Abstract
3-Deoxy-d-manno-2-octulosonic acid-8-phosphate (Kdo-8-P) synthase catalyzes the condensation of phosphoenolpyruvate with d-arabinose-5-phosphate to yield Kdo-8-P. Kdo-8-P is the phosphorylated precursor of Kdo, a rare sugar only found in the rhamnogalacturonan II pectic fraction of the primary cell walls of higher plants and of cell wall polysaccharides of some green algae. A cDNA named LekdsA (accession no. AJ294902) encoding tomato (Lycopersicon esculentum) Kdo-8-P synthase has been isolated. The recombinant protein rescued a kdsA thermosensitive mutant of Salmonella typhimurium impaired in the synthesis of a functional Kdo-8-P synthase. Using site-directed mutagenesis of LekdsA cDNA, the tomato Kdo-8-P synthase was shown to possess the same essential amino acids that form the active sites in the bacterial enzymes. The tomato kdsA gene expression and the relevant Kdo-8-P synthase activity were preferentially associated to dividing cells, in the course of the early development of tomato fruit and in meristematic tissues. Furthermore, the transcription of the kdsA gene was found to oscillate during the cell cycle in tobacco (Nicotiana tabacum) Bright-Yellow 2 synchronized cells with a maximum during mitosis.
Cell wall pectin consists of three structurally well characterized polysaccharides: homogalacturonan, rhamnogalacturonan (RG)-I, and RG-II (Willats et al., 2001). Despite a complex structure, RG-II is evolutionarily conserved in the plant kingdom because it is present in the primary cell wall of all higher plants predominantly in the form of a dimer that is cross-linked by a borate di-ester (dRG-II-B) between two apiosyl residues (O'Neill et al., 1996; Ishii et al., 1999). Nevertheless the precise function of RG-II remains to be fully established. RG-II is the only known borate-binding polysaccharide in the primary cell wall, sequestering up to 80% of the cellular boron (B; Matoh et al., 1996). B deficiency results in altered plant growth and changes in cell wall architecture (Fleischer et al., 1998, 1999; Ishii et al., 2001). Thus, B-mediated cross-linking of RG-II generates a covalently cross-linked pectic network that is involved in the regulation of cell wall properties and plant growth (Fleischer et al., 1999; Ishii et al., 1999, 2001).
The isolation of plant mutants with specifically altered RG-II structure is required to demonstrate the function of RG-II in the cell wall and its relationship with growth. The Arabidopsis mur1 mutant is the first known mutant to be altered in the glycosyl residue composition of RG-II (Reiter et al., 1993; O'Neill et al., 2001). The aerial parts of mur1 plants are defective in an isozyme of GDP-d-Man-4,6-dehydratase that is involved in the synthesis of GDP-l-Fuc. mur1 plants are dwarfed and display altered growth of their rosette leaves (O'Neill et al., 2001). Leaf cell walls of mur1 contain normal amounts of RG-II, but only one-half exists as dRG-II-B. In mur1, the formation of dRG-II-B is slower than in wild type and requires higher concentrations of B. When mur1 plants are supplied with additional B, the formation of dRG-II-B and normal growth are restored (O'Neill et al., 2001), thus demonstrating a direct relationship between RG-II dimerization promoted by B and normal plant growth.
RG-II has an α-1,4-linked homogalacturonan backbone that is substituted with four structurally different oligosaccharide side chains. Eleven different glycosyl residues are present in RG-II. Among these residues are the seldom-observed sugars: aceric acid, apiose, 3-deoxy-d-lyxo-heptulosonic acid, and 3-deoxy-d-manno-octulosonic acid (Kdo) (O'Neill et al., 1996; Pérez et al., 2000). It was long believed that Kdo was synthesized only by Gram-negative bacteria as a component of the lipopolysaccharides present in the outer leaflet of the outer membrane (Rick, 1987). However, Kdo is now known to be a component of the primary cell walls of higher plants and of cell wall polysaccharides of some green algae (York et al., 1985a; Becker et al., 1995).
In Gram-negative bacteria, the synthesis pathway of Kdo involves three sequential reactions (Rick, 1987): (a) the isomerization of d-Rib-5-P into d-Ara-5-P catalyzed by Ara-5-P isomerase, (b) the condensation of phosphoenolpyruvate (PEP) with Ara-5-P to yield 3-deoxy-d-manno-2-octulosonic acid-8-phosphate (Kdo-8-P) and inorganic phosphate catalyzed by Kdo-8-P synthase, and (c) the dephosphorylation of Kdo-8-P by Kdo-8-P phosphatase. The resulting Kdo is then activated by coupling to CMP before its incorporation into the lipopolysaccharide in a reaction catalyzed by CMP-Kdo synthetase. In plants, the recent identification of a CMP-Kdo synthetase gene from maize (Zea mays; Royo et al., 2000a) and Kdo-8-P synthase genes from pea (Pisum sativum; Brabetz et al., 2000) have led to the suggestion that Kdo synthesis and activation are conserved between plants and bacteria (Royo et al., 2000b).
In higher plant cells, a new wall originates from the cell plate. The cell plate itself is formed by the fusion of Golgi-derived vesicles transported by the phragmoplast microtubules during cytokinesis, the final stage of cell division (Cosgrove, 1997; Verma, 2002). Hence, assuming that Kdo is solely found in the RG-II pectic fraction of the plant cell wall (York et al., 1985a; O'Neill et al., 1996; Pérez et al., 2000), it is expected that its synthesis may be associated to the formation of the cell plate and putatively to cell cycle progression especially during mitosis. Nevertheless, the coupling to the cell cycle of gene expression and enzymatic activities involved in cell wall pectic polysaccharides are still poorly documented.
Here, we describe the isolation of a tomato (Lycopersicon esculentum) cDNA, LekdsA, encoding a functional ortholog of bacterial Kdo-8-P synthase. Site-directed mutagenesis experiments of the LekdsA cDNA identified amino acid residues essential for the enzyme activity. We provide evidence that the Kdo-8-P synthase gene expression and enzyme activity are preferentially associated with young tomato fruits and vegetative organs displaying meristematic activity. Furthermore, we demonstrate that Kdo-8-P synthase gene expression and Kdo-8-P synthesis oscillate during the cell cycle with a maximum at the M phase.
RESULTS
Isolation of a cDNA Clone Coding for Tomato Kdo-8-P Synthase
Differential plaque hybridization was performed using a `young tomato fruit' cDNA library (Joubès et al., 1999) to screen for genes preferentially expressed at the cell division phase of early fruit development. The nucleotide sequences of the selected positive cDNAs were determined, and their translation product was deduced and used for protein alignments using the BLAST software (Altschul et al., 1997). Among these cDNAs, we identified a 1,216-bp-long cDNA encoding a putative protein of 290 amino acids (Mr = 31,700), which displayed significant similarities with plant and bacterial Kdo-8-P synthases (Fig. 1). This tomato protein shared 86%, 80%, and 45%, respectively, of identical residues with pea (Brabetz et al., 2000), Arabidopsis, and E. coli Kdo-8-P synthases. Thus, it was identified as being a homolog of Kdo-8-P synthase, and the cDNA was named Le-kdsA according to the bacterial gene name (Woisetschläger and Högenauer, 1987).
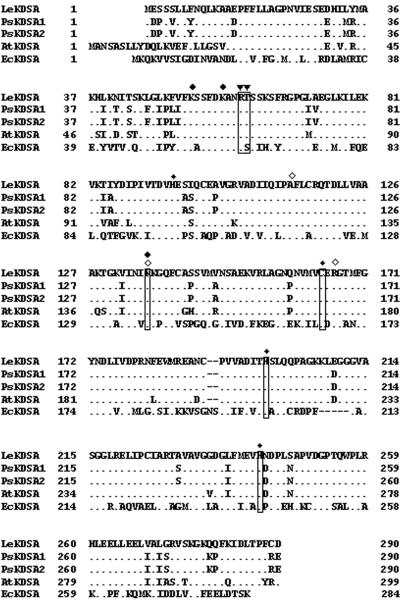
Figure 1.
