS1 subunits dropped both in roots and nodules, with a fairly good correlation with the changes in GS activity. However, because a limited number of transformed plants were obtained, the authors suspected that a fully active antisense RNA was lethal. Unfortunately, the effects of reduced GS activity on both the physiology and the development of the survivors were not presented (Temple et al., 1994). In the second set of experiments, aiming to block the expression of two genes encoding GS1, the specific 3′-untranslated regions were used to make antisense constructs and introduced individually into alfalfa. The two constructs were effective in lowering the level of the corresponding transcripts in the various organs of alfalfa. However, transgenic plants with up to 80% reduction in the transcript level corresponding to each gene class showed no reduction in GS activity and GS1 polypeptides (Temple et al., 1998).
One of the most interesting findings of this study, which fits with our previous investigations, is the correlation between the level of Asn synthesis and the relative activity of GS in the nodules. In contrast, in the present study, the effect of reduced nodule GS activity using an antisense approach on plant biomass production is not so clear-cut. Although an increase in plant biomass was observed when expressed on a fresh weight basis in the three transformed lines studied, this increase was not so obvious when expressed on a dry weight basis. Nevertheless, nodule fresh weight or dry weight was always greater in transgenic plants compared with the controls. When GS1 was overexpressed in legume nodules, this type of compensation has been noted previously in a number of studies (Harrison et al., 2000). In one of our previous investigations, we showed that nodulated Lotus corniculatus plants overexpressing constitutively a soybean GS gene encoding cytosolic exhibited a surprising 40% decrease in total nodule GS activity, although the soybean GS transgene was expressed at a very high level. The decrease in GS activity was accompanied by a 50% increase in biomass of shoots, roots, and nodules. A large increase in amino acids (mostly Asn) concentration concomitant to a decrease in the soluble carbohydrate content revealed more efficient assimilation in the nodules of transformed plants. Although a clear explanation of this spectacular effect could not be provided, results from this study showed that the increases in plant biomass followed the onset of symbiotic N fixation, which remained similar in the transgenic plants compared with the untransformed controls. After these observations, it was proposed that regulatory mechanisms, as yet unknown, control the balance between nodule GS activity and nodule biomass for optimal NUE and plant growth (Hirel et al., 1997).
In another study, the overexpression of GS specifically in the nodule-infected cells of L. japonicus led to a severe decrease in plant biomass production. In transformed plants placed under atmospheric N-fixing conditions, no differences were seen in the concentrations of sugars of the various plant parts, except that the free ammonium content of the nodules was reduced by approximately 50%. In addition, the amino acid content of the nodules was severely decreased due mostly to lower Asn content (Hirel et al., 1997).
Because Asn is one of the major long-distance N transport compounds, especially in temperate legumes such as L. japonicus, it was hypothesized that the capacity of the nodule to synthesize this amino acid would be a determinant factor in the control of plant biomass production. A similar conclusion has been drawn from tobacco plants ectopically expressing the enzyme AS. This result suggests that, even in nonlegumes, an enhanced production of mobile forms of organic N toward sink organs would be beneficial for plant performance (Brears et al., 1993) as also revealed in maize genotypes exhibiting different capacities to export Asn (Lohaus et al., 1998).
After the incorporation of ammonium into the amide position of Gln, the N can be transferred directly to the same position in Asn by AS. However, because less GS activity promotes an increase in the Asn content (or vice versa), it is very unlikely that a lower flux of Gln would enhance its synthesis. Because the enzyme is also able to use ammonium as a substrate although the Km value is 40-fold higher than Gln (Hirel and Lea, 2001), one can hypothesize that part of ammonium derived from N fixation could directly and more efficiently be assimilated by AS when GS is limiting. The increase in both AS protein and transcripts in the nodules of transformed plants also support this hypothesis. This would be a means of saving energy by bypassing the GS/GOGAT pathway. It is also well known that Asn synthesis can function in detoxification (Sieciechowicz et al., 1988).
