Abstract
Insect herbivore-induced plant volatile emission and the subsequent attraction of natural enemies is facilitated by fatty acid-amino acid conjugate (FAC) elicitors, such as volicitin [N-(17-hydroxylinolenoyl)-l-glutamine], present in caterpillar oral secretions. Insect-induced jasmonic acid (JA) and ethylene (E) are believed to mediate the magnitude of this variable response. In maize (Zea mays) seedlings, we examined the interaction of volicitin, JA, and E on the induction of volatile emission at different levels of nitrogen (N) availability that are known to influence E sensitivity. N availability and volicitin-induced sesquiterpene emission are inversely related as maximal responses were elicited in N-deficient plants. Plants with low N availability demonstrated similar volatile responses to volicitin (1 nmol plant-1) and JA (100 nmol plant-1). In contrast, plants with medium N availability released much lower amounts of volicitin-induced sesquiterpenes compared with JA, suggesting an alteration in volicitin-induced JA levels. As predicted, low N plants exhibited greater sustained increases in wound- and volicitin-induced JA levels compared with medium N plants. N availability also altered volicitin-E interactions. In low N plants, E synergized volicitin-induced sesquiterpene and indole emission 4- to 12-fold, with significant interactions first detected at 10 nL L-1 E. Medium N plants demonstrated greatly reduced volicitin-E interactions. Volicitin-induced sesquiterpene emission was increased by E and was decreased by pretreatment the E perception inhibitor 1-methylcyclopropene without alteration in volicitin-induced JA levels. N availability influences plant responses to insect-derived elicitors through changes in E sensitivity and E-independent JA kinetics.
In response to mechanical damage and insect attack, plants undergo a complex series of chemical and biochemical changes that can aid in the prevention of further tissue losses (Karban and Baldwin, 1997). Induced responses triggered by mechanical damage and insect herbivory often reduce the nutritional quality of the tissues and include the accumulation of proteinase inhibitors, phenolics, and alkaloids (Green and Ryan, 1972; Schultz and Baldwin, 1982; Baldwin, 1988). In addition to mechanical damage, caterpillar herbivory also results in insect-specific plant responses (Korth and Dixon, 1997; Reymond et al., 2000), including the induction of volatile emission (Paré and Tumlinson, 1999). Insect-induced volatiles can function as indirect plant defenses through the attraction of natural enemies (Dicke and Sabelis, 1988; Turlings et al., 1990; Vet and Dicke, 1992), a process that is now recognized as regulating important multitrophic interactions in agricultural and natural systems (DeMoraes et al., 1998; Kessler and Baldwin, 2001).
How plants perceive insect herbivory as different from mechanical damage is an active area of research. The first nonenzymatic elicitor of plant volatile emission, N-(17-hydroxylinolenoyl)-l-Gln, was identified from beet armyworm (BAW; Spodoptera exigua) oral secretions (OS) and termed volicitin (Alborn et al., 1997). Since this discovery, several related fatty acid-amino acid conjugates (FACs) have been found in caterpillar OS that also possess volatile-inducing activity in plant bioassays (Paré et al., 1998; Pohnert et al., 1999; Alborn et al., 2000; Halitschke et al., 2001). Before the discovery of FACs, pharmacological applications of jasmonic acid (JA) implicated a possible role of oxylipin signals in triggering induced volatile emission (Hopke et al., 1994; Boland et al., 1995). In maize (Zea mays), quantitative relationships between endogenous JA levels induced by BAW herbivory and volatile emission add additional support for the role of oxylipin signals (Schmelz et al., 2003a). Early evidence that factors present in caterpillar OS influence wound hormone levels, such as JA, came from examinations of interactions between tobacco hornworm (Manduca sexta) and Nicotiana sylvestris (McCloud and Baldwin, 1997). A rapid and transient increase in the induced levels of foliar JA was detected when tobacco hornworm OS was applied to mechanical damage sites. It has now been demonstrated that FAC elicitors, present in hawkmoth and BAW OS, induce JA and volatiles when applied to the wounded leaves of tobacco (Nicotiana attenuata) and maize plants (Halitschke et al., 2001; Schmelz et al., 2003b). Much evidence supports a role for JA in triggering insect-induced volatile emission; however, mechanical damage also triggers JA production with little or no induced volatile emissions. How relatively small changes in elicitor-induced JA levels account for dramatic increases in volatile emission remains unclear.
Insect herbivory is known to promote an increase in JA accumulation and ethylene (E) emission (Kahl et al., 2000; Arimura et al., 2002; Schmelz et al., 2003a). Interestingly, antagonistic and synergistic interactions have been described between jasmonates and E in the expression of plant defenses against pathogens and insects (Xu et al. 1994; Penninckx et al. 1998; Kahl et al., 2000). For example, in tobacco, hawkmoth herbivory induces an E burst that partially inhibits the wound-induced accumulation of nicotine regulated by JA (Kahl et al., 2000; Winz and Baldwin, 2001). In maize, E production or perception influences the magnitude of insect-induced volatile emission. During BAW herbivory on maize seedlings, endogenous JA levels correlate with the induced emission of indole, sesquiterpenes, and E; however, removal of E perception with the inhibitor 1-methylcyclopropene (1-MCP) results in a 2- to 4-fold decrease in BAW-induced volatile emission (Schmelz et al., 2003a). When applied to the wounded leaf surface, volicitin demonstrates only modest volatile-inducing activity on intact plants (Schmelz et al., 2001). Unlike caterpillar herbivory, volicitin treatments fail to elicit ethylene emission; however, exogenous E can synergize volicitin-induced volatile emission (Schmelz et al., 2003b). Based on the magnitude of volatile emission, volicitin alone does not appear to fully reproduce the plant response after actual herbivory. Enantiomerically pure FAC elicitors have been reported to be inactive in lima bean (Phaseolus lunatus) and cotton (Gossypium hirsutum; Spiteller et al., 2001). Identification of conditions and additional interacting factors that mediate the activity of FAC elicitors, such as volicitin, is clearly needed (Kessler and Baldwin, 2002). Recent evidence suggests that interactions between insect-derived elicitors and insect-induced ethylene are critical in the stimulation of volatile emission (Schmelz et al., 2003a, 2003b).
In addition to responses to biotic agents, important developmental processes are also regulated by E production and perception. A well-described E-induced response in monocots is the initiation of programmed cell death (PCD) and aerenchyma formation in roots (Drew et al., 2000). In maize seedlings, root sensitivity to E and subsequent aerenchyma formation can be increased by 100-fold during periods of nitrogen (N) deficiency (He et al., 1992), even though actual E production is suppressed (Drew et al., 1989). E has also been proposed to potentiate the wound-induced accumulation of JA in tomato (Lycopersicon esculentum), suggesting that induced JA levels may be partly E dependent (O'Donnell et al., 1996). To clarify the phytohormone signals and interactions that regulate of FAC elicitor activity, we altered N availability and examined the linkage between volicitin, JA, E sensitivity, and induced volatile emission. If E plays a critical role in phytohormone signaling between perception and response to insect elicitors, then N deficiency-induced increases in E sensitivity should amplify the activity of volicitin as measured in subsequent volatile emission. By controlling N levels in the form of nitrate (NO3), we asked the following questions: Does N availability alter the magnitude of volicitin-induced volatile emission? Does N availability have the same effect on volicitin- and JA-induced volatile emission? Does N availability affect volicitin-induced JA levels? Does N availability influence interactions between E, volicitin, and subsequent volatile emission? Does exogenous E increase volicitin-induced JA levels? Does the E perception inhibitor, 1-MCP, decrease volicitin-induced JA levels or volatile emission?