Comparison of amino acid sequences of plant Kdo-8-P synthases. Multiple alignment of the deduced amino acid sequences of Kdo-8-P synthase was realized using tomato (LeKDSA, AJ294902), pea (PsKDSA1, Y14272; and PsKDSA2, Y14273), and Arabidopsis (AtKDSA, AAD34685) sequences and the Escherichia coli Kdo-8-P synthase (EcKDSA, U18555). Dots, Identical residues between LeKDSA and the different Kdo-8-P synthases. Residues expected to be essential for activity are specified as follows: ▾, binding site for Ara-5-P; ♦, binding site for PEP carboxylate; ⋄, binding site for PEP phosphate; asterisk, structurally important residues for maintenance of active sites. Positions of the mutagenized amino acids used for the subsequent analysis are boxed. Gaps (-) were introduced for maximizing the alignments.
LekdsA Encodes a Functional Homolog of Bacterial Kdo-8-P Synthase
As shown for the pea enzyme (Brabetz et al., 2000), compelling evidence that the LeKDSA protein catalyzed the formation of Kdo-8-P was obtained by expressing the tomato protein in the thermosensitive mutant kdsAts of Salmonella enterica serovar Typhimurium (strain AG701i50) impaired in the synthesis of a functional Kdo-8-P synthase (Rick and Osborn, 1977). The plasmid pLEKdoS expressing the tomato Kdo-8-P synthase was able to rescue the thermosensivity of the kdsAts AG701i50 strain (Fig. 2A). All strains harboring pLEKdoS grew well at 42°C but to a lower density than that of the AG701 controls, suggesting a slight toxic effect to bacteria of the over-expressed tomato Kdo-8-P synthase as was observed for the maize CMP-Kdo synthetase (Royo et al., 2000a).
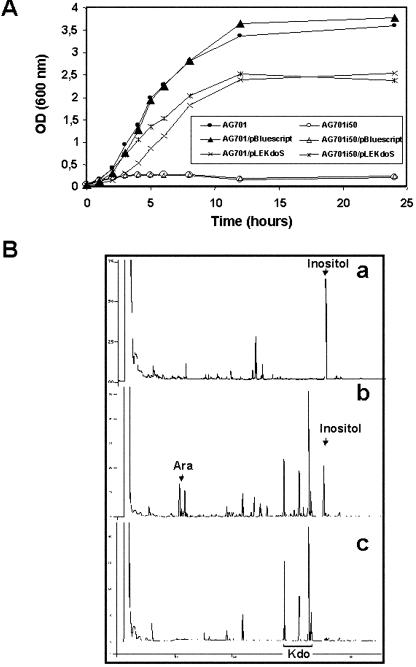
Figure 2.
Functional complementation of a kdsAts mutation in S. enterica serovar Typhimurium by LekdsA. A, Growth curves of the different S. enterica serovar Typhimurium strains at the restrictive temperature (42°C). B, Gas chromatography (GC) profiles of trimethylsilyl-methyl-ester-methyl derivatives of the enzymatic products obtained after incubation with cell extracts of AG701i50 strain transformed with pLEKdoS without (a) or with (b) Ara-5-P compared with a standard of the trimethylsilylmethyl-ester-methyl glycoside derivative of Kdo (c).
In vitro Kdo-8-P synthase enzymatic activities were determined from the transformed S. enterica strains (Table I). In cell extracts from strain AG701 hosting pLEKdoS, the specific activity of Kdo-8-P synthase measured at 30°C was 2.5-fold higher than that of the untransformed strain. In the mutant strain AG701i50, the presence of pLEKdoS led to a 50-fold increase in Kdo-8-P synthase activity. When AG701-pLEKdoS and AG701i50-pLEKdoS cell extracts were incubated at 42°C, we observed a 4-fold stimulation in the specific activity that was comparable with that obtained when the untransformed wild-type AG701 strain was shifted from 30°C to 42°C. Hence, the specific activity measured at 42°C for the AG701i50 strain harboring pLEKdoS was about 200-fold higher than that obtained at 30°C in the untransformed mutant AG701i50 strain.
Table I.
The specific activity of recombinant tomato Kdo-8-P synthase expressed in S. enterica sv. Typhimurium strains
| Strain
|
Growth
|
Specific Activitya
|
||||
|---|---|---|---|---|---|---|
| 30°C
|
42°C
|
Arabinose-5-P
|
Erythrose-4-P
|
Rib-5-P
|
||
| 30°C | 42°C | 42°C | 42°C | |||
| milliunits mg−1 protein | ||||||
| AG701 | + | + | 6.1 ± 0.2 | 18.7 ± 2.4 | ND | ND |
| AG701i50 | + | − | 1.3 ± 0.2 | 0.4 ± 0.1 | ND | ND |
| AG701-pBluescript | + | + | 5.2 ± 1.1 | 18.8 ± 1.5 | 0.99 ± 0.64 | 2.34 ± 0.35 |
| AG701i50-pBluescript | + | − | 1.2 ± 0.2 | 0.9 ± 0.2 | ND | ND |
| AG701-pLEKdoS | + | + | 16.4 ± 0.9 | 67.5 ± 2.8 | 0.67 ± 0.17 | 6.21 ± 0.42 |
| AG701i50-pLEKdoS | + | + | 60.5 ± 0.3 | 232.3 ± 11 | 0.83 ± 0.59 | 6.98 ± 1.07 |
| AG701-R61A/T62A | + | + | 1.3 ± 0.3 | 1,8 ± 0,5 | ND | ND |
| AG701i50-R61A/T62A | + | − | 0.7 ± 0.3 | 0.4 ± 0.1 | ND | ND |
| AG701-K136G | + | + | 3 ± 0.2 | 7.6 ± 0.8 | ND | ND |
| AG701i50-K136G | + | + | 1.2 ± 0.3 | 2.4 ± 0.5 | ND | ND |
| AG701-C164A | + | + | 9 ± 0.6 | 38.6 ± 4.3 | ND | ND |
| AG701i50-C164A | + | + | 7.4 ± 1.3 | 46 ± 8.9 | ND | ND |
| AG701-H198G | + | + | 5.3 ± 0.2 | 14.4 ± 0.5 | ND | ND |
| AG701i50-H198G | + | + | 0.5 ± 0.2 | 1.6 ± 0.4 | ND | ND |
| AG701-H242G | + | + | 3.7 ± 0.4 | 12.3 ± 0.5 | ND | ND |
| AG701i50-H242G | + | + | 0.8 ± 0.1 | 1.7 ± 0.2 | ND | ND |
a Each value is the mean of four independent measurements ± SD. ND, Not done.
Because the assay used for enzymatic activity monitors the formation of 3-deoxy-ald-2-ulosonic acids (Ray, 1980), it was necessary to show that the recombinant tomato Kdo-8-P synthase was specific for Ara-5-P. d-Erythrose-4-P and d-Rib-5-P were assayed as substrates with protein extracts from the transformed S. enterica strains at 42°C (Table I). Using d-erythrose-4-P, the value of residual 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase activity in AG701i50-pLEKdoS cell extracts was not significantly different from that of the residual Kdo-8-P synthase activity in AG701i50 or AG701i50-pBlue-script. A small activity was measured with d-Rib-5-P that may originate from an Ara-5-P isomerase activity present in the bacterial cell extracts as mentioned by Brabetz et al. (2000). This activity was not significantly different between AG701 and AG701i50 overproducing the recombinant tomato Kdo-8-P synthase at 42°C. Hence, these data indicated that the enzymatic activity of the recombinant tomato Kdo-8-P synthase was specific for Ara-5-P.