A concomitant decrease in the Glc content of the nodules is consistent with a more active utilization of C skeletons exported from the shoots and further channeled through the oxaloacetate-Asp pathway (Rawstone et al., 1980). The relatively modest increase in free ammonium in nodules of the transformed plants in comparison with the decrease in GS activity and the fact that biological N2 fixation releasing a constant flux of ammonium was not affected also support this hypothesis. This hypothesis also fits well with the finding that AS expression in the nodules is independent of nitrogenase activity (Shi et al., 1997). In a recent study, an increase in Asn also has been observed in leaves when photorespiratory ammonium is accumulating as the result of impaired GOGAT activity, suggesting that alternative metabolic pathways may be activated to avoid ammonium accumulation (Ferrario-Méry et al., 2002). The reaction catalyzed by GDH may also be one of these alternative pathways that may be induced when the ammonium concentration reaches a certain threshold (Dubois et al., 2003). Interestingly, increased GDH protein activity was observed in the nodules of transformed plants as already seen in leaves of plants impaired for Fd-GOGAT activity (Ferrario-Méry et al., 2002). However, it remains to be demonstrated clearly whether ammonium can be directly assimilated by the enzyme under these physiological conditions or if the increase in GDH activity is the consequence of a more general stress response due, for example, to the lack of carbohydrates (Paczek et al., 2002; Dubois et al., 2003).
The major role of GS in controlling the flux of reduced N and its optimal utilization by the plant for growth and development has been revealed in an increasing number of studies either using transgenic plants with altered capacity for ammonium assimilation (Vincent et al.; 1997; Gallardo et al., 1999; Fuentes et al., 2001; Oliveira et al., 2002).
One of the challenges for improving NUE in plants will be to identify if in a given plant species and/or in a given organ or tissue alternative metabolites are present that may participate in NUE when either GS or GOGAT activity are not optimum. This possibility has been revealed recently through the exploitation of genetic variability using a wide range of lines or genotypes in which the efficiency of ammonium assimilation in particular and N use in general is either enhanced or decreased (Limami et al., 1999; Hirel et al., 2001). Studying both the molecular and physiological changes associated to this variability will be another way of identifying which of the main or alternative N assimilatory pathways are important for plant productivity.
MATERIALS AND METHODS
Construction of the Chimeric LBC3-LjGS1As Construct and Plant Transformation and Regeneration
The HindIII/BamHI fragment containing the leghemoglobin promoter from soybean (Glycine max; Sullivan et al., 1981) was removed from pUC18 and subcloned into HindIII/SmaI cut pGPTV basic binary vector (Becker et al., 1992a) to obtain the LBC3-pGPTV vector. A Lotus japonicus full-length cDNA encoding cytosolic GS cloned in the EcoRI site of BlueScript vector (Ljgln1) was then subcloned in the antisense orientation into the LBC3-pGPTV vector using the flanking SacI and EcoRV sites of the cloning vector and the SmaI and SacI sites of the pGPTV transformation vector to obtain the pGPTV-LBC3-Ljgln1As construct. The full-length cDNA isolated from an L. japonicus roots cDNA library was a gift from Prof. David T. Clarkson (Long-Ashton Research Station, Bristol, UK). The pGPTV vector containing the LBC3-Ljgln1AS construct in Escherichia coli DH5 was transferred to Agrobacterium tumefaciens (strain LBA4404) via triparental mating using pRK2013 as a helper (Ditta et al. 1980). Transformed L. japonicus (ecotype Gifu B-129) plants containing the pGPTVLBC3-Ljgln1AS construct were obtained using the transformation procedure described by Handberg and Stougaard (1992).