RESULTS
N Availability and Volicitin-Induced Volatile Emission
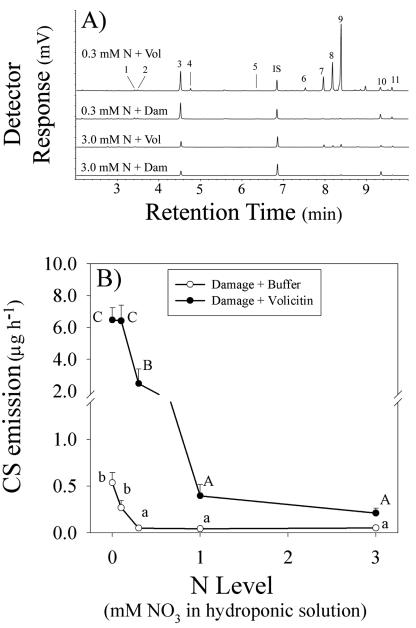
Reduced N availability results in increased levels of induced sesquiterpene emission after mechanical damage and volicitin treatment. Typical of induced volatiles described from soil-grown maize (Gouinguené et al., 2001), volicitin-treated hydroponic plants produce β-myrcene, (Z)-3-hexenyl acetate, linalool, (3E)-4,8-dimethyl-1,3,7-nonatriene, geranyl acetate, β-caryophyllene, (E)-α-bergamotene, (E)-β-farnesene, (E)-nerolidol, and (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (Fig. 1A). Similar to BAW herbivory (Schmelz et al., 2003a), the combination β-caryophyllene, (E)-α-bergamotene, and (E)-β-farnesene volatiles account for 75% to 80% of the total quantity of induced volatiles (Fig. 1A). To simplify data presentation, we refer to the summation of these three predominant induced volatiles as combined sesquiterpenes (CS). Maximal amounts of CS (6.5 μg h-1) were induced at the lowest N levels by volicitin application and mechanical damage treatments (0.5 μg h-1; Fig. 1B). The largest fold increases in volicitin-induced CS, compared with the mechanical damage responses, also occurred at these reduced N levels (0-0.3 mm NO3; Fig. 1B). In this experiment, indole emission was low (<0.03 μg h-1) in all treatment groups (Fig. 1A).
Figure 1.
A, Chromatographic profiles of volatiles from hydroponic maize seedlings grown at 0.3 or 3.0 mm NO3 and damaged (Dam) in the presence and absence of volicitin (Vol) as described below. Commonly detected volatiles in numerical order include β-myrcene, (Z)-3-hexenyl acetate, linalool, (3E)-4,8-dimethyl-1,3,7-nonatriene, indole (trace amount), geranyl acetate, β-caryophyllene, (E)-α-bergamotene, (E)- β-farnesene, (E)-nerolidol, and (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene. IS represents the internal standard n-nonyl-acetate. B, Mean (+se; n = 4) combined sesquiterpene (CS, β-caryophyllene + (E)-α-bergamotene, + (E)-β-farnesene) emission (micrograms per hour) of maize seedlings mechanically damaged and treated with buffer or volicitin (1 nmol plant-1)at 6:00 pm with volatiles collected for 1 h at the beginning of the following photoperiod. All seedlings were grown in hydroponic solution that contained 0, 0.1, 0.3, 1.0, or 3.0 mm NO3. Within damage and volicitin treatments, different letters (a and b or A, B, and C) represent significant differences (P < 0.05, Tukey correction for multiple comparisons).
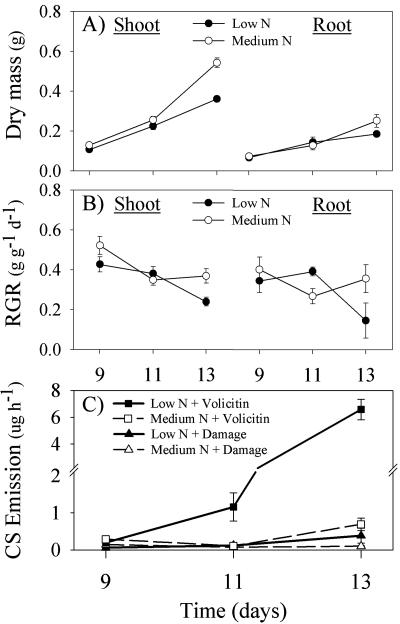
Based on N-mediated differences in volicitin-induced CS emission, median hydroponic solution concentrations of 0.2 and 2.0 mm NO3, termed low and medium N, were selected for further comparisons. After 4 to 6 d in hydroponics, the low N treatment begins to negatively affect root and shoot growth, and this period corresponds to the 11- to 13-d-old plants examined throughout the present study (Fig. 2, A and B). Growth of maize seedlings in hydroponic solutions containing 2.0 and 20 mm N did not differ; thus, it is concluded that the NO3 in the medium N level is not limiting within this time period (data not shown). Given increased volicitin-induced CS emission with reduced N availability (Fig. 1B), plants transferred into low N hydroponic solution are predicted to be increasingly N limited over time and thus increasingly responsive to volicitin. As anticipated, the increased plant volatile response to volicitin in low N plants is maximal at d 13 during reduced growth (Fig. 2C); however, an increased response is detectable at d 11 before significant decreases in root and shoot relative growth rates (RGRs; Fig. 2, A-C). As with the previous experiment, indole emission was low (<0.04 μg h-1) in all treatment groups (Fig. 1A).
Figure 2.
Mean (±se; n = 4) accumulation of maize shoot and root dry mass (in grams; A) and RGR (grams per gram per day) of low and medium N plants (B), corresponding to 0.2 and 2.0 mm NO3, transplanted into hydroponics at d 7. C, Mean (±se; n = 4) damage- and volicitin-induced CS emission in seedlings 2, 4, and 6 d (9-, 11-, and 13-d-old plants) after transfer to low and medium N hydroponic solution at d 7. Seedlings were mechanically damaged and treated with buffer or volicitin (1 nmol plant-1) at 6:00 pm with volatiles collected for 1 h at the beginning of the following photoperiod.
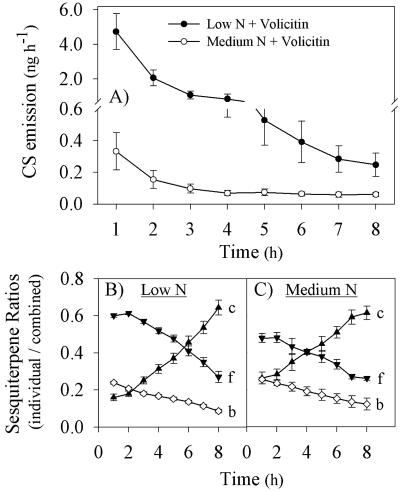
To characterize volatile emission kinetics, 13-d-old plants grown under low and medium N were treated with 1 nmol plant-1 volicitin at the end of the light cycle and volatiles were collected every hour for 8 h beginning at the start of the following light cycle. In both N groups, maximal CS emission occurred within the first 1-h collection period (Fig. 3A); thus, this previously used 1-h collection period was selected for all further comparisons. As the induced emission of CS volatiles declines over time, relative proportions of the three dominant components [β-caryophyllene, (E)-α-bergamotene, and (E)-β-farnesene] change significantly. During the first collection period, (E)-β-farnesene is the predominant sesquiterpene, whereas 6 to 8 h later, β-caryophyllene becomes proportionally dominant (Fig. 3, B and C). This pattern of shifting sesquiterpene ratios is highly consistent between low and medium N plants despite the 10-fold difference in the magnitude of volicitin-induced CS volatile emission.
Figure 3.