Finally, the unequivocal identification of Kdo-8-P synthase encoded by LekdsA was given by GC measurements of Kdo-8-P overproduction in the complemented S. enterica strain (Fig. 2B). A protein extract was prepared from AG701i50-pLEKDOS cells grown at 42°C. The bacterial extract was then incubated with PEP in the absence (Fig. 2B, a) or presence (Fig. 2B, b) of Ara-5-P. The resulting products were lyophilized, submitted to methanolysis and silylation, and the derivatives were then analyzed by GC. As shown in Figure 2B, in addition to the remaining Ara, four chromatographic peaks were only detected after incubation of the protein extract with both Ara-5-P and PEP (Fig. 2B, b) and identified as α- and β-anomers of the two cyclic forms of Kdo by comparison with a GC profile of a Kdo standard (Fig. 2B, c). This confirmed that the tomato enzyme is able to synthesize Kdo-8-P by condensation of PEP onto Ara-5-P.
Site-Directed Mutagenesis of the Tomato Kdo-8-P Synthase
The amino acid residues shown to be essential for E. coli Kdo-8-P synthase activity (Salleh et al., 1996; Sheflyan et al., 1999; Radaev et al., 2000) are conserved in the sequence of the plant enzymes as shown in Figure 1. In the tomato Kdo-8-P synthase, residue K136 may be involved in the binding of PEP, residues R61 and T62 may be involved in the binding of Ara-5-P, and residues C164, H198, and H242 may represent structurally important residues for maintenance of active sites. To investigate the role of these residues for the tomato enzyme activity, mutants were generated by PCR-directed mutagenesis using the cDNA LekdsA as a template and specific oligonucleotides introducing point mutations (Table II). The functionality of the different mutagenized tomato Kdo-8-P synthases was tested by their ability to complement the AG701i50 kdsAts mutation, and the specific activity of the mutagenized Kdo-8-P synthase was determined (Table I).
Table II.
Sets of oligonucleotides used for the site-directed mutagenesis of tomato Kdo-8P synthase encoded by pLEKdoS
| Targeted Protein Structure | Targeted Amino Acids | Primer Sequence (5′ to 3′)a | Resulting Amino Acids |
|---|---|---|---|
| Arabinose-5-P-binding site | R61T62 | GACAAGGCTAATGGAGGCTCGTCTAAATCATTCC | A61A62 |
| GGAATGATTTAGACGAGCCTCCATTAGCCTTGTC | |||
| PEP-binding site | K136 | GGAAAAGTTATTAACATTGGGAAGGGCC | G136 |
| GGCCCTTCCCAATGTTAATAACTTTTCC | |||
| Maintenance of active sites | C164 | GTCATGGTGGCTGAGAGAGG | A164 |
| CCTCTCTCAGCCACCATGAC | |||
| Maintenance of active sites | H198 | CTGATATAACTGGTTCATTACAAC | G198 |
| GTTGTAATGAACCAGTTATATCAG | |||
| Maintenance of active sites | H242 | ATGGAGGTGGGCAATGATCC | G242 |
| GGATCATTGCCCACCTCCAT |
a Nucleotides introducing site-directed mutations in the coding sequence of LekdsA are underlined.
The double mutation R61A/T62A resulted in a complete inactivation of the tomato Kdo-8-P synthase. As a result of this loss of activity, the double mutant was unable to grow at the nonpermissive temperature of 42°C. Moreover, the R61A/T62A mutation also affected the endogenous bacterial Kdo-8-P synthase activity in the wild-type strain because we observed a 10-fold decrease in the specific activity. Because the active bacterial enzyme is a homotetramer (Radaev et al., 2000), this reduction may result from the ability of the tomato Kdo-8-P synthase protein subunit to associate and compete with the bacterial subunits to form oligomers and, thus, impair the functionality of the endogenous enzyme. In all other mutants, the tomato Kdo-8-P synthase activity was dramatically reduced, but the residual enzymatic activity was sufficient to sustain growth of the AG701i50 transformants at 42°C.
LekdsA mRNAs Are Preferentially Expressed in Dividing Tissues during Fruit Development
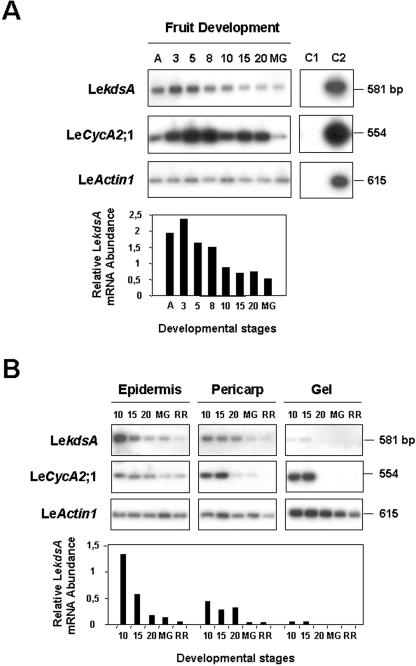
The relative transcript level corresponding to Le-kdsA was analyzed during fruit development by semiquantitative reverse transcription (RT)-PCR as described previously (Joubès et al., 2000b). In the course of fruit development (Fig. 3A), the LekdsA transcripts were highly expressed between anthesis and 8 DPA with a maximum peaking at 3 DPA, i.e. when mitotic activities are maximal during the early growth of tomato fruit (Gillaspy et al., 1993; Joubès et al., 1999). Their abundance decreased during the cell expansion phase (from 8-20 DPA) and until the onset of ripening (MG stage). Then, we investigated the expression of LekdsA in the different tissues of the developing fruit (Fig. 3B). In accordance with our previous works showing the temporal and spatial regulation of mitotic activity and cell cycle gene expression in developing tomato fruit (Joubès et al., 1999, 2000b), the accumulation of LekdsA transcripts was associated with cell divisions occurring to the onset of maturation (MG stage) in the epidermis, until 20 DPA in the pericarp, and up to 15 DPA in the gel (locular tissue). During fruit development (in whole fruit or dissected tissues), the expression profile of LekdsA paralleled that of LeCycA2;1 used as a cell division-associated gene (Joubès et al., 2000b), whereas the expression of the LeActin1 cDNA was found to be constitutive.
Figure 3.
Expression analysis of LekdsA in developing tomato fruits. A, Semiquantitative RTPCR analysis of LekdsA expression during fruit development. The expression of LekdsA, LeCycA2;1, and LeActin1, respectively, used as controls of cell division-specific expression and constitutive expression, were assayed using RTPCR products originating from total RNA isolated from fruits harvested at the following developmental stages: anthesis (A), 3, 5, 8, 10, 15, and 20 DPA, and mature green (MG) stage, as described in `Materials and Methods.' C1, PCR-negative control without adding DNA matrix; C2, PCR-positive control using purified cDNA inserts. The accumulation of LekdsA transcripts was quantified by image scanning of the auto-radiogram using the Quantity One software from Bio-Rad Laboratories (Hercules, CA). The relative LekdsA mRNA abundance was normalized toward that of LeActin1 and expressed as a ratio of arbitrary units for pixel intensities. B, Semiquantitative RT-PCR analysis of LekdsA expression in the different fruit tissues during development. Total RNA was isolated from dissected fruit tissues (epidermis, pericarp, and gel) at the following developmental stages: 10, 15, and 20 DPA and MG and Red Ripe stages. Quantification of the relative LekdsA mRNA abundance was as in A.