Plant Growth Conditions
Plants were taken as small cuttings from parental plants (T0 or T1 generation), grown in sand, and watered with a complete nutrient solution containing 10 mm NO3- plus 2 mm NH4+ (Coïc and Lesaint, 1971) until 2- to 3-cm-long roots had developed. Plants were then placed in hydroponic units in a growth chamber with 16-h-light/8-h-dark periods and day/night aerial temperatures were 25°/19°C. A photosynthetic photon flux density of 220 mmol m-2 s-1 was provided by metal halide lamps. Relative humidity was 60% of the saturation. During the 1st week of hydroponic culture, the plants received the same nutrient solution as for the growth on sand but containing 1 mm NO3-. To obtain nodules, 2-week-old L. japonicus cuttings were grown aeroponically (Deroche et al., 1983) for 4 weeks on an N-free solution (Coïc et al., 1972) and inoculated with Rhizobium loti (strain L08, a gift from Dr. Anick Petit, Institut des Sciences Végétales du Centre National de la Recherche Scientifique, Gif sur Yvette, France). Plants were then harvested 4 weeks after inoculation at 11 am either directly into liquid N for GS, GDH assays, and RNA extractions, or lyophilized for 42 h for dry weight and metabolite determinations. Eight replicate cuttings from each parental plant were used for the study.
Metabolite Analysis
After lyophilization plants were ground to a fine powder. Around 20 mg of this powder was then extracted in 1 mL of 80% (v/v) ethanol for 1 h. During extraction, the samples were continuously agitated; then, they were spun for 5 min at 10, 000g. The supernatant was recuperated, and the pellet was subjected to further extractions in 1 mL of 60% (v/v) ethanol and finally in 1 mL of water. All supernatants were combined to form the hydroalcoholic extract. The ethanol-water fractions were combined and stored at -80°C. Glc, Fru, and Suc in the ethanol-water extract were determined by an enzymatic method using the test of Boehringer Mannheim/Roche (Basel; Bergmeyer, 1974). Free NH4+ was determined by the phenol hypochlorite assay (Berthelot reaction). Amino acids were separated by a Biotronik LC5001 ion chromatograph (Eppendorf, Hamburg, Germany) with lithium citrate buffers and ninhydrin post-column derivatization. They were identified using a standard mixture of amino acids (Benson standard Physiological, Acidic, Neutral and Basic) and quantified using the 2100 software (P.E. Nelson, Cupertino, CA).
Protein Extraction, Fractionation, Enzymatic Assay, and Western Blotting
Protein extraction and western-blot analysis were carried out as described earlier (Hirel et al., 1987) using tobacco GS antibodies (Hirel et al., 1984), which recognize both cytosolic and plastidic GS isoenzymes subunits with a similar efficiency (Becker et al., 1992b). AS protein was detected with the antibodies raised against the nodule enzyme from alfalfa (Medicago sativa; Shi et al., 1997). Proteins were visualized using peroxidaseconjugated goat anti-rabbit antibodies (Towbin et al., 1979). GS activity was assayed using the biosynthetic activity based on γ-glutamyl-hydroxamate synthesis as described by O'Neal and Joy (1973). GDH activity and protein gel-blot analysis were performed as described previously (Masclaux et al., 2000). Proteins were quantified using the Bradford method (Bradford, 1976).
Isolation of Total RNA and Northern-Blot Analysis
Total RNA isolation and northern-blot analysis were carried out as described earlier (Becker et al., 1992b). Hybridization was performed with the insert of the L. japonicus Ljgln1 (a gift from Prof. Davis Clarkson, University of Bristol, UK) or LJAS2 (Waterhouse et al., 1996) full-length cDNA probes. The probes were labeled with 32P-dCTP, and hybridization was carried out at 65°C in 50 mm Tris-HCl (pH 7.6), 2% (w/v) bovine serum albumin, 0.2% (w/v) polyvinylpyrrolidone, 0.2% (w/v) Ficoll (400 kD), 0.2% (w/v) SDS, 0.1% (w/v) sodium pyrophosphate, 6% (w/v) NaCl, and 0.1 mg mL-1 denatured calf thymus DNA. Final washes were performed using 0.1× sodium, TRIS, EDTA (10 mm Tris-HCl [pH 8.0], 100 mm NaCl, and l mm EDTA), and 0.1% (w/v) SDS at 65°C, and filters were exposed to x-ray film at -80°C.