A, Time course of CS emission in 13-d-old low and medium N plants seedlings mechanically damaged and treated with volicitin (1 nmol plant-1) at 6:00 pm with volatiles collected for eight 1-h intervals at the beginning of the following photoperiod. Change in the ratios of volicitin-induced sesquiterpenes over time in low (B) and medium (C) N plants. The ratio of β-caryophyllene (c, ▴), (E)-α-bergamotene (b, ⋄), and (E)-β-farnesene (f, ▾) was calculated by the division of individual sesquiterpene amounts by the CS.
N Availability Differentially Effects Volicitin and JA-Induced Volatile Emission
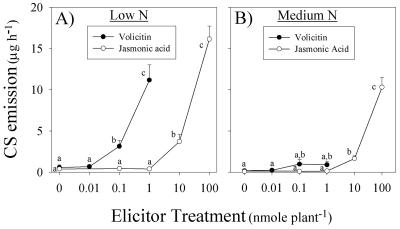
Volicitin is an elicitor of JA; thus, to ascertain if N availability influences volicitin-induced JA signaling, both compounds were applied separately over a range of doses to determine amounts that trigger similar responses. In low N plants, volicitin (1 nmol plant-1) and JA (100 nmol plant-1) stimulated statistically similar levels of CS volatiles corresponding to 11.2 and 16.2 μg h-1, respectively (Fig. 4A). In contrast, the activity of volicitin and JA in medium N plants was significantly different and resulted in 0.9 and 10.3 μg h-1 CS, respectively (Fig. 4B). On a mean basis, JA (100 nmol plant-1) stimulated 1.4-fold greater sesquiterpene emission than volicitin (1 nmol plant-1) in low N plants (Fig. 4A); however, this same amount of JA stimulated 11.4-fold greater sesquiterpene emission than volicitin in medium N plants (Fig. 4B). Indole emission was measurable in low N plants treated with volicitin (1 nmol plant-1) and in low and medium N plants treated with JA (100 nmol plant-1) averaging 0.12, 0.58, and 0.20 μg indole h-1, respectively. Indole emission was below 0.02 μg h-1 in all other groups. Increasing N levels from low to medium resulted in a significant preferential decrease in volicitin-induced CS emission compared with the JA-induced response. A possible explanation for this result is that N availability may affect volicitin-induced JA accumulation more than perception of exogenous JA.
Figure 4.
Mean (+se; n = 4) CS emission of maize seedlings mechanically damaged and treated with volicitin (0, 0.01, 0.1, and 1.0 nmol plant-1) or jasmonic acid (0, 0.1, 1, 10, and 100 nmol plant-1) at 6:00 pm with volatiles collected for 1 h at the beginning of the following photoperiod. All seedlings were grown in hydroponic solution containing low N (A) or medium (B) N levels. Within N levels, different letters (a, b, and c) represent significant differences (P < 0.05, Tukey correction for multiple comparisons).
N Deficiency Prolongs Volicitin-Induced JA Accumulation
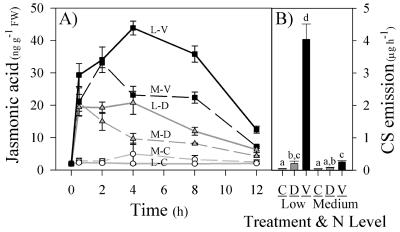
N deficiency results in elevated and prolonged increases in wound- and volicitin-induced JA levels. Consistent with earlier studies, volicitin induced significantly greater JA than mechanical damage alone 2 h after treatment (F5,23 = 58.93, P < 0.05). Comparisons between low and medium N plants indicate that during the 0.5- and 2-h time points, JA levels within each control, mechanical damage, and volicitin treatment group were statistically identical (Fs5,23 < 30.10, P > 0.05; Fig. 5A). In contrast to these short-term similarities, volicitin-induced JA levels in low N plants exceeded those in comparable medium N plants between 4 and 12 h (Fs5,23 > 32.47, P < 0.05; Fig. 4A). Likewise, in response to volicitin, low N plants emitted over 10-fold greater quantities of CS volatiles than medium N plants (Fig. 5B). Indole emission was consistently below 0.02 μg h-1 and did not differ between treatment groups. This result is consistent with the hypothesis that the levels and timing of induced JA partly regulate the magnitude of volicitin-induced CS volatile emission. Induced JA levels at 0.5, 4, and 12 h in mechanically damaged low N plants and volicitin-treated medium N plants were identical. These same two treatments also resulted in statistically similar levels of sesquiterpene volatile emission (Fig. 5B).
Figure 5.
Mean (±se; n = 4) JA (nanograms per gram of fresh weight; A) levels and CS emission (micrograms per hour; B) of untreated control (C), mechanically damaged (D), and volicitin (V, 1 nmol plant-1)-treated maize seedlings grown in hydroponic solution containing low (L) or medium (M) N levels. All plants were treated at the end of the photoperiod (6:00 pm) and were successively harvested for JA analysis, whereas volatiles were collected for 1 h at the beginning of the following photoperiod (6:00 am). Different letters (a, b, c, and d) represent significant differences in volatile emission (P < 0.05, Tukey correction for multiple comparisons).
N Availability Alters E Sensitivity and Interactions with Volicitin
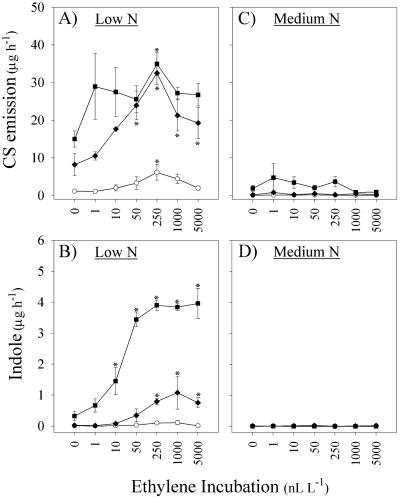
N deficiency results in strong synergistic interactions between volicitin and E, indicated by the induction of sesquiterpene and indole volatile emission in low N plants. In contrast, volicitin-induced volatiles are greatly reduced in medium N plants and interactions with E are largely unapparent. In low N plants, synergies were first detected at 0.1 nmol plant-1 volicitin and 50 nL L-1 E in sesquiterpene emission (Fig. 6A). Similarly, volicitin (1 nmol plant-1) synergies with indole emission were demonstrable at E concentrations as low as 10 nL L-1 in low N plants (Fig. 6B). In this experiment, interactions between volicitin and E in medium N plants were not significant (Fig. 6C) for CS or were entirely absent in the case of indole emission (Fig. 6D). The altered volicitin-E interaction, demonstrated in low and medium N plants through changes in the magnitude of induced volatile emission, is consistent with increases in E sensitivity known to occur during N deficiency.
Figure 6.
Mean (±se; n = 3) CS (A and C) and indole (B and D) emission (micrograms per hour) of 13-d-old maize plants grown in hydroponic solution containing low N or medium N levels and mechanically damaged followed by buffer treatment (○) or two different levels of volicitin (♦, 0.1 nmol plant-1; ▪, 1.0 nmol plant-1). Immediately after treatments at 9:00 pm, all plants were placed into individual 7-liter Plexiglas chambers and were treated with E at 0, 1, 10, 50 250, 1,000, or 5,000 nL L-1. Volatiles were collected at the beginning of the following photoperiod. Asterisks denote significant increases above the zero E addition control (Dunnett's test).