LekdsA Is Down-Regulated during Cell Expansion in the Hypocotyl
The transcript level of LekdsA was determined during hypocotyl growth used as a model system for cell elongation (Gendreau et al., 1997). After 10 d of growth in the light or in the dark, the different parts of tomato seedlings were dissected before RNA extraction as indicated in Figure 4A. We determined the mean cell length and width in each sample of etiolated hypocotyl and in the corresponding parts of the light-grown hypocotyl and found similar results as those published by Gendreau et al. (1997) for Arabidopsis hypocotyls, i.e. a cell elongation process along an acropetal gradient (data not shown). The expression of LekdsA was then measured by semiquantitative RTPCR and compared with that of LeCycA2;1 used as a cell division-expressed gene and LeCDKA;1 used as a constitutively expressed gene in cell division and differentiation (Hemerly et al., 1993; Fig. 4B).
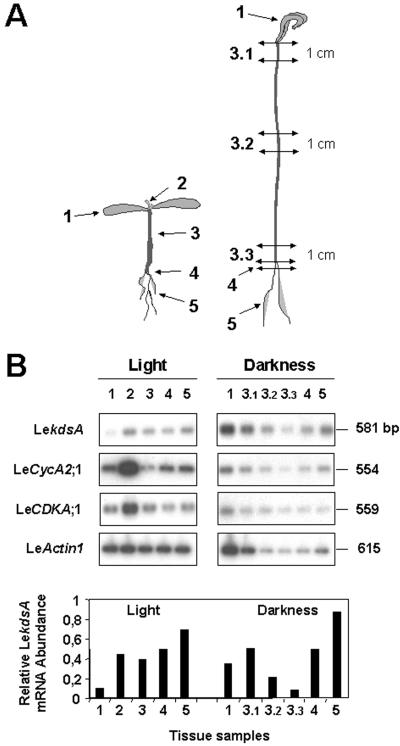
Figure 4.
Expression analysis of LekdsA in light- and dark-grown tomato seedlings. A, Description of the tissue samples of seedlings harvested for total RNA extraction. Samples from light-grown seedlings (left) were from: 1, fully developed colyledons; 2, apical meristem; 3, hypocotyl; 4, crown; and 5, roots. Samples from dark-grown seedlings (right) were from: 1, apical cross; 3.1, top part of hypocotyl (1 cm); 3.2, middle part of hypocotyl (1 cm); 3.3, basal part of hypocotyl (1 cm); 4, crown; and 5, roots. B, Semiquantitative RT-PCR analysis of LekdsA expression in light- and dark-grown tomato seedlings. Total RNA to be used for RT was isolated from the indicated samples in A. The RT-PCR analysis and the determination of relative LekdsA mRNA abundance normalized toward that of LeCDKA;1 were performed as described in Figure 3.
In 10-d-old seedlings grown in the light, the expression of LekdsA was found to be associated with the root system and the apical meristem similarly to LeCycA2;1 and LeCDKA;1. In fully developed cotyledons, the expression of LekdsA was insignificant. These data suggests that LekdsA is preferentially expressed in dividing cells rather than in differentiated cells and confirmed what was observed in developing fruits (Fig. 3). In dark-grown seedlings, the expression of LeActin1 was deeply affected as a consequence of metabolic repression of the gene expression (Joubès et al., 2001). Because the expression of LeCDKA;1 was found to be constitutive in all seedling parts, it was used to normalize that of Le-kdsA. The maximum of LekdsA expression was observed in the root system. The level of LekdsA mRNAs accumulated in the upper part of the etiolated hypocotyl and then decreased gradually toward the base, i.e. inversely to the acropetal gradient of cell elongation. Although LeCDKA1;1 was constitutively expressed in differentiated tissues, the expression of LeCycA2;1 and LeActin1 displayed similar patterns than that of LekdsA, confirming the preferential expression of LekdsA in dividing cells rather than in expanding cells.
Measurement of Kdo-8-P Synthesis in Tomato Plant Tissue Extracts
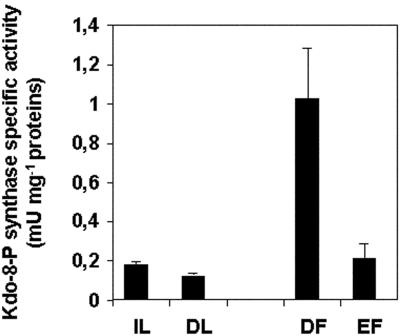
The Kdo-8-P synthase-specific activity was determined in extracts from tomato leaves and fruits (Fig. 5). Immature leaves and fully differentiated leaves were harvested as well as fruits at the two developmental stages, 8 and 20 DPA, to discriminate between cell division and cell expansion, respectively. In fully differentiated leaves, we could measure a basal Kdo-8-P synthase-specific activity that was 1.5-fold lower than that of immature leaves. This latter one was not significantly different from the specific activity determined in 20-DPA fruits. However, in young 8-DPA (dividing) fruits, the Kdo-8-P synthase-specific activity was increased to a 4- to 5-fold level. Hence, the Kdo-8-P synthase activity was preferentially associated with cell division in young organs and, thus, followed the kinetics of transcript accumulation.
Figure 5.
Kdo-8-P synthase-specific activity in tomato. The specific activity of tomato Kdo-8-P synthase was determined in tomato from immature (IL) and fully differentiated (DL) leaves and from 8-DPA dividing (DF) and 20-DPA expanding (EF) fruits. Data represent the mean and sd of four independent experiments.
The Expression of the Tobacco (Nicotiana tabacum) kdsA Gene Is Associated with the M Phase of the Cell Cycle
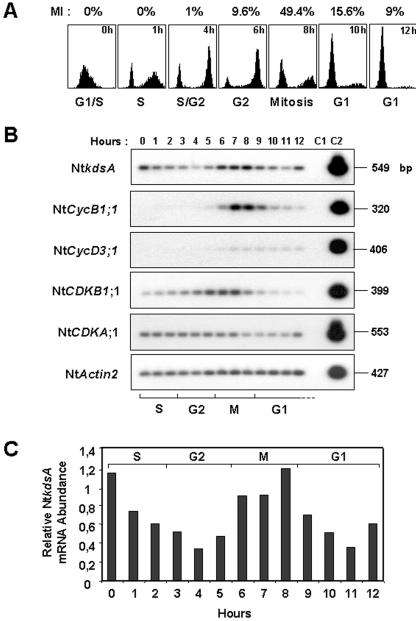
The cell cycle specificity of the kdsA gene expression was investigated next using synchronized Bright-Yellow 2 (BY-2) cell suspensions (Fig. 6). A 550-bp-long cDNA probe specific for the tobacco kdsA gene (NtkdsA; accession no. AJ538191) was generated by RT-PCR. Then, the expression of NtkdsA was measured by semiquantitative RT-PCR using total RNAs extracted from synchronized BY-2 cell suspensions and compared with that of different cell cycle genes used as phase-specific markers: Nt-CycB1;1 for M, NtCycD3;1 for G1, NtCDKB1;1 for G2/M transition, and NtCDKA;1 and NtActin2 as constitutively cell cycle-expressed genes (Fig. 6).
Figure 6.
Kdo-8-P synthase gene expression in synchronized BY-2 cell suspensions. BY-2 cells were synchronized with aphidicolin (see `Materials and Methods'), and samples were taken every hour. A, Cell cycle progression monitored by flow cytometry and by measurement of the mitotic index (MI). B, Semiquantitative RT-PCR analysis of NtkdsA expression. Total RNA to be used for RT was isolated from the indicated samples in A. The RT-PCR analysis was performed as described in Figure 3. Specific cDNA probes were prepared for NtCDKA;1 (L77082), NtCDKB1;1 (AF289465), NtCycB1;1 (Z37978), NtCycD3;1 (AJ011893), and NtActin2 (U60490). C, Quantification of NtkdsA transcript accumulation. Signals obtained after autoradiography from B were quantified as described in Figure 3. The determination of relative NtkdsA mRNA abundance was normalized toward that of NtActin2.