Acknowledgments
We gratefully acknowledge Dr. Helena Carvahlo (Instituto de Biolgia Molecular e Cellular, Porto, Portugal) for performing western-blot analysis using AS antibodies kindly provided by Dr. Carol Vance (University of Minnesota, Minneapolis).
This work was supported by the Biotechnology and Biological Science Research Council-Institut National de la Recherche Agronomique (postdoctoral fellowship to J.H.).
References
- Becker D, Kemper E, Schell J, Masterson R (1992a) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20: 1195-1197 [DOI] [PubMed] [Google Scholar]
- Becker TW, Caboche M, Carrayol E, Hirel B (1992b) Nucleotide sequence of a tobacco cDNA encoding plastidic glutamine synthetase and light-inducibility, organ specificity and diurnal rhythmicity in the expression of the corresponding genes of tobacco and tomato. Plant Mol Biol 19: 367-379 [DOI] [PubMed] [Google Scholar]
- Beevers L (1976) Nitrogen Metabolism in Plants. EJW Barrington, AJ Willis, eds, E Arnold, London, UK pp 1-333
- Benes V, Pekny V, Skorepa J, Vrba J (1989) Impact of diffuse nitrate pollution sources on groundwater quality-some examples from Czechoslovakia. Environ Health Perspect 83: 5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU (1974). Methods of Enzymatic Analysis, Vol. 3. Academic Press, New York, pp 1176-1179 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Brears T, Liu C, Knight TJ, Coruzzi GM (1993) Ectopic overexpression of asparagine synthetase in transgenic tobacco. Plant Physiol 103: 1285-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvahlo H, Lopes-Cardoso I, Lima L, Melo P, Cullimore JV (2003) Nodule specific modulation of glutamine synthetase in transgenic Medicago truncatula leads to inverse alteration in asparagines synthetase expression. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Coïc Y, Lesaint C (1971) Comment assurer une bonne nutrition en eau et en ions minéraux en horticulture. Hortic Française 8: 11-14 [Google Scholar]
- Coïc Y, Tendille C, Lesaint C (1972) La nutrition azotée du tournesol (Helianthus annuus): action sur le rendement et la composition biochimique de la graine. Agrochimica 16: 254-263 [Google Scholar]
- Cren M, Hirel B (1999) Glutamine synthetase in higher plants: regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol 40: 1187-1193 [Google Scholar]
- Deroche ME, Carrayol E, Jolivet E (1983) Phosphoenolpyruvate carboxylase in legume nodules. Physiol Vég 21: 1075-1081 [Google Scholar]
- Dilworth MJ (1974) Dinitrogen fixation. Annu Rev Plant Physiol 25: 81-114 [Google Scholar]
- Ditta G, Stanfield S, Corbin D, Helinski DR (1980) Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA 77: 7347-7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois F, Tercé-Laforgue T, Gonzalez-Moro MB, Estavillo JM, Sangwan R, Gallais A, Hirel B (2003) Glutamate dehydrogenase in plants: is there a new story for an old enzyme? Plant Physiol Biochem 141: 565-576 [Google Scholar]
- Ferrario-Méry S, Valadier MH, Godefroy N, Miallier D, Hirel B, Foyer CH, Suzuki A (2002) Diurnal changes in ammonium assimilation in transformed tobacco plants expressing ferredoxin-dependent glutamate synthase mRNA in the antisense orientation. Plant Sci 163: 59-67 [Google Scholar]
- Fuentes SI, Allen DJ, Ortiz-Lopez A, Hernandez G (2001) Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J Exp Bot 52: 1071-1081 [DOI] [PubMed] [Google Scholar]