E Addition Does Not Alter Volicitin-Induced JA Accumulation
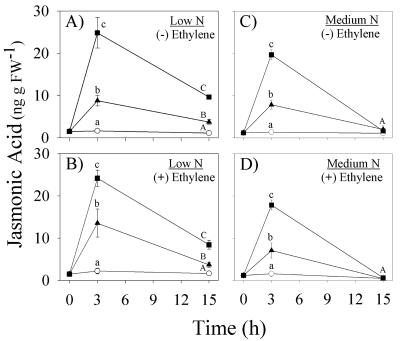
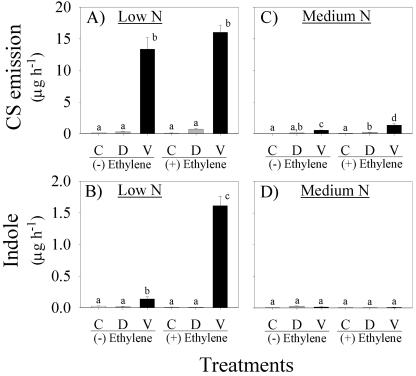
Previous experiments demonstrated that N availability influences the kinetics of JA accumulation and the synergistic interactions between volicitin and E. We confirm that increased volatile emissions from low N plants are associated with long-term increases in volicitin-induced JA levels; however, E does not increase volicitin-induced JA levels. Low N plants treated with volicitin (1 nmol plant-1) and (-) or (+) 50 nL L-1 E resulted in identical JA levels at 3 h (24.8 vs. 24.2 ng g-1 fresh weight) and both maintained 2.3- to 2.5-fold increases in JA above mechanical damage 15 h later (Fig. 7, A and B). In low N plants, E strongly synergized volicitin-induced indole emission (Fig. 8B), yet did not influence volicitin-induced JA levels (Fig. 7, A and B). Consistent with the previous experiment, the addition of low levels of E (50 nL L-1) to low N plants treated with volicitin (1 nmol plant-1) resulted in greater average CS emission levels; however, this specific treatment effect was not statistically significant. As with low N plants, E had no detectable effect on induced JA levels in medium N plants. (+) and (-) E treatments demonstrated 2.5-fold increases in volicitin-induced JA levels above mechanical damage alone after 3 h (Fig. 7, C and D). Once again, wound- and volicitin-induced JA levels in medium N plants returned to control levels sooner than low N plants (Fig. 7, C and D). A small yet significant volicitin-E interaction with resulting CS emission was detected in medium N plants (Fig. 8C).
Figure 7.
Mean (±se; n = 4) JA (nanograms per gram of fresh weight) levels of 12-d-old maize plants grown in hydroponic solution containing low N (A and B) or medium N (C and D) and treated as controls (○), mechanically damaged with buffer (▴), or 1.0 nmol plant-1 volicitin (▪). Immediately after leaf treatments (6:00 pm), all plants were placed into individual 7-liter Plexiglas chambers and were treated with E at 0 or 50 nL L-1, denoted as (-) and (+) E, respectively. Within each time point, different letters (a, b, and c or A, B, and C) represent significant differences (P < 0.05, Tukey correction for multiple comparisons).
Figure 8.
Mean (+se; n = 4) CS (A and C) and indole (B and D) emission (micrograms per hour) of 12-d-old maize plants grown in hydroponic solution containing low N or medium N levels and treated as controls (C) or mechanically damaged with the addition of buffer (D) or volicitin (V, 1.0 nmol plant-1). Immediately after leaf treatments (6:00 pm), all plants were placed in individual 7-liter Plexiglas chambers and were treated with E at 0 or 50 nL L-1, denoted as (-) and (+) E, respectively. Volatiles were collected for 1 h early in the following photoperiod. Within each figure different letters (a, b, c, and d) represent significant differences (P < 0.05, Tukey correction for multiple comparisons).
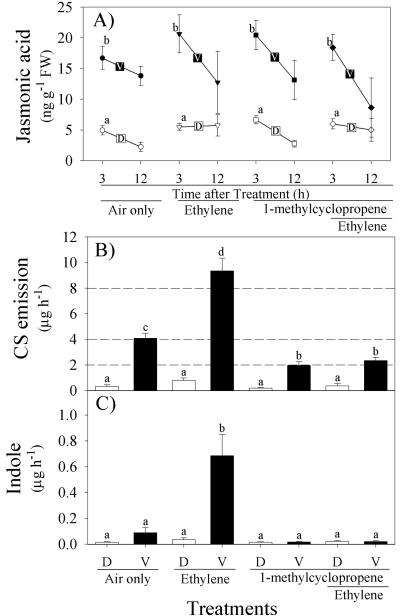
Volicitin-Induced JA Accumulation Is Independent of E Perception
1-MCP is a highly specific gaseous inhibitor of E receptors (Sisler et al., 1996). By pretreating subsets of plants with 1-MCP, we examined whether or not E perception mediates volicitin-induced JA levels and the degree of linkage between volicitin, JA, and sesquiterpene emission. In low N plants, E and 1-MCP act in a push-pull manner on sesquiterpene emission without respectively increasing or decreasing volicitin-induced JA levels. Independent of treatment with air, E, 1-MCP, or 1-MCP + E, volicitin promoted the induction of JA levels 3.1- to 3.7-fold above mechanical damage alone after 3 h (Fig. 9A). Likewise, 12 h after treatments, no evidence was found for an E-promoted increase in volicitin-induced JA or a 1-MCP repression of volicitin-induced JA (Fig. 9A). Similar to volicitin-induced JA, mechanical damage-induced JA levels were also unaffected by treatments with E and/or 1-MCP (Fig. 9A). In the presence of air only, E, and 1-MCP, volicitin triggered emission of 4.1, 9.4, and 2.0 μg h-1 of CS, respectively (Fig. 9B). Thus, E and 1-MCP had a push-pull effect, E increased volicitin-induced sesquiterpene emission by 2.3-fold, and 1-MCP pretreatment decreased sesquiterpene emission by 2.1-fold (Fig. 9B). Under these conditions, volicitin-induced indole emission was entirely dependent on exogenous E with emission abolished by pretreatment with 1-MCP (Fig. 9C). This demonstrates that E and E perception can regulate the magnitude of volicitin-induced volatile emission without acting directly on JA levels.
Figure 9.
A, Mean (±se; n = 6) JA (nanograms per gram of fresh weight); B, CS (+se; n = 9); C, indole emission (micrograms per hour) from low N maize seedlings damaged and treated with buffer (D) or volicitin (V, 1 nmol plant-1). All plants were enclosed in separate 7-liter Plexiglas chambers (6:00 pm) and a subset of them was pretreated with the gaseous E action inhibitor 1-MCP for 3 h before leaf treatments and E addition (250 nL L-1) at 9:00 pm. Plants for JA analysis were harvested 3 and 12 h later. Early in the following photoperiod, plants were removed from the Plexiglas chambers for volatile collection (1 h). Within each figure, different letters (a, b, c, and d) represent significant differences (P < 0.05, Tukey correction for multiple comparisons).
DISCUSSION
In this study, we demonstrate that N availability greatly influences the magnitude and, thus, variation of plant volatile responses to FAC elicitors. Decreased N availability and an associated reduction in plant growth correspond with increases in volicitin-induced CS volatiles. Elevated N levels were found to reduce volicitin-induced CS emission to a greater extent than JA-induced CS emission. From this result, we hypothesized that volicitin-induced JA accumulation may be greater in plants with reduced N availability. This idea was supported as low N plants displayed significantly higher JA levels 4 to 12 h after volicitin treatment compared with medium N plants. Under low N conditions, maize seedlings also displayed strong synergistic interactions between volicitin and E, as indicated by increases in induced CS and indole emissions. In medium N plants, interactions were greatly reduced, demonstrating that N availability effects E sensitivity in foliar interactions with volicitin. In plants with low N availability, the volicitin-E synergy and reciprocal repression with 1-MCP pretreatment accounted for approximately 80% of the total variation in magnitude of volicitin-induced CS emission. Examination of volicitin-induced JA levels demonstrated that E addition did not increase JA and 1-MCP pretreatment did not suppress JA levels. In maize, N deficiency appears to influence volicitin-induced sesquiterpene emission by increasing the duration and magnitude of induced JA levels and by altering the interaction between E and JA signals through changes in E sensitivity.