The level of NtkdsA transcripts was shown to fluctuate during the cell cycle. During the S phase, the accumulation of NtkdsA transcripts decreased to achieve a basal level at the G2 phase. Then, it increased and reached a maximum during the M phase. At the exit of mitosis and in early G1 phase, the level of NtkdsA transcripts dropped again but seemed to be re-induced in G1 progressing cells (at 12 h).
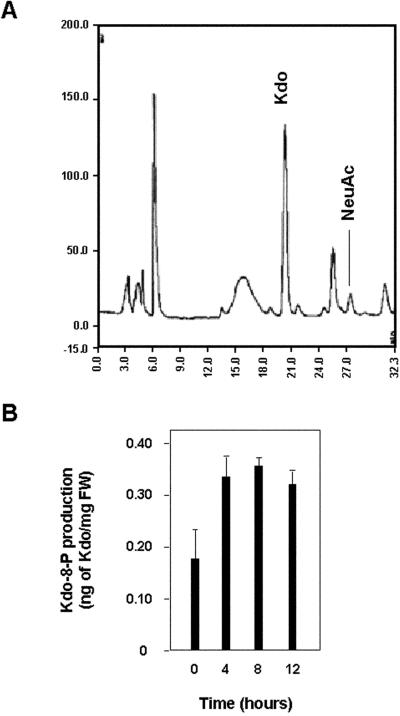
Next, we measured the accumulation of Kdo-8-P in synchronized BY-2 cell extracts corresponding to the different phases of the cell cycle (Fig. 7). Cytosolic extracts were treated with mild acid to remove the phosphate groups and then with DMB, a reagent able to specifically transform α-ketoacids, such as Kdo or sialic acids, into fluorescent derivatives (Hara et al., 1989). N-acetylneuraminic acid was added to the cell extracts before derivatization as an internal standard for quantification of Kdo. Figure 7, A and B, respectively, show an HPLC profile as an illustration and values obtained for synchronized BY-2 cell extracts prepared at 0 h, i.e. right after the release from aphidicolin block in the S phase, 4 h in the G2 phase, 8 h at the peak of mitosis, and 12 h in the G1 phase. Although we did not observe strong variations in Kdo amounts, the maximum of Kdo production seemed to be associated to the M phase.
Figure 7.
Kdo-8-P synthesis in synchronized BY-2 cell suspensions. A, HPLC profile of cytosolic monosaccharides derivatised with 1,2-diamino-4,5-methylene dioxybenzene (DMB). NeuAc, N-acetyl neuraminic acid used as internal standard for quantification of Kdo. B, Quantification of Kdo in synchronized BY-2 cell suspensions. Data represent the mean and sd of three independent experiments.
DISCUSSION
In plants, the new primary cell wall is synthesized in the cell plate that is linked to cytokinesis, the final stage of mitosis. To understand the synthesis of primary cell wall at the molecular, biochemical, and cellular levels, the availability of genes or enzymes involved in this process is required.
In this report, we describe the isolation of the cDNA LekdsA from tomato that codes for Kdo-8-P synthase. Kdo-8-P synthase synthesizes the phosphorylated precursor of Kdo, a rare sugar only found in the RG-II pectic fraction of plant cell wall (Pérez et al., 2000). From the literature, it has been reported previously that Kdo-8-P synthase activity exists in plants (Doong et al., 1991), and a cDNA for Kdo-8-P synthase in pea was isolated (Brabetz et al., 2000). However, neither physiological nor developmental data dealing with the Kdo-8-P synthase gene expression and enzyme activity are available.
In a first step, we provided the unequivocal identification of the tomato LekdsA-encoded protein. This was based on similar functional properties between the plant and bacterial Kdo-8-P synthases: (a) the ability to complement a bacterial mutant, (b) the substrate specificity of the enzyme, and (c) the conservation in the tomato enzyme of essential amino acids for the prokaryotic Kdo-8-P synthase activity. Because Kdo is essential for the survival of Gram-negative bacteria, only conditionally lethal Kdo biosynthesis mutants could be isolated (Lehmann et al., 1977; Rick and Young, 1982). Thus, we showed that LekdsA is able to complement and rescue the phenotype of a Kdo-8-P synthase-defective kdsAts mutant in S. enterica at the nonpermissive temperature (Fig. 2). The specific activity of tomato Kdo-8-P synthase expressed in the transformed S. enterica AG701i50 mutant strain (232 milliunits mg-1 protein) is quite comparable with that of the recombinant pea enzyme (180 milliunits mg-1 protein; Brabetz et al., 2000). This slightly different value for the tomato enzyme activity may originate from the use of 42°C as a restrictive temperature for complementing the mutant, whereas Brabetz et al. (2000) analyzed their recombinant strains for activity at 37°C. In our hands, the thermosensitivity of the kdsAts mutant was only observed when the temperature was increased to 42°C. The specificity of enzymatic activity of the recombinant tomato Kdo-8-P synthase was ascertained by measuring the accumulation of the reaction product formed in cell extracts of the transformed S. enterica AG701i50 mutant strain (Fig. 2B). Furthermore, we could demonstrate the substrate specificity for d-Ara-5-P in accordance with Brabetz et al. (2000): The tomato Kdo-8-P synthase could not accept d-erythrose-4-P or d-Rib-5-P as a substrate (Table I). On the contrary to their bacterial counterparts, Kdo-8-P synthase and 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase from tomato are not only two structurally (Gorlach et al., 1993) but also two functionally distinct enzymes. Finally, the biochemical characterization of the recombinant tomato Kdo-8-P synthase and its mutagenized forms indicated that despite the changes at the level of primary sequence, the overall structure of Kdo-8-P synthase has been conserved during evolution between prokaryotes and plants and, as a consequence, the enzymatic activity and mechanism of reaction (Table I; Fig. 5). The determination of the crystal structure of E. coli Kdo-8-P synthase provided information on the three-dimensional organization of the bacterial enzyme subunit, the interactions between subunits, the conformation of active sites, and the mechanism for the catalyzed reaction (Radaev et al., 2000; Wagner et al., 2000; Asojo et al., 2001). Mutations in the putative binding sites for the substrates totally inactivate (R61/T62 for binding of Ara-5-P) or greatly affect the enzyme activity (K136 for binding of PEP). Similarly, substituting H198, H242, and C164 in the tomato Kdo-8-P synthase greatly impairs the tomato enzyme activity. This suggests that these residues share the same function as their bacterial counterparts: H198 may behave as a general base in the catalytic mechanism, establishing salt bridges between its positive charge and a phosphate oxygen of PEP (Sheflyan et al., 1999; Howe et al., 2000; Asojo et al., 2001); H242 may be involved in the maintenance of the correct local environment at the active site (Sheflyan et al., 1999); and C164 may be required for the normal folding of the protein (Salleh et al., 1996).