- Gallardo F, Fu J, Canton FR, Garcia-Gutierez A, Canovas F, Kirby E (1999) Expression of a conifer glutamine synthetase gene in transgenic poplars. Planta 210: 19-26 [DOI] [PubMed] [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487-496 [Google Scholar]
- Harrison J, Brugière N, Phillipson B, Ferrario-Méry S, Becker T, Limami A, Hirel B (2000) Manipulating the pathway of ammonium assimilation through genetic engineering and breeding: consequences on plant physiology and development. Plant Soil 221: 81-93 [Google Scholar]
- Hirel B, Bertin P, Quillere I, Bourdoncle W, Attagnant C, Dellay C, Gouy A, Cadiou S, Retailliau C, Falque M et al. (2001) Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol 125: 1258-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Bouet C, King B, Layzell D, Jacobs F, Verma DPS (1987) Glutamine synthetase genes are regulated by provided externally or by symbiotic nitrogen fixation. EMBO J 6: 1167-1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Lea PJ (2001) Ammonium assimilation. In PJ Lea, JF Morof Gaudry, eds, Plant Nitrogen. Springer-Verlag, Berlin, pp 79-99
- Hirel B, Phillipson B, Murchie E, Suzuki A, Kunz C, Ferrario S, Limami A, Chaillou S, Deléens E, Brugière N et al. (1997) Manipulating the pathway of ammonium assimilation in transgenic non-legumes and legumes. Z Pflanzenernähr Bodenk 160: 283-290 [Google Scholar]
- Hirel B, Weatherley C, Cretin C, Bergounioux D, Gadal P (1984) Multiple subunit composition of chloroplastic glutamine synthetase of Nicotiana tabacum L. Plant Physiol 74: 448-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TJ, Langston-Unkefer PJ (1988) Enhancement of symbiotic dinitrogen fixation by a toxin-releasing plant pathogen. Science 241: 951-954 [DOI] [PubMed] [Google Scholar]
- Lara M, Cullimore JV, Lea PJ, Miflin BJ, Johnston AWB, Lamb JW (1983) Appearance of a novel form of glutamine synthetase during nodule development in Phaseolus vulgaris. Planta 157: 254-258 [DOI] [PubMed] [Google Scholar]
- Lea PJ, Ireland RJ (1999) Plant amino acids. In BK Singh, ed, Nitrogen Metabolism in Higher plants. Marcel Dekker Inc., New York, pp 1-47
- Limami A, Phillipson B, Ameziane R, Pernollet N, Jiang Q, Roy R, Deleens E, Chaumont-Bonnet M, Gresshoff PM, Hirel B (1999) Does root glutamine synthetase control plant biomass production in Lotus japonicus L. Planta 209: 495-502 [DOI] [PubMed] [Google Scholar]
- Lohaus G, Buker M, Hussman M, Soave C, Heldt H (1998) Transport of amino acids with special emphasis on the synthesis and transport of asparagine in the Illinois low protein and Illinois high protein strains of maize. Planta 205: 181-188 [Google Scholar]
- Masclaux C, Valadier MH, Brugière N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211: 510-518 [DOI] [PubMed] [Google Scholar]
- McNally SF, Hirel B, Gadal P, Mann AF, Stewart GR (1983) Glutamine synthetase in higher plants: evidence for a specific isoform content related to their possible physiological role and their compartmentation within the leaf. Plant Physiol 72: 22-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller H, Landt J, Pederson E, Jensen P, Autrup H, Jensem OM (1990) Endogenous nitrosation in relation to nitrate exposure from drinking water and diet in a Danish rural Population. Cancer Res 49: 3117-3121 [PubMed] [Google Scholar]
- Oaks A (1992) A re-evaluation of nitrogen assimilation in roots. Bioscience 42: 103-110 [Google Scholar]