Many studies of insect-induced volatile emission have detected substantial variability in the magnitude of plant responses measured within treatment groups (Loughrin et al., 1994; Mattiacci et al., 1994). Plant genetic differences are clearly important, as induced volatile emission varies greatly between genotypes (Loughrin et al., 1995; Gouinguené et al., 2001); however, within cultivars, genetically based variation is likely to be small. Differences in abiotic factors, including light, humidity, temperature, timing of treatments, and fertilization, may account for part of this variability in elicitor- and insect-induced volatile emission (Takabayashi et al., 1994; Schmelz et al., 2001; Gouinguené and Turlings, 2002). In soil-grown maize, Gouinguené and Turlings (2002) reported that seedlings given a complete nutrient solution had greater volatile emission in response to Egyptian cotton worm (Spodoptera littoralis) OS than those supplied with only water; however, the role of N could not be inferred, as amounts of all nutrients where simultaneously changed. In this study, small differences in N availability are shown to have large effects on the variation in the magnitude of elicitor-induced CS volatile emission (Fig. 1). Numerous abiotic effects have been previously demonstrated; however, few, if any, studies have considered changes in phytohormone signal transduction as a potential mechanistic basis for this source of variation in volatile response.
In maize seedlings, N deficiency stimulates an increase in E sensitivity as measured by a decreased threshold of E required to promote root aerenchyma formation (He et al., 1992; Drew et al., 2000). Transcriptional regulation of E receptor genes is believed to regulate tissue responsiveness to E in tomato (Ciardi et al., 2000; Klee and Tieman, 2002). In lima bean leaves, exogenous applications of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid, increase the activity of JA in the stimulation of volatile emission (Horiuchi et al., 2001). One proposed mechanism of E action is the modulation of endogenous levels of induced JA (O'Donnell et al., 1996). Given our findings that low N plants display greater increases in induced JA levels over time and greater volicitin-E interactions, we tested the hypothesis that E acts to elevate induced JA levels or prolong their presence. In low N plants, E synergized volicitin-induced volatile emission; however, JA levels were not enhanced by the additional presence of E. Likewise, pretreatment of plants with the inhibitor 1-MCP repressed volatile emission without a decrease in JA levels. Thus, volicitin-induced JA accumulation appears to be independent of E signaling. This supports our previous findings that BAW herbivory on 1-MCP-treated plants have reduced volatile emission yet typical induced JA levels (Schmelz et al., 2003a). During BAW herbivory, maize seedlings demonstrate increases in JA before induced E production is observed (Schmelz et al., 2003a). Given the temporal delay in E emission and that E and 1-MCP do not significantly alter volicitin-induced JA levels, E appears to act downstream of JA as regulator of induced volatile emission.
As an essential macronutrient, N greatly influences primary and secondary plant metabolism (Stitt, 1999; Coruzzi and Zhou, 2001). In a hydroponic maize seeding assay similar to the present study, He et al. (1992) reported N concentrations in root and shoot tissues from N-starved plants to be less than 50% of those grown in a complete N solution after only 4 d. This raises the question: How do N-deficient plants produce greater elicitor-induced defense responses? N deficiency commonly results in increased levels of leaf Glc, Fru, Suc, and starch (Rufty et al., 1988; Paul and Driscoll, 1997). In addition to providing an increased source of nonstructural carbohydrates to potentially fuel volatile biosynthesis, elevated levels of soluble sugars can enhance the expression of wound- and jasmonate-induced genes encoding proteinase inhibitors and vegetative storage proteins (Johnson and Ryan, 1990; Mason et al., 1992).
The influence of N availability on rapidly induced responses has been examined for furanocoumarins in parsnip (Pastinaca sativa; Zangerl and Berenbaum, 1995), protease inhibitors in tomato (Stout et al., 1998), and alkaloids in tobacco (Baldwin and Hamilton, 2000). Comparisons are difficult as ranges of N availability vary between experiments; however, in each case, N availability demonstrated little or no effect on the induced concentrations of these defenses. Substrate/supply-based hypotheses, such as the carbon nutrient balance hypothesis (Bryant et al., 1983), used to predict patterns of carbon and N resource allocation to rapidly induced secondary metabolites commonly fail and thus have provided only limited mechanistic explanations (Reichardt et al., 1991; Hamilton et al., 2001). For constitutive terpenoid production, reduced N availability promoted increased synthesis and accumulation of sesquiterpenes in leaves of Heterotheca subaxillaris, increased allocation of monoterpenes to dill (Anethum graveolens) seeds, and increased rubber concentration in guayule (Parthenium argentatum; Mihaliak and Lincoln, 1989; Wander and Bouwmeester, 1998; Bonner and Galston, 1947). In black spruce (Picea mariana), monoterpene emission correlated with N concentration within field sites, yet idiosyncratically varied from positive to negative and had no correlation between different field sites (Lerdau et al., 1997). How N availability may affect dynamic responses, such as the rapid induction of terpenoids after elicitation, has not been established.
JA is a regulator of conifer terpenoid production (Martin et al., 2002), elicitor-induced terpenoid phytoalexin accumulation (Nojiri et al., 1996; Singh et al., 1998; Mandujano-Chávez et al., 2000), and insect-induced volatile emission (Hopke et al., 1994; Dicke et al., 1999; Schmelz et al., 2003a). Recent research also supports a role for JA in regulation of FAC elicitor-induced volatile emission (Halitschke et al., 2001; Schmelz et al., 2003b). BAW caterpillar herbivory and volicitin application result in an induced accumulation of JA; however, only BAW herbivory significantly increases E production (Schmelz et al., 2003a, 2003b). As predicted from pharmacological studies (Boland et al., 1995; Dicke et al., 1999), endogenous JA levels induced by insect herbivory and FAC elicitors display positive quantitative relationships with induced sesquiterpene emissions (Schmelz et al., 2003a, 2003b). Given this relationship, the prolonged increase in volicitin-induced JA demonstrated in low N plants is consistent with increased sesquiterpene emission. Plant responses to JA treatments are often similar yet different from more complex stimuli such as pathogen elicitation or insect attack, suggesting the interaction of other signals. In addition to JA, E can also regulate terpenoid metabolism. E addition and reciprocal E perception inhibition with 1-MCP has been demonstrated to increase and decrease sesquiterpene emission during fruit ripening, respectively (Puig et al., 1995; Rupasinghe et al., 2001). The interactions between volicitin and exogenous E on CS and indole volatile emission in low N plants demonstrate a role for JA and E in regulating induced-volatile emission in maize leaves.