Early development of tomato fruit offers an interesting model for studying plant organogenesis, particularly the regulation of cell division and cell expansion phenomena that appear to account for two distinct developmental phases (Gillaspy et al., 1993; Joubès et al., 1999). Thus, the spatiotemporal expression of LekdsA was investigated in the course of tomato fruit development. The expression of LekdsA is predominantly associated with cell proliferation (Fig. 3). We could demonstrate that the level of Kdo-8-P synthase transcripts decreased considerably during expansion-dependent cell differentiation because the fruit enlarges through cell expansion (Gillaspy et al., 1993; Joubès et al., 1999). This was especially true within the locular tissues, which are characterized by an extensive cell enlargement and hypervacuolarization, responsible for the jelly-like aspect of these tissues. Concomitant to the expansion process, the nuclear DNA content in cells from the locular tissue increases according to the endoreduplication process (Joubès et al., 1999). Because endoreduplication corresponds to a partial cell cycle in which mitosis is omitted (Joubès and Chevalier, 2000), genes expressed at the G2/M transition and in the M phase such as G2/M-specific CDKBs (Joubès et al., 2000a, 2001) and mitotic cyclins (Joubès et al., 2000b) are switched off. LekdsA transcripts are no more detected in the gel tissue after 15 DPA (Fig. 3B) similarly to the mRNAs corresponding to these mitotic-specific genes. Furthermore, the LekdsA gene expression decreased in the expanding cells of the hypocotyl of etiolated plantlets, thus confirming that it is specific of cell division (Fig. 4). Using tobacco BY-2 synchronized cells, we showed the kdsA gene expression to be cell cycle regulated with a strong peak of transcript accumulation at the M phase (Fig. 6). As expected from the kdsA gene expression data (Figs. 3, 4, and 6), the Kdo-8-P synthase activity was shown to be enhanced in dividing tissues (Fig. 5). However, we could not establish a correlation with the maximum of transcript accumulation and enzyme activity and the Kdo-8-P synthesis because we could not obtain significantly different values for Kdo-8-P production between dividing and expanding tissues on one hand (data not shown) and between the G2 phase and mitosis in BY-2 synchronized cells on the other hand (Fig. 7). This is probably due to the rapid conversion of free cytosolic Kdo into CMP-Kdo transported into the Golgi apparatus (Royo et al., 2000a), thus leading to the underestimation of Kdo production in our analyses.
Our data showing the preferential gene expression, enzyme activity, and presumably Kdo accumulation during cell division at the M phase of the cell cycle are consistent with the synthesis of the new primary cell wall and, thus, synthesis of pectins and mRG-II, occurring at the end of mitosis. Thus, at the mRNA level, the expression of LekdsA is down-regulated during cell expansion (Figs. 3 and 4); however, a slight Kdo-8-P synthase activity still persists in differentiating tissues as shown in Figure 5, i.e. in the absence of cell division. Cell growth involves the incorporation of new materials into the existing cell wall. This could be illustrated in Figure 6 because the kdsA gene seems to be re-induced in late G1 in synchronized BY-2 cells. Before entry in the S phase and subsequent mitosis, the cell must reach a critical size partly controlled at the G1/S cell cycle checkpoint (Jacobs, 1997; Polymenis and Schmidt, 1999). As a result, the synthesis of Kdo and mRG-II may be needed during G1 in association with cell growth before the commitment into the DNA replication process.
Recently, a pectin biosynthetic gene (NpGUT1) encoding a glucuronyltransferase has been discovered (Iwai et al., 2002). Disruption of this gene in T-DNA-tagged plants modifies the polysaccharidic composition of RG-II and causes instability of the RG-II dimer alike the Arabidopsis mur1 mutation (O'Neill et al., 2001). Although these two mutations do not affect the apiose residue actually involved in the binding of B, their physiological consequences magnify the importance of RG-II structure, and its B-mediated cross-linking in normal plant growth. Thus, the discovery of plant cDNA for Kdo-8-P synthases opens the way to manipulate the biosynthesis of Kdo in planta and offers the means to alter the RG-II glycosyl composition and investigate whether it may also affect growth.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Cherry tomato (Lycopersicon esculentum Mill. cv WV106) plants were grown in a growth chamber under a photoperiod of 15/9 h and a thermo-period of 25°C/20°C (day/night) with a light intensity of 400 μmol m-2 s-1. Tomato fruits were harvested at various developmental stages determined according to DPA and fruit diameter. Before protein or RNA extraction, fruits were frozen quickly in liquid nitrogen, ground to fine powders, and stored at -80°C.
Synchronization of Tobacco (Nicotiana tabacum L. cv BY-2) Cell Suspension Culture
Tobacco BY-2 suspension was cultured and synchronized with aphidicolin as described (Porceddu et al., 2001). Mitotic index was determined by UV light microscopic analysis of 500 cells stained with 0.1 μg mL-1 4′,6-diamino-2-phenylindole (Sigma, Saint Quentin Fallavier, France) in the presence of 0.2% (v/v) Triton X-100. Flow cytometry analysis of isolated nuclei from tobacco BY-2 protoplasts was performed as described (Porceddu et al., 2001) using a Flow cytometer (Partec GmbH, Münster, Germany).
cDNA Library Screening
Approximately 50,000 plaques from a `young tomato fruit' cDNA library (Joubès et al., 1999) were plated and transferred to nitrocellulose filters (Schleicher & Schull GmbH, Dassel, Germany). Duplicate membranes were differentially screened by hybridization to radioactively labeled probes prepared as described by Chevalier et al. (1996) from total cDNA of 3- to 6-DPA fruits (`division cDNA') and of 10- to 20-DPA fruits (`expansion cDNA'). Clones showing a signal only, or a stronger signal, with the `division cDNA' probe were selected and submitted to a second screening. Positive cDNAs clones were isolated and rescued from the UniZAP XR vector following the manufacturer's instructions (Stratagene, La Jolla, CA). Nucleotide sequences were then determined, translated in the six frames, and compared against the National Center for Biotechnology Information (Bethesda, MD) nonredundant protein database using the BLAST software (Altschul et al., 1997).
Plasmid Construction and Complementation Study in Salmonella enterica
All plasmid constructions were generated in Escherichia coli XL1-blue cells before transfer into S. enterica strains. The open reading frame for tomato Kdo-8-P synthase was PCR amplified from the LekdsA cDNA using the 5′ primer PET265 (CTCCTCGGATCCATGGAATCATCATCACTG) and the 3′ primer PET263 (CTCCTCCTCGAGCTAGTCACAAAATGGAGTG), respectively, introducing restriction sites for BamHI and XhoI. After digestion, the PCR product subsequently was ligated into BamHI-XhoI restricted pBluescript vector (Stratagene) to generate the pBXLED26 plasmid. The E. coli kdsA promoter was PCR amplified from E. coli genomic DNA using the 5′-specific primer CTCCTCGGATCCGAACATATGCTGGACG and the 3′-specific primer CTCCTCGGATCCGCCTTAATCTTGAG introducing BamHI restriction sites upstream of nucleotides 5,906 to 5,921 and downstream of nucleotides 6,287 to 6,300 of the promoter sequence (Strohmaier et al., 1995). This 395-bp DNA fragment comprised the -35 and -10 promoter boxes and the Shine-Dalgarno sequence at its 3′ end. It was recombined with the BamHI-linearized pBXLED26 plasmid generating plasmid pLEKdoS in which the tomato LekdsA open reading frame was placed under the control of the sense-positioned E. coli kdsA promoter.
Plasmid pLEKdoS and pBluescript used as a control were introduced into the wild-type S. enterica serovar Typhimurium AG701 strain and the kdsAts mutant S. enterica serovar Typhimurium AG701i50 strain by electroporation. Transformed bacteria were selected by growing overnight at 30°C on Luria-Bertani-agar plates containing ampicillin. Independent isolates were then cultured in liquid Luria-Bertani medium in the presence of ampicillin for 12 h at 30°C, further plated in duplicate, and incubated at either the restrictive temperature (42°C) or the permissive temperature (30°C). Complementation studies were performed by growing the recombinant AG701i50 mutant at 42°C to rescue a wild-type phenotype.