- Oliveira IC, Brears T, Knight TJ, Clark A, Coruzzi GM (2002) Overexpression of cytosolic glutamine synthetase: relation to nitrogen, light and photorespiration. Plant Physiol 129: 1170-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira IC, Coruzzi GM (1999) Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiol 121: 301-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D, Joy KD (1973) Glutamine synthetase of pea leaves: I. Purification, stabilisation and pH optima. Arch Biochem Biophys 159: 113-122 [DOI] [PubMed] [Google Scholar]
- Ortega JL, Roche D, Sengupta-Gopalan C (1999) Oxidative turnover of soybean root glutamine synthetase. In vitro and in vivo studies Plant Physiol 119: 1483-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczek V, Dubois F, Sangwan R, Morot-Gaudry JF, Roubelakis-Angelakis KA, Hirel B (2002) Cellular and subcellular localisation of glutamine synthetase and glutamate dehydrogenase in grapes gives new insights on the regulation of C and N metabolism. Planta 216: 245-254 [DOI] [PubMed] [Google Scholar]
- Rawstone S, Michin FR, Summerfield RJ, Cookson C, Coombs J (1980) Carbon and nitrogen metabolism in legume root nodules. Phytochemistry 19: 341-355 [Google Scholar]
- Robertson JG, Farnden KJF, Warburton MP, Banks JAM (1975) Induction of glutamine synthetase during nodule development in lupin. Aust J Plant Physiol 2: 265-272 [Google Scholar]
- Shantharam S, Mattoo AK (1997) Enhancing biological nitrogen fixation: an appraisal of current and alternative technologies for N input into plants. Plant Soil 194: 205-216 [Google Scholar]
- Shi L, Twary SN, Yoshioka H, Gregerson RG, Miller SS, Samac D, Gantt JS, Unkeffer PJ, Vance CP (1997) Nitrogen assimilation in alfalfa: isolation and characterization of an asparagine synthetase gene showing enhanced expression in root nodules and dark adapted leaves. Plant Cell 9: 1339-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieciechowicz K, Joy KW, Ireland RJ (1988) The metabolism of asparagine in plants. Phytochemistry 27: 663-671 [Google Scholar]
- Sullivan D, Brisson N, Goodshild B, Verma DPS, Thomas DY (1981) Molecular cloning and organization of two leghemoglobin genomic sequences of soybean. Nature 289: 516-518 [Google Scholar]
- Temple SJ, Bagga S, Sengupta-Gopalan C (1994) Can glutamine synthetase activity be modulated in transgenic plants by the use of recombinant DNA technology? Transgenic plants and biochemistry. Biochem Soc Trans 22: 915-920 [DOI] [PubMed] [Google Scholar]
- Temple SJ, Bagga S, Sengupta-Gopalan C (1998) Down regulation of specific members of the glutamine synthetase gene family in alfalfa by antisense RNA technology. Plant Mol Biol 37: 535-547 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA 76: 4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R, Fraisier V, Chaillou S, Limami MA, Deléens E, Phillipson B, Douat C, Boutin JP, Hirel B (1997) Overexpression of a soybean gene encoding cytosolic glutamine synthetase in shoots of transgenic Lotus corniculatus L. plants triggers changes in assimilation and plant development. Planta 201: 424-433 [DOI] [PubMed] [Google Scholar]
- Waterhouse RN, Smyth AJ, Massoneau A, Prosser IM, Clarkson DT (1996) Molecular cloning and characterisation of asparagine synthetase from Lotus japonicus: dynamics of asparagines synthesis in N-sufficient plants. Plant Mol Biol 30: 883-897 [DOI] [PubMed] [Google Scholar]
- Waters JK, Hughes IIBL, Purcell LC, Gerhardt KO, Mawhinney TP, Emerich DW (1998) Alanine, not ammonium, is excreted from N2-fixing soybean nodule bacteroids. Proc Natl Acad Sci USA 95: 12038-12042 [DOI] [PMC free article] [PubMed] [Google Scholar]