When phytohormones and elicitors are exogenously applied to plants, special attention should be given to the physiological significance of the levels used. In this system, volicitin application of 1 nmol plant-1 represents a fraction of the amount of active FAC elicitors found in the OS of a single Noctuid caterpillar (Mori et al., 2001). With regards to E, basal levels of E emission from maize seedlings increase by 3 to 4 nL g-1 h-1 within 8 h of BAW herbivory (Schmelz at al., 2003a). In cotton, Beyer and Morgan (1971) determined that an E production rate of 2.5 nL g-1 h-1 equated to an internal E concentration of 500 nL L-1 in the leaf tissue. We find volicitin (1 nmol plant-1) interactions with exogenous E beginning at 10 nL L-1 and reaching a maximum at 250 nL L-1 in low N plants, indole being more responsive to E than the CS. Assuming that applied E equilibrates with plant air spaces and that internal E levels are important, we estimate that our E treatments are within physiologically relevant levels. Relating to exogenous E, the regulation of indole emission is particularly interesting. When maize seedlings are treated with volicitin early in the light cycle, indole emission is rapid and transient, with peak production often within 3 h (Turlings et al., 1998; Frey et al., 2000). In the current series of experiments, plants are treated in the evening and no volatiles are collected during the 12-h scotophase; thus, detected indole may represent a long-term induction. Under these conditions, the regulation of volicitin-induced indole emission is consistent. High levels of indole (>0.5 μg h-1) are only found in low N plants under the combined treatment of volicitin and E. E perception is essential as 1-MCP completely abolished induced indole emission and could not be recovered by further additions of E. Volicitin stimulates indole emission, in part, by increasing transcript levels of indole-3-glycerol phosphate lyase, which catalyzes the conversion of indole-3-glycerol phosphate to free indole (Frey et al., 2000). The current bioassay appears well suited for further investigations on the regulation of indole production in whole plants.
In N-deficient maize plants, low levels of E trigger an increase the activity of cell wall dissolution enzymes, such as cellulases, leading to PCD and aerenchyma formation (He et al., 1994; Drew et al., 2000). In tomato suspension cells treated with a topo isomerase-I inhibitor, E promotes H202 production and PCD, whereas E perception inhibitors diminish these responses (de Jong et al., 2002). Curiously, a major enzyme secreted onto the dietary source from labial salivary glands during caterpillar herbivory is Glc oxidase (GOX), which reacts with Glc to form H202 and gluconic acid (Eichenseer et al., 1999; Musser et al., 2002). Although a possible role of insect GOX on E signaling or volatile emission remains unclear, GOX does inhibit wound-induced nicotine accumulation in tobacco (Musser et al., 2002), a response similar to insect herbivory (McCloud and Baldwin, 1997). In tobacco, an insect-induced ethylene burst is believed to repress the wound-induced expression of root putrescine N-methyltransferase genes and thus reduce the increase of nicotine biosynthesis triggered by mechanical damage during herbivory (Winz and Baldwin, 2001). A possible explanation for the reduced activity of volicitin on medium N plants is the absence other such interacting components present during actual caterpillar herbivory. Unlike well-fertilized plants, N deficiency increases sensitivity to ozone and subsequent formation of reactive oxygen species (Landolt et al., 1997; Langebartels et al., 2002). Key signaling events, such as those acted on by E or reactive oxygen species, may already exist in a sensitized state in low N plants, thus requiring a reduced input of caterpillar-derived bioactive materials to trigger a strong plant response.
Our current work demonstrates that N availability influences JA signaling, E sensitivity, and the level of the plant volatile responses to FAC elicitors. Importantly, these findings provide insight into the phytohormone interactions that regulate the magnitude of FAC elicitor-induced volatile emission. The elucidation of regulatory signals and metabolic control points will be important as terminal terpenoid biosynthetic enzymes appear not to be transcriptionally up-regulated in this system (Schnee et al., 2002). This study demonstrates that intact maize seedlings with low N availability produce a large volatile response to volicitin that is similar in magnitude to the robust plant responses after BAW herbivory (Schmelz et al., 2003a). BAW herbivory induces E emission, yet mechanical damage and volicitin treatments do not (Schmelz et al., 2003a, 2003b). How insect herbivory alters E emission or E sensitivity remains an important unsolved component in elucidating the mechanism behind differential plant perception and response to mechanical damage and insect attack.
MATERIALS AND METHODS
Plant Growth Conditions
Seeds of Zea mays cv Delprim were acquired from Delley Seeds and Plants (Delley, Switzerland), were germinated in vermiculite, and were then transferred to hydroponic containers after 6 to 7 d (see Schmelz et al., 2001). The hydroponic nutrient solution consisted of 0.75 mm MgSO4-7H2O, 0.5 mm KH2PO4, 0.25 mm NaCl, 0.25 mm K2SO4, 60 μm Fe-Na EDTA, 50 μm H3BO3, 15 μm MnCl2-4H20, 2 μm ZnSO4-7H20, 0.25 μm CuSO4-5H20, and 0.2 μm Na2MoO4-2H20. N was supplied in a 2:1 ratio of KNO3:Ca(NO3)2-4 H2O with 3 mm total N used as the initial base level. As N concentrations were varied, a 2:1 ratio of KCl:CaSO4 was substituted to replace the missing K and Ca at the same molarity. All plants were maintained in a 12-h photoperiod (6:00 am-6:00 pm) with 350 μmol m-2 s-1 of photosynthetically active radiation supplied by a mixture of high pressure sodium and metal halide lamps (400W, GE Lucalox; General Electric, Fairfield, CT) with 70% relative humidity and a temperature cycle of 22°C/28°C (night/day). Treatments and volatile analyses were performed on 11-to 13-d-old plants that contained three to four leaves.
Physical and Chemical Leaf Treatments
For mechanical damage treatments, each of the oldest three leaves of individual plants received two superficial damage sites using a razor to scratch the abaxial surface of the leaves perpendicular to but not including the midrib vasculature. The mechanical damage sites (normally 2 × 10 mm) were approximately equidistant between the base and tip of the leaf, but were laterally staggered by 2 cm with one on each side of the midrib. This treatment disrupted the waxy cuticle and epidermal cells and allowed applied buffer solutions to cling to the leaf surface. A total of 10 μL of 50 mm sodium phosphate (pH 8.0) buffer was distributed evenly between all mechanical damage sites on each plant immediately after wounding. The quantity of volicitin or JA dissolved in buffer and applied to each plant is specified in each experiment. Unless indicated otherwise, leaf treatments were performed immediately before the end of the photophase (6:00 pm). All maize seedlings in experiments involving E and 1-MCP were placed in sealed 7-liter cylindrical Plexiglas chambers (12 × 62 cm) fitted with rubber septa for the introduction of E and 1-MCP with air-tight syringes. For experiments using 1-MCP, a 3-h pretreatment of plants occurred between 6:00 pm and 9:00 pm with air containing 10 μL L-1 1-MCP. This gaseous inhibitor was generated by dissolving EthylBloc (0.43% [w/w] 1-MCP; BioTechnologies for Horticulture, Inc., Walterboro, SC) in EthylBloc releasing buffer and trapping the evolved gas. Pretreatment necessitated removing plants from chambers, performing elicitor treatments, and then replacing plants back into the chambers. E (Scotty II Gases; Alltech, Deerfield, IL) addition was always performed immediately after elicitor treatments and was likewise maintained until plant analyses at specified times.
Analysis of Volatiles and JA
Collection and gas chromatography analysis of volatiles was performed as described in Schmelz et al. (2001). Unless otherwise indicated, plant volatile responses were collected for 1 h at the beginning of the following photoperiod, typically 12 h after treatment. This period corresponds with maximal volatile emission (Schmelz et al., 2001). The term CS is used to describe the summation of the three major insect-induced sesquiterpenes in maize (var Delprim), namely β-caryophyllene, (E)-α-bergamotene, and (E)- β-farnesene. Gas chromatography/mass spectrometry quantification of trans-JA, the predominant isomer, was performed as described in Engelberth et al. (2003) with modification; trimethylsilyldiazomethane was used as the methylating agent.