Site-Directed Mutagenesis of the Tomato Kdo-8-P Synthase
Site-directed mutagenesis of the tomato Kdo-8-P synthase was performed by PCR according to the protocol described by Cormack (2000). For each point mutation to be introduced, two specific and complementary oligonucleotides were designed (see Table II). In two separate PCR reactions using LekdsA as a template and Pfu Turbo DNA Polymerase (Stratagene), the 5′ primer on the coding strand and the 3′ primer on the complementary strand were used, respectively, in conjunction with PET263 and PET265. The two amplified fragments were then purified and mixed together to serve as templates in a new PCR reaction using PET265 and PET263. The mutagenized fragments resulting from amplification were then cloned in E. coli as described above and sequenced in their entirety to verify the presence of the intended nucleotide substitutions and the absence of unintentional mutations. The recombinant plasmids were then introduced in AG701 and AG701i50 S. enterica strains to test the functionality of the mutagenized tomato Kdo-8-P synthase.
Protein Extract Preparation and Kdo-8-P Synthase Enzymatic Assay
All procedures were carried out at 0°C to 4°C. Protein extracts from the different S. enterica strains were prepared according to Strohmaier et al. (1995). Protein extracts from tomato plant tissues (leaves and fruits at various developmental stages) were prepared as follows. Tomato plant tissues (1 g fresh weight) were frozen in liquid nitrogen, ground to a fine powder, and thawed in 4 mL of extraction buffer consisting of 50 mm Tris-HCl (pH 7.2), 10 mm dithiothreitol, 5% (w/v) polyvinylpolypyrroli-done, and supplemented with 100 μL of Protease Inhibitor Cocktail (Sigma; catalog no. P-2714). The mixture was homogenized by polytron for 30 s at moderate speed (setting 5-6) three times. The cell debris was discarded after a 10-min centrifugation at 25,000g, and the resulting supernatant was de-salted by G25-Sephadex column chromatography. The specific enzymatic activity of Kdo-8-P synthase was determined by the thiobarbituric acid assay as described by Ray (1980) using crude bacterial extracts of the different S. enterica strains or tomato plant tissue extracts.
Sugar Composition
Sugar compositions were determined by GC analysis of their trimethylsilyl methyl ester methyl glycoside derivatives according to York et al. (1985b) using inositol as internal standard. The gas chromatograph was equipped with a flame ionization detector, a WCOT fused silica capillary column (25-m length, 0.25-mm i.d., Chrompack, Raritan, NJ) with CP-Sil 5 CB as stationary phase and helium as gas vector. The oven temperature program was: 2 min at 120°C, 10°C min-1 to 160°C, 1.5°C min-1 to 220°C, and then 20°C min-1 to 280°C.
Quantification of Cytosolic Kdo
Five-milliliter samples were taken from the BY-2 tobacco cell suspension culture. Cells were harvested by filtration. After weighing, cells were suspended in 70% (v/v) ethanol and heated at 70°C for 15 min to inactivate enzymes. Cells were then ground in a potter homogenizer, and the homogenate was washed twice with hot 70% (v/v) ethanol at 70°C for 15 min. After centrifugation (5,000g for 5 min), the three ethanol fractions were combined, lyophilized, and then dissolved in 2 mL of water. This solution was purified by elution in water through a C18 Bond Elut cartridge (Varian, Sugarland, TX), lyophilized, and then treated with 200 μL of 2 m acetic acid at 80°C for 5 h to hydrolyze the Kdo-8-P and CMP-Kdo into free Kdo. The solution was neutralized with 200 μL of 1 m NH4OH before being dried again. To quantify the Kdo, 400 ng of neuraminic acid was added prior derivatization with DMB.
A 14 mm solution of DMB (Sigma) was prepared by dissolving DMB in an aqueous solution of 80 mm β-mercaptoethanol, 40 mm sodium hydro-sulphite, and 2.8 m acetic acid. The sample containing cytosolic monosaccharides and neuraminic acid was solubilized in 90 μL of deionized water and 90 μL of the DMB solution. The mixture was heated at 50°C for 2.5 h in the dark. Twenty microliters of the resulting solution was injected in a liquid chromatograph (Kontron, Milan) equipped with a reverse-phase C18 column (300 × 4.5 mm) and an SFM-25 fluorescence spectrophotometer (Kontron). Elution of the DMB derivatives was performed at a flow rate of 0.7 mL min-1 at room temperature using solvent A (acetonitrile:methanol:water, 4:6:90 [v/v]) and solvent B (acetonitrile:methanol:water, 11:7:82 [v/v]), with an A to B linear gradient from 50:50 to 0:100 (v/v) over 30 min. The DMB derivatives were detected by fluorescence using excitation and emission wavelengths of 373 and 448 nm, respectively.
Extraction of Total RNA and Expression Analysis
Total RNA from fruits and various organs of tomato plants or BY-2 synchronized cell suspensions was extracted as described (Chevalier et al., 1996). To determine the relative transcript levels of LekdsA, RT-PCR assays were performed using the following oligonucleotides: GGAGACATCTTCTCTGC as a 3′ primer (located within the 3′-untranslated region sequence, positions 965-981), combined with CTGCTTTCTTATGTAGGC as a 5′ primer (located within the coding sequence, positions 400-417). The RT-PCR protocol and cDNAs and primers used as controls were as described previously (Joubès et al., 2000b). A cDNA fragment corresponding to the tobacco Kdo-8-P synthase gene (NtkdsA) was isolated from synchronized BY-2 cells by RT-PCR using the tomato cDNA-derived oligonucleotides GTCGAGCTTTGACAAG as a 5′ primer and TCCATCTCCTCCTACAGCAACAG as a 3′ primer.
Acknowledgments
We wish to express our deepest thanks to Professor Gregor Högenauer (University of Graz, Austria) for providing the S. enterica AG701 and AG701i50 strains.
This work was supported by the Ministère de la Recherche et de la Technologie (France; grant no. 00 -512858 to F.D.), by the Region Aquitaine and Action Incitative Programmée Agraf-Institut National de la Recherche Agronomique (`Elaboration de la Qualité des Fruits' funding to Villenave d'Ornon), by the Centre National de la Recherche Scientifique, by the University of Rouen, and by Medicago Inc. (Quebec; funding to Mont Saint Aignan).