Effect of N Availability on Induced Volatile Emission and Growth
Immediately after transferal from vermiculite, maize seedlings (n = 40) were supplied with hydroponic solutions that contained 0, 0.1, 0.3, 1.0, or 3.0 mm N. Eight 12-d-old seedlings from each of the five N levels were split into two groups (n = 4), were mechanically damaged at 6:00 pm, and were treated with buffer or buffer + volicitin (1 nmol plant-1). Volatiles were collected for 1 h at the beginning of the following photoperiod. Based on the differential responses of plants grown under 0.1 to 0.3 and 1.0 to 3.0 mm N, two median N levels designated as low (0.2 mm NO3) and medium (2.0 mm NO3) were selected for further comparisons. To characterize plant growth and responses under these conditions, seedlings (n = 88) were transferred to low and medium N hydroponic solutions on d 7 and were successively harvested for biomass accumulation and RGR determination at d 7, 9, 11, and 13 (0, 2, 4, and 6 d later). A destructively sampled subset of low and medium N plants (n = 4) were treated on d 8, 10, and 12 with buffer or buffer + volicitin (1 nmol plant-1) at 6:00 pm with volatiles collected for 1 h at the beginning of the following photoperiod. To confirm the period maximal volatile emission, a separate set of low and medium N plants (n = 4) was treated with volicitin (1 nmol plant-1) at 6:00 pm on d 12 with an 8-h time course of volatile emission at 1-h intervals examined the next day. Volicitin is an elicitor of JA accumulation (Schmelz et al., 2003b); thus, we examined if N availability alters volicitin- and JA-induced volatile emission equally. Leaves of 13-d-old plants (n = 72) grown under low and medium N were treated with a range of volicitin (n = 4; 0, 0.01, 0.1, and 1 nmol plant-1) and JA (n = 4; 0, 0.1, 1, 10, and 100 nmol plant-1) doses.
N Availability and the Kinetics of Volicitin-Induced JA Accumulation
An experiment was designed to characterize the interaction of low and medium N availability, volicitin-induced JA levels, and volatile emission. Eleven-day-old maize seedlings grown under low (n = 76) and medium N (n = 76) availability were separated into three treatments (n = 4), including undamaged controls and mechanical damage with buffer or volicitin (1 nmol plant-1). At 6:00 pm, all treatments, including an additional set of “time zero” undamaged controls (n = 4), were initiated and successively harvested 0, 0.5, 2, 4, 8, or 12 h later. Tissue samples for JA quantification were frozen in liquid N2 and stored at -80°C before analysis. Volatiles were collected from a separate group of plants (n = 4).
Effect of N Availability on the Volicitin-E Interaction
An experiment combining four consecutive trials (n = 42 × 4) was designed to examine the interaction of N availability, volicitin, and E on induced volatile emission. Volatile emission was examined under low and medium N after mechanical damage with buffer or volicitin (0.1 and 1 nmol plant-1) applied under seven different E concentrations (0, 1, 10, 50, 250, 1,000, and 5,000 nL L-1). Within each trial, volatile emission was measured at a single volicitin dose and N level over the full range of E concentrations (n = 3, 0-5,000 nL L-1). A full series of mechanical damage + buffer controls were repeated for each trial. Leaf treatments were performed on 13-d-old plants at 9:00 pm.
N Availability, E Interactions, and Volicitin-Induced JA Accumulation
To test the hypothesis that E can regulate the induction of JA accumulation (O'Donnell et al., 1996), 13-d-old plants were grown under low and medium N and were treated (n = 4) at 6:00 pm as undamaged controls or they were mechanically damaged with buffer or volicitin (1 nmol plant-1) in the absence (-) or presence (+) of additional E (50 nL L-1). Tissues for JA analysis were harvested 3 and 15 h after leaf treatments. Not including the time zero control (n = 4), three sets of plants (n = 48 × 3) were required for the JA analyses (n = 4) and volatile collection (n = 4). A second experiment using the E perception inhibitor, 1-MCP, was performed on 11-d-old low N plants treated with mechanical damage and buffer or volicitin (1 nmol plant-1). Four additional treatments were imposed including: air only, E (250 nL L-1), 1-MCP pretreatment, and 1-MCP pretreatment + E, totaling eight groups All plants were in sealed 7-literL chambers from 6:00 pm to 9:00 pm during the 1-MCP pretreatment of the selected groups with all additional treatments beginning at 9:00 pm. Separate groups of treated plants (n = 6) were harvested for JA analysis 3 and 12 h after volicitin application and volatile collection (n = 9).
Statistical Analysis
Analyses of variance were performed on JA levels and volatiles. Significant treatment effects were investigated when the main effects of the Analyses of variance were significant (P < 0.05). Where appropriate, Tukey tests were used to correct for multiple comparisons between control and treatment groups. Dunnett's tests were used to examine significant increases in treatments groups compared with selected controls. Before statistical analysis, all data was subjected to square root transformation to compensate for elevated variation associated with larger mean values (Zar, 1996). The analysis was accomplished with JMP 3.0 statistical discovery software (SAS Institute, Cary, NC).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requester. The provision of novel materials used in this manuscript will be limited due to substantial effort required for synthesis and isolation.
Acknowledgments
We thank Julia Meredith and Elizabeth Rondon for help in plant growth and maintenance. We also thank Harry J. Klee and two anonymous reviewers for their time and shared insights that significantly improved the manuscript.
This work was supported by the U.S. Department of Agriculture-Agricultural Research Service and by the Defense Advanced Research Project Agency.
References
- Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH (2000) Identification and synthesis of volicitin and related components from beet armyworm oral secretions. J Chem Ecol 26: 203-220 [Google Scholar]
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276: 945-949 [Google Scholar]
- Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kuhnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J 29: 87-98 [DOI] [PubMed] [Google Scholar]
- Baldwin IT (1988) The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia 77: 378-381 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Hamilton W (2000) Jasmonate-induced responses of Nicotiana sylvestris results in fitness costs due to impaired competitive ability for nitrogen. J Chem Ecol 26: 915-952 [Google Scholar]
- Beyer EM, Morgan PW (1971) Abscission: the role of ethylene modification of auxin transport. Plant Physiol 48: 208-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland W, Hopke J, Donath J, Nuske J, Bublitz F (1995) Jasmonic acid and coronatine induce odor production in plants. Angew Chem Int Ed Engl 34: 1600-1602 [Google Scholar]
- Bonner J, Galston AW (1947) The physiology and biochemistry of rubber formation in plants. Bot Rev 13: 543-596 [Google Scholar]
- Bryant JP, Chapin FS III, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40: 357-368 [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123: 81-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi GM, Zhou L (2001) Carbon and nitrogen sensing and signaling in plants: emerging “matrix effects.” Curr Opin Plant Biol 4: 247-253 [DOI] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214: 537-545 [DOI] [PubMed] [Google Scholar]
- DeMoraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570-573 [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25: 1907-1922 [Google Scholar]
- Dicke M, Sabelis MW (1988) How plants obtain predatory mites as bodyguards. Netherl J Zool 38: 148-165 [Google Scholar]
- Drew MC, He CJ, Morgan PW (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen-starvation or phosphate-starvation in adventitious roots of Zea mays. Plant Physiol 91: 266-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trend Plant Sci 5: 123-127 [DOI] [PubMed] [Google Scholar]
- Eichenseer H, Mathews MC, Bi JL, Murphy JB, Felton GW (1999) Salivary glucose oxidase: multifunctional roles for Helicoverpa zea? Arch Insect Biochem Physiol 42: 99-109 [DOI] [PubMed] [Google Scholar]
- Engelberth J, Schmelz EA, Alborn HT, Cardoza YJ, Huang J, Tumlinson JH (2003) Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal Biochem 312: 242-250 [DOI] [PubMed] [Google Scholar]
- Frey M, Stettner C, Pare PW, Schmelz EA, Tumlinson JH, Gierl A (2000) An herbivore elicitor activates the gene for indole emission in maize. Proc Natl Acad Sci USA 97: 14801-14806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouinguené S, Degen T, Turlings TCJ (2001) Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11: 9-16 [Google Scholar]
- Gouinguené SP, Turlings TCJ (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129: 1296-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TR, Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: possible defense mechanism against insects. Science 175: 776-777 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR (2001) The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett 4: 86-95 [Google Scholar]
- He CJ, Drew MC, Morgan PW (1994) Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiol 105: 861-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC (1992) Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol 98: 137-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopke J, Donath J, Blechert S, Boland W (1994) Herbivore-induced volatiles: The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a β-glucosidase and jasmonic acid. FEBS Lett 352: 146-150 [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Arimura G, Ozawa R, Shimoda T, Takabayashi J, Nishioka T (2001) Exogenous ACC enhances volatiles production mediated by jasmonic acid in lima bean leaves. FEBS Lett 509: 332-336 [DOI] [PubMed] [Google Scholar]
- Johnson R, Ryan CA (1990) Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol 14: 527-536 [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gäbler R, Kühnemann F, Preston CA, Baldwin IT (2000) Herbivore-induced ethylene suppresses a direct defense but not an indirect defense against an adapted herbivore. Planta 210: 336-342 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 292: 2141-2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299-328 [DOI] [PubMed] [Google Scholar]
- Klee H, Tieman D (2002) The tomato ethylene receptor gene family: form and function. Physiol Plant 115: 336-341 [DOI] [PubMed] [Google Scholar]
- Korth KL, Dixon RA (1997) Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol 115: 1299-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt W, Günthardt-Goerg MS, Pfenninger I, Einig W, Hampp R, Maurer S, Matyssek R (1997) Effect of fertilization on ozone-induced changes in the metabolism of birch (Betula pendula) leaves. New Phytol 137: 389-397 [DOI] [PubMed] [Google Scholar]
- Langebartels C, Wohlgemuth H, Kschieschan S, Grün S, Sandermann H (2002) Oxidative burst and cell death in ozone-exposed plants. Plant Physiol Biochem 40: 567-575 [Google Scholar]
- Lerdau M, Litvak M, Palmer P, Monson R (1997) Controls over monoterpene emissions from boreal forest conifers. Tree Physiol 17: 563-569 [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Tumlinson JH (1995) Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol 21: 1217-1227 [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH (1994) Diurnal cycle of emission of induced volatile terpenoids herbivore-injured cotton plants. Proc Natl Acad Sci USA 91: 11836-11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandujano-Chávez A, Schoenbeck MA, Ralston LF, Lozoya-Gloria E, Chappell J (2000) Differential induction of sesquiterpene metabolism in tobacco cell suspension cultures by methyl jasmonate and fungal elicitor. Arch Biochem Biophys 381: 285-294 [DOI] [PubMed] [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HS, DeWald DB, Creelman RA, Mullet JE (1992) Coregulation of soybean vegetative storage protein gene expression by methyl jasmonate and soluble sugars. Plant Physiol 98: 859-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA (1994) Induction of parasitoid attracting synomone in Brussels sprout plants by feeding of Pieris-brassicae larvae: role of mechanical damage and herbivore elicitor. J Chem Ecol 20: 2229-2247 [DOI] [PubMed] [Google Scholar]
- McCloud ES, Baldwin IT (1997) Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta 203: 430-435 [Google Scholar]
- Mihaliak CA, Lincoln DE (1989) Changes in leaf monoterpene and sesquiterpene metabolism with nitrate availability and leaf age in Heterotheca subaxillaris. J Chem Ecol 15: 1579-1588 [DOI] [PubMed] [Google Scholar]
- Mori N, Alborn HT, Teal PEA, Tumlinson JH (2001) Enzymatic decomposition of elicitors of plant volatiles in Heliothis virescens and Helicoverpa zea. J Insect Physiol 47: 749-757 [DOI] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: Caterpillar saliva beats plant defences: a new weapon emerges in the evolutionary arms race between plants and herbivores. Nature 416: 599-600 [DOI] [PubMed] [Google Scholar]
- Nojiri H, Sugimori M, Yamane H, Nishimura Y, Yamada A, Shibuya N, Kodama O, Murofushi N, Omori T (1996) Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol 110: 387-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914-1917 [DOI] [PubMed] [Google Scholar]
- Paré PW, Alborn HT, Tumlinson JH (1998) Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA 95: 13971-13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325-331 [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Driscoll SP (1997) Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ 20: 110-116 [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103-2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohnert G, Jung V, Haukioja E, Lempa K, Boland W (1999) New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 55: 11275-11280 [Google Scholar]
- Puig DG, Perez ML, Fuster MD, Ortuno A, Sabater F, Porras I, Lidon AG, Delrio JA (1995) Effect of ethylene on naringin, narirutin and nootkatone accumulation in grapefruit. Planta Medica 61: 283-285 [DOI] [PubMed] [Google Scholar]
- Reichardt PB, Chapin FS III, Bryant JP, Mattes BR, Clausen TP (1991) Carbon/nutrient balance as a predictor of plant defense in Alaskan balsam poplar: potential importance of metabolite turnover. Oecologia 88: 401-406 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty TW, Huber SC, Volk RJ (1988) Alterations in leaf carbohydrate metabolism in response to nitrogen stress. Plant Physiol 88: 725-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasinghe HPV, Almquist KC, Paliyath G, Murr DP (2001) Cloning of hmg1 and hmg2 cDNAs encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase and their expression and activity in relation to α-farnesene synthesis in apple. Plant Physiol Biochem 39: 933-947 [Google Scholar]
- Schmelz EA, Alborn HT, Banchio E, Tumlinson JH (2003a) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216: 665-673 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171-179 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2003b) Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in Zea mays. Physiol Plant 117: 403-412 [DOI] [PubMed] [Google Scholar]
- Schnee C, Kollner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E, E)-farnesol after herbivore damage. Plant Physiol 130: 2049-2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217: 149-151 [DOI] [PubMed] [Google Scholar]
- Singh G, Gavrieli J, Oakey JS, Curtis WR (1998) Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyamus muticus in root cultures. Plant Cell Rep 17: 391-395 [DOI] [PubMed] [Google Scholar]
- Sisler EC, Dupille E, Serek M (1996) Effect of 1-methylcyclopropene and methylenecyclopropene on ethylene binding and ethylene action on cut carnations. Plant Growth Regul 18: 79-86 [Google Scholar]
- Spiteller D, Pohnert G, Boland W (2001) Absolute configuration of volicitin, an elicitor of plant volatile biosynthesis from lepidopteran larvae. Tetrahedron Lett 42: 1483-1485 [Google Scholar]
- Stitt M (1999) Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2: 178-186 [DOI] [PubMed] [Google Scholar]
- Stout MJ, Brovont RA, Duffey SS (1998) Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J Chem Ecol 24: 945-963 [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20: 1329-1354 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207: 146-152 [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251-1253 [DOI] [PubMed] [Google Scholar]
- Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37: 141-172 [Google Scholar]
- Wander JGN, Bouwmeester HJ (1998) Effects of nitrogen fertilization on dill (Anethum graveolens L.) seed and carrone production. Ind Crops Prod 7: 211-216 [Google Scholar]
- Winz RA, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: Insect-induced ethylene suppresses jasmonate-induced accumulation of nicotine biosynthesis transcripts. Plant Physiol 125: 2189-2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6: 1077-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl AR, Berenbaum MR (1995) Spatial, temporal, and environmental limits on xanthotoxin induction in wild parsnip foliage. Chemecology 1: 37-42 [Google Scholar]
- Zar JH (1996) Biostatistical Analysis, Ed 3. Prentice-Hall, Upper Saddle River, NJ