References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389-3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asojo O, Friedman J, Adir N, Belakhov V, Shoham Y, Baasov T (2001) Crystal structures of KDOP synthase in its binary complexes with the substrate phosphoenolpyruvate and with a mechanism-based inhibitor. Biochemistry 40: 6326-6334 [DOI] [PubMed] [Google Scholar]
- Becker B, Lommerse JPM, Melkonian M, Kamerling JP, Vliegenthart JFG (1995) The structure of an acidic trisaccharide component from a cell wall polysaccharide preparation of the green alga Tetraselmis striata Butcher. Carbohydr Res 267: 313-321 [Google Scholar]
- Brabetz W, Wolter FP, Brade H (2000) A cDNA encoding 3-deoxy-d-manno-oct-2-ulosate-8-phosphate synthase of Pisum sativum L. (pea) functionally complements a kdsA mutant of the Gram-negative bacterium Salmonella enterica. Planta 212: 136-143 [DOI] [PubMed] [Google Scholar]
- Chevalier C, Bourgeois E, Just D, Raymond P (1996) Metabolic regulation of asparagine synthetase gene expression in maize (Zea mays L.) root tips. Plant J 9: 1-11 [DOI] [PubMed] [Google Scholar]
- Cormack B (2000) Directed mutagenesis using the polymerase chain reaction. In FA Ausubel, R Brent, RE Kingston, DD Moore, JG Seidman, JA Smith, K Struhl, eds, Current Protocols in Molecular Biology. John Wiley & Sons, New York, pp 8.5.7-8.5.10
- Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13: 171-201 [DOI] [PubMed] [Google Scholar]
- Doong RL, Ahmad S, Jensen RA (1991) Higher plants express 3-deoxy-d-manno-octulosonate 8-phosphate synthase. Plant Cell Environ 14: 113-120 [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R (1999) The pore size of nongraminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol 121: 829-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer A, Titel C, Ehwald R (1998) The boron requirement and cell wall properties of growing and stationary suspension-cultured Chenopodium album L. cells. Plant Physiol 117: 1401-1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5: 1439-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach J, Beck A, Henstrand JM, Handa AK, Herrmann KM, Schmid J, Amrhein N (1993) Differential expression of tomato (Lycopersicon esculentum L.) genes encoding shikimate pathway isoenzymes: I. 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase. Plant Mol Biol 23: 697-706 [DOI] [PubMed] [Google Scholar]
- Hara S, Yamaguchi M, Takemori Y, Furuhata K, Ogura H, Nakamura M (1989) Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem 179: 162-166 [DOI] [PubMed] [Google Scholar]
- Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inzé D (1993) cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell 5: 1711-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DL, Duewel HS, Woodard W (2000) Histidine 268 in 3-deoxy-d-arabino-heptulosonic acid 7-phosphate synthase plays the same role as histidine 202 in 3-deoxy-d-manno-octulosonic acid 8-phosphate synthase. J Biol Chem 275: 40258-40265 [DOI] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T, Hayashi N (2001) Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol 126: 1698-1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T, Pellerin P, O'Neill MA, Darvill AG, Albersheim P (1999) The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J Biol Chem 274: 13098-13104 [DOI] [PubMed] [Google Scholar]
- Iwai H, Masaoka N, Ishii T, Satoh S (2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci USA 99: 16319-16324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs T (1997) Why do plant cells divide? Plant Cell 9: 1021-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubès J, Chevalier C (2000) Endoreduplication in higher plants. Plant Mol Biol 43: 737-747 [DOI] [PubMed] [Google Scholar]
- Joubès J, Chevalier C, Dudits D, Heberle-Bors E, Inzé D, Umeda M, Renaudin J-P (2000a) CDK-related protein kinases in plants. Plant Mol Biol 43: 607-620 [DOI] [PubMed] [Google Scholar]
- Joubès J, Lemaire-Chamley M, Delmas F, Walter J, Hernould M, Mouras A, Raymond P, Chevalier C (2001) A new C-type cyclin-dependent kinase from tomato expressed in dividing tissues does not interact with mitotic and G1 cyclins. Plant Physiol 126: 1403-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubès J, Phan T-H, Just D, Rothan C, Bergounioux C, Raymond P, Chevalier C (1999) Molecular and biochemical characterization of the involvement of cyclin-dependent kinase CDKA during the early development of tomato fruit. Plant Physiol 121: 857-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubès J, Walsh D, Raymond P, Chevalier C (2000b) Molecular characterization of the expression of distinct classes of cyclins during the early development of tomato fruit. Planta 211: 430-439 [DOI] [PubMed] [Google Scholar]
- Lehmann V, Rupprecht E, Osborn J (1977) Isolation of mutants conditionally blocked in the biosynthesis of the 3-deoxy-d-manno-octulosonic acid-lipid A part of liposaccharides derived from Salmonella typhimutirum. Eur J Biochem 76: 41-49 [DOI] [PubMed] [Google Scholar]
- Matoh T, Kawaguchi S, Kobayashi M (1996) Ubiquity of a borate-rhamnogalacturonan II complex in the cell walls higher plants. Plant Cell Physiol 37: 636-640 [Google Scholar]
- O'Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P (1996) Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem 271: 22923-22930 [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846-849 [DOI] [PubMed] [Google Scholar]
- Pérez S, Mazeau K, Hervé du Penhoat C (2000) The three-dimensional structures of the pectic polysaccharides. Plant Physiol Biochem 38: 37-55 [Google Scholar]
- Polymenis M, Schmidt EV (1999) Coordination of cell growth with cell division. Curr Opin Genet Dev 9: 76-80 [DOI] [PubMed] [Google Scholar]
- Porceddu A, Stals H, Reichheld J-P, Segers G, De Veylder L, de Pinho Barroco R, Casteels P, Van Montagu M, Inzé D, Mironov V (2001) A plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. J Biol Chem 276: 36354-36360 [DOI] [PubMed] [Google Scholar]
- Radaev S, Dastidar P, Patel M, Woodard RW, Gatti DL (2000) Structure and mechanism of 3-deoxy-d-manno-octulosonate-8-phosphate synthase. J Biol Chem 275: 9476-9484 [DOI] [PubMed] [Google Scholar]
- Ray PH (1980) Purification and characterization of 3-deoxy-d-mannooctulosonic 8-phosphate synthase from Escherichia coli. J Bacteriol 141: 635-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W-D, Chapple CCS, Sommerville CS (1993) Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science 261: 1032-1035 [DOI] [PubMed] [Google Scholar]
- Rick P (1987) Lipopolysaccharide biosynthesis. In FC Neidhardt, ed, Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Publishers, Washington, DC, pp 648-662
- Rick PD, Osborn MJ (1977) Lipid A mutants of Salmonella typhimurium. J Biol Chem 252: 4895-4903 [PubMed] [Google Scholar]
- Rick PD, Young DA (1982) Isolation and characterization of a temperature-sensitive lethal mutant of Salmonella typhimutirum that is conditionally defective in 3-deoxy-d-manno-octulosonate-8-phosphate synthesis. J Bacteriol 150: 447-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo J, Gomez E, Hueros G (2000a) A maize homologue of the bacterial CMP-Kdo synthetases: similar pathways operate in plants and bacteria for the activation of Kdo prior to its incorporation into cellular envelopes. J Biol Chem 275: 24993-24999 [DOI] [PubMed] [Google Scholar]
- Royo J, Gomez E, Hueros G (2000b) CMP-KDO synthetase, a plant gene borrowed from Gram-negative eubacteria. Trends Genet 16: 432-433 [DOI] [PubMed] [Google Scholar]
- Salleh HM, Patel MA, Woodard RW (1996) Essential cysteines in 3-deoxy-d-manno-octulosonic acid 8-phosphate synthase from Escherichia coli: analysis by chemical modification and site-directed mutagenesis. Biochemistry 35: 8942-8947 [DOI] [PubMed] [Google Scholar]
- Sheflyan GY. Duewel HS, Chen G, Woodard RW (1999) Identification of essential histidine residues in 3-deoxy-d-manno-octulosonic acid 8-phosphate synthase: analysis by chemical modification with diethyl pyrocarbonate and site-directed mutagenesis. Biochemistry 38: 14320-14329 [DOI] [PubMed] [Google Scholar]
- Strohmaier H, Remler P, Renner W, Högenauer G (1995) Expression of genes kdsA and kdsB involved in 3-deoxy-d-manno-octulosonic acid metabolism and biosynthesis of enterobacterial lipopolysaccharide is growth phase regulated primary at the transcriptional level in Escherichia coli K-12.J Bacteriol 177: 4488-4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma DSP (2002) Cytokinesis and building of the cell-plate in plants. Annu Rev Plant Physiol Plant Mol Biol 52: 751-784 [DOI] [PubMed] [Google Scholar]
- Wagner T, Kretsinger RH, Bauerle R, Tolbert WD (2000) 3-deoxy-d-manno-octulosonate-8-phosphate synthase from Escherichia coli: model of binding phosphoenolpyruvate and d-arabinose-5-phosphate. J Mol Biol 301: 233-238 [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9-27 [PubMed] [Google Scholar]
- Woisetschläger M, Högenauer G (1987) The kdsA gene coding for 3-deoxy-d-manno-octulosonic acid 8-phosphate synthase is part of an operon in Escherichia coli. Mol Gen Genet 20: 369-373 [DOI] [PubMed] [Google Scholar]
- York WS, Darvill AG, McNeil M, Albersheim P (1985a) 3-desoxy-d-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res 138: 109-126 [Google Scholar]
- York WS, Darvill AG, McNeil M, Albersheim P (1985b) Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol 118: 3-40 [Google Scholar]